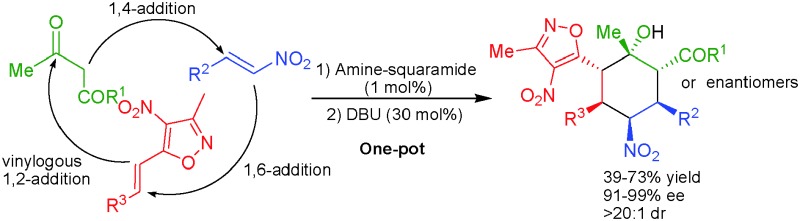
 A new stereoselective organocatalyzed one-pot 1,4-/1,6-/1,2-addition reaction sequentially catalyzed by low loading of a squaramide and an achiral base provide a direct entry to isoxazole bearing cyclohexanes with six contiguous stereogenic centers in good yields and excellent stereoselectivities.
A new stereoselective organocatalyzed one-pot 1,4-/1,6-/1,2-addition reaction sequentially catalyzed by low loading of a squaramide and an achiral base provide a direct entry to isoxazole bearing cyclohexanes with six contiguous stereogenic centers in good yields and excellent stereoselectivities.
Abstract
An unprecedented stereoselective organocatalytic one-pot 1,4-/1,6-/1,2-addition sequence between β-dicarbonyl compounds, β-nitroalkenes and 4-nitro-5-styrylisoxazoles sequentially catalyzed by low loading of a squaramide catalyst and an achiral base has been developed. The protocol opens an efficient entry to isoxazole bearing cyclohexanes with six consecutive stereogenic centers including one tetrasubstituted carbon in good yields and excellent diastereo- and enantioselectivities.
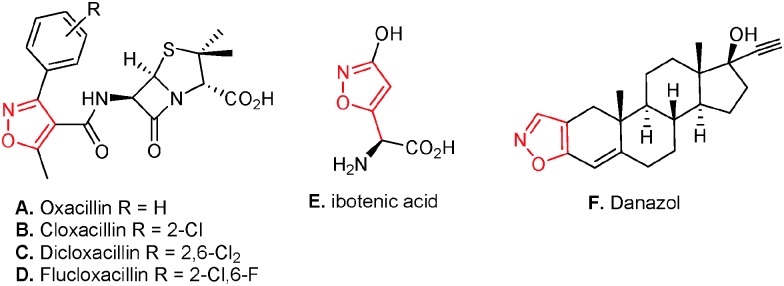
Over the last ten years, asymmetric organocatalytic cascade reactions have emerged as a powerful strategy for the synthesis of complex molecules bearing multiple stereogenic centers in a highly stereocontrolled fashion.1 These one-pot organocatalytic reactions were successfully employed for the creation of cyclohexane ring systems bearing up to six stereocenters.2 Most of these triple cascade reactions are governed by more common 1,4-/1,4-/1,2 addition sequences. Another important class of addition reactions involving the enantioselective 1,6-addition to control the formation of a remote stereocenter is more challenging and less explored in comparison to the other addition variants.3 Moreover, organocatalytic cascade reactions using all possible types of addition reactions, i.e. 1,4-/1,6-/1,2-addition reactions, are not known so far. Hence we took the challenge to develop a new stereoselective one-pot organocascade sequence using 1,4-/1,6-/1,2-additions (Scheme 1).
Scheme 1. Enantioselective strategies for the construction of cyclohexane rings bearing multiple stereogenic centers.
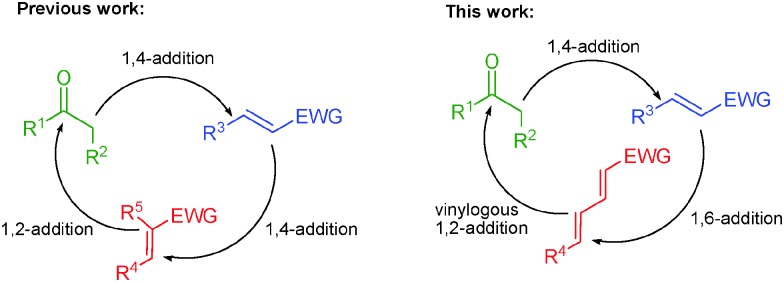
In addition, the isoxazole core is present in various important naturally occurring and synthetic bioactive molecules (Fig. 1). For example, compounds A–D are β-lactamase-resistant antibiotics,4 while an isoxazole containing natural product E is a powerful neurotoxin, which is used as a brain-lesioning agent.5 A synthetic androgenic steroid danazol D bearing an isoxazole ring suppresses the production of gonadotrophins and also has some weak androgenic effects.6 Moreover, isoxazoles serve as precursors for the synthesis of various synthetically useful organic compounds.7 Thus, the development of efficient asymmetric methods for the synthesis of isoxazole ring containing molecules can provide a new series of potentially bioactive molecules.
Fig. 1. Enantiopure drugs and bioactive natural products bearing an isoxazole ring.
Recently, organo- and metal-catalyzed 1,6-additions to 4-nitro-5-styrylisoxazoles emerged as an efficient method to generate enantiopure isoxazole derivatives bearing one or two stereocenters.8,9 However, the 4-nitro-5-styrylisoxazoles remained less explored substrates in stereoselective cascade reactions.9d,g Very recently, Jørgensen's group utilized 4-nitro-5-styrylisoxazoles in trienamine-mediated asymmetric [4+2] cycloaddition reactions to afford cyclohexene products bearing three vicinal stereocenters.10 Herein we report a novel cascade reaction involving a 1,4-/1,6-/vinylogous 1,2-addition sequence to access enantiopure cyclohexane rings bearing as many as six contiguous stereogenic centers, sequentially catalyzed by low loading of a cinchona derived squaramide11 and an achiral base.
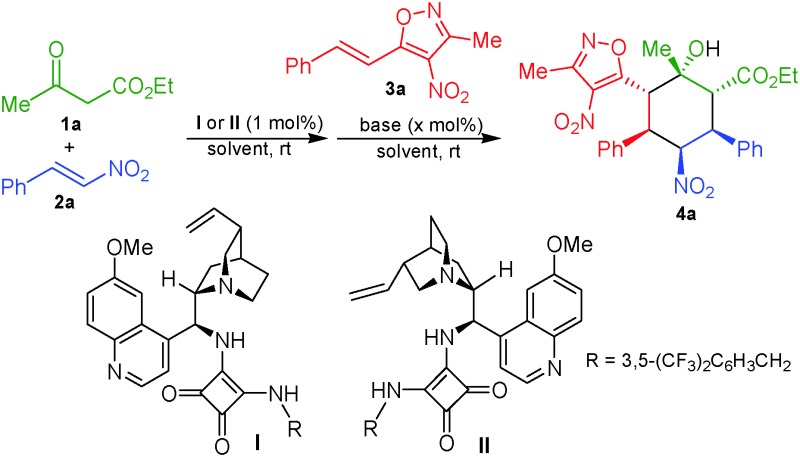
Initially, we started our investigation with a squaramide I (1 mol%) catalyzed one-pot three component reaction between ethyl acetoacetate (1a), β-nitrostyrene (2a) and 4-nitro-5-styrylisoxazole (3a) (Table 1, entry 1). However our attempt to obtain the desired cyclohexane ring failed completely, and only the formation of the Michael adduct was observed.12 We envisaged that the squaramide catalyst was not enough active to generate a nitronate anion in the corresponding Michael adduct to initiate a domino 1,6-/vinylogous 1,2-addition sequence. Thus, a sequential reaction was performed involving a squaramide I catalyzed Michael addition of the β-ketoester 1a to the β-nitrostyrene 2a, followed by the addition of 3a and a catalytic amount of DBU (20 mol%) (entry 2). To our delight, the desired cyclohexane 4a was obtained in 46% yield with excellent stereoselectivity (98% ee and >20 : 1 dr). Further optimization of the reaction conditions by screening different solvents (entries 3–5) and bases (entries 6–11) showed that 30 mol% of DBU in CH2Cl2 provides a maximum yield of 62% and excellent stereoselectivity (entry 6). The use of a quinidine derived squaramide catalyst II led to the opposite enantiomer of the cyclohexane ent-4a with a similar yield, ee and dr (entry 12).
Table 1. Optimizations of the reaction conditions a .
| |||||
| Entry | Base (x mol%) | Solvent | Time b (h) | Yield c (%) | ee d (%) |
| 1 e | — | CH2Cl2 | 24 | — | — |
| 2 | DBU (20) | CH2Cl2 | 24 + 24 | 46 | 98 |
| 3 | DBU (20) | CHCl3 | 24 + 24 | 35 | 98 |
| 4 | DBU (20) | Toluene | 24 + 24 | 44 | 98 |
| 5 | DBU (30) | THF | 24 + 24 | 36 | 98 |
| 6 f | DBU (30) | CH2Cl2 | 24 + 48 | 62 | 98 |
| 7 f | DBN (30) | CH2Cl2 | 24 + 48 | 36 | 97 |
| 8 f | TEA (30) | CH2Cl2 | 24 + 48 | Traces | n.d. |
| 9 f | TBD (30) | CH2Cl2 | 24 + 48 | 29 | 98 |
| 10 f | DABCO (30) | CH2Cl2 | 24 + 48 | — | — |
| 11 f | Piperidine (30) | CH2Cl2 | 24 + 48 | Traces | n.d. |
| 12 f | DBU (30) | CH2Cl2 | 24 + 48 | 58 | 96 g |
aReaction conditions: 0.2 mmol of 1a, 0.2 mmol of 2a, 1 mol% of I, 0.24 mmol of 3a and x mol% of base (0.1 M in solvent).
bTime in hours for both reaction steps.
cYield of isolated 4a after column chromatography.
dEnantiomeric excess of the major diastereomer (>20 : 1 dr) determined by HPLC analysis on a chiral stationary phase.
eAll the reactants were added in one step.
f2 equivalents of 3a were used.
gee value of ent-4a synthesized by using catalyst II.
Once equipped with optimized reaction conditions, we evaluated the substrate scope at a 0.5 mmol scale of the β-dicarbonyl compounds and the β-nitrostyrenes (Table 2). The various nitroalkenes bearing electron withdrawing and electron donating groups gave rise to the corresponding isoxazole products 4b–e in 55–67% yield and excellent stereoselectivities (>20 : 1 dr and 93–99% ee). The nitroalkenes bearing a heteroaromatic group also worked well in this cascade sequence to provide the desired product 4f in 61% yield and 91% ee. Further screening of different 4-nitro-5-styrylisoxazoles bearing electron withdrawing and electron releasing substituents on the aryl ring as well as heteroaryl group provided a direct access to the corresponding cyclohexanes 4g–m in good yields and high enantioselectivities (95–99% ee). The methyl acetoacetate and acetyl acetone were also tolerated under this one-pot protocol to give rise to the respective products 4n and 4o in good yields and excellent stereoselectivities. Employing a pseudo-enantiomeric amino-squaramide catalyst II successfully led to the formation of the enantiomers of 4a–f, 4h and 4l in very good yields (51–69%) and again excellent asymmetric inductions (>20 : 1 dr and 95–98% ee).
Table 2. Substrate scope a .
| |||||
| 4/ent-4 | R1 | R2 | R3 | Yield b (%) | ee c (%) |
| 4a | OEt | Ph | Ph | 61 | 98 |
| 4b | OEt | 4-FC6H4 | Ph | 64 | 99 |
| 4c | OEt | 4-ClC6H4 | Ph | 55 | 99 |
| 4d | OEt | 4-MeC6H4 | Ph | 63 | 93 |
| 4e | OEt | 4-MeOC6H4 | Ph | 67 | 97 |
| 4f | OEt | 2-Thienyl | Ph | 61 | 91 |
| 4g | OEt | Ph | 4-FC6H4 | 60 | 98 |
| 4h | OEt | Ph | 4-ClC6H4 | 61 | 97 |
| 4i | OEt | Ph | 3-ClC6H4 | 69 | 97 |
| 4j | OEt | Ph | 4-MeC6H4 | 73 | 99 |
| 4k | OEt | Ph | 2-MeC6H4 | 49 | 95 |
| 4l | OEt | Ph | 4-MeOC6H4 | 39 | 96 |
| 4m | OEt | Ph | 2-Thienyl | 50 | 97 |
| 4n | OMe | Ph | Ph | 58 | 97 |
| 4o | Me | Ph | Ph | 50 | 96 |
| ent-4a | OEt | Ph | Ph | 69 | 96 |
| ent-4b | OEt | 4-FC6H4 | Ph | 63 | 97 |
| ent-4c | OEt | 4-ClC6H4 | Ph | 51 | 95 |
| ent-4d | OEt | 4-MeC6H4 | Ph | 64 | 98 |
| ent-4e | OEt | 4-MeOC6H4 | Ph | 66 | 95 |
| ent-4f | OEt | 2-Thienyl | Ph | 59 | 96 |
| ent-4h | OEt | Ph | 4-ClC6H4 | 60 | 97 |
| ent-4k | OEt | Ph | 2-MeC6H4 | 50 | 96 |
aReaction conditions: 0.5 mmol of 1, 0.5 mmol of 2, 1 mol% of I (entry 1–17) or II, 1.0 mmol of 3 and 30 mol% of DBU (0.1 M in CH2Cl2).
bYield of isolated product after column chromatography.
cEnantiomeric excess of the major diastereomer determined by HPLC analysis on a chiral stationary phase.
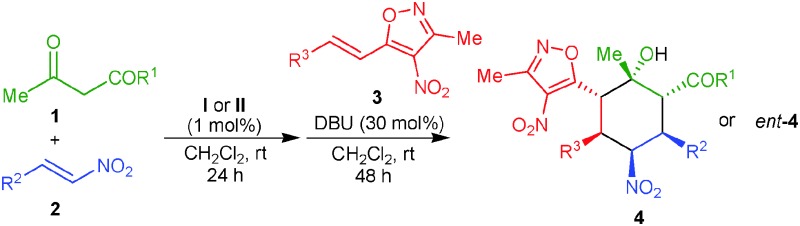
The absolute configuration of the products 4a–o can be assigned as (1S), (2S), (3R), (4S), (5S) and (6R) on the basis of the X-ray crystallographic analysis of 4a (Fig. 2).13
Fig. 2. X-ray structure of 4a.
To demonstrate the practical and preparative application of this new organocascade 1,4-/1,6-/1,2-addition sequence, we performed a gram-scale reaction between 1a, 2a and 3a using a lower loading (0.5 mol%) of the squaramide I (Scheme 2). The desired product 4a was obtained in 57% yield with unchanged ee and dr values. The enantiomeric purity could be enriched to >99% ee after a single crystallization of the product.
Scheme 2. Gram-scale 1,4-/1,6-/1,2-addition sequence.
In conclusion, we have developed a novel 1,4-/1,6-/1,2-addition cascade sequence catalyzed sequentially by low loading of a cinchona-derived squaramide and a commercially available achiral base to afford a series of highly substituted cyclohexane derivatives bearing six consecutive stereogenic centers in good yields and excellent stereoselectivities. The enantiomeric cyclohexanes are also easily synthesized on a same level of asymmetric induction by employing a pseudo-enantiomeric squaramide catalyst. A successful gram-scale reaction documents the preparative utility of this organocascade protocol.
Support from the European Research Council (ERC Advanced Grant 320493 “DOMINOCAT”) is gratefully acknowledged. We thank BASF SE for the donation of chemicals.
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1037530. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc09730k
References
- For selected reviews on organocatalytic domino/cascade reactions, see: ; (a) Enders D., Grondal C., Hüttl M. R. M. Angew. Chem., Int. Ed. 2007;46:1570. doi: 10.1002/anie.200603129. [DOI] [PubMed] [Google Scholar]; (b) Yu X., Wang W. Org. Biomol. Chem. 2008;6:2037. doi: 10.1039/b800245m. [DOI] [PubMed] [Google Scholar]; (c) Grondal C., Jeanty M., Enders D. Nat. Chem. 2010;2:167. doi: 10.1038/nchem.539. [DOI] [PubMed] [Google Scholar]; (d) Albrecht Ł., Jiang H., Jørgensen K. A. Angew. Chem., Int. Ed. 2011;50:8492. doi: 10.1002/anie.201102522. [DOI] [PubMed] [Google Scholar]; (e) Moyano A., Rios R. Chem. Rev. 2011;111:4703. doi: 10.1021/cr100348t. [DOI] [PubMed] [Google Scholar]; (f) Grossmann A., Enders D. Angew. Chem., Int. Ed. 2012;51:314. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]; (g) Pellissier H. Adv. Synth. Catal. 2012;354:237. [Google Scholar]; (h) Volla C. M. R., Atodiresei I., Rueping M., Chem. Rev., 2014, 114 , 2390 , ; For a recent highlight, see: . [DOI] [PubMed] [Google Scholar]; (i) Chauhan P., Enders D. Angew. Chem., Int. Ed. 2014;53:1485. doi: 10.1002/anie.201309952. [DOI] [PubMed] [Google Scholar]
- For a recent review, see: ; (a) Goudedranche S., Raimondi W., Bugaut X., Constantieux T., Bonne D., Rodriguez J., Synthesis, 2013. , 1909 , ; for selected examples, see: . [Google Scholar]; (b) Enders D., Hüttl M. R. M., Grondal C., Raabe G. Nature. 2006;441:861. doi: 10.1038/nature04820. [DOI] [PubMed] [Google Scholar]; (c) Enders D., Hüttl M. R. M., Runsink Y., Raabe G., Wendt B. Angew. Chem., Int. Ed. 2007;46:467. doi: 10.1002/anie.200603434. [DOI] [PubMed] [Google Scholar]; (d) Enders D., Hüttl M. R. M., Raabe G., Bats J. W. Adv. Synth. Catal. 2008;350:267. [Google Scholar]; (e) McGarraugh P. G., Brenner S. E. Org. Lett. 2009;11:5654. doi: 10.1021/ol9024293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang Y., Han R.-G., Zhao Y.-L., Yang S., Xu P.-F., Dixon D. J. Angew. Chem., Int. Ed. 2009;48:9834. doi: 10.1002/anie.200905014. [DOI] [PubMed] [Google Scholar]; (g) Jiang K., Jia Z.-J., Chen S., Wu L., Chen Y.-C. Chem. – Eur. J. 2010;16:2852. doi: 10.1002/chem.200903009. [DOI] [PubMed] [Google Scholar]; (h) Baslé O., Raimondi W., Sanchez Duque M. M., Bonne D., Constantieux T., Rodriguez J. Org. Lett. 2010;12:5246. doi: 10.1021/ol102289g. [DOI] [PubMed] [Google Scholar]; (i) Enders D., Schmid B., Erdmann N. Synthesis. 2010:2271. [Google Scholar]; (j) Rueping M., Haack K. L., Ieawsuwan W., Sundén H., Blanco M., Schoepke F. R. Chem. Commun. 2011;47:3828. doi: 10.1039/c1cc10245a. [DOI] [PubMed] [Google Scholar]; (k) Cassani C., Tian X., Escudero-Adán E. C., Melchiorre P. Chem. Commun. 2011;47:233. doi: 10.1039/c0cc01957g. [DOI] [PubMed] [Google Scholar]; (l) Zea A., Alba A.-N. R., Mazzanti A., Moyano A., Rios R. Org. Biomol. Chem. 2011;9:6519. doi: 10.1039/c1ob05753g. [DOI] [PubMed] [Google Scholar]; (m) Enders D., Greb A., Deckers K., Selig P., Merkens C. Chem. – Eur. J. 2012;18:10226. doi: 10.1002/chem.201201493. [DOI] [PubMed] [Google Scholar]; (n) Enders D., Urbanietz G., Cassens-Sasse E., Keeß S., Raabe G. Adv. Synth. Catal. 2012;354:1481. [Google Scholar]; (o) Raimondi W., Sanchez Duque M. M., Goudedranche S., Quintard A., Constantieux T., Bugaut X., Bonne D., Rodriguez J. Synthesis. 2013:1659. [Google Scholar]; (p) Zeng X., Ni Q., Raabe G., Enders D. Angew. Chem., Int. Ed. 2013;52:2977. doi: 10.1002/anie.201209581. [DOI] [PubMed] [Google Scholar]; (q) Chauhan P., Urbanietz G., Raabe G., Enders D. Chem. Commun. 2014;50:6853. doi: 10.1039/c4cc01885k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Chauhan P., Mahajan S., Loh C. C. J., Raabe G., Enders D. Org. Lett. 2014;16:2954. doi: 10.1021/ol501093v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Sun P., Meng C.-Y., Zhoua F., Li X.-S., Xie J.-W. Tetrahedron. 2014;70:9330. [Google Scholar]; (t) Martínez J. I., Villar L., Uria U., Carrillo L., Reyes E., Vicario J. L. Adv. Synth. Catal. 2014;355:3627. [Google Scholar]
- For selected reviews and highlights on 1,6-addition reactions, see: ; (a) Csaký A. G., de la Herrán G., Murcia M. C. Chem. Soc. Rev. 2010;39:4080. doi: 10.1039/b924486g. [DOI] [PubMed] [Google Scholar]; (b) Biju A. T. ChemCatChem. 2011;3:1847. [Google Scholar]; (c) Silva E. M. P., Silva A. M. S. Synthesis. 2012:3109. [Google Scholar]; (d) Lear M. J., Hayashi Y. ChemCatChem. 2013;5:3499. [Google Scholar]; (e) Jurberg I. D., Chatterjee I., Tannert R., Melchiorre P., Chem. Commun., 2013, 49 , 4869 , ; for recent examples, see: . [DOI] [PubMed] [Google Scholar]; (f) Tian X., Liu Y., Melchiorre P. Angew. Chem., Int. Ed. 2012;51:6439. doi: 10.1002/anie.201202392. [DOI] [PubMed] [Google Scholar]; (g) Dell'Amico L., Albrecht Ł., Naicker T., Poulsen P. H., Jørgensen K. A. J. Am. Chem. Soc. 2013;135:8063. doi: 10.1021/ja4029928. [DOI] [PubMed] [Google Scholar]; (h) Chu W.-D., Zhang L.-F., Bao X., Zhao X.-Y., Zeng C., Du J.-Y., Zhang G.-B., Wang F.-X., Ma X.-Y., Fan C.-A. Angew. Chem., Int. Ed. 2013;52:9229. doi: 10.1002/anie.201303928. [DOI] [PubMed] [Google Scholar]; (i) Caruana L., Kniep F., Johansen T. K., Poulsen P. H., Jørgensen K. A. J. Am. Chem. Soc. 2014;136:15929. doi: 10.1021/ja510475n. [DOI] [PubMed] [Google Scholar]; (j) Akagawa K., Nishi N., Sen J., Kudo K. Org. Biomol. Chem. 2014;12:3581. doi: 10.1039/c4ob00565a. [DOI] [PubMed] [Google Scholar]
- (a) Sutherland R., Croydon E. A. P., Rolinson G. N. Br. Med. J. 1970;4:455. doi: 10.1136/bmj.4.5733.455. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miranda-Novales G., E Leaños-Miranda B., Vilchis-Pérez M., Solórzano-Santos F. Ann. Clin. Microbiol. Antimicrob. 2006;5:25. doi: 10.1186/1476-0711-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Isacson O., Brundin P., Kelly P. A. T., Gage F. H., Björklund A. Nature. 1984;311:458. doi: 10.1038/311458a0. [DOI] [PubMed] [Google Scholar]; (b) Becker A., Grecksch G., Bernstein H.-G., Höllt V., Bogerts B. Psychopharmacology. 1999;144:333. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Neumann H. C., Potts G. O., Ryan W. T., Stonner F. W. J. Med. Chem. 1970;13:948. doi: 10.1021/jm00299a034. [DOI] [PubMed] [Google Scholar]
- Baraldi P. G., Barco A., Benetti S., Pollini G. P., Simoni D. Synthesis. 1987:857. [Google Scholar]
- For selected examples of 4-nitro-5-styrylisoxazoles in non-enantioselective 1,6-addition reactions, see: ; (a) Chimichi S., Sio F. D., Donatic D., Fantoni P. S., Adamo M. F. A. Tetrahedron Lett. 2002;43:4157. [Google Scholar]; (b) Bruschi S., Suresh S., Piras L., Adamo M. F. A. Tetrahedron Lett. 2008;49:7406. [Google Scholar]; (c) Nagabelli M., Adamo M. F. A. Tetrahedron Lett. 2007;48:4703. [Google Scholar]; (d) Konda V. R., Adamo M. F. A. Org. Lett. 2007;9:303. doi: 10.1021/ol0627698. [DOI] [PubMed] [Google Scholar]; (e) Fini F., Naabelli M., Adamo M. F. A. Adv. Synth. Catal. 2010;352:3163. [Google Scholar]; (f) Illera D. S., Suresh S., Moccia M., Bellini G., Saviano M., Adamo M. F. A. Tetrahedron Lett. 2012;53:1808. [Google Scholar]; (g) Reddy M. N., Reddy K. G., Krishna S. R., Rajanarendar E. Tetrahedron Lett. 2012;53:2909. [Google Scholar]
- For selected examples of 4-nitro-5-styrylisoxazoles in enantioselective 1,6-addition reactions, see: ; (a) Nagabelli M., Adamo M. F. A. Org. Lett. 2008;10:1807. doi: 10.1021/ol800397z. [DOI] [PubMed] [Google Scholar]; (b) Baschieri A., Bernardi L., Ricci A., Suresh S., Adamo M. F. A. Angew. Chem., Int. Ed. 2009;48:9342. doi: 10.1002/anie.200905018. [DOI] [PubMed] [Google Scholar]; (c) Pei Q.-L., Sun H.-W., Wu Z.-J., Du X.-L., Zhang X.-M., Yuan W.-C. J. Org. Chem. 2011;76:7849. doi: 10.1021/jo2012779. [DOI] [PubMed] [Google Scholar]; (d) Sun H.-W., Liao Y.-H., Wu Z.-J., Wang H.-Y., Zhang X.-M., Yuan W.-C. Tetrahedron. 2011;67:3991. [Google Scholar]; (e) Fiandra C. D., Piras L., Fini F., Disetti P., Moccia M., Adamo M. F. A. Chem. Commun. 2012;48:3863. doi: 10.1039/c2cc30401e. [DOI] [PubMed] [Google Scholar]; (f) Zhang J.-L., Liu X.-H., Ma X.-J., Wang R. Chem. Commun. 2013;49:9329. doi: 10.1039/c3cc44059a. [DOI] [PubMed] [Google Scholar]; (g) Chew R. J., Huang Y., Li Y., Pullarkat S. A., Leung P.-H. Adv. Synth. Catal. 2013;355:1403. [Google Scholar]; (h) Liu X.-L., Han W.-Y., Zhang X.-M., Yuan W.-C. Org. Lett. 2013;15:1246. doi: 10.1021/ol400183k. [DOI] [PubMed] [Google Scholar]
- Li Y., López-Delgado F. J., Jørgensen D. K. B., Nielsen R. P., Jiang H., Jørgensen K. A. Chem. Commun. 2014;50:15689. doi: 10.1039/c4cc08171d. [DOI] [PubMed] [Google Scholar]
- For reviews on squaramides, see: ; (a) Alemán J., Parra A., Jiang H., Jørgensen K. A. Chem. – Eur. J. 2011;17:6890. doi: 10.1002/chem.201003694. [DOI] [PubMed] [Google Scholar]; (b) Storer R. I., Aciro C., Jones L. H. Chem. Soc. Rev. 2011;40:2330. doi: 10.1039/c0cs00200c. [DOI] [PubMed] [Google Scholar]
- (a) Malerich J. P., Hagihara K., Rawal V. H. J. Am. Chem. Soc. 2008;130:14416. doi: 10.1021/ja805693p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bae H. Y., Some S., Oh J. S., Lee Y. S., Song C. E. Chem. Commun. 2011;47:9621. doi: 10.1039/c1cc13637b. [DOI] [PubMed] [Google Scholar]; (c) Wang Y.-F., Chen R.-X., Wang K., Zhang B.-B., Lib Z.-B., Xu D.-Q. Green Chem. 2012;14:893. [Google Scholar]
- CCDC 1037530 (for 4a)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


