Abstract
Untreated HIV disease is associated with chronic immune activation and CD4+ T cell depletion. A variety of mechanisms have been invoked to account for CD4+ T cell depletion in this context, but the quantitative contributions of these proposed mechanisms over time remains unclear. We turned to the DO11.10 TCR transgenic (tg) mouse model, where OVA is recognized in the context of H-2d, to explore the impact of chronic antigenic stimulation on CD4+ T cell dynamics. To model dichotomous states of persistent antigen exposure in the presence or absence of proinflammatory stimulation, we administered OVA peptide (OVAp) to these mice on a continuous basis with or without the prototypic proinflammatory cytokine, interleukin 1β (IL-1β). In both cases, circulating antigen-specific and non-specific CD4+ T cells were depleted. However, in the absence of IL-1β, there was limited proliferation and effector/memory conversion of antigen-specific T cells, depletion of peripheral CD4+ T cells in hematolymphoid organs, and systemic induction of regulatory FoxP3+CD4+ T cells, as often observed in late-stage HIV disease. By contrast, when OVAp was administered in the presence of IL-1β, effector/memory phenotype T cells expanded and the typical symptoms of heightened immune activation were observed. Acknowledging the imperfect and incomplete relationship between antigen-stimulated DO11.10 TCR tg mice and HIV-infected humans, our data suggest that CD4+ T cell depletion in the setting of HIV disease may reflect, at least in part, chronic antigen exposure in the absence of proinflammatory signals and/or appropriate antigen-presenting cell functions.
INTRODUCTION
Persistent immune activation is a defining characteristic of HIV infection, both in the case of untreated and treated disease (1–3). Although the causes of such immune activation are not fully understood, they are thought to reflect changes in the mucosal barrier of the gut (4), and to underlie the loss of CD4+ T cells in untreated HIV-infected individuals (1–3) as well as the lack of full CD4+ T cell reconstitution during antiretroviral therapy (ART) (5, 6). However, given the intrinsic difficulties associated with longitudinal studies of hematolymphoid organs in humans, the impact, relative contribution, and fundamental definition of “activation” in the context of HIV disease remain unclear.
To better clarify the role of immune activation in HIV-mediated CD4+ T cell depletion, we turned to the DO11.10 TCR transgenic (tg) mouse model, in which >60% of peripheral CD4+ T cells express a transgenic TCR that recognizes OVA323–339 peptide (OVAp) in the context of H-2d. We reasoned that continuous administration of OVAp to these animals might, to a certain degree, mimic the state of chronic antigen exposure found in HIV-infected humans. Accordingly, we conducted a careful analysis of T cell production and destruction across a full range of phenotypic subsets in multiple hematolymphoid organs, and quantified the fractional representation and absolute numbers of such cells as a function of time, comparing the effects of continuous antigen exposure in the presence or absence of proinflammatory stimulation provided by interleukin (IL)-1β to recapitulate chronic activation of the innate immune system (7–9). We observed CD4+ T cell loss in the peripheral blood with ongoing exposure to OVAp, whether or not IL-1β was provided concomitantly. In the absence of IL-1β, however, we found a state of T cell depletion analogous to that observed in HIV-infected individuals, with limited expansion of effector memory T cells, depletion of CD4+ T cells in hematolymphoid organs, and induction of regulatory T cells (TREGS). These results are discussed with respect to the known and inferred pathophysiological mechanisms implicated in untreated and treated HIV disease.
MATERIALS & METHODS
Mice
Male and female OVA TCR tg mice (DO11.10) (10), 6–12 weeks of age at the beginning of each experiment, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in the mouse facility at the University of California, San Francisco (UCSF). All data shown are from female mice aged six weeks. As these mice are not bred on a RAG−/− background, they have a variable (2–32%) fraction of non-OVA-specific CD4+ T cells, dependent on age and location; lower fractions are present in younger mice and in peripheral lymph nodes (5–10% of CD4+ T cells) than in older mice and in the spleen (7–15% of CD4+ T cells). All experiments and procedures were approved by the UCSF Institutional Animal Care and Use Committee.
Procedures
Mice were studied longitudinally for up to seven weeks. Blood was acquired at varying time points by phlebotomy of the saphenous vein (without anesthetic). Surgery was performed under general anesthesia, using ketamine/xylazine (Wyeth, Madison, NJ, USA and Lloyd Labs Inc., Shenandoah, IA, USA). Mice were given buprenorphine (Reckitt & Colman Pharmaceuticals Inc., Richmond, VA, USA) post-operatively for pain relief. Alzet® mini-osmotic pumps (Durect Corporation, Cupertino, CA, USA) containing PBS alone, OVA323–339 peptide (ISQAVHAAHAEINEAGR; Biopeptide, San Diego, CA, USA) in PBS, or OVA323–339 peptide together with murine recombinant IL-1β (PeproTech Inc., Rocky Hill, NJ, USA) in PBS were placed subcutaneously (s.c.) between the scapulae of DO11.10 mice. Both 14-day (model 1002) and 28-day (model 2004) pumps were used, each releasing 0.2–0.25 µl/h of PBS or a solution containing either 0.25–1.25 µg/µl OVAp in PBS (for all data shown, the concentration of OVAp was 1.25 µg/µl and the total amount of peptide released per day was 6.6–7 µg) or 0.25–1.25 µg/µl OVAp in PBS together with IL-1β (10 µg IL-1β / pump model 1002 and 20 µg IL-1β / pump model 2004 providing approximately 0.6 µg IL-1β / day). Pumps were equilibrated in sterile PBS according to the manufacturer’s instructions for up to 48 h prior to implantation. For cross-sectional analyses of lymphoid tissue, mice were sacrificed by CO2 asphyxiation followed by cervical dislocation. Thymus, spleen, and lymph nodes (inguinal, brachial, and axillary) were harvested and passed through mesh filters in sterile PBS with 2% FBS to obtain single cell suspensions. The absolute number of cells per organ was determined using a Coulter Counter (Beckman Coulter, Fullerton, CA, USA).
Flow cytometry
Cells from heparinized blood, lymph nodes, spleen, and thymus were stained for flow cytometric analyses using combinations of the following antibodies: (i) anti-CD4-Alexa Fluor 700 (clone L3T4), anti-CD4-allophycocyanin-Cy7 (clone L3T4), anti-CD25-PE (clone PC61), anti-CD25-allophycocyanin-Cy7 (clone PC61), anti-Ki67-FITC (clone B56), and mouse FcBlock™ (purified rat anti-mouse CD16/CD32; clone 2.4G2) (BD Pharmingen, San Jose, CA, USA); (ii) anti-CD8-Pacific Blue (clone 5H10) and anti-TCR DO11.10-allophycocyanin (clone KJ1–26) (Caltag Laboratories, Burlingame, CA, USA); and (iii) anti-FoxP3-PE (clone FJK-16s), anti-CD62L–PE-Cy7 (clone MEL-14), and anti-CD44-PE-Cy5.5 (clone IM7) (eBioscience, San Diego, CA, USA). The Live/DEAD® fixable Aqua Dead Cell Stain Kit for 405 nm excitation (Molecular Probes/Invitrogen, Eugene, Oregon, USA) was used in conjunction with Annexin V to discriminate between live, apoptotic, and dead cells. TruCount tubes (BD Biosciences, San Jose, CA, USA) were used according to the manufacturer’s instructions to measure absolute peripheral blood T cell numbers. Cells were fixed after staining with either BD™ Lysing Solution (BD Biosciences, San Jose, CA, USA) for TruCount analysis or with PBS containing 2% FBS and 1% paraformaldehyde for flow cytometric analysis. For intracellular staining, cells were permeabilized with BD™ Phosflow Perm/Wash Buffer 1 (BD Biosciences, San Jose, CA, USA), which contains saponin to avoid cell fixation. Using this buffer, the incubation time for intracellular FoxP3 detection could be reduced to 1 h instead of overnight. Cells for sorting remained unfixed. Events were analyzed and/or sort-purified using an LSRII, a Digital Vantage, or a BD FACSAria™ flow cytometer (BD Biosciences, San Jose, CA, USA). Frequencies of OVA-specific (KJ1–26+) CD4+ T cell subsets were determined using FlowJo software (Tree Star Inc., Ashland, OR, USA).
In vivo T cell turnover
To measure the proliferation rate and lifespan of OVAp-stimulated T cells in vivo, mice with similar weight distributions across all three groups (Suppl. Fig. 1A) received a loading dose of 2H2O (Cambridge Isotope Laboratories Inc., Andover, MA, USA) to reach 5% body water enrichment at the time of pump implantation, followed by 8% 2H2O orally (ad libitum; to reach 5% body water enrichment) for a total of three days. This three-day administration protocol was designed to label C-H bonds in the deoxyribose moiety of replicating DNA in dividing cells, as described previously (11). Re-utilization of labeled 2H-deoxyribose for new DNA synthesis in purine deoxyribonucleotides is minimal (11, 12). Accordingly, this protocol results in pulse-chase incorporation of label into replicating DNA in vivo and allows accurate measurement of the lifespan of labeled cells. At days 3, 6, 12, and 20 after pump implantation, three mice per group were euthanized. Thymuses, lymph nodes, and spleens were harvested after blood collection from the saphenous vein. Cells were counted and stained as above, and naive (TN: CD62LhighCD44low) and effector memory (TEM: CD62LlowCD44high) OVA-specific CD4+ T cells (Suppl. Fig. 1B) were sort-purified using separated gates (Suppl. Fig. 2A) so that 2H-labeling in DNA could be measured by mass spectrometry. All analyzed cell populations were ≥98% pure (Suppl. Fig. 2B).
Measurement and analysis of 2H enrichment in T cell DNA
The stable isotope-based method for measuring T cell proliferation has been described in detail previously (12–14). Additional precautions and controls required for working with low cell numbers have also been developed (15). DNA from sorted cell subsets was extracted using a QIAmp DNA Micro Kit (Qiagen, Valencia, CA, USA) and hydrolyzed according to standard protocols (12–15). Deoxyribonucleosides were derivatized using pentafluorobenzyl hydroxylamine (PFBHA) solution (Sigma-Aldrich, St. Louis, MO, USA). Analysis of the derivatized deoxyribose was performed by quadrupole gas chromatography/mass spectrometry (GC/MS, Agilent 5873/6980) in negative chemical ionization mode (NCI), with helium as the carrier and methane as the reagent gas, using a DB-17 or DB-225 column (J&W Scientific, Agilent, Foster City, CA, USA). The parent ion of the pentafluoro tri-acetate (PFTA) derivative of deoxyribose has a mass-to-charge ratio (m/z) of 435 and the M1 mass isotopomer has an m/z of 436. The mole fraction of the M1 mass isotopomer was calculated as the ratio of peak areas, M1/(M0 + M1). Label incorporation in experimental samples was measured as the excess mole fraction of M1 (EM1) above the M1 mole fraction in abundance-matched unlabeled standards: [M1/(M0 + M1)sample] – [M1/(M0 + M1)standard]. The value of f, the fraction of newly synthesized DNA strands or, equivalently, the fraction of newly divided cells, was calculated as EM1/EM1*, where EM1* represents the maximal or asymptotic EM1 enrichment possible in 100% newly divided cells (13, 14). EM1* values were calculated from measured 2H enrichments in bone marrow cells (12–14).
Calculations of the fraction of labeled cells and the number of labeled cells per organ
The fraction (f) of labeled cells present in each organ was measured as described above. The absolute number of labeled cells per organ was calculated by multiplying the fraction of labeled cells by the total number of cells for each phenotype as determined by flow cytometry.
Statistical analysis
The two-tailed Mann-Whitney U test was used to evaluate differences between two groups. A two-way repeated measures ANOVA with the Tukey post-test to correct for multiple comparisons was used to evaluate differences across more than two groups.
RESULTS
Continuous antigenic stimulation in the presence or absence of IL-1β results in loss of circulating T cells
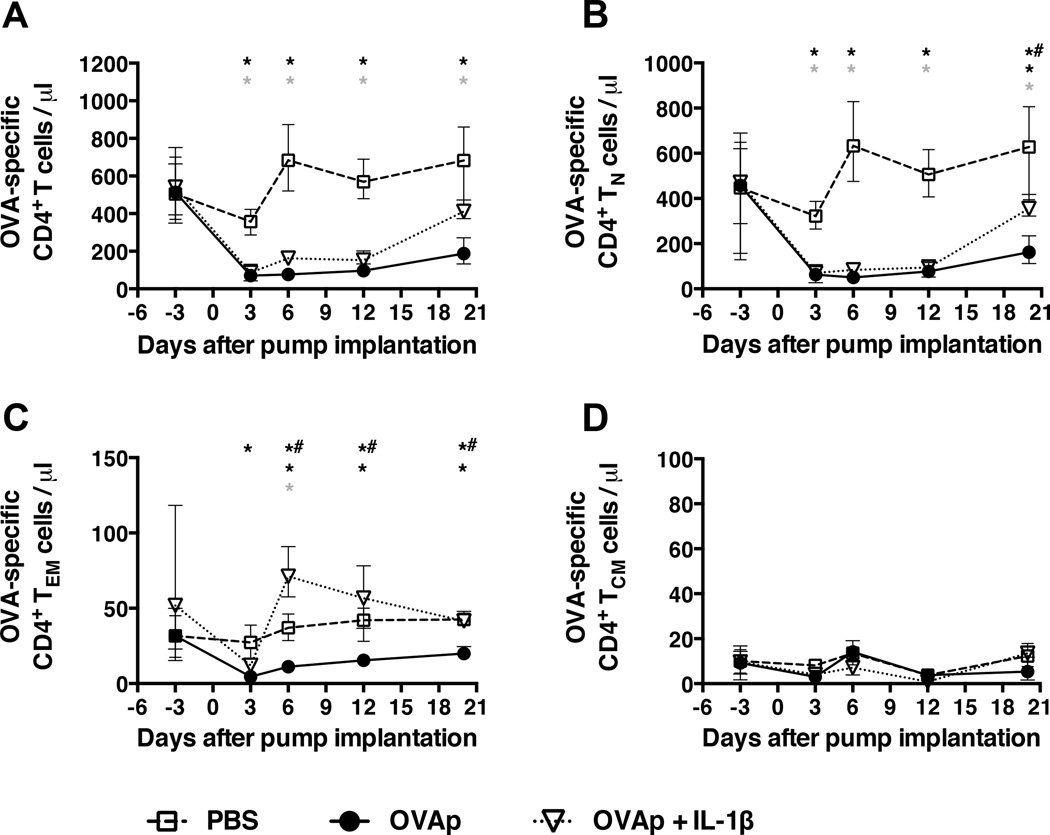
The DO11.10 TCR transgenic mouse model was used to evaluate the effects of chronic antigenic stimulation on the CD4+ T cell compartment. These mice have a large (>60%) peripheral pool of CD4+ T cells that recognize OVA323–339 peptide in the context of H-2d. OVAp was administered over a period of 20 days via s.c. mini-osmotic pumps delivering ∼6.8 µg peptide/day. Absolute numbers of OVA-specific CD4+ T cells in the peripheral blood dropped precipitously and significantly (from ∼400 cells/µl to <100 cells/µl) by day 3, and remained low for the duration of the experiment (Fig. 1A). By contrast, only a small transient dip was observed in control mice, reflecting surgical pump implantation. These patterns held across all experiments despite batch-to-batch variability in absolute cell counts (Suppl. Fig. 3). Concomitant declines were observed in the circulating OVA-specific CD4+ TN and TEM subsets (Figs. 1B and 1C), while the corresponding central memory (TCM) cells remained numerically stable (Fig. 1D).
Figure 1. Continuous antigenic stimulation in the presence or absence of IL-1β results in loss of circulating CD4+ T cells.
Mini-osmotic pumps containing either PBS, OVAp in PBS, or OVAp together with IL-1β in PBS were implanted s.c. between the scapulae of DO11.10 TCR tg mice and, at the indicated time points, the numbers of (A) OVA-specific (KJ1–26+) CD4+ T cells, (B) OVA-specific CD4+ TN cells, (C) OVA-specific CD4+ TEM cells, and (D) OVA-specific CD4+ TCM cells were assessed per µl of peripheral blood using Trucount tubes (BD Biosciences). Data shown are from three mice per group per time point, and are representative of three similar experiments for the PBS and OVAp groups (Suppl. Fig. 3). Shown are means and ranges. *, p<0.05 PBS versus OVAp; grey asterix, p<0.05 PBS versus OVAp + IL-1β; *#, p<0.05 OVAp versus OVAp + IL-1β. Statistical analysis was conducted using a two-way ANOVA with the Tukey post-test to correct for multiple comparisons.
As this profound decline in peripheral CD4+ T cell numbers could reflect insufficient activation of antigen-specific cells by peptide stimulation alone, we co-administered IL-1β with OVAp in a separate group of mice. IL-1β was chosen as the adjuvant for these experiments because it can be delivered continuously by s.c. mini-osmotic pumps, and because it is known to enhance the antigen-driven differentiation and expansion of CD4+ T cells in this mode (9). It also acts downstream of aluminum-containing adjuvants, which are routinely used and licensed for use in human vaccines (16). Again, a rapid and significant drop in circulating OVA-specific CD4+ T cells was observed by day 3, paralleled by a decline in the corresponding TN cell subset (Figs. 1A and 1B). In contrast to mice receiving OVAp alone, however, OVA-specific CD4+ TEM cell numbers increased significantly at day 6, and then declined by day 12 to the levels observed in control mice (Fig. 1C). No changes were observed in OVA-specific TCM cell numbers (Fig. 1D). Continuous s.c. administration of cognate peptide therefore depletes circulating OVA-specific CD4+ T cells in the presence or absence of IL-1β, which is nevertheless required to observe an increase in the corresponding TEM cell subset.
T cell depletion in the spleen and lymph nodes only occurs in the absence of IL-1β
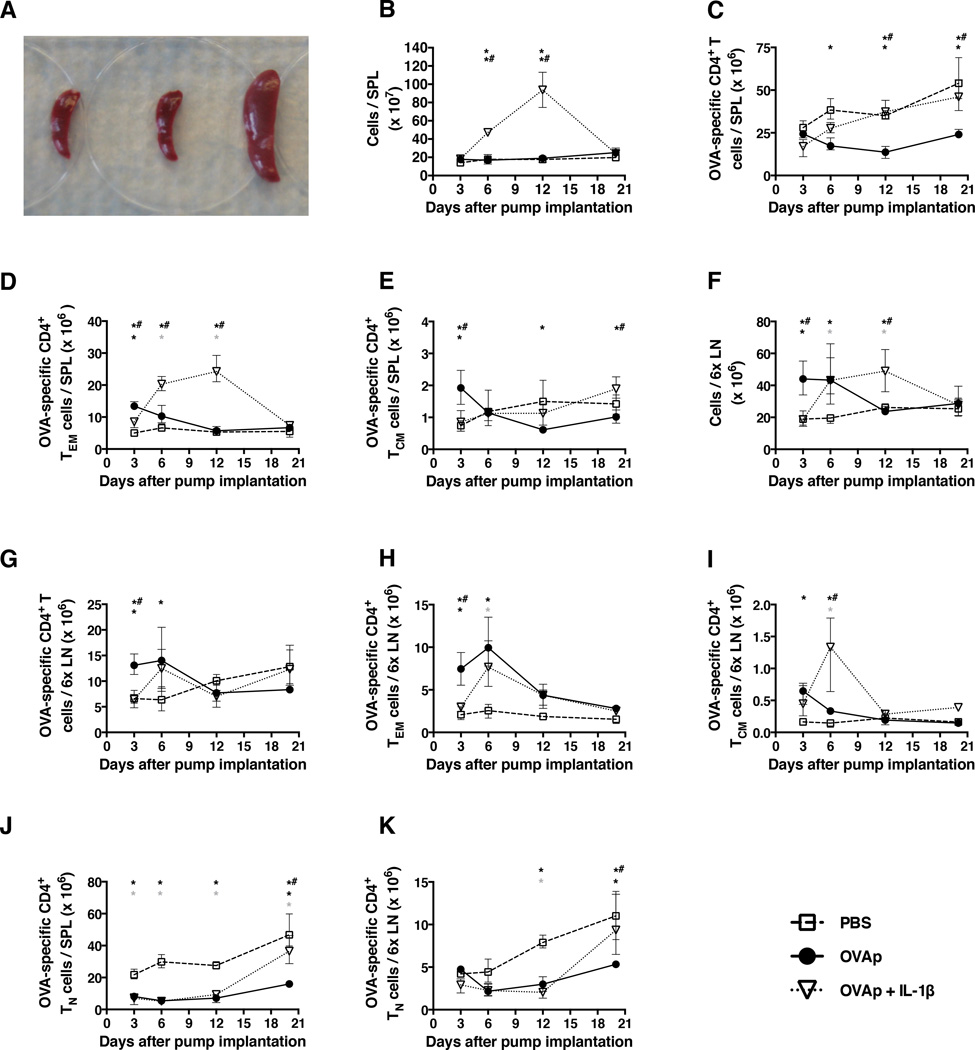
As circulating T cells are thought to comprise only a small fraction (<2–3%) of the total body T cell compartment, we examined the impact of chronic antigenic stimulation on CD4+ T cell subsets in the spleen and peripheral lymph nodes (LNs). The latter comprised pooled cells from the two brachial, axillary, and inguinal chains in each mouse. OVAp was administered with or without IL-1β via s.c. mini-osmotic pumps and mice were euthanized for tissue harvest at days 3, 6, 12, and 20 (n=3 mice/group/time point). Splenomegaly was evident when OVAp was delivered in the presence but not the absence of IL-1β (Fig. 2A), reflecting a statistically significant five-fold increase in the total number of cells per spleen (Fig. 2B). Many (35–40%) but not all of these cells were OVA-specific CD4+ T cells (Fig. 2C) of the TEM phenotype (Fig. 2D). TCM cell numbers remained low, but appeared to increase between day 12 and day 20 (Fig. 2E). At day 20, TCM cell numbers were significantly higher in the spleens of mice receiving OVAp with IL-1β compared to those receiving OVAp alone (Fig. 2E). Of note, the content-releasing ends of the mini-osmotic pumps were expelled after day 12 in mice that received OVAp with IL-1β, which may explain why CD4+ T cell numbers in lymphoid organs normalized to some extent in these mice by day 20. Data comparisons at the final time point are therefore strictly valid only for control mice versus those receiving OVAp in the absence of IL-1β.
Figure 2. CD4+ T cell depletion in the spleen and lymph nodes only occurs in the absence of IL-1β.
Mice were implanted with mini-osmotic pumps as per the legend for Fig. 1. (A) Representative spleens from PBS (far left), OVAp (middle), and OVAp with IL-1β mice collected at day 12. Splenomegaly was already observed at day 6 in mice receiving OVAp with IL-1β. The spleens are shown on a 6-well tissue culture plate. Numbers of (B) spleen cells, (C) OVA-specific CD4+ T cells per spleen, (D) OVA-specific CD4+ TEM cells per spleen, (E) OVA-specific CD4+ TCM cells per spleen, (F) pooled peripheral LNs (2x brachial, 2x axillary, 2x inguinal) cells, (G) OVA-specific CD4+ T cells per peripheral LN pool, (H) OVA-specific CD4+ TEM cells per peripheral LN pool, (I) OVA-specific CD4+ TCM cells per peripheral LN pool, (J) OVA-specific CD4+ TN cells per spleen, and (K) OVA-specific CD4+ TN cells per peripheral LN pool. Data shown are from the same experiment shown in Fig. 1. Shown are means and ranges. *, p<0.05 PBS versus OVAp; grey asterix, p<0.05 PBS versus OVAp + IL-1β; *#, p<0.05 OVAp versus OVAp + IL-1β. Statistical analysis was conducted using a two-way ANOVA with the Tukey post-test to correct for multiple comparisons.
Similar patterns were observed with pooled peripheral LNs from mice receiving OVAp and IL-1β, with a statistically significant increase in total cell numbers by days 6–12 (Fig. 2F), of which about 25% were OVA-specific CD4+ T cells (Fig. 2G). These cells were especially enriched for the TEM and TCM phenotypes at day 6 (Fig. 2H and 2I), whereas mice receiving OVAp alone had higher numbers of antigen-specific TEM cells at day 3 compared to those receiving IL-1β in addition (Fig. 2H). Administration of either OVAp alone or OVAp with IL-1β led to a decrease in OVA-specific TN cells in the spleen and peripheral LNs, although a degree of recovery was noted at day 20 (Fig. 2J and 2K).
Collectively, these observations underscore the fact that depletion of OVA-specific CD4+ T cells in the peripheral blood is not reflective of cell numbers and/or composition in the lymphoid organs. Co-administration of OVAp with IL-1β caused a durable increase in OVA-specific CD4+ TEM cells in the spleen and peripheral LNs, while administration of OVAp alone induced only a transient increase in OVA-specific CD4+ TEM cells in the peripheral LNs.
Loss of thymic function does not explain changes in the peripheral OVA-specific CD4+ T cell compartment
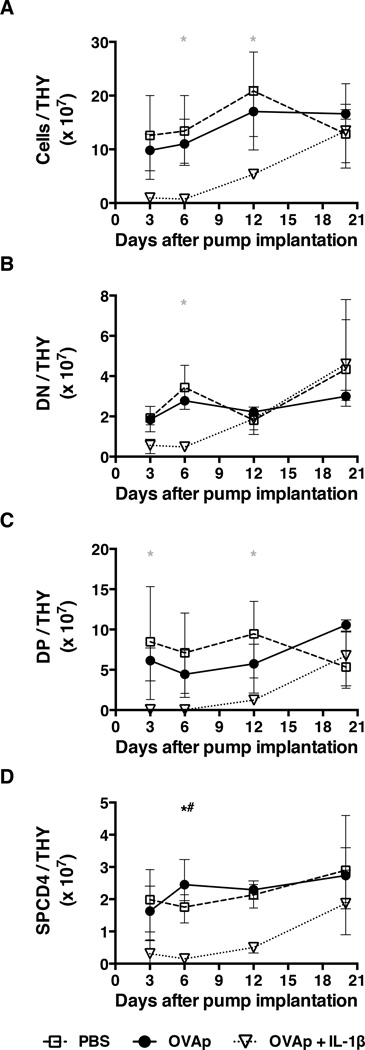
Given previous reports that administration of cognate peptide to TCR tg mice induces antigen-specific thymocyte depletion (10), we studied the impact of chronic OVAp exposure on thymocyte populations over time. During the same period that administration of OVAp alone resulted in a rapid loss of circulating CD4+ T cells (Fig. 1A), the total numbers of thymocytes (Fig. 3A) and of CD4−CD8− (DN), CD4+CD8+ (DP), and CD4+CD8− (SPCD4) thymocytes (Figs. 3B–3D) remained unchanged. By contrast, at a time (days 3–12) when mice receiving OVAp and IL-1β demonstrated an increase in OVA-specific CD4+ T cells in the spleen and peripheral LNs, there was evidence of thymocyte depletion (Figs. 3A–3D). Although it is not clear how such depletion occurred, these observations reveal no consistent association between the cellularity of the thymus and the number and/or composition of OVA-specific CD4+ T cells in the periphery.
Figure 3. Loss of thymic function does not explain changes in the peripheral OVA-specific CD4+ T cell compartment.
Mice were implanted with mini-osmotic pumps as per the legend for Fig. 1. At four time points thereafter, thymuses (THY) were removed from individual mice to count the numbers of (A) total cells, (B) DN thymocytes, (C) DP thymocytes, and (D) SPCD4 thymocytes. Data shown are from the same experiment shown in Fig. 1. Shown are means and ranges. *, p<0.05 PBS versus OVAp; grey asterix, p<0.05 PBS versus OVAp + IL-1β; *#, p<0.05 OVAp versus OVAp + IL-1β. Statistical analysis was conducted using a two-way ANOVA with the Tukey post-test to correct for multiple comparisons.
The kinetics of FoxP3+CD4+ TREG induction are similar but not identical in the presence or absence of IL-1β
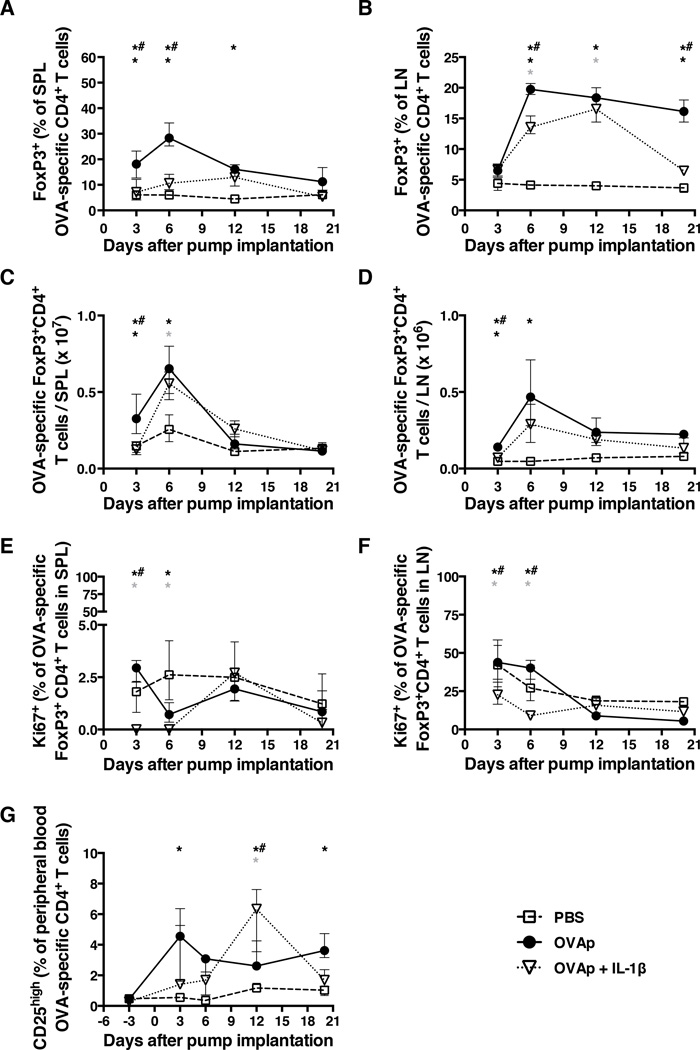
As FoxP3+CD4+ TREGS may influence peripheral CD4+ T cell homeostasis (17–19), we evaluated the fraction and absolute number of such cells as a function of time (representative flow cytometric data are shown in Suppl. Fig. 1C). In the spleen, an early (day 3–6) and significant increase in the proportion of OVA-specific (KJ1–26+) TREGS was observed in mice receiving OVAp alone, but not in those receiving OVAp with IL-1β (Fig. 4A). Likewise, the proportion of TREGS in pooled peripheral LNs increased significantly at an early time point (day 6), more markedly and for a longer period of time (out to day 20) in mice receiving OVAp alone compared to those receiving IL-1β in addition (Fig. 4B). When plotted as numbers per organ, these TREG increases in the spleen and peripheral LNs were equivalent in the presence or absence IL-1β (Figs. 4C and 4D). The fractional TREG increase in the spleens of mice receiving OVAp alone compared to those receiving OVAp and IL-1β was preceded by increased proliferation of TREGS as measured by intracellular Ki67 staining (Fig. 4E and Suppl. Fig. 1D). The frequency of OVA-specific TREGS expressing Ki67 in the peripheral LNs was also higher at early time points (days 3 and 6) in mice receiving OVAp alone compared to those receiving OVAp and IL-1β (Fig. 4F). Moreover, a persistent fractional increase in peripheral OVA-specific CD25highCD4+ T cells was observed in mice receiving OVAp alone, whereas the frequency of these cells increased only transiently at day 12 in mice receiving OVAp with IL-1β (Figs. 4G). These data reveal important differences in the proportional representation and proliferation of OVA-specific TREGS induced by administration of OVAp in the presence or absence of IL-1β, and suggest that TREG induction in mice treated with OVAp alone may contribute to OVA-specific CD4+ T cell depletion in the periphery.
Figure 4. The kinetics of FoxP3+CD4+ TREG induction are similar but non-identical in the presence or absence of IL-1β.
Mice were implanted with mini-osmotic pumps as per the legend for Fig. 1. At four time points thereafter, peripheral blood and the indicated tissues were collected to determine (A) the frequency of OVA-specific FoxP3+CD4+ T cells in the spleen, (B) the frequency of OVA-specific FoxP3+CD4+ T cells in peripheral LNs, (C) the number of OVA-specific FoxP3+CD4+ T cells in the spleen, (D) the number of OVA-specific FoxP3+CD4+ T cells in peripheral LNs, (E) the frequency of OVA-specific FoxP3+CD4+ T cells expressing Ki67 in the spleen, (F) the frequency of OVA-specific FoxP3+CD4+ T cells expressing Ki67 in peripheral LNs, and (G) the frequency of OVA-specific CD25highCD4+ T cells in the peripheral blood. Data shown are from the same experiment shown in Fig. 1. Shown are means and ranges. *, p<0.05 PBS versus OVAp; grey asterix, p<0.05 PBS versus OVAp + IL-1β; *#, p<0.05 OVAp versus OVAp + IL-1β. Statistical analysis was conducted using a two-way ANOVA with the Tukey post-test to correct for multiple comparisons.
Continuous antigenic stimulation induces OVA-specific CD4+ T cells to enter cell cycle and divide
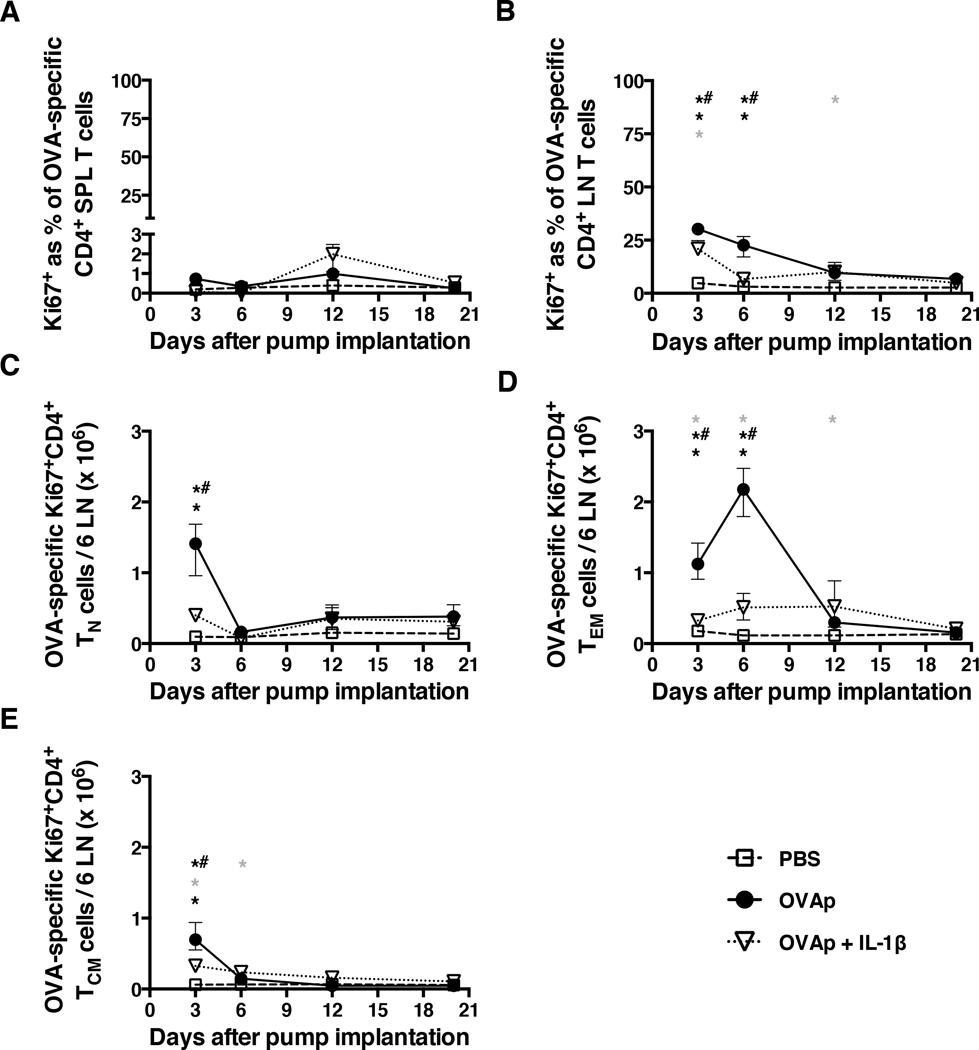
To test the possibility that co-administration of IL-1β might induce increased OVA-specific CD4+ T cell proliferation, cells were examined for expression of Ki67. Counterintuitively, Ki67 expression by OVA-specific CD4+ T cells in the spleen was very low (≤ 3%) in all groups of mice (Fig. 5A). At days 3 and 6, Ki67 expression by OVA-specific CD4+ T cells in the peripheral LNs was highest in mice receiving OVAp alone (Fig. 5B). High levels of Ki67 expression in the same compartment were also observed at day 3 in mice receiving OVAp with IL-1β but these were not sustained and fell steeply to baseline levels by day 6 (Fig. 5B). In the spleen, too few Ki67+ OVA-specific CD4+ TN, TEM, and TCM cells were detected to allow reliable quantification within each phenotypic subset. Sufficient cell yields were obtained from the peripheral LNs, however, and the number of Ki67+ OVA-specific CD4+ TN cells was found to be significantly elevated at day 3 in mice receiving OVAp (Fig. 5C). At days 3 and 6, the number of Ki67+ OVA-specific CD4+ TEM cells in the peripheral LNs was dramatically increased in mice receiving OVAp alone (Fig. 5D). There was a small but significant increase in the corresponding cells at days 3, 6, and 12 in mice receiving OVAp with IL-1β (Fig. 5D). The number of Ki67+ OVA-specific CD4+ TCM cells increased at day 3 in mice receiving OVAp with or without IL-1β (Fig. 5E). This increase was smaller than that observed for OVA-specific CD4+ TN cells (Fig. 5C) and fell by day 6 close to baseline levels present in mice receiving PBS (Fig. 5E). The expression of Ki67, a putative marker of cell division, was therefore higher overall in OVA-specific CD4+ T cell populations from mice receiving OVAp alone.
Figure 5. Continuous antigenic stimulation induces OVA-specific CD4+ T cells to enter cell cycle.
Mice were implanted with mini-osmotic pumps as per the legend for Fig. 1. At the indicated time points, spleens and peripheral LNs were collected to determine (A) the percentage of Ki67+ OVA-specific CD4+ T cells in the spleen, (B) the percentage of Ki67+ OVA-specific CD4+ T cells in peripheral LNs, (C) the number of Ki67+ OVA-specific CD4+ TN cells in peripheral LNs, (D) the number of Ki67+ OVA-specific CD4+ TEM cells in peripheral LNs, and (E) the number of Ki67+ OVA-specific CD4+ TCM cells in peripheral LNs. Data shown are from the same experiment shown in Figure 1. Shown are means and ranges. *, p<0.05 PBS versus OVAp; grey asterix, p<0.05 PBS versus OVAp + IL-1β; *#, p<0.05 OVAp versus OVAp + IL-1β. Statistical analysis was conducted using a two-way ANOVA with the Tukey post-test to correct for multiple comparisons.
In vivo proliferation of OVA-specific CD4+ TN and TEM cells is higher in mice receiving OVAp in the presence of IL-1β compared to those receiving OVAp alone
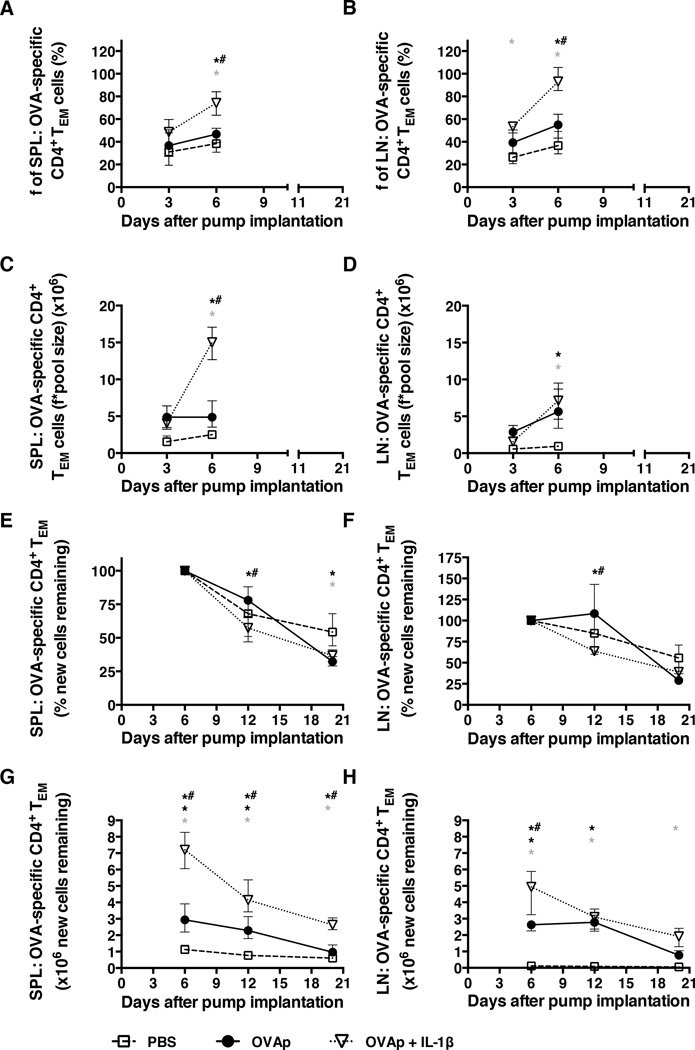
As Ki67 expression only signifies movement into the cell cycle, mice were loaded with deuterated water (2H2O) to enable the in vivo measurement of new DNA synthesis (and, hence, cell division). OVA-specific CD4+ TEM (CD62LlowCD44high) and TN (CD62LhighCD44low) cells were then sort-purified from spleen or pooled peripheral LNs at multiple time points, and 2H-enriched DNA was quantified by GC-MS. Bone marrow cell 2H enrichments were determined as a reference (Suppl. Fig. 4E) and used to calculate the fraction of labeled cells. OVA-specific CD4+ TCM cells were not sorted, as they constituted a very small population (Fig. 1D, 2E and 2I). Although OVAp alone increased the proliferation of OVA-specific CD4+ TEM cells compared to mice receiving PBS, a significantly higher proportion of these cells in the spleen and peripheral LNs incorporated 2H by day 6 in mice receiving OVAp with IL-1β (Fig. 6A and 6B). In each organ, this cell division led to a statistically significant increase in the total number of newly divided TEM cells (Figs. 6C and 6D). As this is one of the cell populations that increased most markedly over time (Figs. 2D and 2H), it seems likely that this difference can be attributed to the induction of proliferation by IL-1β.
Figure 6. In vivo proliferation of OVA-specific TEM cells is higher in mice receiving OVAp and IL-1β compared to those receiving OVAp alone.
After an intraperitoneal loading dose of 2H2O to reach 5% body water enrichment, mice were labeled orally with 2H2O provided in drinking water for the first 72 h after mini-osmotic pump implantation as per the legend for Fig. 1. (A) The fraction of labeled OVA-specific CD4+ TEM cells in the spleen. (B) The fraction of labeled OVA-specific CD4+ TEM cells in peripheral LNs. (C) The number of labeled OVA-specific CD4+ TEM cells in the spleen. (D) The number of labeled OVA-specific CD4+ TEM cells in peripheral LNs. (E) The percentage of new OVA-specific CD4+ TEM cells remaining in the spleen. (F) The percentage of new OVA-specific CD4+ TEM cells remaining in peripheral LNs. (G) The number of new OVA-specific CD4+ TEM cells remaining in the spleen. (H) The number of new OVA-specific CD4+ TEM cells remaining in peripheral LNs. Data shown are from the same experiment shown in Fig. 1. Shown are means and ranges. *, p<0.05 PBS versus OVAp; grey asterix, p<0.05 PBS versus OVAp + IL-1β; *#, p<0.05 OVAp versus OVAp + IL-1β. Statistical analysis was conducted using a two-way ANOVA with the Tukey post-test to correct for multiple comparisons.
Reciprocally, the administration of OVAp in the absence of IL-1β may trigger movement into the cell cycle, as evidenced by increased Ki67 expression in peripheral LN-resident TEM cells (Figure 5D), but only limited proliferation. In support of this model, the percentage and number of new TEM cells remaining did not decrease between days 6 and 12 in the peripheral LNs of mice receiving OVAp alone (Fig. 6F and 6H), and the decrease in labeled TEM cells in the spleen appeared to be less than that observed in control mice receiving PBS (Fig. 6E and 6G). These data are consistent with a lack of input from unlabeled, newly divided cells between days 6 and 12. Concomitantly, labeled TEM cells in the spleen and peripheral LNs declined to a significantly greater extent in mice receiving OVAp with IL-1β (Fig. 6E and 6F), indicating continued input of newly divided, unlabeled cells with IL-1β treatment. Moreover, at days 12–20, a loss of total labeled TEM cells in the spleen and peripheral LNs was observed both in mice receiving OVAp alone and in mice receiving OVAp with IL-1β (Fig. 6G and 6H), indicating death of newly divided cells consistent with the dissipation of IL-1β effects and normalization of cell counts subsequent to pump expulsion in the latter group by day 12. 2H enrichments in TN cells from the peripheral LNs were similar and increased at day 3 together with the fraction of labeled TN cells in both the OVAp alone and OVAp with IL-1β groups (Suppl. Fig. 4A and 4C). By contrast, the fraction of labeled TN cells in the spleen did not increase significantly despite apparent 2H enrichments at day 3 both in mice receiving OVAp alone and in mice receiving OVAp with IL-1β (Suppl. Fig. 4B and 4D).
As expected, the overall fractions of labeled TN cells were lower than the corresponding fractions of labeled TEM cells (Fig. 6A and 6B, Suppl. Fig. 4C and 4D). The increase in the fraction of labeled TN cells in the peripheral LNs was still apparent at day 6 in mice receiving OVAp with IL-1β (Suppl. Fig. 4C). As the number of TN cells decreased, it is most likely that they converted to TEM cells with further division. 2H incorporation in TN cells from mice receiving PBS alone increased over time (Suppl. Fig. 4A and 4C). Similar findings have been reported for TN cells derived from human peripheral blood (20), almost certainly reflecting slow division rates that lead to the progressive accumulation of labeled cells in this compartment.
Collectively, these kinetic results reinforce the cell count findings and show that de novo cell division in this model depends on the presence of both OVAp and IL-1β.
DISCUSSION
One of the unanswered questions regarding the pathogenesis of HIV infection is whether chronic antigenic stimulation per se leads to CD4+ T cell depletion. Here, we provide data gathered from a mouse model suggesting that antigen-specific CD4+ T cells are indeed lost during a period of continuous antigenic exposure, but that such loss may only occur within hematolymphoid organs when antigen is provided without engagement of important direct and indirect contributions from the innate immune system (e.g., those induced by concomitant administration of the prototypic proinflammatory cytokine IL-1β. We chose DO11.10 TCR tg mice for these experiments because they harbor high frequencies of CD4+ T cells that recognize OVA in the context of H-2d. This trait allowed a systematic study of circulating and tissue-resident CD4+ T cells responding to a defined antigenic stimulus over time. Using implanted s.c. mini-osmotic pumps, these mice were exposed continuously to OVA323–339 peptide (OVAp) for 20 days, with or without IL-1β. In both instances, OVA-specific CD4+ T cell counts dropped in the peripheral blood. In the presence of IL-1β, however, such depletion of the circulating CD4+ T cell pool was accompanied by a marked and significant increase in the absolute number of OVA-specific CD4+ T cells found in the spleen, which in turn was associated with a higher absolute number of dividing TEM cells (as evidenced by increased incorporation of deuterated water into newly synthesized DNA). By contrast, mice receiving OVAp in the absence of IL-1β showed no such increase in the absolute number of OVA-specific CD4+ T cells in the spleen (although they did show a transient increase in LN cellularity due to an increase in OVA-specific CD4+ T cells, mostly of the TEM phenotype). Cell division was initiated in mice receiving OVAp alone, as evidenced by increased Ki67 expression and deuterium incorporation, but this did not lead to an increase in OVA-specific CD4+ TEM cell numbers in the spleen. These changes in T cell homeostasis were accompanied by evidence of greater FoxP3+ TREG induction in mice receiving OVAp in the absence of IL-1β. In sum, the presence of IL-1β was associated with cell division and an increase in the absolute number of OVA-specific CD4+ TEM cells, even though CD4+ T cell depletion was observed in the peripheral blood. By contrast, continuous exposure to antigen in the absence of innate immune activation resulted in conditions most analogous to those found in the setting of HIV disease. Notably, none of these changes were easily relatable to the size and function of the thymus in these young adult mice.
Substantial differences clearly exist between antigen-stimulated DO11.10 TCR tg mice and HIV-infected humans. In particular, most of the CD4+ T cells lost in the context of HIV infection are not specific for the virus, which itself is a major cause of the observed immunopathology (21). Moreover, a range of mechanisms beyond direct viral cytopathogenicity likely contribute to the process of CD4+ T cell depletion in HIV-infected individuals, including the diverse effects of other proinflammatory cytokines (e.g., IFNα, TNF) (22, 23), type I interferons (24), and microbial translocation (25, 26), as well as alternative forms of cell death (e.g., pyroptosis) (27). These multifactorial causes of CD4+ T cell lymphopenia are not recapitulated in DO11.10 TCR tg mice. Nonetheless, this simplified model allowed an unfettered evaluation of CD4+ T cell dynamics in the presence of continuous antigenic stimulation. Acknowledging the many caveats, how might our findings relate to the disposition and fate of CD4+ T cells in the setting of HIV disease?
It has long been noted that the number of circulating CD4+ T cells declines inexorably as a function of disease progression in most HIV-infected individuals, providing a remarkably accurate barometer for the ultimate susceptibility of the patient to opportunistic infections and, historically, a definition of “AIDS” when the circulating CD4+ T cell count falls below 200 cells/µl (28, 29). However, it remains unclear whether and to what extent the circulating CD4+ T cell count reflects the number of CD4+ T cells found within fixed hematolymphoid compartments. Indeed, lymphadenopathy is a pathognomonic clinical sign of progressive disease (30) and early radiographic studies noted evidence of abdominal lymphadenopathy in HIV-infected patients as well (31), suggesting that CD4+ T cells are sequestered to lymphoid tissues. Alternatively, or in addition, CD4+ T cells may be lost from the circulation due to: (i) loss of progenitor activity in the bone marrow and/or thymus; (ii) loss of homeostatic mechanisms that normally sustain T cell proliferation in the periphery; (iii) sequestration/redistribution of CD4+ T cells into extravascular spaces that are not usually assessed in HIV-infected individuals; (iv) killing of HIV-infected CD4+ T cells by HIV-specific cytotoxic CD8+ T cells; and (v) killing of “bystander” CD4+ T cells by unknown mechanisms (32). Finally, although there have been numerous studies over the past 20 years to document the proliferation and activation status of T cells in the setting of HIV disease (33), there are scant quantitative or qualitative data showing that “activated” cells are actually moving through multiple rounds of proliferation. Thus, the fraction of cells positive for Ki67 (or other markers of cell division) has been found to be high at times when the circulating CD4+ T cell count is low, leading to the inference that CD4+ T cell division is high (34, 35). On the other, the absolute number of CD4+ T cells positive for Ki67 or incorporating deuterated glucose is inappropriately low in late-stage disease (13), suggesting a block on cell division instead.
Our observations in the OVA TCR tg model raise several interesting possibilities that might be pertinent to the pathophysiology of HIV disease. First, it is clear that continuous antigenic exposure results in depletion of circulating CD4+ T cells in this model, whether or not IL-1β is present. Yet, it is only in the absence of IL-1β that total body CD4+ T cell loss is observed. Such loss is associated with evidence that cells can begin to move through the cell cycle (i.e., express Ki67) but that they then do not divide (i.e., incorporate 2H into new strands of DNA), as has been observed in the circulating cells of HIV-infected subjects by Sieg and colleagues (36). At the same time, there is a relative increase in CD4+ TREGS, as has also been observed in those infected with HIV (37). In the presence of IL-1β, by contrast, T cell division is enhanced in the spleen and peripheral LNs, consistent with a qualitatively distinct mechanism of T cell homeostasis.
As the difference between the two states is related to the presence or absence of IL-1β, it is tempting to speculate that continuous antigen exposure in the absence of IL-1β (or, more generally, co-stimulation) leads to total body CD4+ T cell depletion. In this scenario, both the cytopathic effects of HIV on CD4+ T cells and the deleterious impact of HIV infection on CD4+ myeloid cells (including antigen-presenting cells) capable of IL-1β production (38) might cumulatively lead to altered homeostasis of the T cell lineage, with less cell division over time. Although further work is clearly required to extend these findings to humans (e.g., in studies examining IL-1β blockade in the context of treated HIV disease) (39), our data suggest that interventions designed to restore innate immunity in general (or the production of IL-1β in particular) might help to reinstate complete T cell immunity in patients treated with ART.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank George Chkhenkeli and José Rivera for technical help, Cheryl Stoddart, Sofiya Galkina, and Mary Beth Moreno for advice and resource provision, and Marty Bigos, Tomasz Poplonski, Valerie Stepps and Ck Poon for flow cytometry services.
Abbreviations used in this paper
- TN
naive T cells
- TCM
central memory T cells
- TEM
effector memory T cells
- TREG
regulatory T cells
- tg
transgenic
- AICD
activation-induced cell death
- THY
thymus
- SPL
spleen
- LNs
lymph nodes
Footnotes
This work was supported in part by National Institutes of Health Grant RO1 AI43866 (to M.K.H.), European Molecular Biology Organization Grant ALTF 254–2002 (to M.D.H.), and National Institutes of Health Awards U01 AI43641 and R37 AI40312 (to J.M.M.), who is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research and the National Institutes of Health Director’s Pioneer Award, part of the National Institutes of Health Roadmap for Medical Research, through Grant DPI OD00329. Funding for the optimization of low-count techniques by mass spectrometry was provided by KineMed Inc. (Emeryville, CA, USA). D.A.P. is a Wellcome Trust Senior Investigator. The Clinical and Translational Science Institute Clinical Research Center was supported by Grant UL1 RR024131-01 from the National Center for Research Resources, a component of the National Institutes of Health, and the National Institutes of Health Roadmap for Medical Research.
REFERENCES
- 1.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 2.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi JV. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 3.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 6.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, Crane HM, Willig J, Mugavero M, Saag M, Martin JN, Deeks SG. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48:787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 11.Macallan DC, Fullerton CA, Neese RA, Haddock K, Park SS, Hellerstein MK. Measurement of cell proliferation by labeling of DNA with stable isotope-labeled glucose: studies in vitro, in animals, and in humans. Proc Natl Acad Sci U S A. 1998;95:708–713. doi: 10.1073/pnas.95.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCune JM, Hanley MB, Cesar D, Halvorsen R, Hoh R, Schmidt D, Wieder E, Deeks S, Siler S, Neese R, Hellerstein M. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest. 2000;105:R1–R8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune JM. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 14.Neese RA, Siler SQ, Cesar D, Antelo F, Lee D, Misell L, Patel K, Tehrani S, Shah P, Hellerstein MK. Advances in the stable isotope-mass spectrometric measurement of DNA synthesis and cell proliferation. Anal Biochem. 2001;298:189–195. doi: 10.1006/abio.2001.5375. [DOI] [PubMed] [Google Scholar]
- 15.Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc. 2007;2:3045–3057. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- 16.Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25:4575–4585. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 18.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 19.Dahlberg PE, Schartner JM, Timmel A, Seroogy CM. Daily subcutaneous injections of peptide induce CD4+ CD25+ T regulatory cells. Clin Exp Immunol. 2007;149:226–234. doi: 10.1111/j.1365-2249.2007.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellerstein MK, Hoh RA, Hanley MB, Cesar D, Lee D, Neese RA, McCune JM. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J Clin Invest. 2003;112:956–966. doi: 10.1172/JCI17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating SM, Jacobs ES, Norris PJ. Soluble mediators of inflammation in HIV and their implications for therapeutics and vaccine development. Cytokine Growth Factor Rev. 2012;23:193–206. doi: 10.1016/j.cytogfr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB, Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonell KB, Chmiel JS, Poggensee L, Wu S, Phair JP. Predicting progression to AIDS: combined usefulness of CD4 lymphocyte counts and p24 antigenemia. Am J Med. 1990;89:706–712. doi: 10.1016/0002-9343(90)90210-5. [DOI] [PubMed] [Google Scholar]
- 29.Chang SW, Kate MH, Hernandez SR. The new AIDS case definition. Implications for San Francisco. JAMA. 1992;267:973–975. [PubMed] [Google Scholar]
- 30.Redfield RR, Wright DC, Tramont EC. The Walter Reed staging classification for HTLV-III/LAV infection. N Engl J Med. 1986;314:131–132. doi: 10.1056/NEJM198601093140232. [DOI] [PubMed] [Google Scholar]
- 31.Knollmann FD, Maurer J, Grunewald T, Schedel H, Vogl TJ, Pohle HD, Felix R. Abdominal CT features and survival in acquired immunodeficiency. Acta Radiol. 1997;38:970–977. doi: 10.1080/02841859709172112. [DOI] [PubMed] [Google Scholar]
- 32.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 33.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254:326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anthony KB, Yoder C, Metcalf JA, DerSimonian R, Orenstein JM, Stevens RA, Falloon J, Polis MA, Lane HC, Sereti I. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33:125–133. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro RM, Mohri H, Ho DD, Perelson AS. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: why are CD4+ but not CD8+ T cells depleted? Proc Natl Acad Sci U S A. 2002;99:15572–15577. doi: 10.1073/pnas.242358099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sieg SF, Bazdar DA, Lederman MM. S-phase entry leads to cell death in circulating T cells from HIV-infected persons. J Leukoc Biol. 2008;83:1382–1387. doi: 10.1189/jlb.0907643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonetta F, Bourgeois C. CD4+FOXP3+ Regulatory T-Cell Subsets in Human Immunodeficiency Virus Infection. Front Immunol. 2013;4:215. doi: 10.3389/fimmu.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz AM, DiNapoli SR, Brenchley JM. Macrophages Are Phenotypically and Functionally Diverse across Tissues in Simian Immunodeficiency Virus-Infected and Uninfected Asian Macaques. J Virol. 2015;89:5883–5894. doi: 10.1128/JVI.00005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Botsios C. Safety of tumour necrosis factor and interleukin-1 blocking agents in rheumatic diseases. Autoimmun Rev. 2005;4:162–170. doi: 10.1016/j.autrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.