Abstract
P63 is a p53 family member involved in multiple facets of biology, including embryonic development, cell proliferation, differentiation, survival, apoptosis, senescence and aging. The p63 gene encodes multiple protein isoforms either with (TAp63) or without (ΔNp63) the N-terminal transactivation domain. Amounting evidence suggests that p63 can function as a tumor suppressor, yet the precise molecular mechanisms, and particularly the specific roles of TAp63 and ΔNp63 in cancer progression, are still largely unclear. Here, we demonstrated that ΔNp63α, the predominant isoform expressed in epithelial cells and squamous cell carcinomas, inhibits cell invasion. Affymetrix gene expression profiling, combined with gain- and loss-of-function analyses and chromatin immunoprecipitation, indicated that cluster of differentiation 82 (CD82), a documented metastasis suppressor, is a direct transcriptional target of ΔNp63α. Expression of ΔNp63α inhibited outgrowth in Matrigel and cancer cell invasion, which was largely reversed by specific ablation of CD82. Conversely, ΔNp63α knockdown led to increased cell invasion, which was reversed by ectopic expression of CD82. Moreover, inhibition of glycogen synthase kinase-3β (GSK3β) by either pharmacological inhibitors or by RNA interference resulted in the downregulation of ΔNp63α and CD82 expression, concomitant with increased cell invasion, independently of β-catenin. Furthermore, decreased expression of p63 and CD82 is correlated with cancer progression. Taken together, this study reveals that ΔNp63α upregulates CD82 to inhibit cell invasion, and suggests that GSK3β can regulate cell invasion by modulating the ΔNp63α–CD82 axis.
Keywords: p63, CD82, KAI1, GSK3β, β-catenin, cell invasion
The p63 gene is a member of the p53 family that is expressed as multiple isoforms with pleiotropic functions. TAp63 isoforms contain an N-terminal transactivation domain homologous to that of p53, whereas ΔNp63 isoforms bear no resemblance to the p53 transactivation domain. Alternative splicing generates five C termini (α, β, γ, δ and ɛ) for a total of 10 isoforms described to date. All p63 isoforms share the same DNA-binding domain (DBD) and oligomerization domain. In addition, p63α isoforms contain a sterile alpha motif (SAM), which is important for protein–protein interaction, and a transactivation inhibitory domain. TAp63 and ΔNp63 proteins have important roles in the regulation of multiple processes, including cell proliferation, survival, apoptosis, differentiation, senescence and aging.1, 2 Heterozygous autosomal mutations in the p63 gene are associated with human developmental diseases, such as EEC (ectrodactyly ectodermal dysplasia clefting) and AEC (ankyloblepharon-ectodermal dysplasia clefting) syndromes, which are characterized by orofacial and limb malformations.3, 4 More marked phenotypes are found in p63-null mice, which succumb to early postnatal lethality owing to the lack of stratified epithelia, limb truncations and craniofacial defects.5, 6 TAp63 isoforms are highly expressed in oocytes, where they are crucial for inducing apoptosis upon DNA damage independently of p53.7, 8 On the other hand, ΔNp63α is the predominant isoform in the proliferative, basal compartment of stratified epithelia, and has been shown to be essential for maintaining the regenerative potential of epithelial stem cells.9, 10
Unlike p53, mutations in the p63 gene are rarely found in human cancers.11, 12 Rather, ΔNp63α is usually overexpressed in a wide range of human squamous cell carcinomas (SCCs).13, 14 Multiple studies have described oncogenic functions for ΔNp63α, such as inhibition of TAp73-mediated apoptosis in SCC15, 16 and cooperation with Ras to induce tumor formation in mouse xenografts.17 However, accumulating evidence strongly suggests that p63 proteins have an important role as metastasis suppressors. For example, p53+/−;p63+/− mice display a much higher rate of metastasis than p53+/− mice,18 and TAp63-deficient mice develop highly metastatic cancers.19 Moreover, disrupting p63 expression results in the upregulation of mesenchymal genes and in enhanced cell motility in tissue culture.20, 21 Taken together, these studies strongly implicate p63 as a metastasis inhibitor. However, the molecular mechanisms underlying these effects are not yet fully understood.
Cluster of differentiation 82 (CD82; also known as KAI1) is a transmembrane protein belonging to the tetraspanin superfamily. Tetraspanins form specialized membrane microdomains believed to interact with other transmembrane proteins and microdomains, thus forming signaling networks involved in a wide variety of biological processes, including cell migration, fusion, adhesion and proliferation.22 CD82 was initially assigned a role in metastasis suppression by a genetic screen for metastasis-suppressing genes in prostate cancer.23 CD82 has been shown to downregulate p130Cas, thereby preventing Rac1 activation and leading to decreased cell migration,24 and to inhibit EGFR/Her2-induced migration by preventing ligand binding and heterodimerization.25, 26 Furthermore, p53 binds the CD82 promoter and promotes its transcription, thus suggesting a tumor suppressor role for CD82.27, 28
Glycogen synthase kinase 3β (GSK3β) is a serine/threonine kinase that is involved in multiple cellular functions, including metabolism, cell growth and proliferation, differentiation and apoptosis.29 GSK3β phosphorylates the cotranscriptional activator β-catenin, thus targeting it for ubiquitination and degradation. Activation of Wnt signaling inhibits GSK3β activity, leading to nuclear β-catenin accumulation and increased gene expression, including oncogenes such as cyclin D1 and Myc.30 GSK3β has also been recognized as an important player in the regulation of epithelial-to-mesenchymal transition (EMT) by targeting Snail to proteasomal degradation.31 Snail induces EMT by repressing E-cadherin expression, thereby promoting metastasis.32
In this study, we found that ΔNp63α inhibits cell invasion without significantly affecting the expression of EMT markers. We identified the metastasis suppressor CD82 as a direct ΔNp63α transcriptional target, and found that CD82 has an important role in mediating inhibition of cell invasion by ΔNp63α. Moreover, we discovered that silencing GSK3β results in the downregulation of both ΔNp63α and CD82, leading to increased cell invasion.
Results
ΔNp63α inhibits Matrigel outgrowth and invasion of human Hs-578T cells
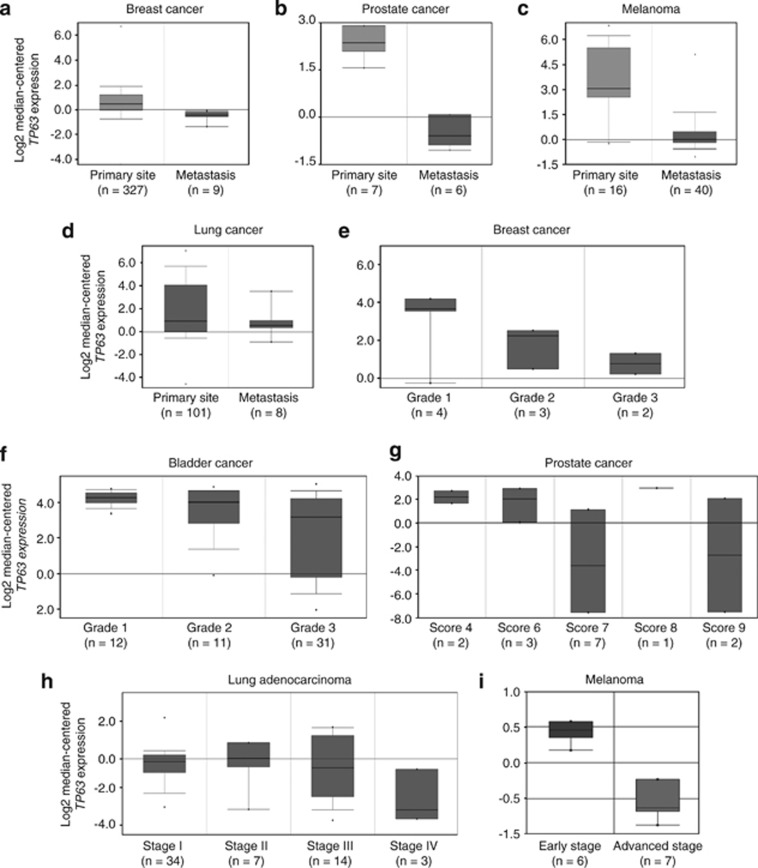
To analyze the role of p63 in cancer development, we used Oncomine, an online cancer-profiling database, to investigate a potential correlation between p63 expression and cancer progression. As shown in Figures 1a–d, p63 expression was significantly decreased in biopsy samples from metastatic lesions, compared with samples from primary tumors in breast, prostate, lung cancer and melanoma. Moreover, p63 expression was decreased progressively in breast, bladder and prostate cancers of higher pathological grade or stage (Figures 1e–i). These data clearly indicate that p63 downregulation correlates with cancer progression from primary tumor to metastatic dissemination.
Figure 1.
P63 expression inversely correlates with cancer progression in human cancers. Box plots representing p63 gene (TP63) expression from microarray analysis of human tumor biopsy samples. The top and bottom of each box represent the first and third quartiles, and the band inside the box represents the median value. The error bars represent 1 S.D. above and below the mean value. Oncomine (Compendia Bioscience) was used for analysis and visualization. (a–d) Clinical specimens removed from primary tumor (primary site) or metastatic nodule (metastasis). (a) Bittner breast data set. Fold change: −2.168; P=2.00e−5. (b) Verambally Prostate data set. Fold change: −7.334; P=3.54e−8. (c) Riker Melanoma data set. Fold change: −7.005; P=7.26e−5. (d) Bittner Lung data set. Fold change: −2.231; P=0.026. (e–g) Tumor biopsies were classified pathologically by grade. (e) Ma Breast 4 data set. (f) Stransky Bladder data set. (g) Luo Prostate 2 data set. Data were classified by Gleason score. (h and i) Samples were classified pathologically by tumor stage. (h) Bild Lung data set. (i) Smith Skin data set
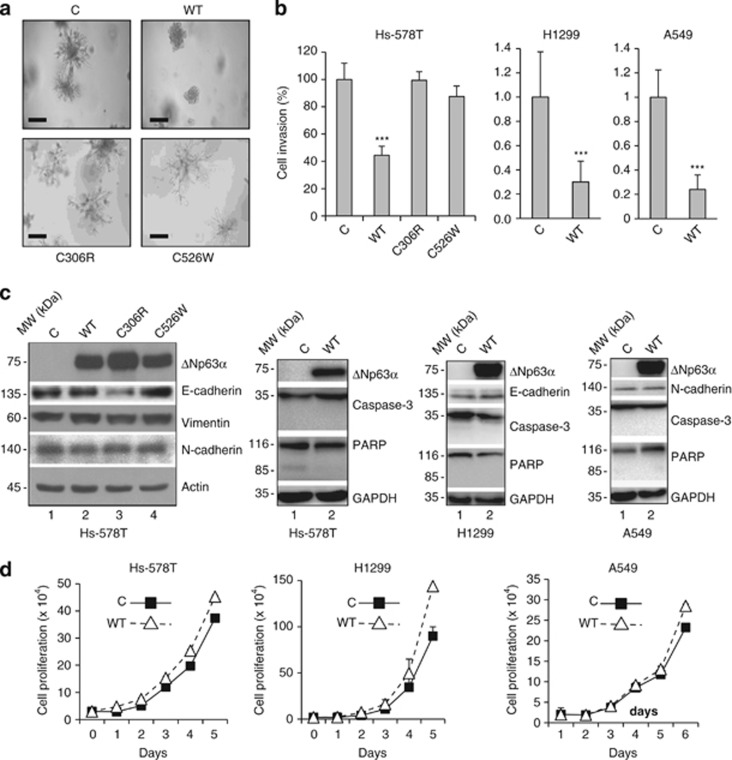
As ΔNp63α is the predominant protein isoform expressed in the majority of epithelial and cancer cells,14, 33, 34 we investigated the role of ΔNp63α in cell invasion. We chose human breast cancer Hs-578T cells because they lack detectable p63 protein expression, are highly invasive and exhibit branching morphogenesis in the Matrigel.35 We infected Hs-578T cells with retrovirus expressing either wild-type (WT) ΔNp63α or a disease-derived mutant. ΔNp63α(C306R) harbors a point mutation in the DBD that impairs DNA binding and is associated with EEC syndrome,3 whereas C526W is a point mutation in the SAM domain that affects protein–protein interaction and is associated with AEC syndrome.4 Control Hs-578T cells exhibited characteristic outgrowth and branching morphogenesis in the Matrigel, whereas the expression of WT ΔNp63α markedly inhibited outgrowth. By contrast, expression of either mutant ΔNp63α had little effect on Matrigel outgrowth (Figure 2a). In addition, WT ΔNp63α, but not the mutant derivatives, significantly inhibited cell invasion (Figure 2b and Supplementary Figure S1a). To examine whether ΔNp63α could inhibit cell invasion in other cell types, we analyzed human non-small-cell lung carcinoma H1299 cells and human lung adenocarcinoma A549 cells stably expressing WT ΔNp63α. Consistently, ΔNp63α markedly inhibited cell invasion in both H1299 and A549 cells (Figure 2b and Supplementary Figure S1b). Taken together, these results indicate that ectopic ΔNp63α expression inhibits cell invasion, and that both direct DNA binding and interaction with cellular proteins via the SAM domain are required for ΔNp63α to exert this function. Moreover, no significant changes in the expression of classic EMT markers (i.e. E-cadherin, N-cadherin and vimentin) were observed in cells expressing ΔNp63α (Figure 2c). These results suggest that ΔNp63α expression inhibits cell invasion independently of modulation of typical EMT markers. We further investigated whether ΔNp63α expression affected apoptosis or cell proliferation. ΔNp63α expression had little effect on cell proliferation (Figure 2d) and on apoptosis, as assessed by cleaved PARP and cleaved caspase-3 levels (Figure 2c).
Figure 2.
ΔNp63α inhibits Hs-578T cell outgrowth in Matrigel and cell invasion. Hs-578T, H1299 and A549 cells were infected with a recombinant retrovirus expressing a vector control (C), WT murine ΔNp63α (WT) or a mutant ΔNp63α derivative (C306R or C526W), as shown, and selected by puromycin resistance. (a) Stable Hs-578T cells were seeded into 96-well plates for Matrigel outgrowth assay, and photographed after 7 days to assess morphology. Representative pictures from three independent experiments are shown. Scale bar=400 μm. (b) Stable Hs-578T, H1299 and A549 cells were subjected to transwell assays for cell invasion. Twenty-four hours after plating, invading cells were fixed and stained with crystal violet, and quantitated as described in the Materials and Methods section. Results are presented as means and S.E. from three independent experiments. ***P<0.001. (c) Whole-cell lysates from stable cells were subjected to western blotting, as indicated. (d) Stable cells were subjected to cell proliferation analysis by counting the number of cells every 24 h with a hemacytometer, as described in the Materials and Methods section. Results are presented as means and S.E. from three independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PARP, poly (ADP-ribose) polymerase
CD82 is upregulated by WT ΔNp63α, but not by its two disease-associated mutants
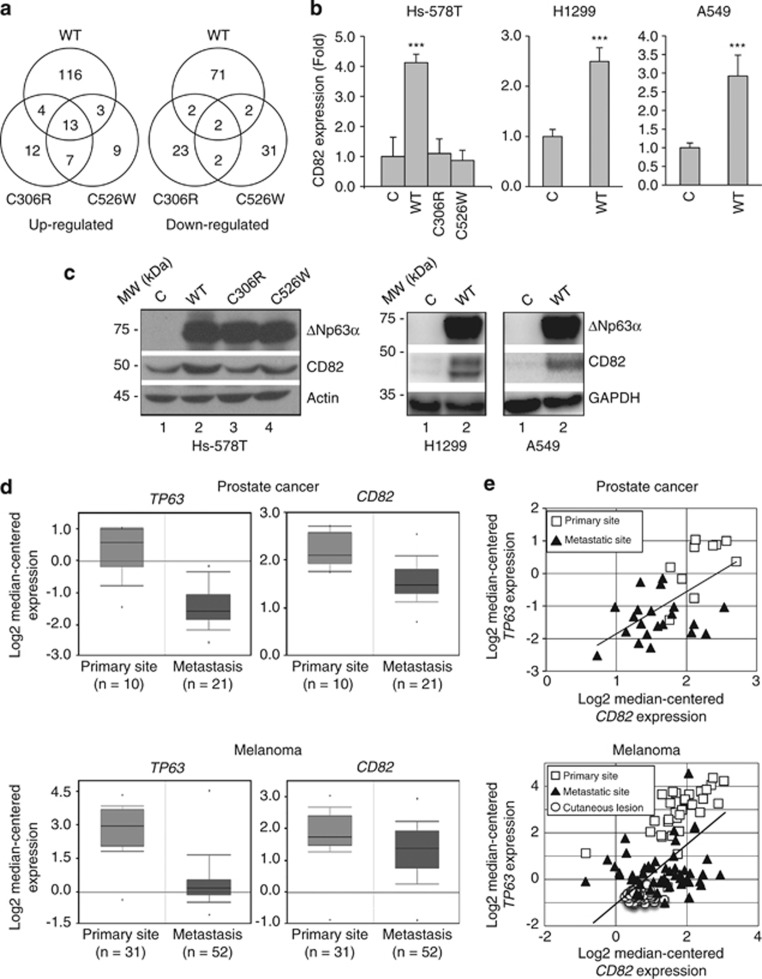
To identify molecular mechanisms by which ΔNp63α inhibits cell invasion, we profiled gene expression of Hs-578T cells expressing WT or mutant ΔNp63α. As shown in Figure 3a and Supplementary Figure S2a, ΔNp63α expression upregulated 136 genes and downregulated 77 genes. Out of these genes, 17 were also upregulated and 4 downregulated by ΔNp63α(C306R), suggesting that, in some instances, ΔNp63α may act as a cotranscriptional regulator independently of direct DNA binding. Likewise, ΔNp63α(C526W) upregulated 16 genes and downregulated 4 genes overlapping with WT ΔNp63α, suggesting that protein–protein interaction via the SAM domain is required for the regulation of yet another subset of genes. In addition, WT and both mutant ΔNp63α proteins upregulated 13 overlapping genes and downregulated 2 overlapping genes to a similar extent. These data suggest that both direct DNA binding and interaction via the SAM domain may be dispensable for the regulation of certain genes. On the other hand, both ΔNp63α(C306R) and ΔNp63α(C526W) regulated a unique set of non-overlapping genes. Of note, our microarray analyses showed upregulation of several previously published p63 downstream targets (Supplementary Table S1).
Figure 3.
ΔNp63α upregulates CD82 expression. Stable Hs-578T cells expressing WT murine ΔNp63α (WT), a mutant derivative (C306R or C526W), or a vector control (C) as described in Figure 2 were used. (a) Stable cells were subjected to gene expression profiling using Affymetrix human genome U133A 2.0 arrays in two independent arrays. A P-value lesser than 0.05 was used as the cutoff for significance, and genes exhibiting ≥2-fold changes compared with the vector control were selected (also see Supplementary Figure S1 and Supplementary Tables S1–3). Venn diagrams indicate the number of upregulated (left) and downregulated (right) genes for each ΔNp63α construct (WT, C306R or C526W). Note that expression of some genes overlaps, whereas that of other genes are uniquely regulated. (b) Stable cells were subjected to Q-PCR analysis for CD82 expression. Results are presented as means and S.E. from three independent experiments performed in triplicate. ***P<0.001. (c) Stable cells were subjected to western blotting, as indicated. (d and e) P63 (TP63) and CD82 gene expression from microarray analysis of human tumor biopsy samples from Chandran Prostate data set and Xu Melanoms data set. Oncomine (Compendia Bioscience) was used for analysis and visualization. (d) Box plots representing TP63 and CD82 expression. The top and bottom of each box represent the first and third quartile, and the band inside the box represents the median value. The error bars represent 1 S.D. above and below the mean value. For prostate cancer samples (top panels): TP63 fold change: −3.194, P=3.07e−5. CD82 fold change: −1.534, P=9.11e−5. For melanoma samples (lower panels): TP63 fold change: −5.582, P=6.43e−16. CD82 fold change: −1.413, P=0.002. (e) Correlation analysis between TP63 and CD82 expression in prostate cancer samples (top panel; r=0.603, P=3.30e−4) and melanoma samples (lower panel; r=0.615, P<1.0e−5). Statistical significance was assessed by Pearson's correlation coefficient (r) followed by a two-tailed probability test (P-value)
Ontological analysis of genes that were affected by WT, but not mutant ΔNp63α, revealed multiple biological functions, including cell communication, development, differentiation, immune system processes, response to stress, regulation of apoptosis, cell proliferation and regulation of cell cycle (Supplementary Tables S2 and S3). In particular, we found a subset of genes involved in cell adhesion or motility/migration. Of these genes, CD82 was upregulated by WT ΔNp63α, but not by the mutant derivatives (Supplementary Figure S2b). CD82 functions as a metastasis suppressor in a variety of human cancers,36 thus making it a likely candidate for acting as an effector of ΔNp63α function in inhibiting cell migration and invasion. We confirmed CD82 induction by WT ΔNp63α in Hs-578T, H1299 and A549 cells via quantitative polymerase chain reaction (Q-PCR) (Figure 3b) and western blotting (Figure 3c). These results suggest that ΔNp63α regulates CD82 at the transcriptional level, and that this effect requires an intact DBD and SAM domain.
Next, we explored the clinical relevance of ΔNp63α-mediated upregulation of CD82. We used Oncomine to analyze CD82 and p63 expression. In prostate cancer and melanoma, expression of both p63 and CD82 was decreased in metastatic lesions compared with primary tumor samples (Figure 3d), and further analysis revealed a clear positive correlation between p63 and CD82 expression (Figure 3e). Taken together, these observations indicate that decreased p63 and CD82 expression is correlated with metastatic cancer progression.
ΔNp63α-mediated inhibition of cell invasion requires CD82
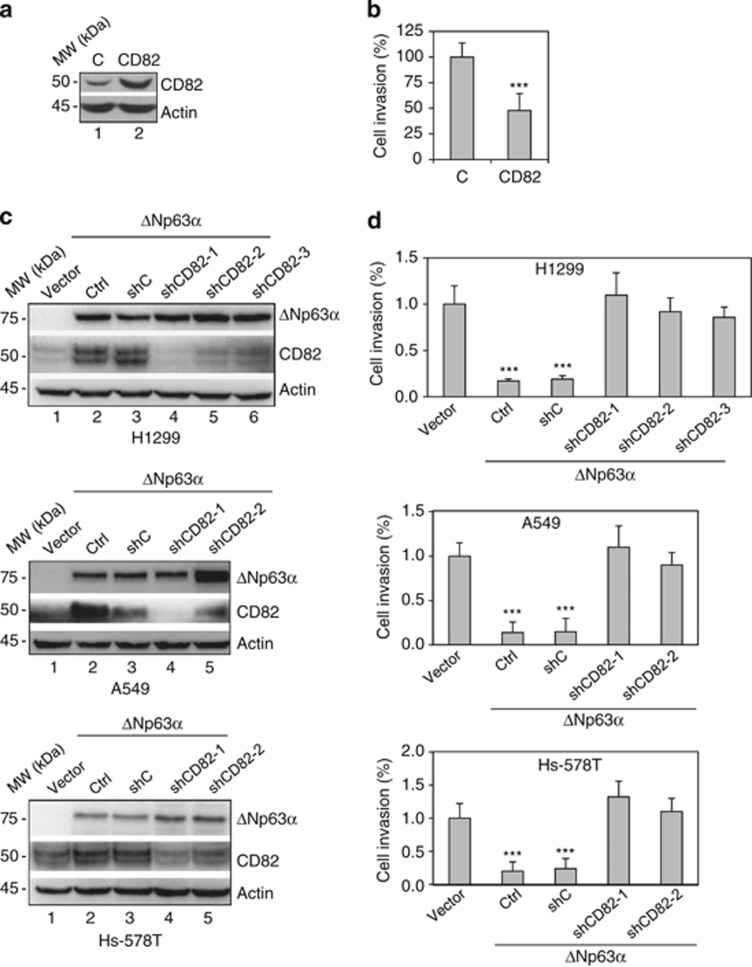
To investigate the role of CD82 in ΔNp63α-induced inhibition of cell migration and invasion, we expressed CD82 in Hs-578T cells (Figure 4a) and showed that expression of CD82 alone significantly reduced cell invasion (Figure 4b and Supplementary Figure S3a). We next examined whether ΔNp63α-mediated inhibition of cell invasion requires CD82 by ablating CD82 in Hs-578T, H1299 and A549 cells stably expressing ΔNp63α (Figure 4c). As shown in Figure 4d and Supplementary Figures S3b–g, ΔNp63α expression markedly inhibited cell invasion, whereas silencing CD82 significantly rescued the invasive phenotype of these cells. Moreover, reintroduction of CD82 into Hs-578T and H1299 cells with concomitant ΔNp63α expression and CD82 ablation effectively reverted cell invasion to levels comparable to ΔNp63α expression alone (Supplementary Figures S4a and b). Taken together, these data strongly suggest that CD82 is an important downstream mediator of ΔNp63α in the regulation of cell invasion.
Figure 4.
CD82 is essential for ΔNp63α-mediated inhibition of cell invasion. (a and b) Hs-578T cells were infected with retrovirus expressing CD82 or an empty vector control (C) and selected by puromycin resistance. (a) Whole-cell lysates from stable cells were subjected to western blotting as indicated. (b) Stable Hs-578T cells were subjected to transwell assays for cell invasion, as described previously. Results from invasion assays were quantitated and presented as means and S.E. from three independent experiments. (c and d) Hs-578T, H1299 and A549 cells stably expressing WT murine ΔNp63α or a vector control were infected with either one of three independent lentivirus expressing shRNA against CD82 (shCD82-1, shCD82-2 and shCD28-3) or a control shRNA (shC). (c) Whole-cell lysates were subjected to western blotting, as indicated. (d) Stable cells were subjected to transwell assays for cell invasion and quantitated as described previously. Results presented as means and S.E. from three independent experiments
ΔNp63α is an endogenous regulator of CD82 in the regulation of cell invasion
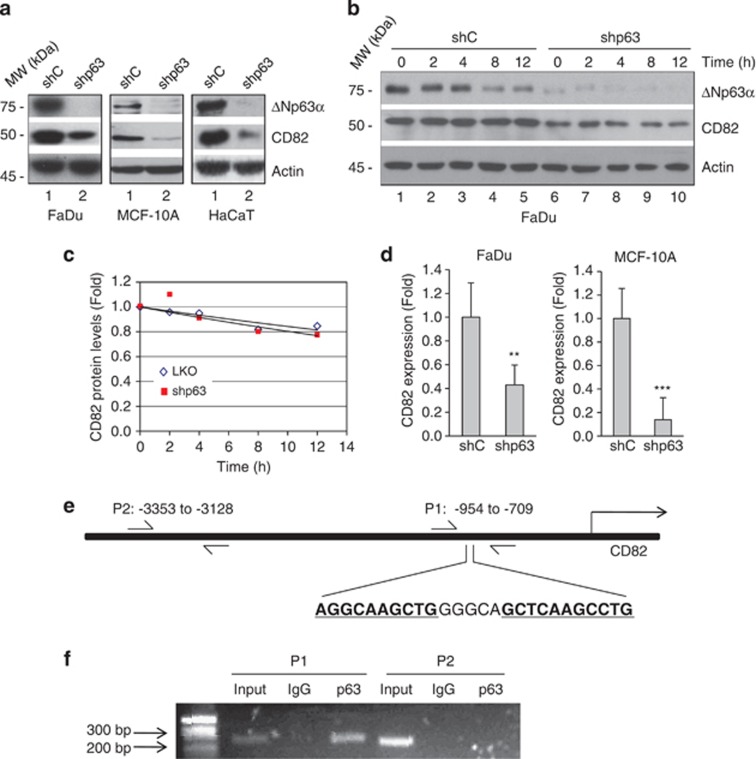
We next examined whether endogenous ΔNp63α affects CD82 expression by ablating pan-p63 isoforms in human-immortalized keratinocyte HaCaT, head and neck SCC FaDu and non-transformed mammary epithelial MCF-10A cells, all of which express ΔNp63α as the major p63 isoform. Silencing p63 led to decreased CD82 protein levels, suggesting that ΔNp63α is a critical regulator of CD82 expression (Figure 5a). To examine whether CD82 protein stability is affected by p63 ablation, we treated p63-silenced FaDu cells with protein synthesis inhibitor cycloheximide. Ablation of p63 did not affect CD82 protein stability (Figures 5b and c). We next investigated whether CD82 is regulated at the transcriptional level. Q-PCR analyses of FaDu and MCF-10A cells showed marked CD82 downregulation upon p63 knockdown (Figure 5d). Moreover, analysis of the CD82 promoter region revealed a putative p63 binding site (P1: −871 to −846; Figure 5e). Chromatin immunoprecipitation followed by PCR demonstrated specific p63 binding to this site in FaDu cells, whereas no PCR product could be detected using primers targeting an irrelevant fragment (P2: −3353 to −3128) upstream of the CD82 gene (Figure 5f). Taken together, these results indicate that CD82 is most likely a direct transcriptional target of ΔNp63α.
Figure 5.
CD82 is a transcriptional target of ΔNp63α. (a) FaDu, HaCaT and MCF-10A cells were infected with lentivirus expressing shRNA specific for pan-p63 (shp63) or a control shRNA (shC), and selected by puromycin resistance. Whole-cell lysates were subjected to western blot analysis, as shown. (b) FaDu cells expressing shp63 or shC were treated with 20 μg/ml cycloheximide for the indicated times. Whole-cell lysates were subjected to western blot analysis, as shown. (c) Quantitation of CD82 protein levels was performed using densitometry scanning. (d) Cells were subjected to Q-PCR analysis for CD82 expression. Results are presented as means and S.E. from three independent experiments performed in triplicate. **P<0.01; ***P<0.001. (e) Diagram of the CD82 gene and promoter locus showing a putative p63-binding element (P1: −954 to −709). An unrelated segment (P2: −3353 to −3128) was used as a negative control. Arrows indicate the position of the primers used for PCR amplification of the immunoprecipitated DNA. (f) Binding of p63 to this putative binding site was assessed in FaDu cells by chromatin immunoprecipitation using a specific p63 antibody (4A4) or a control mouse immunoglobulin G (IgG), followed by PCR amplification of both P1 and P2 sites
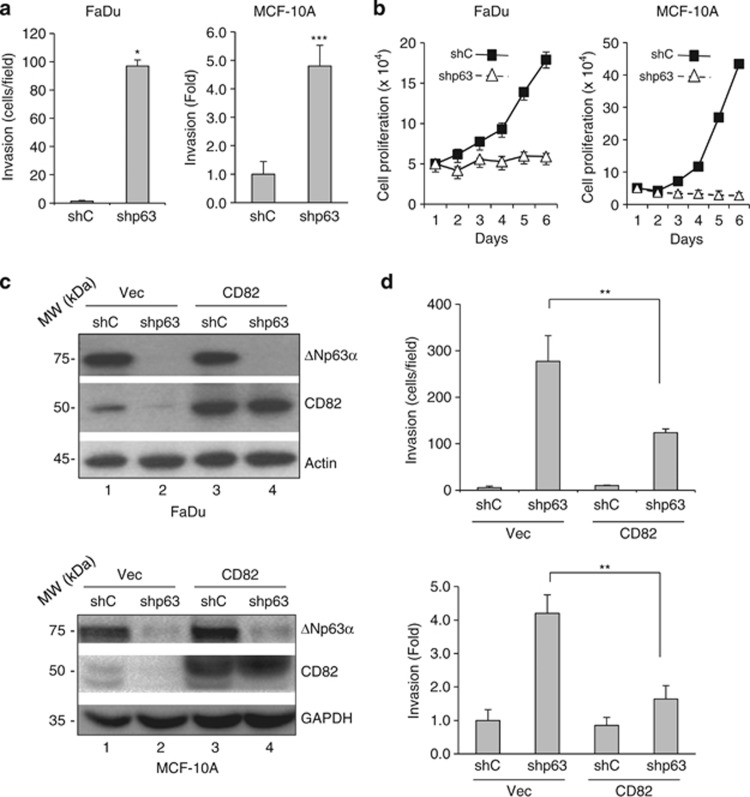
Next, we examined the effect of ΔNp63α ablation on cell invasion. Silencing ΔNp63α in FaDu and MCF-10A cells led to increased cell invasion (Figure 6a and Supplementary Figure S5a), as well as a marked decrease in cell proliferation (Figure 6b), in keeping with previous reports showing the importance of ΔNp63α for epithelial and SCC cell proliferation and survival.16, 37 To examine whether CD82 induction by ΔNp63α is responsible for mediating the function of ΔNp63α in inhibiting cell invasion, we ablated p63 expression and concomitantly expressed CD82 in FaDu and MCF-10A cells (Figure 6c). Again, p63 knockdown markedly induced cell invasion, whereas concomitant CD82 expression significantly reverted cell invasion (Figure 6d and Supplementary Figure S5b), indicating that CD82 is, at least partly, responsible for ΔNp63α-mediated regulation of cell invasion.
Figure 6.
P63 ablation-induced cell invasion is largely mediated by CD82. (a and b) FaDu and MCF-10A cells were infected with lentivirus expressing shRNA against pan-p63 (shp63) or a control shRNA (shC). (a) Cells were subjected to transwell cell invasion assays, as described previously. Results are presented as mean and S.E. from three independent experiments. (b) Cells were subjected to cell proliferation analysis by counting the number of cells every 24 h with a hemacytometer. Results are presented as means and S.E. from three independent experiments. (c and d) FaDu and MCF-10A cells expressing shp63 or shC were infected with retrovirus expressing CD82 or a vector control (Vec). Puromycin-resistant cells were subjected to western blotting (c), or transwell cell invasion assays (d), as described previously. Results from cell invasion assays were quantitated and presented as mean and S.E. from three independent experiments. **P<0.01
Inhibition of GSK3 activity reduces p63 mRNA and protein levels, and promotes cell invasion
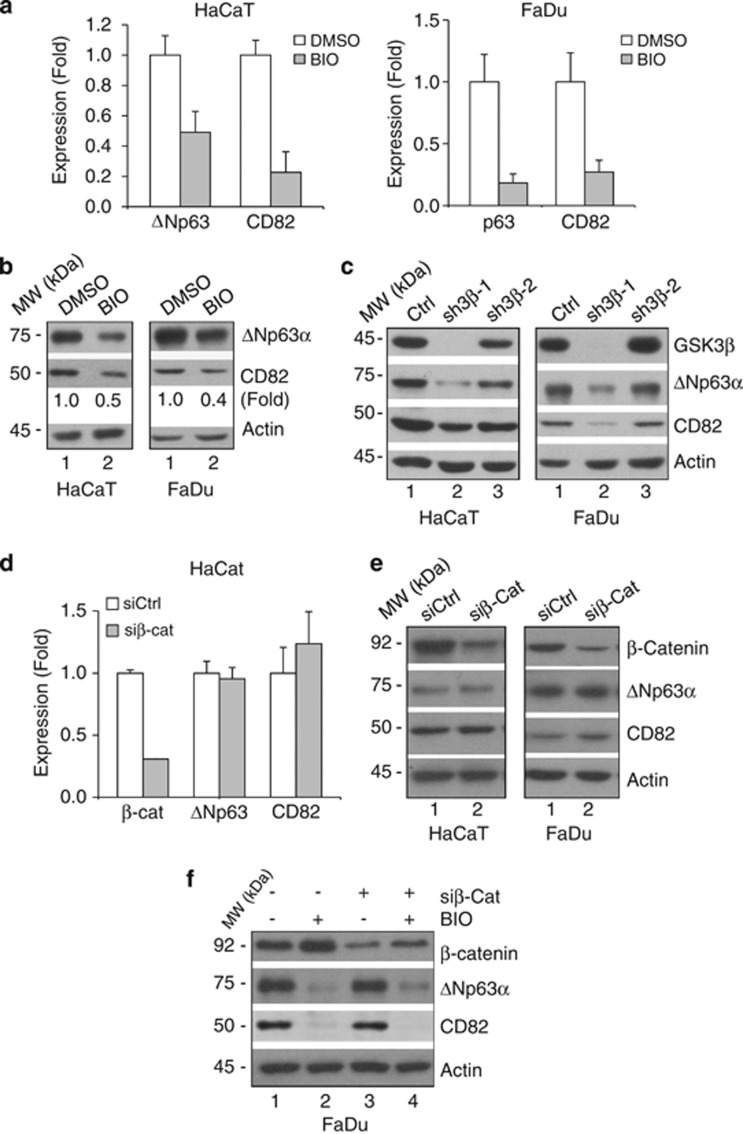
We investigated potential upstream signaling mechanisms that could influence the ΔNp63α-CD82 pathway in cell invasion. We observed that when HaCaT and FaDu cells were treated with a GSK3 inhibitor, 6-bromoindirubin-3′-oxime (BIO), ΔNp63α and CD82 expression was significantly reduced (Figures 7a and b). To confirm the specificity of this inhibition, we silenced GSK3β expression, which led to a decrease in both ΔNp63α and CD82 expression in HaCaT and FaDu cells (Figure 7c). It has been reported that CD82 can be downregulated by a β-catenin–reptin complex, thereby increasing the metastatic potential in prostate cancer.38 As GSK3β is an important negative regulator of β-catenin, we investigated whether the effect of GSK3β inhibition on ΔNp63α and CD82 is mediated by β-catenin. Silencing endogenous β-catenin in HaCaT and FaDu cells did not affect ΔNp63α and CD82 levels (Figures 7d and e). In addition, as β-catenin is activated upon inhibition of GSK3β, we treated FaDu cells with BIO upon β-catenin knockdown. As expected, β-catenin accumulated in control cells after BIO treatment; however, BIO treatment downregulated ΔNp63α and CD82 regardless of β-catenin expression. Of note, downregulation of β-catenin alone did not significantly affect CD82 protein levels in FaDu cells (Figure 7f). Hence, these data indicate that GSK3β regulates ΔNp63α expression independently of β-catenin, and suggest that CD82 downregulation upon GSK3β inhibition is likely mediated by downregulated ΔNp63α, rather than as a result of accumulated β-catenin.
Figure 7.
GSK3β modulates ΔNp63α and CD82 expression independently of β-catenin. (a) HaCaT and FaDu cells were treated with 5.0 μM BIO or vehicle control (DMSO) for 24 h. ΔNp63α and CD82 mRNA levels were analyzed by Q-PCR. Results are presented as means and S.E. from three independent experiments performed in triplicate. (b) Whole-cell lysates were subjected to western blotting, as indicated. (c) HaCaT and FaDu cells were infected with two independent lentivirus shRNA against GSK3β (sh3β-1 and sh3β-2) or a control vector (Ctrl), and selected by puromycin resistance. Whole-cell lysates were subjected to western blot analysis. (d and e) HaCaT cells were transfected with siRNA specific for β-catenin (siβ-cat) or a control siRNA (siCtrl). Forty-eight hours after transfection, cells were collected and subsequently subjected to Q-PCR analyses for ΔNp63α, CD82 and β-catenin gene expression (d), or to western blotting (e). Results from Q-PCR are presented as mean and S.E. from two independent experiments performed in triplicate. (f) Forty-eight hours after FaDu cells were transfected with siRNA against β-catenin (siβ-cat) or a control siRNA (siCtrl), cells were treated with 5 μM BIO for an additional 24 h. Whole-cell lysates were subjected to western blot analysis, as shown
GSK3β ablation promotes cancer cell invasion, which is overcome by CD82 expression
As it has been shown that GSK3β can inhibit cell migration,31 we examined whether CD82 has a role in GSK3β-mediated inhibition of cell invasion. Thus, we silenced GSK3β expression and reintroduced CD82 ectopically in FaDu cells (Figure 8a). While GSK3β knockdown markedly promoted cell invasion, concomitant CD82 expression partially, but significantly, reverted cell invasion to lower levels (Figure 8b and Supplementary Figure S5c). These findings indicate that the ΔNp63α-CD82 pathway is regulated by GSK3β, which may contribute to GSK3β inhibitory function in cancer progression.
Figure 8.
GSK3β ablation induces cell invasion via downregulation of CD82. (a and b) FaDu cells were infected with lentivirus expressing shRNA against GSK3β (sh3β-1) or a control (shC), and subsequently infected with recombinant retrovirus expressing either CD82 or a vector control (c). Puromycin-resistant cells were subjected to western blotting (a), or cell invasion assays (b), as described previously. Results from cell invasion assays were quantitated and presented as means and S.E. from three independent experiments. (c) A working model for ΔNp63α inhibition of cell invasion via CD82 and a role for GSK3β signaling. ΔNp63α upregulates CD82 expression, leading to the inhibition of cell invasion and cancer metastasis. GSK3β is important for ΔNp63α expression, either through an unidentified mechanism (as in the case of this study) or through the downregulation of Snail, which inhibits ΔNp63α expression via Snail-mediated suppression of C/EBP.46 GSK3β also inhibits EMT by targeting Snail to proteasomal degradation, thereby suppressing cell invasion and cancer metastasis
Discussion
ΔNp63α has been shown to have an important role in the regulation of cell proliferation, survival and differentiation. In this study, we showed that ΔNp63α functions as an inhibitor of cancer cell invasion via upregulation of CD82, a documented metastasis repressor. First, we showed that ectopic ΔNp63α potently inhibits cell invasion and branching morphogenesis in Matrigel, whereas silencing ΔNp63α markedly promotes cell invasion. Second, we identified CD82 as a direct ΔNp63α transcriptional target gene, and demonstrated that CD82 is critical in ΔNp63α-mediated regulation of cell invasion. Third, inhibition of GSK3β leads to a significant downregulation of both ΔNp63α and CD82 expression independently of β-catenin. Finally, GSK3β ablation results in increased cell invasion, which is significantly reverted by CD82 expression.
Although ΔNp63α lacks a p53-homologous transactivation domain, the unique 14 N-terminal residues in ΔNp63 isoforms possess transactivation activity.39 Here, we showed that ectopic ΔNp63α expression regulated a specific set of genes, many of which were unaffected by mutant derivatives harboring either EEC- or AEC-associated mutations, implying that these mutations result in the loss of normal transactivation activity. This is consistent with a previous study reporting that ΔNp63α mutations in the DBD and in the SAM domain are unable to transactivate skin-specific promoters.40 We also found that ΔNp63α(C306R) and ΔNp63α(C526W) can each regulate a unique set of genes. Interestingly, mutant p53 proteins have been shown to regulate gene expression differentially and to acquire prometastatic functions via multiple molecular mechanisms.41 Similarly, mutant p63 proteins could gain new functions. For example, an R298Q mutation associated with ADULT (acro-dermato-ungual-lacrimal-tooth) syndrome has been shown to increase ΔNp63γ transactivation capacity,42 and G530V and Q536L mutations in the SAM domain have been shown to increase ΔNp63α binding to the transcription factor SATB2, and to render TAp63α more susceptible to inhibition by SATB2 on transactivation of a PERP gene reporter.43
Interestingly, we demonstrated that ΔNp63α strongly inhibits cell invasion, yet we did not detect significant changes in classical epithelial or mesenchymal markers in cells with altered motility. Notably, the specific role of ΔNp63α in EMT is still unclear. While it has been reported that loss of ΔNp63α results in increased N-cadherin expression in urothelial carcinoma21 and that enforced ΔNp63 expression in prostate cancer cells inhibits ZEB1 (a major positive regulator of EMT) expression,44 it has also been shown that ΔNp63α does not affect either N-cadherin or E-cadherin in SCC.45, 46 These findings suggest that ΔNp63α-mediated regulation of cell invasion and metastasis could involve EMT in some cases, whereas it may also be different and independent from classical EMT in other cases.
We found that inhibition of GSK3β decreases ΔNp63α expression and promotes cell invasion independently of β-catenin. GSK3β has been shown to inhibit EMT by targeting Snail for proteasomal degradation, thus releasing E-cadherin from transcriptional repression by Snail and promoting metastasis.31, 32 Snail has been shown to also promote cell invasion by downregulating ΔNp63α in human SCC via suppression of C/EBPα-dependent transcription.46 Moreover, clinical studies showed that increased Snail levels correlate with ΔNp63α downregulation in SCC.47 Thus, these results suggest multiple and complex signaling pathways in the regulation of ΔNp63α (Figure 8d).
By analyzing clinical data, we found that p63 expression decreases as cancers become more aggressive. In particular, metastatic lesions frequently exhibit lower p63 levels than primary tumors, consistent with previous studies showing that p63 expression is decreased in advanced cases of urothelial, breast and bladder carcinomas.48, 49, 50 As gene expression data reflect levels of all p63 isoforms, the specific contribution of each p63 isoform to cancer progression remains unclear. This is particularly important as it has been reported that both homozygous and heterozygous TAp63-specific knockout mice develop spontaneous tumors that are highly metastatic.19 In addition, p53 may have a critical role in TAp63-mediated regulation of metastasis, as mutant p53 has been shown to induce metastasis, at least partly, by inhibiting TAp63.51, 52 More recently, it was shown that TAp63 deletion in a mouse model of KRAS(G12D)-driven pancreatic cancer does not increase metastasis, whereas simultaneous p53 and TAp63 deletion results in aggressive tumors with higher metastatic potential,53 suggesting that the antimetastatic effects of TAp63 may depend on p53 status. Notably, it has been shown that ΔNp63α expression in epithelial cells is 10-fold to several hundred-fold higher than TAp63 expression.20, 37, 54, 55 Thus, any significant changes in p63 gene expression observed in clinical studies of carcinoma samples should predominantly reflect changes in ΔNp63α levels, which would strongly implicate that ΔNp63α has a role in inhibition of cancer metastasis. In support of this notion, independent groups have shown that enforced expression of ΔNp63α in spindle carcinoma or prostate cancer cells inhibits metastasis to the lungs when injected intravenously into recipient mice.44, 51 Recently, we showed that ΔNp63α, but neither TAp63 nor ΔNp63γ, can revert invasion in cells with pan-p63 ablation.56 In addition, we showed that ΔNp63α deficiency in mutant Her2/Neu-transformed mammary epithelial cells markedly enhances metastatic dissemination in nude mice.56 Taken together, these studies strongly suggest that ΔNp63α is likely responsible for inhibiting cell invasion and metastasis in cancers of epithelial origin. Clearly, given the great variation in p63 protein expression between cell types, it is likely that the specific function of each p63 isoform is cell context-dependent.
Similar to ΔNp63α, CD82 downregulation has been found to be clinically associated with metastasis in a variety of cancers.57, 58, 59, 60 Analogously, CD82 overexpression has been shown to suppress metastatic spread in animal models of melanoma, breast and prostate tumors.61, 62, 63 We revealed that CD82 is an important mediator of ΔNp63α function toward inhibiting cell invasion and metastasis. First, ΔNp63α directly transactivates CD82 expression independently of p53 status, as silencing of ΔNp63α reduces CD82 expression in both MCF-10A (WT p53) and FaDu cells (truncated p53 and DNA-binding deficient p53[R248L]64). Second, CD82 is an important mediator of ΔNp63α-mediated inhibition of cell invasion, as silencing of CD82 significantly reverses inhibition of cell invasion induced by ectopic ΔNp63α expression. Conversely, ectopic CD82 expression can largely revert enhanced cell invasion elicited by ΔNp63α ablation. However, rescuing by CD82 is incomplete, suggesting that CD82 is an important player, but not the sole effector mediating ΔNp63α function in cell invasion. Third, we found a significant correlation between ΔNp63α and CD82 expression in prostate cancer and melanoma. Taken together, these data suggest that CD82 regulation by ΔNp63α may have a clinically relevant and likely important role in the regulation of cancer metastasis.
It is now clear that ΔNp63α regulates multiple genes involved in cell migration and invasion. For example, silencing ΔNp63α in SCC cells has been shown to upregulate genes involved in cell motility, such as N-cadherin, L1 adhesion molecule and Wnt-5A.20 ΔNp63α has also been shown to inhibit invasion via induction of Id-3 expression, which subsequently inhibits Ets-1-mediated MMP2 transcription.45 More recently, we showed that ΔNp63α negatively regulates Erk2 signaling by upregulating MAP kinase phosphatase 3 expression to inhibit cancer cell migration, invasion and metastasis.56 Moreover, here we demonstrated that inhibition of GSK3β impacts on the ΔNp63α–CD82 axis in the regulation of cell invasion. Thus, ΔNp63α seems to act as an integrator of multiple signaling pathways that impact on cell migration and invasion.
Materials and Methods
Cell culture, drug treatment and proliferation analysis
Human non-transformed mammary epithelial MCF-10A cells were maintained in 1 : 1 mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F12 medium with reduced Ca2+ (0.04 mM; Invitrogen Inc., Carlsbad, CA, USA), 20 ng/ml epidermal growth factor (Invitrogen), 100 ng/ml cholera toxin (Sigma, St. Louis, MO, USA), 10 μg/ml insulin (Sigma), 500 ng/ml (95%) hydrocortisone (Sigma) and 5% of Chelex-treated horse serum (Invitrogen). Human-immortalized keratinocyte HaCaT and head and neck SCC FaDu cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen). Human breast cancer Hs-578T cells were cultured in DMEM supplemented with 10% FBS and 10 μg/ml insulin. All cells were grown in media containing 1% penicillin-G/streptomycin sulfate at 37 °C in a humidified incubator under 5% CO2. For cell proliferation analysis, equal numbers of cells were seeded and grown under normal growth conditions. Every 24 h, cells were counted with a hemacytometer.
Plasmid construction, viral infections and RNA interference
To generate pMSCV-ΔNp63α, a PCR fragment containing the mouse ΔNp63α gene using murine pcDNA3-ΔNp63α as a template was subcloned into the retroviral vector pMSCV-puro (Clontech, Palo Alto, CA, USA) digested with BglII and HpaI. The ΔNp63α point mutant derivatives, C306R and C526W, were generated using QuikChange Site-Directed Mutagenesis Kit (Stratagene Inc., La Jolla, CA, USA). To construct pMSCV-CD82, the fragment containing the human CD82 gene from pcDNA3-CD8265 was subcloned into the retroviral vector pMSCV-puro digested with BglII and XhoI. Lentivirus expressing a short hairpin RNA (shRNA) specific for CD82 or GSK3β were constructed using a pLKO.1 vector backbone (AddGene, Cambridge, MA, USA). Targeting shRNA sequences are listed in Supplementary Table S4. All constructs were confirmed by DNA sequencing.
For amplification and preparation of lentivirus, 293FT cells (5 × 106) were co-transfected with 6 μg of psPAX2/pMD2.G (lentiviral packaging plasmids) and either 6 μg of a pLKO.1 shRNA plasmid or control using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, culture media were collected, filtered and subjected to ultracentrifugation at 25 000 r.p.m. for 90 min at 4 °C. Pellets were resuspended in 200 μl DMEM and aliquots were stored at −80 °C. Cells in 6-well plates were infected with 50 μl of recombinant virus particles suspended in 1 ml culture media containing 10 μg/ml polybrene. Forty-eight hours postinfection, cells were selected by puromycin resistance (1 μg/ml for HaCaT, 2 μg/ml for MCF-10A and FaDu and 4 μg/ml for Hs-578T) for an additional 48 h before subsequent experiments. To amplify retrovirus, a process similar to lentivirus was used, except using the pol/gag/env retroviral packaging system for transfection.
β-Catenin small interfering RNA (siRNA) was designed and synthesized by Qiagen Inc. (Valencia, CA, USA). Cells were transfected with 200 nM siRNA using Lipofectamine 2000 in 60 mm cell culture dishes according to the manufacturer's instructions. Twelve to sixteen hours after transfection, media were changed to 5 ml normal growth media. Targeting siRNA sequences are listed in Supplementary Table S4.
Western blot analysis
Cells were lysed in RIPA buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, 2 mg/ml leupeptin and 2 mg/ml aprotinin). Equal amounts of total protein (40–100 μg) were separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (NEN LifeSciences, Waltham, MA, USA) and hybridized to an appropriate primary antibody and HRP-conjugated secondary antibody for subsequent detection by enhanced chemiluminescence (Amersham, Piscataway, NJ, USA). Specific antibodies for p63 (4A4), CD82 (C-16), E-cadherin (H-108), N-cadherin (13A9), β-actin (C-11) and p53 (DO-1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies specific for p21 (SXM-30) and β-catenin (610153) were purchased from BD Biosciences (San Jose, CA, USA). Antibodies for GSK3β (F9332) and vimentin (clone V9) were obtained from Cell Signaling (Danvers, MA, USA) and Lab Vision (Fremont, CA, USA), respectively. Goat anti-mouse IgG-HRP (sc-2005), goat anti-rabbit IgG-HRP (sc-2004) and donkey anti-goat IgG-HRP (sc-2020) secondary antibodies were obtained from Santa Cruz Biotechnology.
Q-PCR
Total RNA was extracted from cultured cells using TRIzol (Gibco, Life Technologies, Rockville, MD, USA) according to the manufacturer's instruction. RNA (5 μg) was reverse transcribed using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen), followed by Q-PCR in 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using QuantiTect SYBR Green PCR Kit (Qiagen, Venlo, Netherlands). The reactions were carried out in a 96-well plate at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 58 °C for 20 s and 72 °C for 30 s. GAPDH expression was used as an endogenous control to normalize target gene expression by the ΔΔCt method. Oligonucleotide primers used for Q-PCR are listed in Supplementary Table S4.
ChIP assays
Chromatin immunoprecipitation (ChIP) assays were performed by using EZ ChIP Kit (Upstate Biotechnology Inc., Waltham, MA, USA). Briefly, cells were crosslinked to chromatin with 1% formaldehyde for 10 min at 37 °C, the p63–DNA complexes were immunoprecipitated using antibodies against p63 (4A4) or anti-mouse IgG, recovered with protein A/G beads and treated with proteinase K to purify DNA. PCR was performed to amplify fragments of the CD82 promoter sequences. The primers targeting CD82 promoter are listed in Supplementary Table S4.
Assays for cell invasion and Matrigel outgrowth
Cell invasion was measured using 24-well, 8 μm polycarbonate transwell systems coated with Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, Hs-578T (1.0 × 105) or FaDu (2.0 × 105) cells suspended in serum-free DMEM were seeded into the inner chamber, and DMEM containing 10% FBS was used as a chemoattractant in the outside chamber. Twenty-four hours after seeding, noninvasive cells were removed with a cotton swab, and invading cells were fixed and stained with 0.5% crystal violet in 70% ethanol for 10 min. Cells were counted in four random fields under a Zeiss light microscope (Carl Zeiss, Jena, Germany) at × 100 magnification. Alternatively, stained cells were lyzed with 2% SDS buffer (in 1 × PBS) and subjected to spectrophotometric analysis at 570 nm.
Matrigel outgrowth was measured in Matrigel-coated 96-well plates. For the bottom layer, 40 μl of 6.3 mg/ml Matrigel in serum-free DMEM was added into a 96-well tissue culture plate and incubated at 37 °C for 30 min to allow the Matrigel to solidify. Two microliters of Hs-578T cell suspension (1.0 × 103 cells) in serum-free medium (DMEM) were mixed with ice-cold 40 μl Matrigel and plated onto the solidified bottom layer. The plates were then incubated at 37 °C for 30 min to allow the Matrigel to solidify. Growth medium was added into the wells and was replaced with fresh medium every 3 days. Following incubation at 37 °C for 5–7 days, cell morphology was photographed using a Zeiss Axiovert 200M light microscope (Carl Zeiss) at × 100 magnification.
Microarray and statistical analysis
Hs-578T cells were infected with retrovirus-expressing WT mouse ΔNp63α, a mutant derivative (ΔNp63α-C306R or ΔNp63α-C526W), or an empty vector. Infected cells were selected by puromycin treatment (4.0 μg/ml). Total RNA was isolated from puromycin-resistant cells and subjected to cDNA array analysis using U133Av2.0 human gene chip (Affymetrix, Santa Clara, CA, USA). Two independent RNA samples were analyzed. The results were analyzed using a mixed-model analysis of variance (ANOVA). Genes showing differential expression (two-fold changes or more, P-value <0.05) in WT ΔNp63α samples relative to control were classified ontologically using DAVID Bioinformatics Resources 6.7.66, 67 Statistical significance was assessed by Student's t-test, ANOVA or Pearson's correlation analysis as appropriate.
Bioinformatic analysis of gene expression
Oncomine (Compendia Bioscience, Ann Arbor, MI, USA) was used for analysis and visualization.
Acknowledgments
We thank Dr. Frank McKeon for providing pcDNA-p63 plasmid and Dr. Xiaoqi Wang for pcDNA-CD82 plasmid. This work was supported by the National Key Basic Research Program (973 Program) of China (2012CB910700), National Natural Science Foundation of China (NSFC) Grants (81330054 and 31171362) and NIH Grant (CA79804) to Z-XX, and NSFC Grant (31350110216) to JB.
Glossary
- AEC
ankyloblepharon-ectodermal dysplasia clefting
- BIO
6-bromoindirubin-3′-oxime
- CD82
cluster of differentiation 82
- DBD
DNA-binding domain
- EEC
ectrodactyly ectodermal dysplasia clefting
- EMT
epithelial-to-mesenchymal transition
- GSK3
glycogen synthase kinase 3
- Q-PCR
quantitative polymerase chain reaction
- SAM
sterile α motif
- SCC
squamous cell carcinoma
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- WT
wild type
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Melino
Supplementary Material
References
- 1Bergholz J, Xiao Z-X. Role of p63 in development, tumorigenesis and cancer progression. Cancer Microenviron 2012; 5: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Candi E, Cipollone R, Rivetti di Val Cervo P, Gonfloni S, Melino G, Knight R. P63 in epithelial development. Cell Mol Life Sci 2008; 65: 3126–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 1999; 99: 143–153. [DOI] [PubMed] [Google Scholar]
- 4McGrath JA, Duijf PH, Doetsch V, Irvine AD, de Waal R, Vanmolkot KR et al. Hay–Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum Mol Genet 2001; 10: 221–229. [DOI] [PubMed] [Google Scholar]
- 5Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. P63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999; 398: 708–713. [DOI] [PubMed] [Google Scholar]
- 6Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT et al. P63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999; 398: 714–718. [DOI] [PubMed] [Google Scholar]
- 7Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009; 15: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 8Suh E-K, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z et al. P63 protects the female germ line during meiotic arrest. Nature 2006; 444: 624–628. [DOI] [PubMed] [Google Scholar]
- 9Senoo M, Pinto F, Crum CP, McKeon F. P63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 2007; 129: 523–536. [DOI] [PubMed] [Google Scholar]
- 10Shalom-Feuerstein R, Lena AM, Zhou H, De La Forest Divonne S, Van Bokhoven H, Candi E et al. ΔNp63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ 2010; 18: 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Hagiwara K, McMenamin MG, Miura K, Harris CC. Mutational analysis of the p63/p73L/p51/p40/CUSP/KET gene in human cancer cell lines using intronic primers. Cancer Res 1999; 59: 4165–4169. [PubMed] [Google Scholar]
- 12Sunahara M, Shishikura T, Takahashi M, Todo S, Yamamoto N, Kimura H et al. Mutational analysis of p51A/TAp63gamma, a p53 homolog, in non-small cell lung cancer and breast cancer. Oncogene 1999; 18: 3761–3765. [DOI] [PubMed] [Google Scholar]
- 13Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA 2000; 97: 5462–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res 2003; 63: 7113–7121. [PubMed] [Google Scholar]
- 15DeYoung MP, Johannessen CM, Leong C-O, Faquin W, Rocco JW, Ellisen LW. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res 2006; 66: 9362–9368. [DOI] [PubMed] [Google Scholar]
- 16Rocco JW, Leong C-O, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 2006; 9: 45–56. [DOI] [PubMed] [Google Scholar]
- 17Keyes WM, Pecoraro M, Aranda V, Vernersson-Lindahl E, Li W, Vogel H et al. ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell 2011; 8: 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Flores ER, Sengupta S, Miller JB, Newman JJ, Bronson R, Crowley D et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 2005; 7: 363–373. [DOI] [PubMed] [Google Scholar]
- 19Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin Y-L et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 2010; 467: 986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Barbieri CE, Tang LJ, Brown KA, Pietenpol JA. Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res 2006; 66: 7589–7597. [DOI] [PubMed] [Google Scholar]
- 21Fukushima H, Koga F, Kawakami S, Fujii Y, Yoshida S, Ratovitski E et al. Loss of DeltaNp63alpha promotes invasion of urothelial carcinomas via N-cadherin/Src homology and collagen/extracellular signal-regulated kinase pathway. Cancer Res 2009; 69: 9263–9270. [DOI] [PubMed] [Google Scholar]
- 22Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005; 6: 801–811. [DOI] [PubMed] [Google Scholar]
- 23Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995; 268: 884–886. [DOI] [PubMed] [Google Scholar]
- 24Zhang XA, He B, Zhou B, Liu L. Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem 2003; 278: 27319–27328. [DOI] [PubMed] [Google Scholar]
- 25Odintsova E, Sugiura T, Berditchevski F. Attenuation of EGF receptor signaling by a metastasis suppressor, the tetraspanin CD82/KAI-1. Curr Biol 2000; 10: 1009–1012. [DOI] [PubMed] [Google Scholar]
- 26Odintsova E, Voortman J, Gilbert E, Berditchevski F. Tetraspanin CD82 regulates compartmentalisation and ligand-induced dimerization of EGFR. J Cell Sci 2003; 116(Part 22): 4557–4566. [DOI] [PubMed] [Google Scholar]
- 27Marreiros A, Dudgeon K, Dao V, Grimm M-O, Czolij R, Crossley M et al. KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cells. Oncogene 2005; 24: 637–649. [DOI] [PubMed] [Google Scholar]
- 28Mashimo T, Watabe M, Hirota S, Hosobe S, Miura K, Tegtmeyer PJ et al. The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc Natl Acad Sci USA 1998; 95: 11307–11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. GSK-3β: a bifunctional role in cell death pathways. Int J Cell Biol 2012; 2012: 930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004; 5: 691–701. [DOI] [PubMed] [Google Scholar]
- 31Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol 2004; 6: 931–940. [DOI] [PubMed] [Google Scholar]
- 32Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84–89. [DOI] [PubMed] [Google Scholar]
- 33Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ 2006; 13: 1037–1047. [DOI] [PubMed] [Google Scholar]
- 34Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V et al. Pp63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998; 2: 305–316. [DOI] [PubMed] [Google Scholar]
- 35Fu SW, Schwartz A, Stevenson H, Pinzone JJ, Davenport GJ, Orenstein JM et al. Correlation of expression of BP1, a homeobox gene, with estrogen receptor status in breast cancer. Breast Cancer Res 2003; 5: R82–R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal 2009; 21: 196–211. [DOI] [PubMed] [Google Scholar]
- 37Carroll DK, Carroll JS, Leong C-O, Cheng F, Brown M, Mills AA et al. P63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 2006; 8: 551–561. [DOI] [PubMed] [Google Scholar]
- 38Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C et al. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature 2005; 434: 921–926. [DOI] [PubMed] [Google Scholar]
- 39Helton ES, Zhu J, Chen X. The unique NH2-terminally deleted (DeltaN) residues, the PXXP motif, and the PPXY motif are required for the transcriptional activity of the DeltaN variant of p63. J Biol Chem 2006; 281: 2533–2542. [DOI] [PubMed] [Google Scholar]
- 40Browne G, Cipollone R, Lena AM, Serra V, Zhou H, van Bokhoven H et al. Differential altered stability and transcriptional activity of ΔNp63 mutants in distinct ectodermal dysplasias. J Cell Sci 2011; 124(Part 13): 2200–2207. [DOI] [PubMed] [Google Scholar]
- 41Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012; 26: 1268–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Duijf PHG, Vanmolkot KRJ, Propping P, Friedl W, Krieger E, McKeon F et al. Gain-of-function mutation in ADULT syndrome reveals the presence of a second transactivation domain in p63. Hum Mol Genet 2002; 11: 799–804. [DOI] [PubMed] [Google Scholar]
- 43Chung J, Grant RI, Kaplan DR, Irwin MS. Special AT-rich binding protein-2 (SATB2) differentially affects disease-causing p63 mutant proteins. J Biol Chem 2011; 286: 40671–40680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci USA 2012; 109: 15312–15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Higashikawa K, Yoneda S, Tobiume K, Saitoh M, Taki M, Mitani Y et al. DeltaNp63alpha-dependent expression of Id-3 distinctively suppresses the invasiveness of human squamous cell carcinoma. Int J Cancer 2009; 124: 2837–2844. [DOI] [PubMed] [Google Scholar]
- 46Higashikawa K, Yoneda S, Tobiume K, Taki M, Shigeishi H, Kamata N. Snail-induced down-regulation of DeltaNp63alpha acquires invasive phenotype of human squamous cell carcinoma. Cancer Res 2007; 67: 9207–9213. [DOI] [PubMed] [Google Scholar]
- 47Herfs M, Hubert P, Suarez-Carmona M, Reschner A, Saussez S, Berx G et al. Regulation of p63 isoforms by Snail and Slug transcription factors in human squamous cell carcinoma. Am J Pathol 2010; 176: 1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Koga F, Kawakami S, Fujii Y, Saito K, Ohtsuka Y, Iwai A et al. Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res 2003; 9: 5501–5507. [PubMed] [Google Scholar]
- 49Urist MJ, Di Como CJ, Lu M-L, Charytonowicz E, Verbel D, Crum CP et al. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol 2002; 161: 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Wang X, Mori I, Tang W, Nakamura M, Nakamura Y, Sato M et al. P63 expression in normal, hyperplastic and malignant breast tissues. Breast Cancer 2002; 9: 216–219. [DOI] [PubMed] [Google Scholar]
- 51Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B et al. A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 2009; 137: 87–98. [DOI] [PubMed] [Google Scholar]
- 52Muller PAJ, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139: 1327–1341. [DOI] [PubMed] [Google Scholar]
- 53Tan EH, Morton JP, Timpson P, Tucci P, Melino G, Flores ER et al. Functions of TAp63 and p53 in restraining the development of metastatic cancer. Oncogene 2014; 33: 3325–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Barbareschi M, Pecciarini L, Cangi MG, Macrì E, Rizzo A, Viale G et al. P63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol 2001; 25: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 55Romano R-A, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One 2009; 4: e5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Bergholz J, Zhang Y, Wu J, Meng L, Walsh EM, Rai A et al. ΔNp63α regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene 2014; 33: 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Liu F-S, Dong J-T, Chen J-T, Hsieh Y-T, Ho ES-C, Hung M-J et al. KAI1 metastasis suppressor protein is down-regulated during the progression of human endometrial cancer. Clin Cancer Res 2003; 9: 1393–1398. [PubMed] [Google Scholar]
- 58Liu FS, Chen JT, Dong JT, Hsieh YT, Lin AJ, Ho ES et al. KAI1 metastasis suppressor gene is frequently down-regulated in cervical carcinoma. Am J Pathol 2001; 159: 1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Liu FS, Dong JT, Chen JT, Hsieh YT, Ho ES, Hung MJ. Frequent down-regulation and lack of mutation of the KAI1 metastasis suppressor gene in epithelial ovarian carcinoma. Gynecol Oncol 2000; 78: 10–15. [DOI] [PubMed] [Google Scholar]
- 60Yang X, Wei L, Tang C, Slack R, Montgomery E, Lippman M. KAI1 protein is down-regulated during the progression of human breast cancer. Clin Cancer Res 2000; 6: 3424–3429. [PubMed] [Google Scholar]
- 61Phillips KK, White AE, Hicks DJ, Welch DR, Barrett JC, Wei LL et al. Correlation between reduction of metastasis in the MDA-MB-435 model system and increased expression of the Kai-1 protein. Mol Carcinogen 1998; 21: 111–120. [PubMed] [Google Scholar]
- 62Takaoka A, Hinoda Y, Sato S, Itoh F, Adachi M, Hareyama M et al. Reduced invasive and metastatic potentials of KAI1-transfected melanoma cells. Jpn J Cancer Res 1998; 89: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Yang X, Welch DR, Phillips KK, Weissman BE, Wei LL. KAI1, a putative marker for metastatic potential in human breast cancer. Cancer Lett 1997; 119: 149–155. [DOI] [PubMed] [Google Scholar]
- 64Eicheler W, Zips D, Dörfler A, Grénman R, Baumann M. Splicing mutations in TP53 in human squamous cell carcinoma lines influence immunohistochemical detection. J Histochem Cytochem 2002; 50: 197–204. [DOI] [PubMed] [Google Scholar]
- 65Zhou B, Liu L, Reddivari M, Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res 2004; 64: 7455–7463. [DOI] [PubMed] [Google Scholar]
- 66Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 67Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.