Abstract
STAT3 activation is associated with poor prognosis in human colorectal cancer (CRC). Our previous data demonstrated that regorafenib (Stivarga) is a pharmacological agonist of SH2 domain-containing phosphatase 1 (SHP-1) that enhances SHP-1 activity and induces apoptosis by targeting STAT3 signals in CRC. This study aimed to find a therapeutic drug that is more effective than regorafenib for CRC treatment. Here, we showed that SC-43 was more effective than regorafenib at inducing apoptosis in vitro and suppressing tumorigenesis in vivo. SC-43 significantly increased SHP-1 activity, downregulated p-STAT3Tyr705 level, and induced apoptosis in CRC cells. An SHP-1 inhibitor or knockdown of SHP-1 by siRNA both significantly rescued the SC-43–induced apoptosis and decreased p-STAT3Tyr705 level. Conversely, SHP-1 overexpression increased the effects of SC-43 on apoptosis and p-STAT3Tyr705 level. These data suggest that SC-43–induced apoptosis mediated through the loss of p-STAT3Tyr705 was dependent on SHP-1 function. Importantly, SC-43–enhanced SHP-1 activity was because of the docking potential of SC-43, which relieved the autoinhibited N-SH2 domain of SHP-1 and inhibited p-STAT3Tyr705 signals. Importantly, we observed that a significant negative correlation existed between SHP-1 and p-STAT3Tyr705expression in CRC patients (P = .038). Patients with strong SHP-1 and weak p-STAT3Tyr705 expression had significantly higher overall survival compared with patients with weak SHP-1 and strong p-STAT3Tyr705 expression (P = .029). In conclusion, SHP-1 is suitable to be a useful prognostic marker and a pharmacological target for CRC treatment. Targeting SHP-1-STAT3 signaling by SC-43 may serve as a promising pharmacotherapy for CRC.
Introduction
Colorectal cancer (CRC) is a leading malignancy worldwide [1]. Regorafenib (BAY 73-4506, commercial name Stivarga) approved by the Food and Drug Administration in September 2012 is a therapy for the patients with metastatic CRC (mCRC) [2] and is a novel oral multikinase inhibitor that targets the oncogenic receptor tyrosine kinases as well as the RAF/MEK/ERK signaling pathway[3]. Based on the clinical trial data, regorafenib significantly improves overall survival (OS) of patients and serves as an effective and safe third-line pharmacotherapeutic option for mCRC patients with a KRAS mutation, and is a third- or fourth-line option form CRC patients with wild-type KRAS [2]. However, it is still necessary to improve OS of CRC patients and provide an effective pharmacotherapeutic option by searching for a novel anti-CRC therapy.
SH2 domain-containing phosphatase 1 (SHP-1) is a nonreceptor protein tyrosine phosphatase (PTP) that was first identified in hematopoietic cells and epithelial cells [4–6]. Notably, SHP-1 has tumor-suppressive potential due to its negative regulation of STAT3 oncogenic signaling during tumor progression [7–10]. It contains two SH2 domains, a catalytic PTP domain, and a C-terminal tail [10]. The tyrosine phosphatase activity of SHP-1 is highly dependent on its structural variability. Most importantly, we demonstrate that SHP-1 is a druggable target of regorafenib in CRC [11]. Regorafenib induces apoptosis of CRC cells in vitro and suppresses tumorigenesis in vivo by relieving the autoinhibition of SHP-1 through the docking potential of regorafenib into the N-SH2 domain of SHP-1 that further downregulates p-STAT3Tyr705 level [11]. These observations are also consistent with the recent findings that regorafenib exerts an antitumor effect by targeting SHP-1–mediated STAT3 inhibition in hepatocellular carcinoma (HCC) [12]. These results therefore indicate that the pharmacological targeting of the SHP-1-STAT3 axis by regorafenib is a potential anticancer therapy.
Previously, our team discovered that the tyrosine phosphatase SHP-1 plays a critical role in mediating a novel kinase-independent antitumor effect of sorafenib [13]. Sorafenib treatment increases the activity of SHP-1 tyrosine phosphatase in HCC cells by directly disrupting the autoinhibited structure of SHP-1 between its N-SH2 and catalytic PTP domain [13]. Based on these findings, we further generated a series of dimer-based derivatives of sorafenib that exert antitumor effects via SHP-1 enhancing without kinase inhibition properties, among which SC-43 is the most potent pharmacological SHP-1 agonist [13]. In HCC cells, we found that SC-43 exerts better in vitro and in vivo antitumor efficiency than sorafenib through targeting SHP-1–dependent STAT3 inactivation in HCC preclinical model [13]. In addition, preclinical tests also showed that SC-43 is a highly active compound against breast cancer [14]. Notably, we also found that regorafenib, a sorafenib analog developed by Bayer, inhibits the survival of colon cancer cells through identical mode of action on SHP-1 as sorafenib and SC-43 [11]. Because SC-43 has a closely related chemical structure with regorafenib with depletion of the kinase inhibition properties, a detailed preclinical testing on SC-43 will help to address the feasibility of targeting SHP-1/STAT3 signaling for the treatment of CRC.
In this study, we discovered that SC-43 exerts more potent drug efficiency than regorafenib and identified SC-43 as an SHP-1 agonist that activates SHP-1–dependent STAT3 inhibition signaling in CRC. These findings suggest that SHP-1 is a pharmacological target and prognostic marker of CRC and also indicate that targeting the SHP-1-STAT3 axis with SC-43 may be a promising therapeutic approach to prevent CRC development.
Materials and Methods
Cell Culture
Colorectal cancer cell lines Hct-15, Hct-116, DLD1, HT-29, and SW480 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 U/ml of penicillin and streptomycin (Invitrogen, Carlsbad, CA). Cells were then incubated at 37°C in a humidified 5% CO2 atmosphere.
Reagents and Plasmids
Regorafenib was kindly provided by Bayer Healthcare Pharmaceuticals. For in vitro studies, regorafenib and SC-43 were dissolved in dimethyl sulfoxide and then added to the cells maintained in RPMI 1640 medium without FBS. For in vivo animal study, regorafenib and SC-43 were suspended in Kolliphor (JT Baker). SHP-1 inhibitor (PTPIII) was purchased from Calbiochem. Smart-pool siRNAs, including control (D-001810-10) and SHP-1 (PTPN6, L-009778-00-0005), were all purchased from Dharmacon (Chicago, IL). Plasmids of human wild-type STAT3 and SHP-1 (PTPN6) were encoded by pCMV6 vector with myc-tag. For mutant-type SHP-1 expression, we generated two plasmids, designated dN1 and D61A, with a truncated N-SH2/PTP domain and aspartic acid at 61 changed to an alanine residue, respectively. Both plasmids were cloned into pCMV6 entry vector. These plasmids or siRNAs were subsequently transfected into cells by using Lipofectamine 2000 Reagent (Invitrogen, CA).
Cell Proliferation Assay
After treatment with regorafenib or SC-43 at the indicated doses for 2 days, cell viability was measured using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells were counted and seeded in 96-well plates and then incubated at 37°C in a humidified 5% CO2 atmosphere. Twenty microliters of MTT reagent (5 mg/ml; Sigma, St. Louis, MO) was added to each well at the end of incubation; 4 hours later, the medium was discarded, and 150 μl of dimethyl sulfoxide was added to each well to dissolve the purple crystal. Then the absorbance at 540 nm was measured. Experiments were performed three times in duplicate.
Sub-G1 Analysis
For the examination of cell population in the sub-G1 phase, both floating and attached cells were collected and centrifuged before being washed with cold phosphate-buffered saline, and then fixed in 70% cold ethanol overnight at − 20°C. Cells were incubated with 50 μg/ml of propidium iodide and 20 μg/ml of Rnase A in the dark at room temperature for 30 minutes and then analyzed by flow cytometry (BD Biosciences, San Jose, CA).
Apoptosis Assay
Apoptotic cells were determined by using an enzyme-linked immunosorbent assay (Cell Death Detection ELISAPLUS Kit; Roche Molecular Biochemicals) according to the manufacturer's instructions. This apoptosis assay is used to measure DNA fragmentation and histone release from the nucleus during the apoptosis process. Briefly, CRC cells (1 × 105) were cultured in 96-well plate for 24 hours and then treated with dose escalation of regorafenib or SC-43 for 24 hours. After removing the supernatant from cells cultured in 96-well plate, 200 μl of lysis buffer per well was added and incubated for 30 minutes at room temperature. Lysates were centrifuged, and then 20 μl of supernatant was transferred into the streptavidin-coated microplate. Eighty microliters of immunoreagent was added to each well and incubated for 2 hours at room temperature. After each well was rinsed with incubation buffer and 100 μl of ABTS solution was added for 20 minutes, the absorbance at 405 nm was measured.
Western Blotting
Whole-cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA) and incubated with the primary antibody, and then incubated with horseradish peroxidase–conjugated secondary antibodies. Specific proteins were detected using enhanced chemiluminescence reagent. The primary antibodies used for Western blotting, including cyclin D1 and PARP, were purchased from Santa Cruz Biotechnology (San Diego, CA); p-STAT3, STAT3, Mcl-1 and survivin were purchased from Cell Signaling (Danvers, MA); SHP-1 and β-actin were purchased from Abcam (Cambridge, MA). For quantification of protein levels on chemiluminescent Western blots, the densitometry Image J software was employed.
SHP-1 Phosphatase Activity
After treatment with SC-43, cell protein extracts were incubated with anti–SHP-1 antibody in immunoprecipitation buffer [20 mM Tris-Hcl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 1% sodium deoxycholate] overnight. Protein G-Sepharose 4 Fast Flow (GE Healthcare Bio-Science, NJ) was added to each sample, followed by incubation for 3 hours at 4°C with rotation. A Rediplate 96 Enzchekr Tyrosine Phosphatase Assay Kit (R-22067) was used for SHP-1 activity assay (Molecular Probes, Invitrogen, CA). For the examination of the SHP-1 activity in the SHP-1–containing immunoprecipitation of cellular extract, 1 mg of whole-cell lysates was incubated with anti–SHP-1 antibody in immunoprecipitation buffer overnight. Then, samples were incubated with Protein G-Sepharose 4 Fast Flow for 3 hours at 4°C with rotation and finally treated with SC-43 for 2 hours at 4°C. The SHP-1 activity in SHP-1–containing immunoprecipitation of cellular extract following SC-43 treatment was determined by using a Rediplate 96 Enzchekr Tyrosine Phosphatase Assay Kit.
Xenograft Tumor Growth
We subcutaneously injected Hct-116 cells (2 × 106) into the posterior flank of nude mice and then treated them with SC-43 [per os (p.o.), 10 mg/kg per day], regorafenib (p.o., 10 mg/kg per day), or vehicle when the tumors became palpable and grew rapidly. The tumor size of SC-43–, regorafenib-, or vehicle-treated tumors was measured on days 0, 4, 7, 11, 14, 18, 21, 24, and 25. On day 25 the tumor weights were measured after tumor excision.
Patient Specimens
Patients with CRC determined according to the World Health Organization criteria were enrolled from January 2002 through January 2014 and classified according to the American Joint Committee on Cancer Staging System (version 6). Clinical data were obtained from the cancer registry. Overall survival was defined as the time from primary resection to death from cancer. The follow-up period ended in January 2014 or at the time of death of the patient. Left colon cancer was defined as a malignancy in the splenic flexure, descending colon, sigmoid colon, rectosigmoid colon, and rectum.
Immunohistochemistry
For tissue microarray and immunohistochemistry, the procedures followed our previous methods and the manufacturer’s instructions [35]. SHP-1 and p-STAT3Tyr705 antibodies were purchased from Abcam (Cambridge, MA). Omission of the primary antibody served as a negative control. Immunopositive results were evaluated by 2 pathologists. The intensity of stained cells was scored as 0, 1, 2, or 3. Percentages of stained cells were counted. A final immunohistochemical score (H-score) was calculated by summing the products of the staining intensities (0-3) and distributions (0%-100%). H-scores ranged from 0 to 300. An H-score equal to or greater than 150 was defined as strongly positive for SHP-1 staining; all others were scored as weakly positive. An H-score greater than 10 was defined as strongly positive for p-STAT3Tyr705 staining (nuclear staining); all others were scored as weakly positive.
Statistical And Survival Analysis
Quantitative data were presented as the mean ± standard deviation (SD) from three independent experiments. The t test was used to compare age distribution. The correlations between clinicopathological variables and immunopositivity were analyzed using the χ2 test or Fisher’s exact test. Survival was estimated using the Kaplan-Meier method. A two-sided P value of less than .05 was regarded as statistically significant. SPSS software (version 16.00; SPSS, Chicago, IL) was used for all the statistical analyses.
Results
SC-43 Is More Effective than Regorafenib at Inhibiting Growth and Inducing Apoptosis in Colon Cancer Cell Lines
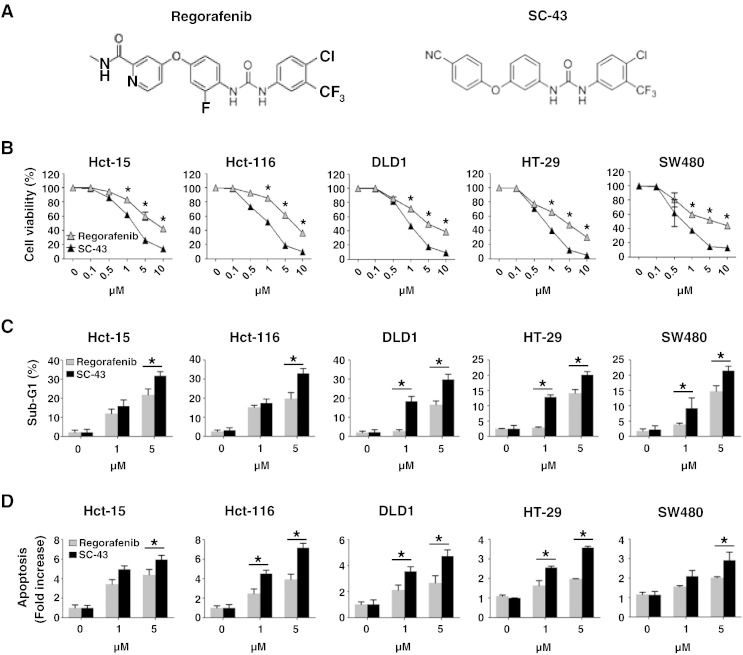
SC-43 is a derivative of regorafenib (Figure 1A). Here, we investigated whether SC-43 exerts better anticancer effects than regorafenib by inhibiting growth and inducing apoptosis in CRC cells. We observed that SC-43 was more effective than regorafenib, showing a gradual decrease in growth 2 days after CRC cells (Hct-15, Hct-116, DLD1, HT-29, and SW480) were treated with dose escalation of SC-43 or regorafenib (Figure 1B). Meanwhile, we also found that CRC cells treated with SC-43 showed more increase in cell population in the sub-G1 phase and more apoptosis than cells treated with regorafenib in a dose-dependent manner (Figure 1, C and D). Taken together, these findings suggest that SC-43 may be more effective than regorafenib as a potential therapeutic drug for CRC treatment.
Figure 1.
SC-43, a derivative of regorafenib, induces more potent apoptosis of CRC cells than regorafenib. (A) Chemical structures of regorafenib and SC-43. (B) MTT assay was performed to measure the cell viability in parental colon cancer cell lines (Hct-15, Hct-116, DLD1, HT-29, and SW480) 2 days after treatment with regorafenib or SC-43 in a dose-dependent manner. The results are shown as mean ± SD of three independent experiments, made in triplicate. *P < .05. (C) Sub-G1 analysis was performed in the cells 2 days after treatment with regorafenib or SC-43 in a dose-dependent manner. The results are shown as mean ± SD of three independent experiments, made in triplicate. *P < .05. (D) Apoptotic cell death was measured in the cells 24 hours after treatment with dose escalation of regorafenib or SC-43. The results are shown as mean ± SD of three independent experiments, made in triplicate. *P < .05.
Loss of p-STAT3Tyr705 Expression Is Associated with SC-43–Induced Apoptosis
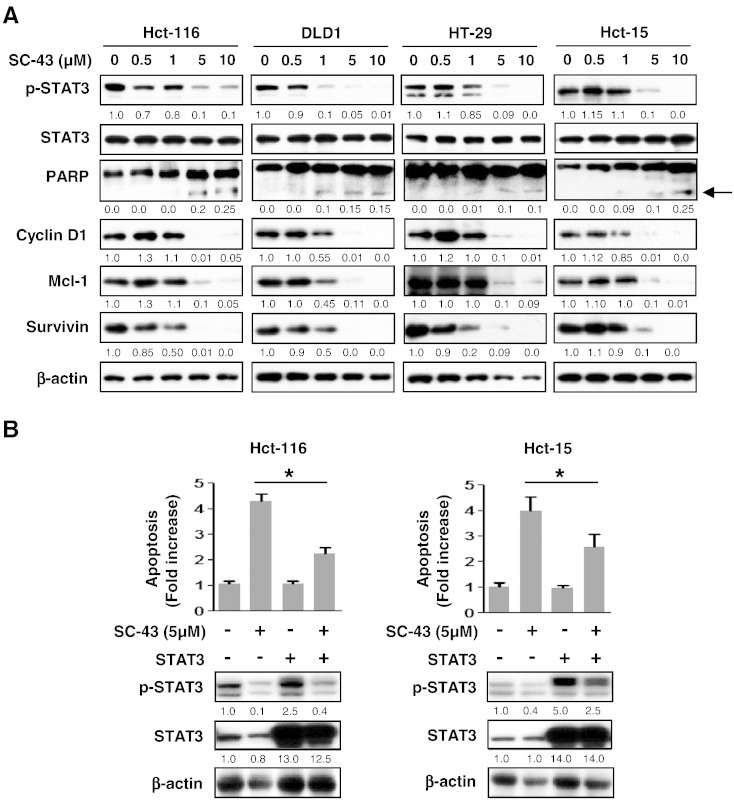
In CRC, STAT3 activation was determined by an elevated level of p-STAT3Tyr705, which was constitutively expressed due to its transcriptional activation in cellular proliferative regulation [15]. Next, we examined whether p-STAT3Tyr705 expression is involved in SC-43–triggered apoptosis. As shown in Figure 2A, dose escalation of SC-43–treated CRC cells, including Hct-116, DLD1, HT-29, and Hct-15, showed a significant gradual decrease in the level of p-STAT3Tyr705and its related survival markers, including cyclin D1, Mcl-1, and survivin. Meanwhile, cleaved fragments of PARP, which are markers of apoptosis, were significantly increased 24 hours after treatment with an escalating dose of SC-43 (Figure 2A). Therefore, downregulation of p-STAT3Tyr705 expression was associated with SC-43–triggered apoptosis in CRC cells. In contrast to cancer cells, SC-43 treatment had little effects on FHC cells (normal colon epithelial cell) and CCD-18Co cells (normal colon fibroblast). The extent of SC-43–induced cell apoptosis and downregulation of p-STAT3Tyr705 and Mcl-1 in FHC and CCD-18Co cells was significantly lower than Hct-116 (Supplementary Figure 1). Notably, overexpression of STAT3 in Hct-116 cells not only increased the level of p-STAT3Tyr705but also significantly inhibited apoptosis induced by treatment with 5 μM SC-43 for 24 hours (Figure 2B, left panels). Importantly, this observation was also seen in Hct-15 cells (Figure 2B, right panels). Taken together, these results indicate that SC-43–triggered apoptosis is dependent on STAT3 inactivation.
Figure 2.
SC-43 triggers apoptosis via p-STAT3Tyr705 downregulation. (A) Western blotting of p-STAT3Tyr705, the cleaved fragments of PARP, and the survival markers (cyclin D1, Mcl-1, and survivin) in cells 24 hours after treatment with dose escalation of SC-43. β-Actin was used as a loading control. The cleaved fragments of PARP are indicated by arrows. (B) Overexpression of STAT3 in Hct-116 and Hct-15 cells rescued apoptosis induced by treatment with 5 μM SC-43 for 24 hours. Data from apoptosis assay are shown as mean ± SD of three independent experiments, made in triplicate. *P < .05.
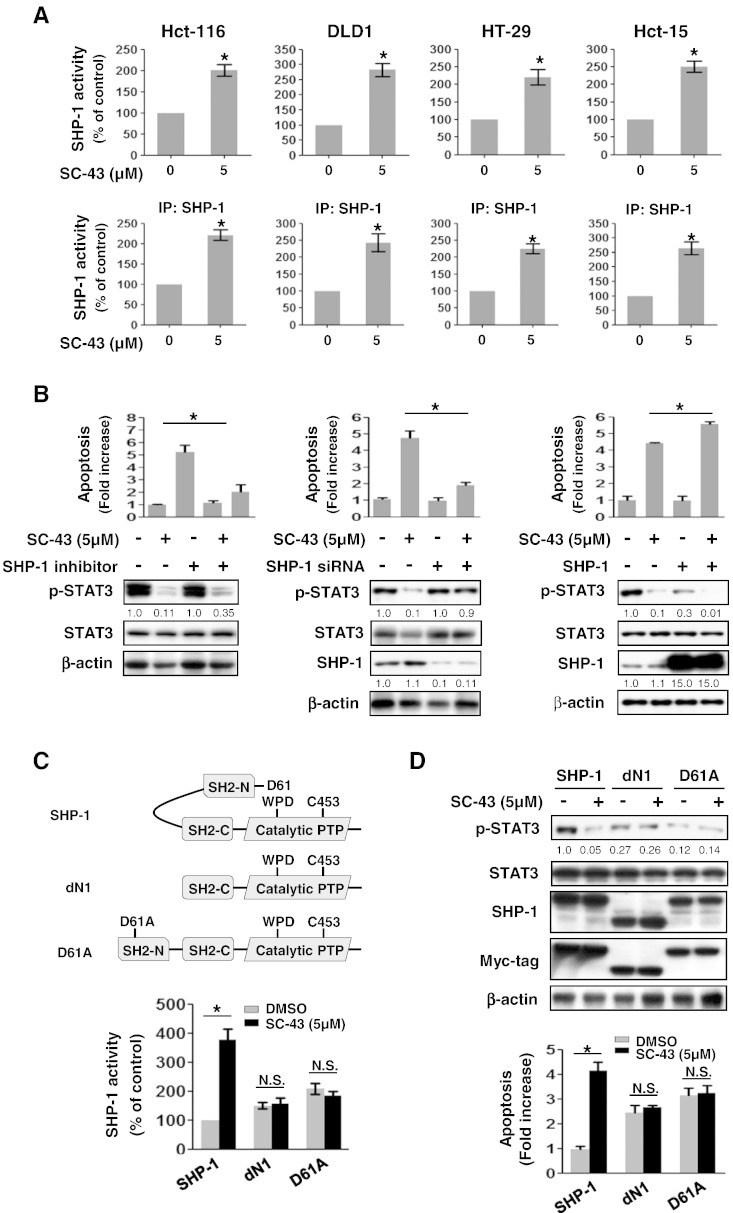
SC-43-Enhanced SHP-1 Tyrosine Phosphatase Activity Is Required for p-STAT3Tyr705 Downregulation and Apoptosis Induced by SC-43
SHP-1 tyrosine phosphatase activity has been reported to have a critical role in induction of apoptosis in different cancer types by depleting the level of p-STAT3Tyr705[14,16–18]. Therefore, we evaluated whether SHP-1 tyrosine phosphatase activity is involved in downregulation of p-STAT3Tyr705and apoptosis of CRC cells triggered by SC-43. Our data revealed that SC-43–treated CRC cells (Hct-116, DLD1, HT-29, and Hct-15) showed a significant increase in SHP-1 tyrosine phosphatase activity after treatment with 5 μM SC-43 for 24 hours (Figure 3A, upper panels). Notably, SC-43–enhanced SHP-1 tyrosine phosphatase activity was further confirmed in SHP-1–containing immunoprecipitation extract incubated with 5 μM SC-43 (Figure 3A, lower panels). Here, we provide evidence that SC-43 serves as an SHP-1 agonist to increase SHP-1 tyrosine phosphatase activity. To understand whether SC-43–induced apoptosis via suppression of p-STAT3Tyr705 is dependent on SHP-1, we used SHP-1 phosphatase-specific inhibitor (PTPIII) to inhibit SHP-1 activity and observed that SC-43–induced apoptosis and a decrease of p-STAT3Tyr705were significantly rescued by treatment with SHP-1 inhibitor (Figure 2B, left panels). Moreover, siRNA-mediated SHP-1 knockdown also significantly restored the effects of SC-43 on apoptosis and p-STAT3Tyr705expression (Figure 2B, middle panels). These results demonstrate that SC-43–induced apoptosis mediated through p-STAT3Tyr705 suppression is dependent on SHP-1 tyrosine phosphatase activity. On the other hand, overexpression of SHP-1 reinforced the effects of SC-43 on apoptosis and p-STAT3Tyr705(Figure 2B, right panels). Taken together, these results show that SC-43–augmented SHP-1 tyrosine phosphatase activity is crucial for SC-43–reduced p-STAT3Tyr705 which results in apoptosis in CRC cells.
Figure 3.
SC-43 enhances SHP-1 tyrosine phosphatase activity by targeting the autoinhibited SH2 domain of SHP-1 to suppress p-STAT3Tyr705signaling and further induce apoptosis. (A, upper panels) SHP-1 tyrosine phosphatase activity was measured in the cells (Hct-116, DLD1, HT-29, and Hct-15) after treatment with or without 5 μM SC-43 for 24 hours. (Lower panels) SC-43 increased the SHP-1 tyrosine phosphatase activity in immunoprecipitation-SHP-1–containing cell extract. The results are shown as mean ± SD of three independent experiments, made in triplicate.*P < .05. (B) SC-43 induced a decrease in p-STAT3Tyr705. Apoptosis occurring in Hct-15 cells was dependent on SHP-1 as assessed by using 20 nM of SHP-1 phosphatase-specific inhibitor (PTPIII) and 25 nM of siRNA specifically depleted SHP-1 as shown in the left panels and middle panels, respectively. (Right panels) Overexpression of SHP-1 in Hct-15 cells treated with 5 μM SC-43 increased the effects of SC-43 on p-STAT3Tyr705 and apoptosis. Data from apoptosis assay are shown as mean ± SD of three independent experiments, made in triplicate.*P < .05. (C, upper panels) A schematic representation of wild-type and mutant SHP-1 (dN1 with a deletion of the N-terminal inhibitory domain, and D61A, a single point mutation). (Lower panels) Two days after Hct-116 cells were treated with 5 μM SC-43, SHP-1 activity was potently increased in wild-type SHP-1–expressing but not in dN1 or D61A mutant SHP-1–expressing cells. Data from SHP-1 activity are shown as mean ± SD of three independent experiments, made in triplicate (*P < .05; nonsignificant). (D, upper panels) Western blotting of p-STAT3Tyr705 and SHP-1 in the wild-type and mutant-type (dN1 And D61A) SHP-1–transfected Hct-116 cells 2 days after treatment with or without SC-43 at 5 μM. (Lower panels) Apoptosis was detected in these cells as shown in upper panels of (D). Data from apoptosis assay are shown as mean ± SD of three independent experiments, made in triplicate (*P < .05; nonsignificant).
SC-43 Increases SHP-1 Tyrosine Phosphatase activity By Directly Impairing the Interaction between N-SH2 and PTP Catalytic Domains of SHP-1
To further investigate the mechanism through which SC-43 increased SHP-1 tyrosine phosphatase activity, we transfected Hct-116 cells with wild-type or mutant SHP-1 (dN1 and D61A). In SHP-1, intramolecular inhibition occurs through association of the N-SH2 domain with the PTP catalytic domain which is stabilized by a salt bridge between Asp61 (D61) and Lys362 (Figure 3C, upper panels).The dN1 has a deletion of the N-SH2 domain of SHP-1, the D61A point mutant mimics the open conformation of SHP-1, and both serve as constitutive activators (Figure 3C, upper panels).The dN1 and D61A mutant SHP-1 showed an increase in SHP-1 tyrosine phosphatase activity (Figure 3C, lower panels) but displayed a marked decrease in p-STAT3Tyr705level compared with wild-type SHP-1 (Figure 3D, upper panels). Importantly, SC-43 significantly increased SHP-1 tyrosine phosphatase activity in wild-type SHP-1–transfected but not in dN1 or D61A mutant SHP-1–transfected Hct-116 cells (Figure 3C, lower panels). This model provides evidence that SC-43 increases SHP-1 tyrosine phosphatase activity through the direct disruption of the interaction between the N-SH2 domain and the PTP catalytic domain of SHP-1 that further relieves the autoinhibition of SHP-1. In addition, wild-type SHP-1–transfected Hct-116 cells showed a marked decrease in p-STAT3Tyr705level (Figure 3D, upper panels) and a significant increase in apoptosis (Figure 3D, lower panels) following SC-43 treatment. However, this observation was not seen in dN1 or D61A mutant SHP-1–transfected Hct-116 cells following treatment with SC-43 (Figure 3D). Our model therefore identifies that SHP-1 is a druggable target of SC-43 verified by the evidence that SC-43 pharmacologically increases SHP-1 activity through the disruption of autoinhibition in SHP-1, leading to reduced p-STAT3Tyr705 level and eventual induction of apoptosis in CRC cells.
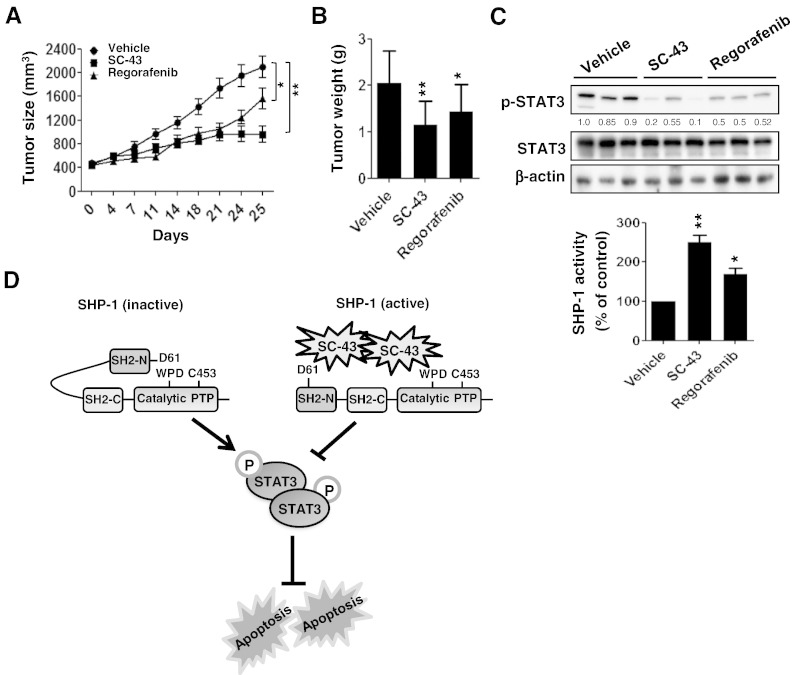
SC-43 Exerts a More Suppressive Effect on Tumor Formation In Vivo than Regorafenib by Targeting SHP-1–Dependent STAT3 Inhibition
Next, to investigate whether SC-43 has a better therapeutic effect on tumorigenesis in vivo than regorafenib, Hct-116 cells (2 × 106) were subcutaneously injected into the posterior flank of nude mice, and then SC-43 (p.o.,10 mg/kg per day), regorafenib (p.o.,10 mg/kg per day), or vehicle was administered when the tumors became palpable and grew rapidly. As shown in figure 4, A and B, mice receiving SC-43 or regorafenib treatment exhibited a reduction in tumor growth and tumor weight compared with vehicle-treated mice. Importantly, the group receiving SC-43 displayed a more effective reduction in tumor growth (Figure 4A) and tumor weight (Figure 4B) than the regorafenib treatment group. Notably, SC-43–treated tumors showed relatively lower p-STAT3Tyr705level but had significantly higher SHP-1 tyrosine phosphatase activity (Figure 4C) compared with regorafenib-treated tumors. The data above demonstrate that SC-43 is more efficient than regorafenib at curbing tumorigenesis in vivo by targeting SHP-1–dependent STAT3 inactivation signaling. Collectively, our data establish SC-43 as a potent pharmacological SHP-1 agonist that enhances SHP-1 activation by relieving the inhibitory N-SH2 domain of SHP-1, leading to STAT3 inactivation and apoptosis in CRC cells (Figure 4D).
Figure 4.
SC-43 shows more effective tumor inhibition than regorafenib in a CRC subcutaneous tumor model. (A) Tumor sizes were measured at the times indicated after mice received regorafenib (10 mg/kg per day), SC-43 (10 mg/kg per day), or vehicle. Points, mean (n = 7); bars, SEM. *P < .05, **P < .01. (B) Tumor weight was measured on day 25 after tumor excision. Columns, mean; bars, SD. *P < .05, **P < .01. (C, upper panels) The Levels of p-STAT3Tyr705 and STAT3 were measured by Western blotting on day 25 after excision of vehicle-, regorafenib-, and SC-43–treated tumors. β-Actin was used as a loading control. (Lower panels) SHP-1 activity was measured in vehicle-, regorafenib-, and sc-43–treated tumors on day 25 after tumor excision. Columns, mean; bars, SD. *P < .05, **P < .01. (D) SC-43 induced potent apoptosis in CRC which was mediated through SC-43–enhanced SHP-1 activity. SC-43 had the potential to dock to the inhibitory N-SH2 domain and the catalytic PTP domain of SHP-1, resulting in the direct relief of autoinhibition of SHP-1. The activity Of SHP-1 tyrosine phosphatase specifically increased the susceptibility to p-STAT3Tyr705, which was the major trigger of apoptosis.
SHP-1 Is Significantly and Negatively Correlated with p-STAT3Tyr705 Expression in CRC Tissues
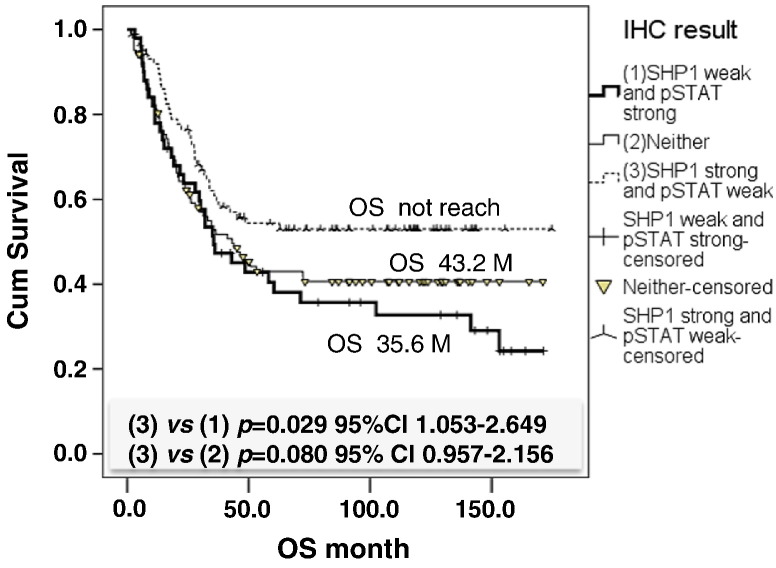
We used immunohistochemistry to examine the expression level of SHP-1 and p-STAT3Tyr705 in CRC tissues and then investigated the clinical relevance of SHP-1 and p-STAT3Tyr705 and the clinicopathological parameters of CRC patients. Two hundred and forty-three CRC patients were enrolled for this clinical analysis. As shown in Table 1, neither SHP-1 nor p-STAT3Tyr705 expression had a significant correlation with age, gender, location, clinical stage, grade, or lymphovascular invasion. Moreover, there was no correlation between SHP-1 expression and pathology. However, a significant positive correlation was observed between p-STAT3Tyr705expression and pathology, especially in mucinous adenocarcinoma (MAC) (P = .036). Importantly, we observed that a significant negative correlation existed between SHP-1 and p-STAT3Tyr705expression (P = .038) (Table 1). The total survival curve for the 243 CRC patients is shown in Figure 5. A Kaplan-Meier analysis showed that patients with strong SHP-1 and weak p-STAT3Tyr705expression had significantly higher OS compared with patients with weak SHP-1 and strong p-STAT3Tyr705 expression (P = .029) (Figure 5). These observations indicate that patients with SHP-1–dependent STAT3 inactivation may have OS benefits and better clinical prognosis and also revealed that SHP-1 and p-STAT3Tyr705are the relevant therapeutic targets as well as suitable prognostic markers for CRC treatment.
Table 1.
The Association between Clinicopathological Parameters and SHP-1 and p-STAT3Tyr705 Expression (N = 243)
| Clinicopathological Parameters | SHP-1 |
p-STAT3Tyr705 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weak |
Strong |
Weak |
Strong |
||||||||
| n | (%) | n | (%) | P | n | (%) | n | (%) | P | ||
| Age (mean/SD) | 62.3 ± 13.0 | 65.6 ± 13.1 | 63.4 ± 13.6 | 65.3 ± 12.4 | |||||||
| Gender | Female | 40 | 47.6 | 44 | 52.4 | .690 | 53 | 63.1 | 31 | 36.9 | .795 |
| Male | 71 | 44.9 | 87 | 55.1 | 97 | 61.4 | 61 | 38.6 | |||
| Location | Left | 57 | 40.1 | 85 | 59.9 | .33 | 90 | 63.4 | 52 | 36.6 | .594 |
| Right | 54 | 54.0 | 46 | 46.0 | 60 | 60.0 | 40 | 40.0 | |||
| Stage | I | 6 | 35.3 | 11 | 64.7 | .309 | 12 | 70.6 | 5 | 29.4 | .864 |
| II | 36 | 53.7 | 31 | 46.3 | 40 | 59.7 | 27 | 40.3 | |||
| III | 29 | 39.7 | 44 | 60.3 | 46 | 63.0 | 27 | 37.0 | |||
| IV | 40 | 47.1 | 45 | 52.9 | 52 | 61.2 | 33 | 38.8 | |||
| Pathology | Adenocarcinoma | 102 | 44.2 | 129 | 55.8 | .097 | 147 | 63.6 | 84 | 36.4 | .036 |
| Carcinoma | 1 | 100.0 | 0 | .0 | 0 | .0 | 1 | 100.0 | |||
| MAC | 7 | 77.8 | 2 | 22.2 | 2 | 22.2 | 7 | 77.8 | |||
| Signet ring cell | 1 | 100.0 | 0 | .0 | 1 | 100.0 | 0 | .0 | |||
| Primary T4 or not | T1-3 | 60 | 45.1 | 73 | 54.9 | .877 | 87 | 65.4 | 46 | 34.6 | .071 |
| T4 | 31 | 46.3 | 36 | 53.7 | 35 | 52.2 | 32 | 47.8 | |||
| Grade | Low | 95 | 44.2 | 120 | 55.8 | .255 | 130 | 60.5 | 85 | 39.5 | .157 |
| High | 12 | 57.1 | 9 | 42.9 | 16 | 76.2 | 5 | 23.8 | |||
| Lymphovascular invasion | Without | 80 | 46.0 | 94 | 54.0 | .761 | 110 | 63.2 | 64 | 36.8 | .955 |
| With | 24 | 43.6 | 31 | 56.4 | 35 | 63.6 | 20 | 36.4 | |||
| p-STAT3Tyr705 | Weak | 61 | 40.7 | 89 | 59.3 | .038 | 150 | 100.0 | 0 | .0 | – |
| Strong | 50 | 54.3 | 42 | 45.7 | 0 | .0 | 92 | 100.0 | |||
| SHP-1 | Weak | 111 | 100.0 | 0 | .0 | – | 61 | 55.0 | 50 | 45.0 | .038 |
| Strong | 0 | .0 | 131 | 100.0 | 89 | 67.9 | 42 | 32.1 | |||
Figure 5.
Overall survival of patients with CRC relative to the expression of SHP-1 and p-STAT3Tyr705. Patients with strong SHP-1and weak p-STAT3 expression had better OS than others.
Discussion
Regorafenib is approved for the treatment of mCRC [2] and is also a promising treatment for gastrointestinal stromal tumors [19]. It serves as a multitargeted tyrosine kinase inhibitor of BRAF, VEGFR-1, VEGFR-2, VEGFR-3, KIT, TIE-2, PDGFR-Β, FGFR-1, RET, and RAF-1, which are involved in oncogenesis, angiogenesis, and the maintenance of the tumor microenvironment [20]. In our earlier study, we reported that SHP-1 is a pharmacological target of regorafenib in CRC [11]. Regorafenib markedly enhances the tyrosine phosphatase activity of SHP-1 that negatively targets p-STAT3Tyr705signaling, resulting in apoptosis in vitro and suppression of tumorigenesis in vivo[11].Here we demonstrated that SC-43, a derivative of regorafenib, inhibits Hct-116 xenograft growth by downregulating p-STAT3Tyr705 level more potently than regorafenib but inducing a more significant increase in SHP-1 activity, suggesting that SC-43 has more therapeutic efficiency than regorafenib by targeting SHP-1–dependent p-STAT3Tyr705inhibition. In addition, we tested the effects of regorafenib and SC-43 on kinase inhibition. Regorafenib, but not SC-43, significantly inhibited the activity of Raf-1 (Supplementary Figure 2), suggesting that the effects of regorafenib and SC-43 on SHP-1–dependent STAT3 inactivation were kinase-independent effects. Most importantly, our present findings demonstrate the mechanism by which SC-43 enhances SHP-1 activity, which is through direct interaction with the inhibitory N-SH2 domain and catalytic PTP domain of SHP-1 to relieve the autoinhibition of SHP-1. The observations were consistent with the mode of action of regorafenib in enhancing SHP-1 activity [11]. Our study, therefore, not only verified that SHP-1 is a pharmacological target of SC-43 but also identified SC-43 as a potent agonist of SHP-1, which negatively regulates p-STAT3Tyr705 signals and consequently induces apoptosis and suppresses xenograft growth in a preclinical CRC model. On the other hand, sufficient data from in vivo animal study are still lacking. Therefore, much more attention should be paid when generating an orthotopic tumor model in place of a xenograft tumor model that will be a good approach to further support our in vitro findings.
To our knowledge, this is the first study to report the clinical relevance of SHP-1 and p-STAT3Tyr705 and the novel relationship between the SHP-1-STAT3 axis and OS of CRC patients. We observed that SHP-1 expression was significantly and negatively correlated with p-STAT3Tyr705 expression in the tissues of 243 CRC patients. This observation further supported our results that SHP-1 is a negative regulator of p-STAT3Tyr705 signaling in a preclinical CRC model. Most importantly, CRC patients with strong SHP-1 and weak p-STAT3Tyr705 expression had significantly higher OS than patients with weak SHP-1 and strong p-STAT3Tyr705expression, suggesting that patients with SHP-1–dependent STAT3 inactivation are likely to have OS benefit and better clinical outcome. Thus, SHP-1 may serve as a useful prognostic marker to determine clinical outcome and may also be a promising pharmacological target for therapeutic intervention for CRC. Overall, our findings present a basis for pharmacologically targeting SHP-1–dependent STAT3 inactivation using SC-43 rather than regorafenib. Use of SC-43 may improve OS of patients and provide an effective pharmacotherapeutic option for patients who have exhausted currently approved and standard therapies.
MAC of CRC is an uncommon histological subtype of adenocarcinoma (approximately 5%-15%) [21] with more than 50% extracellular mucin within the tumor [22]. MAC is one of the most aggressive phenotypes of stage III CRC [23]. The clinical outcome of MAC is extremely poor once recurrence occurs [23] and is usually associated with a modest increase in mortality due to an increase in mucinous differentiation [24]. Moreover, ample evidence has demonstrated a strong correlation between mucin expression and worse prognosis in various other tumor types [23,25–27]. Mucins are known to participate in cellular growth, differentiation, transformation, invasion, and metastasis during cancer development [25,28,29]. Most notably, our data specifically showed that patients with MAC but not other subtypes of CRC had a significant and positive correlation with p-STAT3Tyr705 expression. Importantly, several studies have illustrated that STAT3 activation has a key role in mucin overexpression and cancer development [30–33]. In addition, STAT3 activation has been reported to serve as a marker of poor prognosis in OS of human CRC [34]. Collectively, our findings reveal a tight relationship between p-STAT3Tyr705 and mucinous differentiation in MAC of CRC and further support the assertion that p-STAT3Tyr705 may be a relevant therapeutic target for the treatment of MAC of CRC. Although the mechanism through which p-STAT3Tyr705 signaling increases mucinous differentiation in MAC is still poorly understood in our present study, we provide a new drug, SC-43, that is not only a potent SHP-1 agonist but also a STAT3 inhibitor. We suggest that pharmacologically targeting p-STAT3Tyr705 by SC-43–enhanced SHP-1 activity may improve OS and result in better clinical outcome and will be an effective pharmacotherapy for MAC of CRC.
In conclusion, we investigated the clinical relevance of SHP-1 and p-STAT3Tyr705 in CRC and demonstrated a tight relationship between the SHP-1-STAT3 axis and OS of CRC patients, indicating that SHP-1 is not only a useful prognostic marker but also a good pharmacological target for CRC therapy. Importantly, we discovered that SC-43 is a potent therapeutic agonist of SHP-1 that exerts more effective anti-CRC effects than regorafenib by inhibiting p-STAT3Tyr705signals. Therefore, targeting SHP-1-STAT3 signaling by SC-43 may be an alternative pharmacotherapy for the treatment of CRC.
Acknowledgements
This work was supported by grants MOST 103-2325-B-002-016 and MOST 103-2622-B-002-006 from the Ministry of Science and Technology and NTUH104-P06 from the National Taiwan University Hospital, Taiwan.
Footnotes
Conflict of interest: We have no potential conflicts of interest to disclose.
Financial support: This work was supported by grants MOST 103-2325-B-002-016 and MOST 103-2622-B-002-006 from the Ministry of Science and Technology and NTUH104-P06 from the National Taiwan University Hospital, Taiwan.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2015.08.007.
Appendix A. Supplementary data
Supplementary Figures.
References
- 1.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–378. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 3.Strumberg D, Schultheis B. Regorafenib for cancer. Expert Opin Investig Drugs. 2012;21:879–889. doi: 10.1517/13543784.2012.684752. [DOI] [PubMed] [Google Scholar]
- 4.Banville D, Stocco R, Shen SH. Human protein tyrosine phosphatase 1C (PTPN6) gene structure: alternate promoter usage and exon skipping generate multiple transcripts. Genomics. 1995;27:165–173. doi: 10.1006/geno.1995.1020. [DOI] [PubMed] [Google Scholar]
- 5.Mok SC, Kwok TT, Berkowitz RS, Barrett AJ, Tsui FW. Overexpression of the protein tyrosine phosphatase, nonreceptor type 6 (PTPN6), in human epithelial ovarian cancer. Gynecol Oncol. 1995;57:299–303. doi: 10.1006/gyno.1995.1146. [DOI] [PubMed] [Google Scholar]
- 6.Tsui HW, Hasselblatt K, Martin A, Mok SC, Tsui FW. Molecular mechanisms underlying SHP-1 gene expression. Eur J Biochem. 2002;269:3057–3064. doi: 10.1046/j.1432-1033.2002.02986.x. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Ruiz P, Rodriguez-Ubreva J, Cariaga AE, Cortes MA, Colas B. SHP-1 in cell-cycle regulation. Anticancer Agents Med Chem. 2011;11:89–98. doi: 10.2174/187152011794941154. [DOI] [PubMed] [Google Scholar]
- 8.Witkiewicz A, Raghunath P, Wasik A, Junkins-Hopkins JM, Jones D, Zhang Q, Odum N, Wasik MA. Loss of SHP-1 tyrosine phosphatase expression correlates with the advanced stages of cutaneous T-cell lymphoma. Hum Pathol. 2007;38:462–467. doi: 10.1016/j.humpath.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Guan Q, Wang Y, Zhao ZJ, Zhou GW. SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J Cell Biochem. 2003;90:1026–1037. doi: 10.1002/jcb.10727. [DOI] [PubMed] [Google Scholar]
- 10.Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 11.Fan LC, Teng HW, Shiau CW, Lin H, Hung MH, Chen YL, Huang JW, Tai WT, Yu HC, Chen KF. SHP-1 is a target of regorafenib in colorectal cancer. Oncotarget. 2014;5:6243–6251. doi: 10.18632/oncotarget.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai WT, Chu PY, Shiau CW, Chen YL, Li YS, Hung MH, Chen LJ, Chen PL, Su JC, Lin PY. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res. 2014;20:5768–5776. doi: 10.1158/1078-0432.CCR-14-0725. [DOI] [PubMed] [Google Scholar]
- 13.Tai WT, Shiau CW, Chen PJ, Chu PY, Huang HP, Liu CY, Huang JW, Chen KF. Discovery of novel Src homology region 2 domain-containing phosphatase 1 agonists from sorafenib for the treatment of hepatocellular carcinoma. Hepatology. 2014;59:190–201. doi: 10.1002/hep.26640. [DOI] [PubMed] [Google Scholar]
- 14.Liu CY, Tseng LM, Su JC, Chang KC, Chu PY, Tai WT, Shiau CW, Chen KF. Novel sorafenib analogues induce apoptosis through SHP-1 dependent STAT3 inactivation in human breast cancer cells. Breast Cancer Res. 2013;15:R63. doi: 10.1186/bcr3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen KF, Tai WT, Hsu CY, Huang JW, Liu CY, Chen PJ, Kim I, Shiau CW. Blockade of STAT3 activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur J Med Chem. 2012;55:220–227. doi: 10.1016/j.ejmech.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Chen KF, Chen HL, Liu CY, Tai WT, Ichikawa K, Chen PJ, Cheng AL. Dovitinib sensitizes hepatocellular carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5 antibody, through SHP-1–dependent inhibition of STAT3. Biochem Pharmacol. 2012;83:769–777. doi: 10.1016/j.bcp.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Chen KF, Su JC, Liu CY, Huang JW, Chen KC, Chen WL, Tai WT, Shiau CW. A novel obatoclax derivative, SC-2001, induces apoptosis in hepatocellular carcinoma cells through SHP-1–dependent STAT3 inactivation. Cancer Lett. 2012;321:27–35. doi: 10.1016/j.canlet.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Crona DJ, Keisler MD, Walko CM. Regorafenib: a novel multitargeted tyrosine kinase inhibitor for colorectal cancer and gastrointestinal stromal tumors. Ann Pharmacother. 2013;47:1685–1696. doi: 10.1177/1060028013509792. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 21.Kelemen LE, Kobel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol. 2011;12:1071–1080. doi: 10.1016/S1470-2045(11)70058-4. [DOI] [PubMed] [Google Scholar]
- 22.Bosman F. 4th edition. IARC; Lyon: 2010. WHO Classification of Tumours of the Digestive System. [Google Scholar]
- 23.Ooki A, Akagi K, Yatsuoka T, Asayama M, Hara H, Yamamoto G, Nishimura Y, Yamaguchi K. Inverse effect of mucinous component on survival in stage III colorectal cancer. J Surg Oncol. 2014;110:851–857. doi: 10.1002/jso.23742. [DOI] [PubMed] [Google Scholar]
- 24.Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381–388. doi: 10.1136/jclinpath-2011-200340. [DOI] [PubMed] [Google Scholar]
- 25.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815:224–240. doi: 10.1016/j.bbcan.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–1638. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, Ganti AK, Batra SK. Targeting EGF-receptor(S)-STAT1 Axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6:5164–5181. doi: 10.18632/oncotarget.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, Ouellette MM, Batra SK. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through alpha7nAChR-mediated MUC4 upregulation. Oncogene. 2013;32:1384–1395. doi: 10.1038/onc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mejias-Luque R, Linden SK, Garrido M, Tye H, Najdovska M, Jenkins BJ, Iglesias M, Ernst M, De Bolos C. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene. 2010;29:1753–1762. doi: 10.1038/onc.2009.467. [DOI] [PubMed] [Google Scholar]
- 32.Mejias-Luque R, Peiro S, Vincent A, Van Seuningen I, De Bolos C. IL-6 induces MUC4 expression through Gp130/STAT3 pathway in gastric cancer cell lines. Biochim Biophys Acta. 2008;1783:1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, Fujiyama Y. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol. 2009;183:687–695. doi: 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- 34.Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, Nagayasu T, Sekine I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15:1445–1451. [PubMed] [Google Scholar]
- 35.Teng HW, Yang SH, Lin JK, Chen WS, Lin TC, Jiang JK, Yen CC, Li AF, Chen PC, Lan YT. CIP2A is a predictor of poor prognosis in colon cancer. J Gastrointest Surg. 2012;16:1037–1047. doi: 10.1007/s11605-012-1828-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures.