Abstract
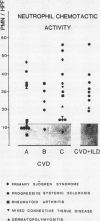
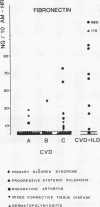
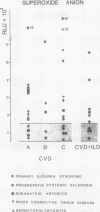
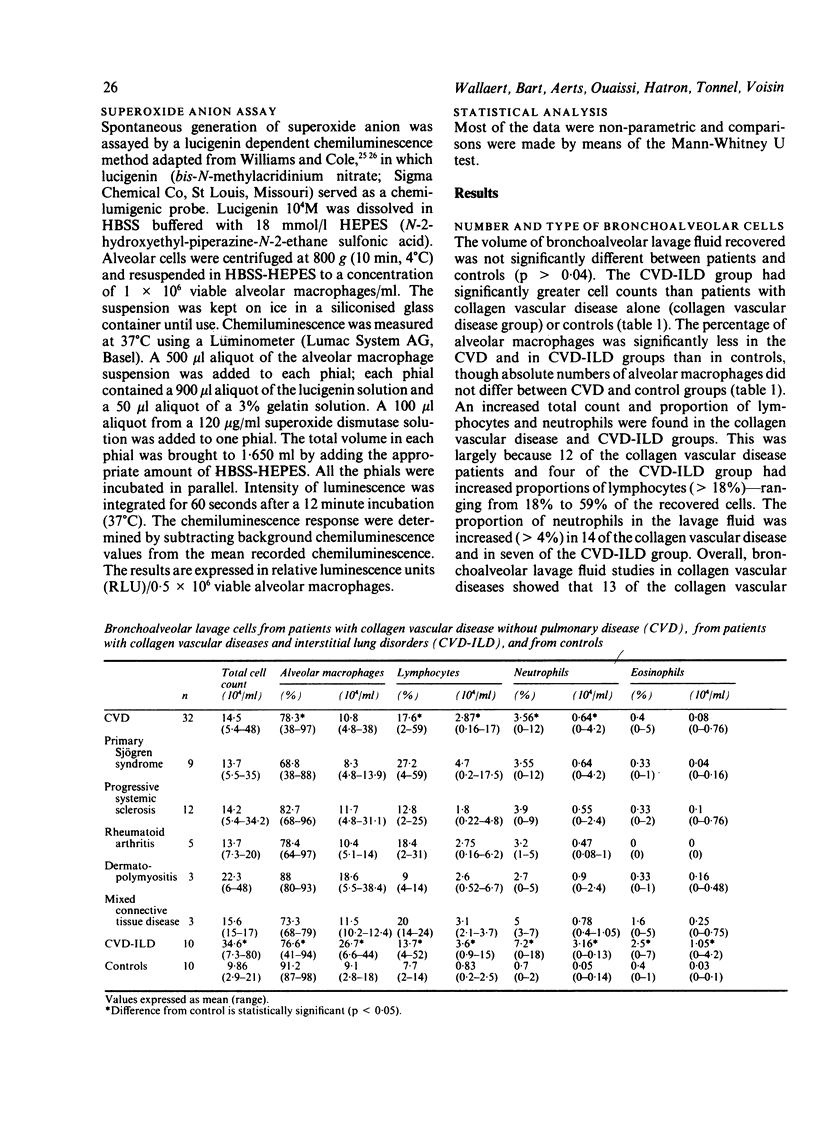
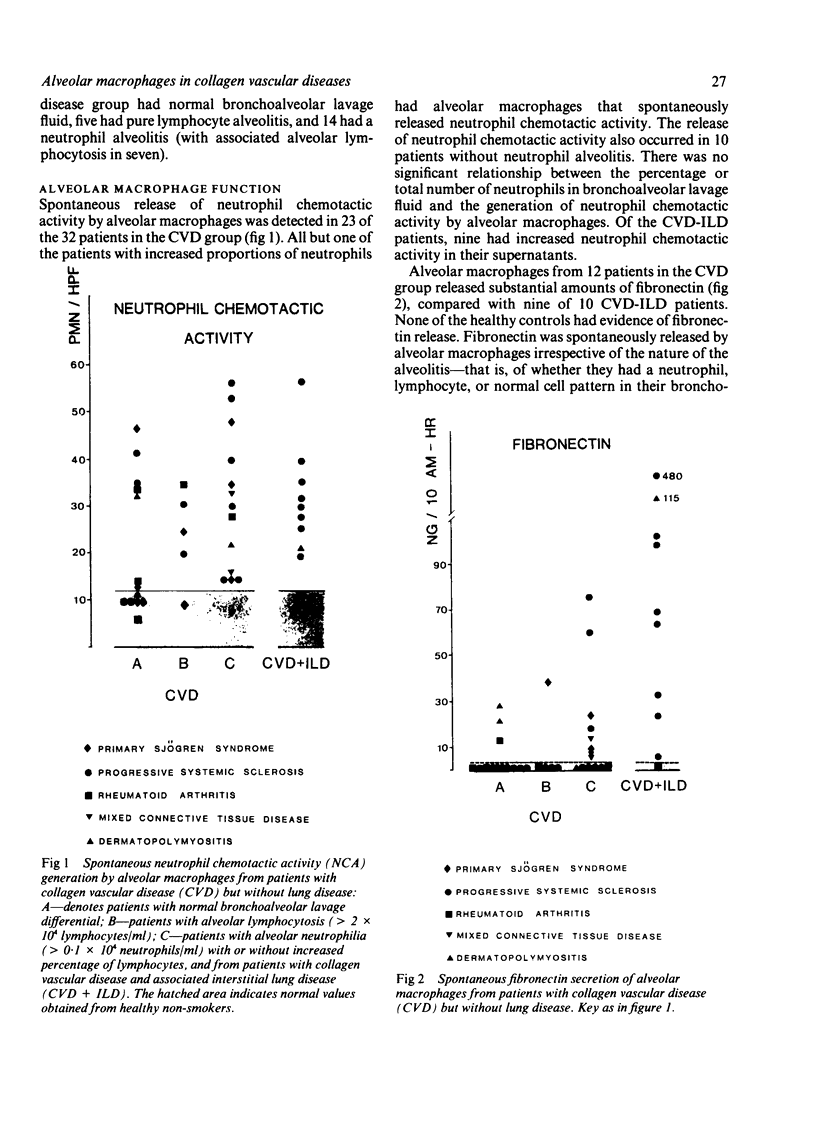
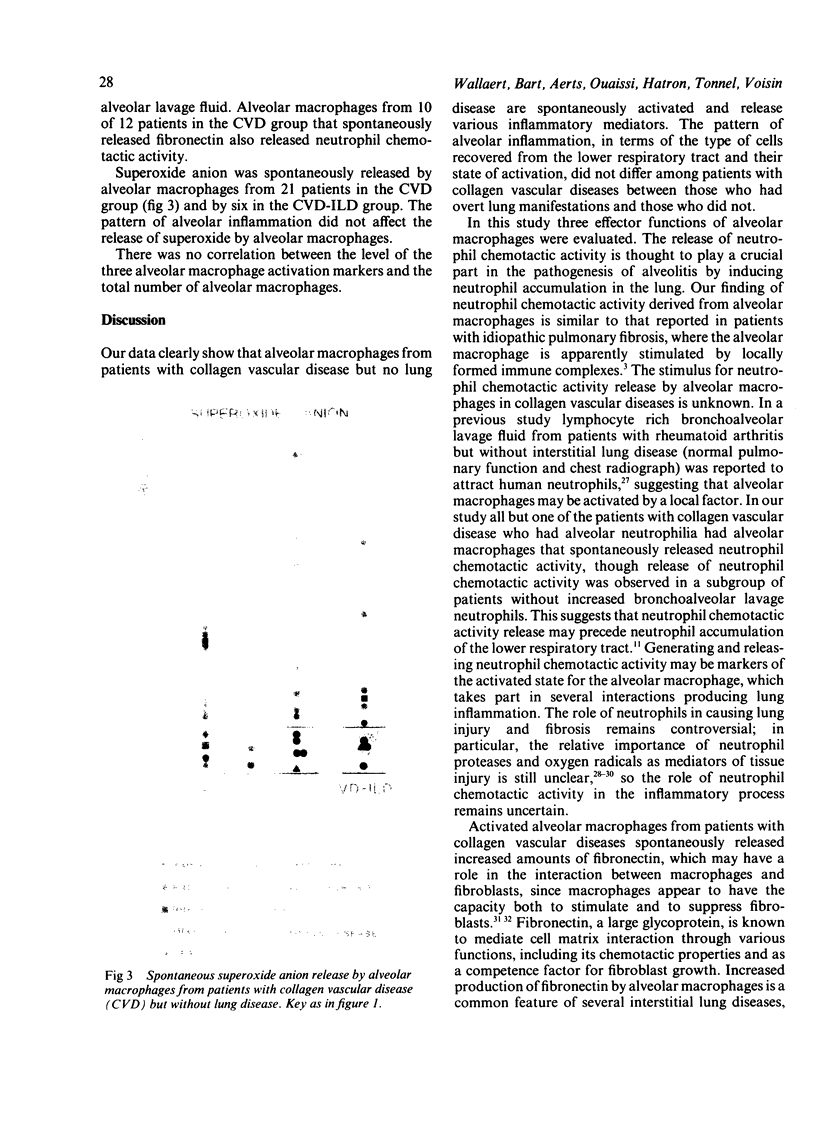
A study was initiated to determine whether alveolar macrophages from patients with collagen vascular diseases but free of pulmonary symptoms were spontaneously activated and whether they released various mediators related to the pathogenesis of pulmonary fibrosis. Alveolar macrophages obtained by bronchoalveolar lavage from 32 patients with proved collagen vascular disease but no evidence of lung disease were compared with those from 10 patients with collagen vascular disease with interstitial lung disease (CVD-ILD) and from 10 healthy controls. The total number of alveolar macrophages did not differ between patients with collagen vascular disease and controls but were substantially increased in the CVD-ILD group. Alveolar macrophages from 31 of the 32 patients with collagen vascular disease and from all 10 in the CVD-ILD group had at least one criterion of activation. Neutrophil chemotactic activity was detected in supernatants from alveolar macrophage culture in 23 of the 32 patients with collagen vascular disease and from nine of the 10 in the CVD-ILD group; fibronectin secretion by alveolar macrophages was increased in 12 of the 32 patients with collagen vascular disease and in nine of the 10 in the CVD-ILD group. Furthermore, alveolar macrophages from 20 of the 32 patients with collagen vascular disease and four of the 10 CVD-ILD patients spontaneously released increased amounts of superoxide anion. Thus alveolar macrophages were spontaneously activated in a high proportion of patients with collagen vascular disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts C., Wallaert B., Grosbois J. M., Voisin C. Release of superoxide anion by alveolar macrophages in pulmonary sarcoidosis. Ann N Y Acad Sci. 1986;465:193–200. doi: 10.1111/j.1749-6632.1986.tb18495.x. [DOI] [PubMed] [Google Scholar]
- Bird J., Pelletier M., Tissot M., Giroud J. P. The modification of the oxidative metabolism of cells derived both locally and at distance from the site of an acute inflammatory reaction. J Leukoc Biol. 1985 Jan;37(1):109–120. doi: 10.1002/jlb.37.1.109. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Keogh B. A., Wewers M. D., Adelberg S., Crystal R. G. Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med. 1986 May 22;314(21):1343–1347. doi: 10.1056/NEJM198605223142103. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Rossman M. D., Zurier R. B., Daniele R. P. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985 Jan;131(1):94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Parhami N., Killam D., Garcia P. L., Keogh B. A. Bronchoalveolar lavage fluid evaluation in rheumatoid arthritis. Am Rev Respir Dis. 1986 Mar;133(3):450–454. doi: 10.1164/arrd.1986.133.3.450. [DOI] [PubMed] [Google Scholar]
- Gellert A. R., Macey M. G., Uthayakumar S., Newland A. C., Rudd R. M. Lymphocyte subpopulations in bronchoalveolar lavage fluid in asbestos workers. Am Rev Respir Dis. 1985 Oct;132(4):824–828. doi: 10.1164/arrd.1985.132.4.824. [DOI] [PubMed] [Google Scholar]
- Gerberick G. F., Jaffe H. A., Willoughby J. B., Willoughby W. F. Relationships between pulmonary inflammation, plasma transudation, and oxygen metabolite secretion by alveolar macrophages. J Immunol. 1986 Jul 1;137(1):114–121. [PubMed] [Google Scholar]
- Gerberick G. F., Willoughby J. B., Willoughby W. F. Serum factor requirement for reactive oxygen intermediate release by rabbit alveolar macrophages. J Exp Med. 1985 Feb 1;161(2):392–408. doi: 10.1084/jem.161.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud J. P., Sheng Y. C., Pelletier M., Florentin I., Bird J. Acute non-specific inflammation and modification of macrophage and lymphocyte functions. Br J Dermatol. 1983 Jul;109 (Suppl 25):41–54. doi: 10.1111/j.1365-2133.1983.tb06817.x. [DOI] [PubMed] [Google Scholar]
- Gosset P., Tonnel A. B., Joseph M., Prin L., Mallart A., Charon J., Capron A. Secretion of a chemotactic factor for neutrophils and eosinophils by alveolar macrophages from asthmatic patients. J Allergy Clin Immunol. 1984 Dec;74(6):827–834. doi: 10.1016/0091-6749(84)90186-6. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis. 1979 Mar;119(3):471–503. doi: 10.1164/arrd.1979.119.3.471. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M., Tonnel A. B., Torpier G., Capron A., Arnoux B., Benveniste J. Involvement of immunoglobulin E in the secretory processes of alveolar macrophages from asthmatic patients. J Clin Invest. 1983 Feb;71(2):221–230. doi: 10.1172/JCI110762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh B. A., Crystal R. G. Alveolitis: the key to the interstitial lung disorders. Thorax. 1982 Jan;37(1):1–10. doi: 10.1136/thx.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Elias J. A., Bashey R. I., Jimenez S. A., Daniele R. P. The regulation of lung fibroblast proliferation by alveolar macrophages in experimental silicosis. Am Rev Respir Dis. 1984 May;129(5):767–771. doi: 10.1164/arrd.1984.129.5.767. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Chused T. M., Mann D. L., Klippel J. H., Fauci A. S., Frank M. M., Lawley T. J., Hamburger M. I. Sjögren's syndrome (Sicca syndrome): current issues. Ann Intern Med. 1980 Feb;92(2 Pt 1):212–226. doi: 10.7326/0003-4819-92-2-212. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaissi M. A., Neyrinck J. L., Capron A. Development of a competitive radioimmunoassay for human plasma fibronectin. Int Arch Allergy Appl Immunol. 1986;81(1):75–80. doi: 10.1159/000234111. [DOI] [PubMed] [Google Scholar]
- Owens G. R., Paradis I. L., Gryzan S., Medsger T. A., Jr, Follansbee W. P., Klein H. A., Dauber J. H. Role of inflammation in the lung disease of systemic sclerosis: comparison with idiopathic pulmonary fibrosis. J Lab Clin Med. 1986 Mar;107(3):253–260. [PubMed] [Google Scholar]
- Pesci A., Bertorelli G., Manganelli P., Ambanelli U. Bronchoalveolar lavage analysis of interstitial lung disease in CREST syndrome. Clin Exp Rheumatol. 1986 Apr-Jun;4(2):121–124. [PubMed] [Google Scholar]
- Rossi G. A., Bitterman P. B., Rennard S. I., Ferrans V. J., Crystal R. G. Evidence for chronic inflammation as a component of the interstitial lung disease associated with progressive systemic sclerosis. Am Rev Respir Dis. 1985 Apr;131(4):612–617. doi: 10.1164/arrd.1985.131.4.612. [DOI] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Snider G. L. Interstitial pulmonary fibrosis--which cell is the culprit? Am Rev Respir Dis. 1983 May;127(5):535–539. doi: 10.1164/arrd.1983.127.5.535. [DOI] [PubMed] [Google Scholar]
- Solal-Céligny P., Laviolette M., Hébert J., Cormier Y. Immune reactions in the lungs of asymptomatic dairy farmers. Am Rev Respir Dis. 1982 Dec;126(6):964–967. doi: 10.1164/arrd.1982.126.6.964. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Phan S. H., McCormick J. R., Ward P. A. The development of bleomycin-induced pulmonary fibrosis in neutrophil-depleted and complement-depleted rats. Am J Pathol. 1981 Oct;105(1):76–81. [PMC free article] [PubMed] [Google Scholar]
- Wallaert B., Colombel J. F., Tonnel A. B., Bonniere P., Cortot A., Paris J. C., Voisin C. Evidence of lymphocyte alveolitis in Crohn's disease. Chest. 1985 Mar;87(3):363–367. doi: 10.1378/chest.87.3.363. [DOI] [PubMed] [Google Scholar]
- Wallaert B., Hatron P. Y., Grosbois J. M., Tonnel A. B., Devulder B., Voisin C. Subclinical pulmonary involvement in collagen-vascular diseases assessed by bronchoalveolar lavage. Relationship between alveolitis and subsequent changes in lung function. Am Rev Respir Dis. 1986 Apr;133(4):574–580. doi: 10.1164/arrd.1986.133.4.574. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Investigation of alveolar macrophage function using lucigenin-dependent chemiluminescence. Thorax. 1981 Nov;36(11):866–869. doi: 10.1136/thx.36.11.866. [DOI] [PMC free article] [PubMed] [Google Scholar]