Abstract
Purpose of review
Recent clinical trials and animal studies indicate that resistant starches (RS) may be beneficial therapeutic tools for the management of metabolic diseases. The purpose of this review is to summarize these findings and discuss the established and proposed mechanisms by which RS exert their benefits. We also examine open questions regarding how RS improve metabolism and propose future research directions for the field.
Recent findings
Data from both humans and animal models clearly support a role for RS in improving a variety of metabolic features; however, discrepancies do exist regarding specific effects. Concomitant improvements in both insulin levels and body fat depots are often reported in rodents fed RS whereas RS feeding in humans improves insulin sensitivity without having a major impact on fat mass. These differences could be explained by the coexistence of several mechanisms (both gut microbiota-dependent and gut microbiota-independent) underpinning the metabolic benefits of RS.
Summary
Together, the studies presented in this review offer new insights into the potential pathways by which RS enhance metabolic health, including modulation of the gut microbiota, gut peptides, circulating inflammatory mediators, innate immune cells, and the bile acid cycle.
Keywords: Resistant starches, insulin sensitivity, gut microbiota, bile acids, macrophages
Introduction
Obesity is rapidly becoming a major global health challenge. From 1980 to 2013, the proportion of overweight adults increased worldwide from 29% to 37% [1]. Abdominal adiposity is the primary risk factor for development of metabolic diseases such as type 2 diabetes (T2D) and cardiovascular disease [2;3].
Traditional approaches to treating obesity and its related diseases have emphasized appropriate dietary choices and increased physical activity. One of the nutritional interventions proposed to counteract metabolic diseases is dietary supplementation with resistant starches (RS). RS include all starch and starch degradation products that escape digestion in the small intestine of healthy individuals because they are physically inaccessible, either within the food matrix (RS1) or within starch granules (RS2) or because they are present as retrograded starch (RS3), which is produced during food manufacture and preparation. In addition, starch can also be chemically modified (RS4) to make it resistant to enzymatic digestion [4*].
RS offer many practical advantages for use in the management of obesity-associated pathologies when compared to other fibers. RS can easily replace regular starch in baked goods and, by being non-digestible, can reduce both the caloric density and glycemic index of food products. However, RS exert physiological effects that go beyond resistance to digestibility, including modulation of satiety perception, dyslipidemia, insulin sensitivity, and glycemic control in both healthy and obese volunteers as well as in patients with metabolic syndrome [5*;6*;7**].
In this review, we provide an overview of recent studies that investigated the potential of various RS types to manage metabolic disorders. We then discuss the established and proposed mechanisms by which RS exert metabolic benefits. Finally, we examine open questions regarding how RS improve metabolism and propose future research directions for the field.
Resistant starches to combat metabolic diseases
RS were first characterized in 1982 and then rapidly studied for their ability to improve colonic health [8]. Since then, considerable effort has also been devoted to investigating the metabolic effects of RS using both human clinical trials and animal models [5*;6*]. In particular, several recent studies have strengthened the concept that RS confer metabolic benefits.
Modulation of insulin resistance and glucose homeostasis
Improved insulin resistance and glucose homeostasis are arguably the best-characterized metabolic benefits associated with RS consumption [6;9]. Although these effects are well documented in healthy individuals and those with metabolic syndrome, information regarding its impact on populations with established metabolic disease is lacking.
In a recent clinical trial, Bodinham and colleagues addressed this issue by assessing the effects of RS2 consumption on insulin sensitivity and glucose control in patients with T2D [6]**]. RS2 improved postprandial glucose levels, but had no impact on hepatic or peripheral insulin sensitivity as assessed by the hyperinsulinemic clamp technique [6]**]. It appears that RS can improve insulin sensitivity in healthy and pre-diabetic individuals but not after T2D has been established.
Recent studies regarding RS feeding and glucose control unfortunately show conflicting results. In particular, Zhou et al. observed significant decreases in blood glucose levels compared to controls when RS2 (8% w/w) was fed to streptozotocin-induced diabetic rats [10]. This was in contrast to Harazaki et al. who reported that feeding RS2 (55% w/w) significantly decreased fasting insulin levels and the index of insulin resistance but did not change glucose tolerance in an OLETF rat model of T2D [11*].
There is clearly good evidence that RS improve glucose metabolism beyond what would be expected from its reduced glycemic index, but to what extent established pathologies can be reversed is still unclear, and some studies do not show a beneficial effect on glycemic variables [12;13*]. Differences in RS intake levels, RS source, timeframe and disease status, among other factors (such as gut microbiota variation), could explain these apparent discrepancies, as discussed later in this review.
Alterations in body weight, body composition, and dyslipidemia
In 2014, Higgins provided a comprehensive review concerning the impact of RS on energy balance, body weight, and body composition [14**]. Since then, Nichenametla and colleagues published a human crossover, dietary intervention comparing effects of RS4-enriched flour with regular flour in healthy individuals and subjects with metabolic syndrome. Patients with metabolic syndrome experienced significant improvements in their blood cholesterol profiles [13*]. Similarly, Dodevska et al. showed that increasing RS intake during a lifestyle intervention for prediabetic overweight and obese adults significantly decreased their total serum cholesterol levels [12]. These two clinical trials provide exciting evidence that RS feeding positively influences dyslipidemia.
Also of interest, Nichenametla et al. found that only healthy persons consuming RS4-enriched flour experienced significant (albeit it minor) effects on their waist circumference and percent body fat; no such effects were observed for individuals with metabolic syndrome [13*]. This is in line with the aforementioned Bodinham et al. study where T2D patients experienced no changes in body weight, fat mass, or body fat depots despite decreases in fasting free fatty acid levels [6]. Dodevska and colleagues did report significant decreases in body weight and waist circumference, but the changes were minor [12]. All told, these recent reports demonstrate that dietary supplementation with RS has little to no impact on body composition in humans with metabolic disease.
Mechanisms underpinning the health effects of RS
The ways in which RS exert their health effects are insufficiently understood, but recent research has provided new insight into potential mechanisms. It has long been recognized that RS essentially function like fermentable fibers as well as bulking agents. To that end, we will examine the potential mechanisms by which RS impact metabolic processes, including those that involve the gut microbiota and those that may not.
The gut microbiota, short-chain fatty acids, and beyond
The community of microbes inhabiting the intestinal tract (the gut microbiota) provides critical control over host energy metabolism, insulin sensitivity, and immune development and regulation [15**–17]. RS remain intact until reaching the distal small and large intestines—here, they can be fermented by the microbiota, ultimately modulating both microbial composition and activity [18–23]. Consequently, the gut microbiota has been proposed as a key component in mediating the metabolic benefits of RS [5*;14**].
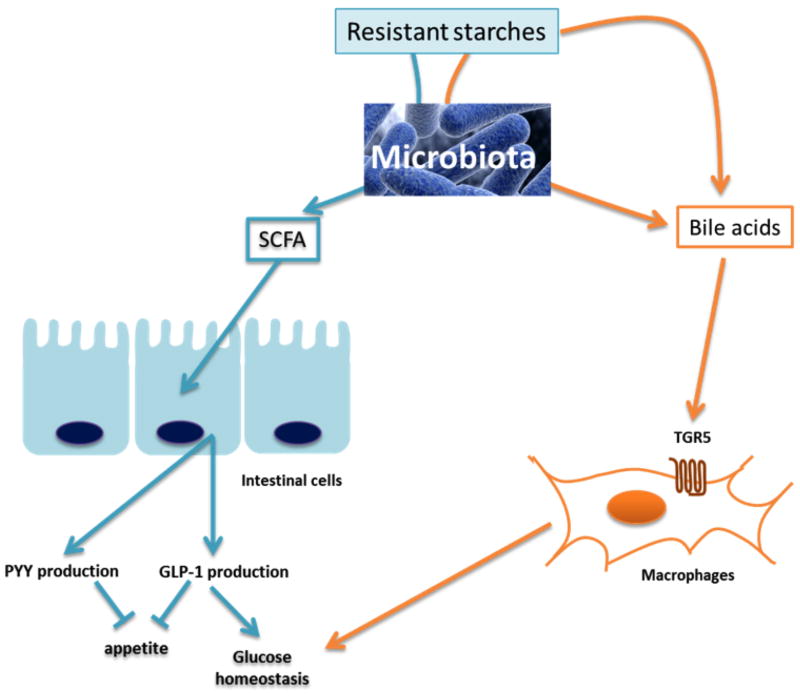
Microbial metabolites that result from RS fermentation, especially short chain fatty acids (SCFAs), have several physiological, metabolic, and immunological effects that could bring about the health benefits of RS. SCFA can trigger the release of gut peptides involved in appetite regulation and glucose homeostasis, such as peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) [24–26] (Figure 1). In accordance with this hypothesis, Zhou and colleagues showed that feeding RS2 to mice (30% w/w) increased both the full cecum and cecal tissue weights (indicative of fermentation), increased cecal expression of PYY and proglucagon (the precursor of GLP-1), and decreased body fat. The anti-obesity effect was dependent on GLP-1R and PYY, as the RS-mediated reduction in body fat was not observed in mice receiving a daily injection of a PYY receptor antagonist or in GLP-1-receptor deficient mice [29**]. Although this study did not address glucose homeostasis, future research should determine if GLP-1 induction is a mechanism by which RS improves glucose control.
Figure 1. Mechanistic insights into the metabolic benefits of RS.
This figure summarizes various hypotheses regarding the mechanisms underlying the beneficial effects conferred by RS consumption. RS could stimulate the microbial production of SCFA, thereby increasing the production of PYY and GLP-1 by intestinal L cells [24]. RS could also alter the bile acid profile via gut microbiota-dependent and independent pathways. Bile acids control glucose homeostasis via binding of the nuclear farnesoid X receptor (FXR) and the cell membrane receptor TGR5 [27]. In particular, recent work has shown that macrophage-specific deletion of Tgr5 increased adipose tissue macrophage accumulation and aggravated insulin resistance in obese animals [28]. GLP-1: glucagon-like peptide 1; PYY: peptide YY, SCFA: short chain fatty acids.
Information regarding the impact of RS2 on GLP-1 in human trials is inconsistent. RS-mediated improvements in insulin sensitivity have been observed without a concomitant impact on GLP-1 levels [5]. In the Bodinham et al. study, feeding RS2 to T2D patients for 12 weeks lowered fasting GLP-1 levels but increased GLP-1 postprandial excursion during a meal tolerance test [6]. The latter result is in contrast with the previous observation that postprandial GLP-1 levels were significantly lower following acute RS2 consumption [30]. Bodinham et al. also reported significant decreases in butyrate and propionate (with no change in acetate) levels in the serum of RS2-fed T2D patients. Although a decreased serum level of these SCFA does not necessarily preclude their production in the intestine, it does, however, exclude these microbial metabolites as systemic drivers of any peripheral effects of RS2 such as lipolysis.
Modulation of bile acids, innate immunity, and inflammatory mediators
By functioning like a fiber, and subsequently bulking and increasing digesta viscosity, RS are also likely to exert some effects independently of their impact on the gut microbiota. One of these alternative mechanisms could involve bile acids. Indeed, various types of RS have been shown to impact bile acid excretion [31]. Bile acids control glucose homeostasis and insulin resistance via binding of the nuclear farnesoid X receptor (FXR) and the cell membrane receptor TGR5 [27]. Indeed, recent work has elegantly shown that macrophage-specific deletion of Tgr5 increased adipose tissue macrophage accumulation and aggravated insulin resistance in obese animals [28]. How RS modulate bile acid profiles remains to be more deeply characterized, but two scenarios can be envisioned. On one hand, RS could influence the bile pool by modulating specific microbial taxa that chemically transform bile acids. On the other, RS may impact the concentration and reabsorption of bile acids via direct binding, bulking, and increasing viscosity [31] (Figure 1).
The notion that RS modulates the immune system and insulin resistance through modulation of the bile acid profile is an underexplored concept that deserves further attention. The aforementioned work by Harazaki et al. describing reduced insulinemia in RS2-fed T2D rats was the first to report a potential link between RS and regulation of immune cell populations. Specifically, feeding RS was associated with decreased adipose tissue expression of CD11c, a marker expressed on antigen presenting cells and pro-inflammatory macrophages [11*]. This finding is of considerable interest, as deletion of CD11c expressing cells has previously been shown to normalize insulin sensitivity in obese, insulin-resistant animals [32]. Macrophages can also secrete a myriad of pro-inflammatory cytokines, including TNF-Kα, IL-6 and IL-β. Bodinham et al. reported a 60% decrease in plasma TNF-α levels following 12 weeks of RS2 feeding. Over expression of TNF-α has long been hypothesized to play a significant role in the pathophysiology of insulin resistance [33]. Regulation of innate immune cells and their inflammatory mediators via bile acids may be a potential mechanism by which RS improves glucose homeostasis and insulin resistance, but additional studies are needed.
Open questions and future research directions
Several challenges remain in regards to understanding how RS improve metabolism. Here, we identify a few key questions for the field and discuss potential answers in light of the latest findings.
What is the exact role of the gut microbiota in the health effects of RS?
Recent evidence implicates the gut microbiota in precipitating the numerous health effects attributed to RS. Like other fibers, RS are fermented by the gut microbiota to SCFA—microbial metabolites whose physiological effects are increasingly recognized [24;25*]. In addition, RS shift the intestinal microbiota composition by enhancing putatively health-promoting taxa. Human trials in healthy volunteers demonstrated that feeding RS2 and RS3 increased the abundance of Ruminococcus bromii and Eubacterium rectale, while RS4 had a major bifidogenic effect [18;34]. In addition, administration of RS3 to pigs increased Faecalibacterium prausnitzii and reduced the abundance of Escherichia coli and Pseudomonas spp. [21]. Eubacterium rectale and Faecalibacterium prausnitzii are major butyrate producers with anti-inflammatory properties [35], and bifidobacteria are generally considered health-promoting organisms, thus RS-induced shifts in microbiota composition, in addition to metabolic effects, might contribute to health outcomes.
Despite these promising findings, the exact role of the gut microbiota in mediating the effects of RS has not been systematically studied. As we discussed above, several of the beneficial effects of RS may occur without microbial contributions. Strong correlations between RS feeding and taxa changes could result simply from host and microbiome responding independently to the diet. Comparing the effects of RS in conventional and germ-free animals would constitute a tractable model to separate direct versus microbiota-mediated effects [36]. Recent work from our own laboratory has revealed that feeding RS2 or RS4 supplemented Western diets to both germ-free and conventionalized mice improved their index of insulin resistance, indicating microbiome-independent mechanisms do exist (LB Bindels, J Walter, and AE Ramer-Tait, unpublished data). As gnotobiotic mouse models and protocols for microbiota transplants become more widely available, this line of research not only has the potential to determine the causative role of the microbiome in the health effects of dietary fiber (which will be critical for determining which fibers qualify for a prebiotic designation [37*]), but it will also facilitate direct testing of cultured strains with specific characteristics to ultimately identify individual bacterial contributors and underlying mechanisms [36].
What accounts for the discrepancies between animal and human studies?
Concomitant improvements in both insulin levels and body fat depots are often reported in rodents fed RS. This is in contrast to studies in healthy humans or patients with metabolic syndrome where RS feeding improves insulin sensitivity without having a major impact on fat mass. Several factors likely contribute to this discrepancy, one of which is the gut microbiota. Human and rodent microbiomes are strikingly dissimilar and may vary widely in their response to RS. Another explanation could be the vastly different RS doses fed during the animal studies, which often range from 30% to 55% of the diet. Unfortunately, these doses are likely not realistic for human diets. Feeding higher doses of RS during animal studies may also explain why increased SCFA production is frequently reported in rodents but not humans, as SCFA production is a dose-dependent phenomenon. SCFA, working to stimulate PYY and GLP-1 secretion, could potentially reduce fat mass whereas other mechanisms (independent of SCFA and GLP-1) may improve insulin sensitivity. This working model would explain why RS feeding does not have a profound impact on fat mass in humans but still enhances insulin sensitivity.
What accounts for the variation and inconsistency among experiments?
Beyond the discrepancies described among the various animal models and human populations, inconsistencies have even been reported within the same experimental design. What causes this variation is not understood. However, interactions between RS and the gut microbiome could potentially contribute, as microbiota composition differs widely among animal facilities, is highly individualized in humans (especially in westernized societies) [38], and is influenced by dietary regimen. If RS do indeed impact host physiology via effects on the microbiome, one would assume these responses to differ among vivaria or people. The ability of RS2 to reduce fat mass in mice has not been detected consistently across experiments and is associated with whether or not a particular cohort of mice fermented the RS [5]. These observations collectively point to functional differences in the microbiome. In addition, human microbiomes also vary in their ability to utilize RS. For example, Ruminoccocus bromii has been shown to be a keystone bacterial species required to begin the RS3 degradation process, and the starch remains untouched in persons who do not harbor this species [39]. There have been attempts to determine microbiome responsiveness to RS3 based on characteristics of an individual’s fecal microbiome composition, especially at baseline [22*]. This concept is still in its infancy, but if the microbiota indeed confers the metabolic effects of RS, variation in the functional response of individuals’ gut microbes will likely impact the outcomes of clinical trials. Considering these characteristics will introduce the microbiome into the realm of personalized nutrition.
What is the functional relevance of the chemical differences among RS types?
We often speak of RS as a whole. However, RS encompass a wide variety of structures, and this heterogeneity could also explain, at least in part, some apparent discrepancies among studies. Several pathways are likely involved in mediating the metabolic benefits associated with consuming RS. It is therefore quite possible that the relative contribution of each of these pathways will vary from one structure to another. Due to their inherent and distinct physicochemical properties, specific types of RS or even specific structures within a type may favor the growth of different gut microorganisms, which relates to disparities in how various RS shape the human microbiome [4;18;34;40]. Thus, systematic comparisons of different types of RS regarding their unique ability to improve metabolic alterations are warranted.
Conclusions
The metabolic benefits of RS for humans have been well-established using gold standard techniques such as the euglycemic-hyperinsulinemic clamp. However, the mechanisms underpinning these effects remain to be elucidated. Progress in obtaining such an understanding requires a combination of clinical trials and experiments in animal models. Specifically, mechanisms must first be investigated using animal models—this information should then inform and be corroborated by clinical trials in which key biomarkers are assessed. In this framework, collecting samples from patients consuming RS to investigate gut microbiota composition and function, bile acid profiles, and SCFA production becomes essential. Such research will ultimately provide the mechanistic insight needed to better design dietary strategies for specific human populations that, when combined with microbiome assessment, exemplify the concept of personalized nutrition.
Key points.
Recent clinical studies show that feeding resistant starches can have a positive effect on insulin sensitivity and dyslipidemia, with little to no impact on body composition.
Several pathways – gut microbiota-dependent and gut microbiota-independent – are likely involved in conferring the metabolic benefits associated with consuming resistant starches.
Despite promising findings, the exact role of the gut microbiota in mediating the metabolic benefits of resistant starches remains to be determined.
Gut microbiota variation among animal facilities, human populations, and even individuals, might contribute to inconsistencies observed regarding the effects of resistant starches.
Resistant starches encompass a wide variety of structures—this heterogeneity must be considered and could explain, at least in part, some apparent discrepancies among studies.
Acknowledgments
Financial support and sponsorship
LBB was awarded a complementary post-doctoral grant by the FSR (Fonds Spécial de Recherche, Université catholique de Louvain). JW acknowledges funds from the Campus Alberta Innovates Program (CAIP) and the University of Alberta. ART receives funding from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM104320), the Crohn’s and Colitis Foundation of America, the Nebraska Corn Board, the Nebraska Research Initiative, and the University of Nebraska-Lincoln.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References and recommended reading
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani PD, Van Hul M. Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr Opin Biotechnol. 2015 Apr;32:21–7. doi: 10.1016/j.copbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- *4.Hamaker BR, Tuncil YE. A Perspective on the Complexity of Dietary Fiber Structures and Their Potential Effect on the Gut Microbiota. J Mol Biol. 2014;426:3838–50. doi: 10.1016/j.jmb.2014.07.028. Elegant review discussing the structure-activity relationship between gut microbes and various dietary fiber, including resistant starches. [DOI] [PubMed] [Google Scholar]
- *5.Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, et al. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr. 2015;6:198–205. doi: 10.3945/an.114.007419. Comprehensive review regarding the impact of resistant starch on adiposity and insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Bodinham CL, Smith L, Thomas EL, Bell JD, Swann JR, Costabile A, et al. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect. 2014;3:75–84. doi: 10.1530/EC-14-0036. First study to assess the therapeutic potential of resistant starch in patients with type 2 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki KC, Phillips AK. Dietary substitutions for refined carbohydrate that show promise for reducing risk of type 2 diabetes in men and women. J Nutr. 2015;145:159S–63S. doi: 10.3945/jn.114.195149. [DOI] [PubMed] [Google Scholar]
- 8.Englyst H, Wiggins HS, Cummings JH. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982;107:307–18. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- 9.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med. 2010;27:391–7. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Wang F, Ren X, Wang Y, Blanchard C. Resistant starch manipulated hyperglycemia/hyperlipidemia and related genes expression in diabetic rats. Int J Biol Macromol. 2015;75:316–21. doi: 10.1016/j.ijbiomac.2015.01.052. [DOI] [PubMed] [Google Scholar]
- *11.Harazaki T, Inoue S, Imai C, Mochizuki K, Goda T. Resistant starch improves insulin resistance and reduces adipose tissue weight and CD11c expression in rat OLETF adipose tissue. Nutrition. 2014;30:590–5. doi: 10.1016/j.nut.2013.10.020. First report of decreased expression of CD11c (a marker of antigen presenting cells and pro-inflammatory macrophages) in the adipose tissue of diabetic rats fed resistant starches. [DOI] [PubMed] [Google Scholar]
- 12.Dodevska MS, Sobajic SS, Djordjevic PB, Dimitrijevic-Sreckovic VS, Spasojevic-Kalimanovska VV, Djordjevic BI. Effects of total fibre or resistant starch-rich diets within lifestyle intervention in obese prediabetic adults. Eur J Nutr. 2015 Jan 15; doi: 10.1007/s00394-015-0831-3. [DOI] [PubMed] [Google Scholar]
- *13.Nichenametla SN, Weidauer LA, Wey HE, Beare TM, Specker BL, Dey M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol Nutr Food Res. 2014;58:1365–9. doi: 10.1002/mnfr.201300829. Well-designed human cross-over dietary intervention where feeding RS4-enriched flour had a positive impact on hyperlipidemia in healthy volunteers and in patients with metabolic syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Higgins JA. Resistant starch and energy balance: impact on weight loss and maintenance. Crit Rev Food Sci Nutr. 2014;54:1158–66. doi: 10.1080/10408398.2011.629352. Comprehensive review of the literature pertaining to resistant starches and weight loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Khan MT, Nieuwdorp M, Backhed F. Microbial Modulation of Insulin Sensitivity. Cell Metab. 2014;20:753–60. doi: 10.1016/j.cmet.2014.07.006. Thoughtful review discussing the interplay between the gut microbiota and regulation of insulin resistance. [DOI] [PubMed] [Google Scholar]
- 16.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith JR, Sartor RB. The role of diet on intestinal microbiota metabolism: downstream impacts on host immune function and health, and therapeutic implications. J Gastroenterol. 2014;49:785–98. doi: 10.1007/s00535-014-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange K, Hugenholtz F, Jonathan MC, Schols HA, Kleerebezem M, Smidt H, et al. Comparison of the effects of five dietary fibers on mucosal transcriptional profiles, and luminal microbiota composition and SCFA concentrations in murine colon. Mol Nutr Food Res. 2015 Apr 25; doi: 10.1002/mnfr.201400597. [DOI] [PubMed] [Google Scholar]
- 20.Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol. 2013;83:299–309. doi: 10.1111/j.1574-6941.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 21.Haenen D, Zhang J, Souza da SC, Bosch G, van der Meer IM, van AJ, et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr. 2013;143:274–83. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- *22.Salonen A, Lahti L, Salojarvi J, Holtrop G, Korpela K, Duncan SH, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–30. doi: 10.1038/ismej.2014.63. Study suggesting that individualized microbiomes can predict dietary responsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umu OC, Frank JA, Fangel JU, Oostindjer M, da Silva CS, Bolhuis EJ, et al. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome. 2015;3:16. doi: 10.1186/s40168-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci. 2013;34:226–32. doi: 10.1016/j.tips.2013.02.002. [DOI] [PubMed] [Google Scholar]
- *25.Puertollano E, Kolida S, Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care. 2014;17:139–44. doi: 10.1097/MCO.0000000000000025. Recent review on short chain fatty acids. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsdottir G, Nyman M, Fak F. Designing future prebiotic fiber to target metabolic syndrome. Nutrition. 2014;30:497–502. doi: 10.1016/j.nut.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31:159–65. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perino A, Pols TW, Nomura M, Stein S, Pellicciari R, Schoonjans K. TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. J Clin Invest. 2014;124:5424–36. doi: 10.1172/JCI76289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Zhou J, Martin RJ, Raggio AM, Shen L, McCutcheon K, Keenan MJ. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol Nutr Food Res. 2015;59:1000–3. doi: 10.1002/mnfr.201400904. This study demonstrated that the impact of resistant starches on adiposity is dependent on a functional GLP-1 receptor and can be partially blocked using a PYY antagonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodinham CL, Al-Mana NM, Smith L, Robertson MD. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br J Nutr. 2013;110:1429–33. doi: 10.1017/S0007114513000731. [DOI] [PubMed] [Google Scholar]
- 31.Ebihara K, Shiraishi R, Okuma K. Hydroxypropyl-modified potato starch increases fecal bile acid excretion in rats. J Nutr. 1998;128:848–54. doi: 10.1093/jn/128.5.848. [DOI] [PubMed] [Google Scholar]
- 32.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miquel S, Leclerc M, Martin R, Chain F, Lenoir M, Raguideau S, et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. MBio. 2015;6:e00300–15. doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter J, Martinez I, Rose DJ. Holobiont nutrition: considering the role of the gastrointestinal microbiota in the health benefits of whole grains. Gut Microbes. 2013;4:340–6. doi: 10.4161/gmic.24707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. Opinion paper wherein the authors proposed to clarify and widen the prebiotic concept. [DOI] [PubMed] [Google Scholar]
- 38.Martinez I, Stegen JC, Maldonado-Gomez MX, Eren AM, Siba PM, Greenhill AR, et al. The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–38. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 39.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–43. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott KP, Antoine JM, Midtvedt T, van HS. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. 2015;26:25877. doi: 10.3402/mehd.v26.25877. [DOI] [PMC free article] [PubMed] [Google Scholar]