Abstract
Irinotecan is a widely used topoisomerase-I-inhibitor with a very narrow therapeutic window because of its severe toxicity. In the current study we have examined the effects of fasting prior to irinotecan treatment on toxicity and anti-tumor activity. FabplCre;Apc15lox/+ mice, which spontaneously develop intestinal tumors, of 27 weeks of age were randomized into 3-day fasted and ad libitum fed groups, followed by treatment with a flat-fixed high dose of irinotecan or vehicle. Side-effects were recorded until 11 days after the start of the experiment. Tumor size, and markers for cell-cycle activity, proliferation, angiogenesis, and senescence were measured. Fasted mice were protected against the side-effects of irinotecan treatment. Ad libitum fed mice developed visible signs of discomfort including weight loss, lower activity, ruffled coat, hunched-back posture, diarrhea, and leukopenia. Irinotecan reduced tumor size in fasted and ad libitum fed groups similarly compared to untreated controls (2.4 ± 0.67 mm and 2.4 ± 0.82 mm versus 3.0 ± 1.05 mm and 2.8 ± 1.08 mm respectively, P < 0.001). Immunohistochemical analysis showed reduced proliferation, a reduced number of vascular endothelial cells, and increased levels of senescence in tumors of both irinotecan treated groups. In conclusion, 3 days of fasting protects against the toxic side-effects of irinotecan in a clinically relevant mouse model of spontaneously developing colorectal cancer without affecting its anti-tumor activity. These results support fasting as a powerful way to improve treatment of colorectal carcinoma patients.
Keywords: APC, colorectal cancer, fasting, irinotecan, mouse models
Introduction
Irinotecan is extensively used in first and second line treatment for unresectable and metastatic disease of colorectal cancer.1 In 1996, approval was obtained in the United States for the second-line treatment of patients with metastatic colorectal carcinoma. Today, irinotecan is approved as a single agent as well as in combination with other drugs (i.e. oxaliplatin, and fluorouracil) for treatment of colorectal cancer.2,3 It is a pro-drug of the topoisomerase I inhibitor SN-38, and is metabolized by CYP3A into inactive metabolites.4 Irinotecan is typically known for its narrow therapeutic window, which explains its unpredictable toxicities, including severe myelosuppression, massive diarrhea, and in some cases even death as a complication of other side-effects.5,6 These side-effects may lead to dose reductions and early discontinuation of treatment, and limit the anti-tumor activity of this chemotherapeutic agent.7
A potentially protective intervention against these severe side-effects is dietary restriction (DR), i.e., a reduction of caloric intake before drug delivery. We previously showed that 3-days of fasting up-regulates cytoprotective and antioxidant enzymes, and induces protection against oxidative stress.8,9
While much information is obtained from studies that revealed beneficial effects of fasting and DR using transplantable tumor models, there is a need to perform these studies in more clinically relevant animal models.10-12 FabplCre;Apc15lox/+ mice express an adenomatous polyposis coli (APC) mutant allele, Apc15lox, based on loxP sites flanking exon 15, and a Cre-mediated knockout by deletion of this exon.13 These mice are genetically predisposed to develop macroscopic adenomas in the distal small and large intestine emerging at approximately 3 months of age. Humans with germ-line inactivating mutations of this gene are also predisposed to develop many adenomatous polyps in the colon and rectum, a hereditary cancer syndrome called familial adenomatous polyposis (FAP). In addition, 80% of the sporadic colorectal tumors are initiated by a germ-line mutation in APC.14,15
To study the role of APC in prevention and treatment of colorectal cancer, many inactivating mutant alleles of the mouse Apc gene were investigated. Morbidity and mortality rates of most of these models were high at a rather young age.16,17 An advantage of the conditional Apc-mutant mouse model discussed here is the slow onset of tumor development, mimicking the growth of a spontaneously developing tumor in a more mature mouse model. Furthermore, this model accounts for neoplasia in the small intestine as well as in the large intestine, which makes it a relevant model to study colorectal cancer.
In this study we examined the effects of fasting before administration of a high dose irinotecan on the occurrence of side-effects, and number and size of tumors in this conditional Apc-mutant mouse model for colorectal carcinoma. In addition, we performed immunohistochemistry on tumor tissue to compare the anti-tumor activity of irinotecan in fasted and ad libitum fed animals.
Results
Adverse side-effects in irinotecan treated Apc-mutant mice
During the first 3 days of the experiment, animals in the fasting group were withheld from food, and had ad libitum access to water. Bodyweight was presented as a relative percentage of the starting weight. Weight loss was 22.5 ± 3.0% during fasting relative to the animals starting weight in both fasting groups. The ad libitum fed groups gained 2.3 ± 1.9% body weight during this period. Directly after the first irinotecan injection, fasted mice were fed ad libitum again. In the ad libitum fed group, mice showed weight loss following the first irinotecan injection, and at the end of the experiment they had lost 22.1 ± 3.7% of their starting weight. In contrast, the fasted mice gained weight following the first injection and at the end of the experiment their weight had returned to baseline levels (94.8 ± 3.5% of their starting weight) (Fig. 1a). From day 3 after the first irinotecan injection, mice in the ad libitum fed group were less active (P < 0.001) and showed a ruffled coat compared with fasted mice treated with irinotecan and untreated controls (P < 0.001). Furthermore, ad libitum fed mice treated with irinotecan had a hunched-back posture (P < 0.001) and suffered from diarrhea from day 3 after the first injection, whereas this was not observed in fasted mice treated with irinotecan and untreated controls (P < 0.001) (Fig. 1b–e). Eight days after the first irinotecan injection the number of leukocytes was determined to measure bone marrow toxicity. Among the mice receiving irinotecan, the ad libitum fed group showed a significant reduction in leukocyte numbers compared to fasted animals (3.4 ± 0.6*106/mL vs. 6.6 ± 0.7*106/mL, P < 0.001) (Fig. 1f). There was no significant difference between the control groups. Thus, fasted mice treated with irinotecan did not show signs of toxicity whereas all ad libitum fed mice did.
Figure 1.
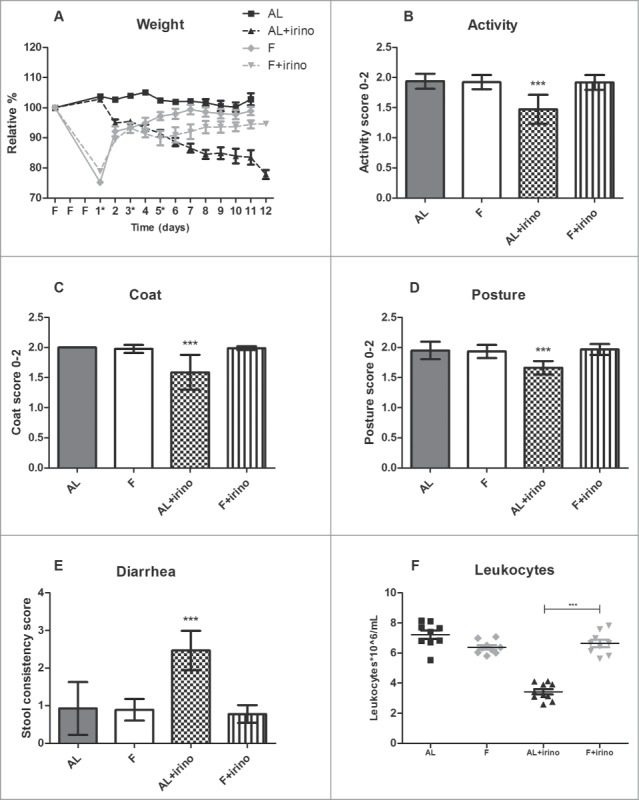
Protective effects of fasting against side-effects of irinotecan in Apc-mutant mice. Side-effects were monitored from the start of the fasting regimen until 11 days after the first irinotecan injection (*). Graphs represent a total of 36 mice: 1. Ad libitum group (n = 9; males (m) n = 4, females (f) n = 5), 2. Fasted group (n = 9; m, n = 4, f, n = 5), 3. Ad libitum group treated with irinotecan (n = 10; m, n = 5, f, n = 5), and 4. Fasted group treated with irinotecan (n = 8; m, n = 4, f, n = 4). (A) Effects of fasting and irinotecan treatment on body weight. Fasted (F) mice lost weight during the fasting regimen, but gained weight during irinotecan treatment. In contrast, ad libitum (AL) fed animals lost weight during irinotecan administration. (B–E) Effects of fasting and irinotecan treatment on activity, coat, posture, and stool. ***Indicates significant difference (P < 0.001) between ad libitum fed animals treated with irinotecan compared to each of the other groups. (F) Effect of fasting and irinotecan treatment on bone marrow toxicity. Number of leukocytes on day 8 after the first irinotecan injection was significantly lower in ad libitum fed animals compared to fasted animals in the irinotecan treated groups. ***P < 0.001.
Number and size of intestinal tumors
At the end of the experiment the entire gastrointestinal tract from each mouse was removed, and the small and large intestine were opened for tumor enumeration. The average number of tumors was 17% lower in the irinotecan-treated groups compared to controls. There were 16.4 ± 3.1 and 17.3 ± 3.3 tumors per mouse in the ad libitum and fasted group without irinotecan treatment, whereas the ad libitum and fasted group with irinotecan treatment mice had 14.0 ± 3.6 and 14.6 ± 2.5 intestinal tumors, respectively (Fig. 2a). Interestingly, the size of the tumors showed a significant difference between irinotecan treated and control groups. Tumor size of ad libitum fed and fasted control groups was 2.8 ± 1.08 mm (range: 0.8 mm – 6.5 mm) and 3.0 ± 1.05 mm (range: 1.0 mm – 6.0 mm) respectively, while tumor size in irinotecan treated groups was 2.4 ± 0.82 mm (range: 1.0 mm – 4.8 mm) and 2.4 ± 0.67 mm (range: 1.0 mm – 4.3 mm) (P < 0.001) (Fig. 2b).
Figure 2.
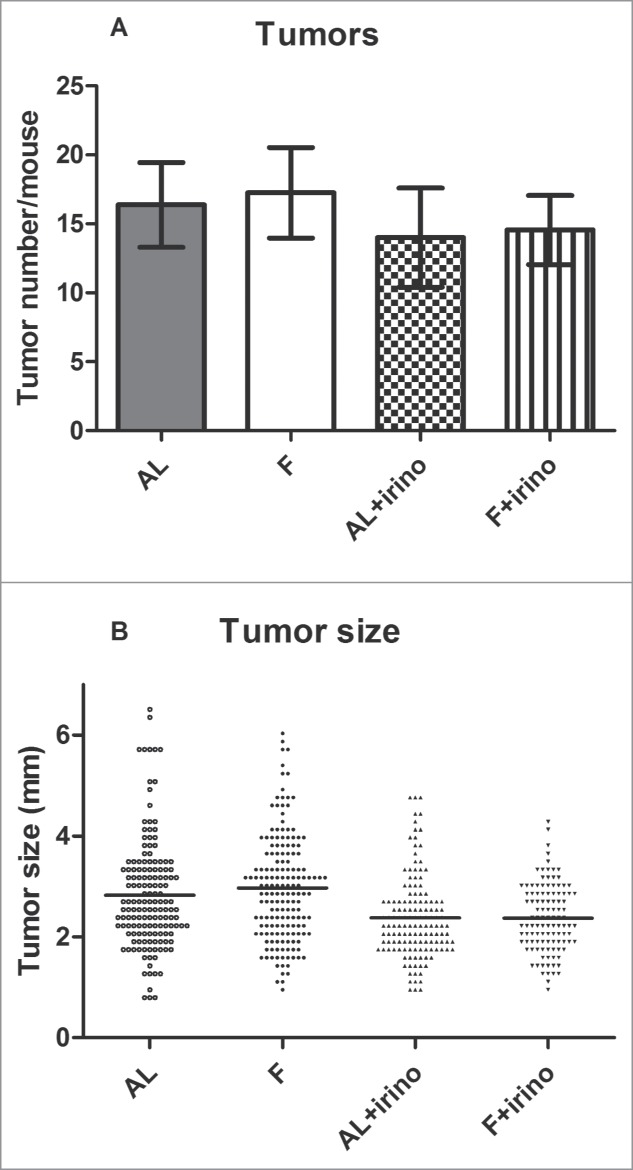
Number and size of intestinal tumors. Tumors from the gastrointestinal tract were counted and measured under a dissection microscope. (A) Number of intestinal tumors per mouse. In the irinotecan treated groups, ad libitum (AL) fed and fasted (F) mice had respectively 14.0 ± 3.6 and 14.6 ± 2.5 tumors per intestine. In control groups, ad libitum (AL) fed and fasted (F) mice had respectively 16.4 ± 3.1 and 17.3 ± 3.3 tumors per intestine. (B) Size of all intestinal tumors. Tumor size in irinotecan treated mice was 2.4 ± 0.82 mm in fasted, and 2.4 ± 0.67 mm in ad libitum fed and fasted mice respectively. In control groups this was 2.8 ± 1.08 mm and 3.0 ± 1.05 mm (P < 0.001).
Antitumor effect of irinotecan
We examined whether fasting changed the effects of irinotecan on the cell cycle, cellular proliferation, induction of senescence, and angiogenesis by immunohistochemistry on 4 randomly chosen tumors per intestinal tract. Cyclin D1, a marker that indicates the G1/S-phase of the cell cycle and that is often seen overexpressed in tumors, showed a significantly lower expression in irinotecan treated animals compared to controls (P < 0.01) (Fig. 3a). This corresponded with a significantly lower expression of the mitosis marker pHiston H3 in irinotecan treated animals (P < 0.001) (Fig. 3b). Ki-67, the proliferation marker that is present in all active phases of the cell cycle was also significantly lower expressed in both irinotecan treated groups (P < 0.01) (Fig. 3c). Expression of the cyclin dependent kinase inhibitor (CDKI) p21, indicating senescence and S/G2-phase arrest, was significantly up-regulated in both irinotecan treated groups (P < 0.05) (Fig. 3d). CD146, present on vascular endothelial cells and a marker for angiogenesis, showed significantly lower expression in both treated groups (P < 0.01) (Fig. 3e). There were no significant differences between irinotecan treated ad libitum fed and fasted animals. Collectively these data show that irinotecan treatment significantly inhibited tumor cell proliferation, and has a comparable anti-tumor effect in both ad libitum fed-, and fasted mice.
Figure 3.
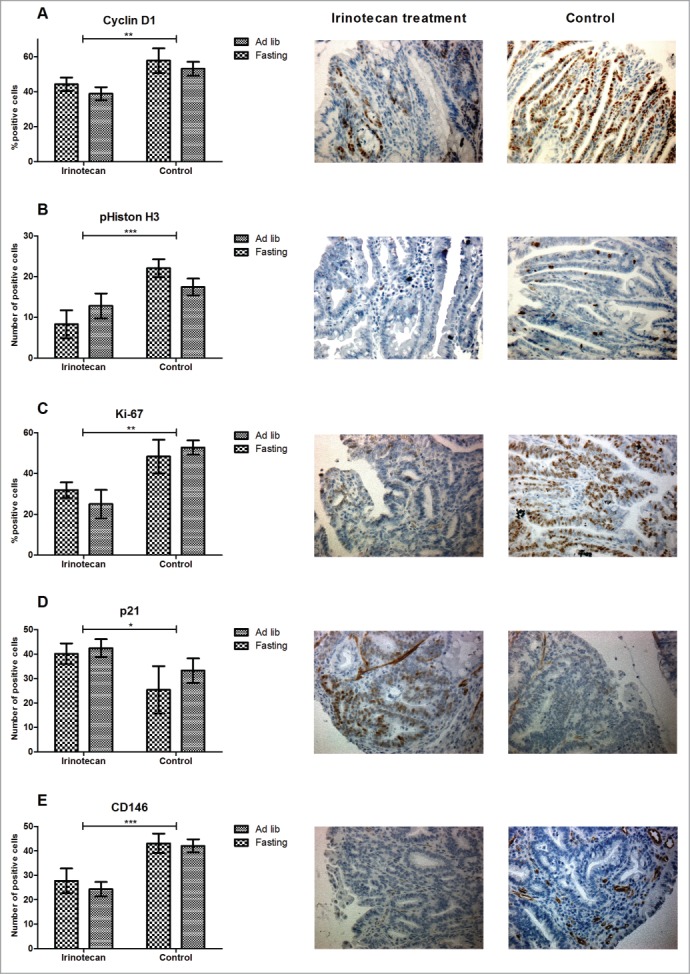
Anti-tumor effect of irinotecan. (A–C) Irinotecan treatment significantly inhibited tumor cell proliferation as shown by differential expression of Cyclin D1, pHiston H3, and Ki-67. (D) This corresponded with an upregulation of the CDKI p21. (E) CD146, a marker for endothelial cells, was significantly down-regulated in irinotecan treated animals. Representative photomicrographs of irinotecan treated (middle panels) and control (right panels) tumor specimens (magnification 200x). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The experiments presented here show that 3-days of fasting prior to treatment with a high dose of the chemotherapeutic agent irinotecan protects against its side-effects in an Apc-mutant mouse model that spontaneously develops intestinal tumors. We observed a similar reduction in tumor size in fasted and ad libitum fed irinotecan treated groups compared to controls. Furthermore, we found a significant decrease in cell cycle activity, proliferation, and angiogenesis, while senescence was increased in both fasted and ad libitum fed irinotecan treated groups, indicating that fasting does not affect the anti-tumor activity of irinotecan.
To our knowledge, this is the first study that shows beneficial effects of fasting on chemotherapy toxicity in a mouse model that spontaneously develops colorectal cancer. In support of these observations, others have shown that long term dietary-restricted rodents are more resistant against a variety of toxic agents.18-21 We showed that beneficial effects seen after long-term dietary restriction, can also be induced rapidly using a 3-day fasting regimen.8 Others have observed the beneficial effects of short-term fasting on chemotherapy treatment as well. Mice injected with neuroblastoma cells and subsequently fasted for 48 hours were protected against side-effects of etoposide, while anti-tumor activity was preserved.11 In previous studies, tumor-cells or tissues were placed subcutaneously, whereas in the current study we used an Apc-mutant mouse model that mimics spontaneous intestinal tumor formation.13 Spontaneously developing tumor models are needed to better develop strategies to control malignant cell growth, including chemotherapy treatment. In this regard, the ApcMin/+ mouse model is one of the most studied models of intestinal tumorigenesis in which tumor growth is suppressed by different anticancer agents.22,23
In the present study we used fasting as a pre-conditioning method to protect against the side-effects of irinotecan. Previously, we reported that 3-days of fasting increases stress-resistance, including upregulation of the Nrf2 pathway, which is involved in the protection against oxidative stress.8,24 In contrast to the up-regulation of protective pathways by normal cells, it is thought that cancer cells are unable to obtain a protected state by dietary restriction. This phenomenon is called differential stress sensitization (DSS) and is based on the fact that cancer cells have acquired a number of mutations that progressively decrease their ability to adapt to diet restriction.25
A key finding in this study is that 3-day fasted Apc-mutant mice do not show any signs of toxicity in response to a very high dose irinotecan while the anti-tumor response is still present. Using immunohistochemistry, we found no significant differences between ad libitum fed- and fasted animals in both the irinotecan treated groups as well as the control groups, indicating that the effects of irinotecan are unimpaired in fasted animals. Phosphorylation of Histone H3 at serine 10 takes place during mitosis of the cell.26 The reduced expression of pHiston H3 in tumors of irinotecan treated mice indicates that mitosis is suppressed. Cyclin D1 regulates the progression of cells into the proliferative stage.27 Both fasted and ad libitum fed irinotecan treated animals showed a similarly reduced expression of cyclin D1. Ki-67, a widely used proliferation marker, confirmed the anti-proliferative effect of irinotecan in all treated animals.
Due to irinotecan induced DNA damage, the p53/p21 pathway is activated and subsequently arrests cells from division and causes cellular senescence.28,29 We evaluated the expression of p21 as a senescence marker, and showed that irinotecan treated tumors express significantly higher levels of p21 compared to controls. The expression of CD146, a marker present on vascular endothelial cells, was significantly reduced in tumors from irinotecan treated mice. The effects of irinotecan on vascular endothelial cells remain largely unknown. Irinotecan decreases hypoxia inducible factor 1α accumulation in tumors, which may inhibit tumor angiogenesis.30 These findings support our observation that irinotecan reduces CD146 expression and thus angiogenesis.
We investigated both male and female FabplCre;Apc15lox/+ mice. The number of male and female mice was evenly distributed between the different groups, and we observed no differences in side-effects, number and size of tumors, and anti-tumor effect of irinotecan between genders. The only difference was seen in bodyweight; at the start of the experiment, at 27 weeks of age, male mice were on average 30% heavier. Therefore, bodyweight is presented as a relative percentage of the starting weight. Several studies have compared gender differences in the ApcMin/+ model. The small molecule compound SHetA2 significantly reduced incidence and size of intestinal polyps, both in males and females.22 In contrast, in a dietary study using the retinoid-x-receptor agonist bexarotene, a greater reduction of intestinal tumors was found in male mice compared to female mice.31 The observation that we did not find differences between genders could be attributed to the fact that we administered a flat-fixed dose to all mice, based on the bodyweight of male mice. Thus, female mice received a higher concentration irinotecan relative to their bodyweight, but are known to be less susceptible to the toxicity induced by irinotecan.32
Based on existing evidence from in vitro and in vivo experiments, fasting has a great potential to be implemented in clinical cancer patients. Although the introduction of fasting remains challenging, a study with self-reported patients showed a decrease in side-effects and an increase in subjective well-being after chemotherapy treatment.33 Therefore, randomized controlled clinical trials are needed to validate the beneficial effects of fasting in a clinical setting.
In conclusion, fasting significantly prevented the occurrence of side-effects of irinotecan in a genetic mouse model that spontaneously develops intestinal neoplasms through an inactivating mutation of the Apc gene. Irinotecan significantly reduced tumor size and proliferation in fasted and ad libitum fed animals, indicating that fasting does not abrogate the anti-tumor response. These results support fasting before irinotecan treatment as a powerful way to improve treatment for colorectal carcinoma patients.
Materials and Methods
Animals
FabplCre;Apc15lox/+ mice (n = 36) with a C57Bl/6 background were generated, bred, and maintained under pathogen-free conditions at a licensed biomedical animal facility, Erasmus University Medical Center, Rotterdam, the Netherlands, as previously reported.13 During 27 weeks they were housed in individually ventilated cages (n = 3–4 animals per cage) where standard laboratory conditions were maintained, i.e. temperature ∼22°C, humidity ∼50%, and a 12 h light/12 h dark cycle. During this period, there were no visible signs of disease in the studied animals. All mice had free access to water and food (Special Diet Services, Witham, UK) unless mentioned otherwise. Previously we have shown that after 27 weeks of age, intestinal tumors were macroscopically present. Mice were divided into 4 groups; 1. Ad libitum group (n = 9; males (m) n = 4, females (f) n = 5), 2. Fasted group (n = 9; m, n = 4, f, n = 5), 3. Ad libitum group with irinotecan treatment (n = 10; m, n = 5, f, n = 5), and 4. Fasted group with irinotecan treatment (n = 8; m, n = 4, f, n = 4). The experimental protocol was approved by the Animal Experiments Committee under the Dutch National Experiments on Animals Act, and complied with the 1986 directive 86/609/EC of the Council of Europe.
Fasting
The two ad libitum fed groups were allowed unrestricted access to food. Before the start of the experiment, all mice were transferred to a clean cage and the 2 fasted groups were withheld food for 3 days starting at 4:00 PM and were fed ad libitum again 3 days later at 10:00 AM. All animals were given continuous access to water. No mortality occurred during fasting.
Chemotherapy
Irinotecan, HCl-trihydrate 20 mg/mL (Hospira, Benelux) was diluted in sodium chloride 0.9% (Braun, Melsungen, Germany) to a final volume of 200 µL per injection, and was given intraperitoneally. The optimal cumulative drug dose (400 mg/kg) was determined in a pilot experiment and defined as the concentration that induces severe toxicity without causing mortality (data not shown). All animals received the same amount of irinotecan, called a flat-fixed dose, based on the average weight of ad libitum fed male mice, which was 25 gram. In the ad libitum fed group as well as in the fasted group mice received 167 µL irinotecan supplemented with 33 µL sodium chloride 0.9% per injection.
Experimental setup
All mice in the irinotecan treated groups received a cumulative flat-fixed dose of 400 mg/kg irinotecan. On days 1,3 and 5 after fasting, mice received 133 mg/kg irinotecan. The control groups received vehicle (sodium chloride 0.9%). Following the first irinotecan injection mice were weighed and inspected daily for adverse side effects by a mouse wellbeing-score protocol adapted from ‘the guidelines for welfare of animals in experimental neoplasia research’ (1988: United Kingdom Co-ordinating Committee on Cancer Research, UKCCCR). Assessing wellbeing by one researcher took 10 minutes per cage with 4 mice. Side-effects were scored independently by 2 experienced researchers. Mouse cages were removed from racks and placed on a bench to facilitate visualization of the mice, but cages were not opened at any point during the scoring process, except for the determination of the stool consistency at the end of the process. Mouse activity level was scored according to the amount each mouse moved in its cage. A score of 2 indicates that an animal moved around the cage normally. A score of 1 indicates that an animal was moving slowly or less frequently and with an altered gait. A score of 0 indicated that an animal was not moving and was taking no more than 5 steps. The appearance of the coat was scored according to the smoothness. A score of 2 indicated a healthy, smooth uninterrupted coat. A score of 1 indicated a slightly fluffy coat. A score of 0 indicated a severe fluffy coat with evident parts of visible skin. Posture was scored as follows; A score of 2 indicated a normal body posture. A score of 1 indicated a moderately hunched posture. A score of 0 indicated a severely hunched posture. Severity of diarrhea was assessed according to the stool consistency score (0: normal, 1: loose stool, 2: loose/some diarrhea, 3: diarrhea, 4: severe watery diarrhea).34 Before every stool consistency measurement clean white tissues were placed at the bottom of the cage to allow determination of the consistency of the stool. Results are expressed as mean ± SD. Leukocyte numbers were determined on day 8 after the first irinotecan injection with a Z series Coulter Counter (Beckman Coulter, Woerden, The Netherlands). Ten days after the first irinotecan injection mice were sacrificed by exsanguination.
Determination of tumor burden
Directly after sacrificing the animals, the entire gastrointestinal tract was removed for dissection but the stomach, duodenum and cecum were omitted from the analysis because of their low tumor incidence. The intestinal tract was opened along the cephalocaudal axis, flushed with phosphate-buffered saline (PBS), cut into 5 segments of approximately equal lengths, and spread out flat on filter paper. These preparations were fixed overnight at 4°C in 10% phosphate-buffered formalin and thereafter stored in 70% EtOH until further analysis. Tumor enumeration was performed using a dissecting microscope. Diameter of each tumor was determined using an ocular micrometer that allows precise measurements with a resolution of 0.1 mm. The smallest detected tumor was 0.8 mm. All tumors were scored by 2 experienced researchers blinded to the treatment.
Immunohistochemistry
After determination of tumor size, 4 tumors per intestinal tract were embedded in paraffin, sectioned at 5 µm, and stained with the following antibodies: 1. polyclonal antibody against pHiston H3 (Ser10; 9701S, dilution 1:200, Cell Signaling, Danvers, MA). 2. monoclonal antibody against Cyclin D1 (VP-RM03, dilution 1:500, Bio-Connect, Huissen, The Netherlands). 3. Monoclonal antibody against Ki-67 (D3B5; 12202S, dilution 1:500, Cell Signaling, Danvers, MA). 4. Monoclonal antibody against CD146 (EPR3208; ab75769, dilution 1:200, Abcam, Cambridge, UK) 5. Monoclonal antibody against p21 (Waf1/Cip1; 12D1; 2947S, dilution 1:500, Cell Signaling, Danvers, MA). All primary antibodies were visualized with a polyclonal horseradish peroxidase-Y-conjugated secondary antibody (goat-anti-rabbit IgG/HRP, P0448, dilution 1:500, DAKO, Denmark). Slides were scored using the number of positive cells for pHiston H3, CD146, and p21. For Cyclin D1, and ki-67 the percentage of positive cells was used. Two independent observers blinded to the treatment scored the slides at a magnification of 200–400x. Five microscopic fields per tumor were measured. Results are expressed as mean ± SD.
Statistical analyses
Categorical data are presented as number (percentage) and continuous variables as mean ± SD. Means between 2 groups were compared using the t-test for parametric data. One-way analysis of variance (ANOVA) in combination with the Bonferroni multiple comparison test was used to assess whether fasting significantly altered irinotecan induced toxicity in treated mice compared to the other groups. Two-way ANOVA was used to assess if fasting or irinotecan treatment significantly influenced expression of immunohistochemical markers. All standard statistical tests were performed using SPSS version 21 for Windows software (Statistical Package for Social Sciences, Chicago, IL), and P < 0.05 was considered to be significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Dutch Cancer Society (EMCR 2009-4506).
References
- 1.Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM.. Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol 2001; 19:1501-18; PMID:11230497 [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, et al.. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998; 352:1413-8; PMID:9807987; http://dx.doi.org/ 10.1016/S0140-6736(98)02309-5 [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al.. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343:905-14; PMID:11006366; http://dx.doi.org/ 10.1056/NEJM200009283431302 [DOI] [PubMed] [Google Scholar]
- 4.Di Paolo A, Bocci G, Danesi R, Del Tacca M. Clinical pharmacokinetics of irinotecan-based chemotherapy in colorectal cancer patients. Curr Clin Pharmacol 2006; 1:311-23; PMID:18666754; http://dx.doi.org/ 10.2174/157488406778249307 [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg ML. Efficacy and toxicity of irinotecan in patients with colorectal cancer. Semin Oncol 1998; 25:39-46; PMID:9786315 [PubMed] [Google Scholar]
- 6.Kim TW, Innocenti F. Insights, challenges, and future directions in irinogenetics. Ther Drug Monit 2007; 29:265-70; PMID:17529881; http://dx.doi.org/ 10.1097/FTD.0b013e318068623b [DOI] [PubMed] [Google Scholar]
- 7.Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, et al.. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998; 352:1407-12; PMID:9807986 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de Jong M, et al.. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010; 9:40-53; PMID:19878145; http://dx.doi.org/ 10.1111/j.1474-9726.2009.00532.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Ginhoven TM, Mitchell JR, Verweij M, Hoeijmakers JH, Ijzermans JN, de Bruin RW.. The use of preoperative nutritional interventions to protect against hepatic ischemia-reperfusion injury. Liver Transpl 2009; 15:1183-91; PMID:19790167; http://dx.doi.org/ 10.1002/lt.21871 [DOI] [PubMed] [Google Scholar]
- 10.Huisman S, Van Den Engel S, Roert H, Bijman-Lachger W, Ijzermans J, De Bruin R.. Fasting protects against the adverse side effects of chemotherapy but does not abrogate anti-tumor activity. Eur Surg Res 2013; 50:3 [Google Scholar]
- 11.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD.. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A 2008; 105:8215-20; PMID:18378900; http://dx.doi.org/ 10.1073/pnas.0708100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, Nguyen H, Vessella R, Pili R.. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 2013; 4:2451-61; PMID:24353195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robanus-Maandag EC, Koelink PJ, Breukel C, Salvatori DC, Jagmohan-Changur SC, Bosch CA, Verspaget HW, Devilee P, Fodde R, Smits R.. A new conditional Apc-mutant mouse model for colorectal cancer. Carcinogenesis 2010; 31:946-52; PMID:20176656; http://dx.doi.org/ 10.1093/carcin/bgq046 [DOI] [PubMed] [Google Scholar]
- 14.Albuquerque C, Bakker ER, van Veelen W, Smits R. Colorectal cancers choosing sides. Biochim Biophys Acta 2011; 1816:219-31; PMID:21855610 [DOI] [PubMed] [Google Scholar]
- 15.Fodde R, Smits R, Clevers H.. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 2001; 1:55-67; PMID:11900252; http://dx.doi.org/ 10.1038/35094067 [DOI] [PubMed] [Google Scholar]
- 16.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF.. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992; 256:668-70; PMID:1350108; http://dx.doi.org/ 10.1126/science.1350108 [DOI] [PubMed] [Google Scholar]
- 17.Zeineldin M, Neufeld KL. Understanding phenotypic variation in rodent models with germline Apc mutations. Cancer Res 2013; 73:2389-99; PMID:23580574; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aidoo A, Desai VG, Lyn-Cook LE, Chen JJ, Feuers RJ, Casciano DA.. Attenuation of bleomycin-induced Hprt mutant frequency in female and male rats by calorie restriction. Mutat Res 1999; 430:155-63; PMID:10592326; http://dx.doi.org/ 10.1016/S0027-5107(99)00197-9 [DOI] [PubMed] [Google Scholar]
- 19.Apte UM, Limaye PB, Desaiah D, Bucci TJ, Warbritton A, Mehendale HM. Mechanisms of increased liver tissue repair and survival in diet-restricted rats treated with equitoxic doses of thioacetamide. Toxicol Sci 2003; 72:272-82; PMID:12655029; http://dx.doi.org/ 10.1093/toxsci/kfg021 [DOI] [PubMed] [Google Scholar]
- 20.Duffy PH, Feuers RJ, Pipkin JL, Berg TF, Leal LMCC, Turturro A, Hart RW.. The effect of dietary restriction and aging on the physiological response of rodents to drugs Dietary restriction: implications for the design and interpretation of toxicity and carcinogenicity studies. Washington, D.C.: ILSI Press; 1995:127-40. [Google Scholar]
- 21.Sun D, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol 2001; 8:1003-11; PMID:11527818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benbrook DM, Guruswamy S, Wang Y, Sun Z, Mohammed A, Zhang Y, Li Q, Rao CV.. Chemoprevention of colon and small intestinal tumorigenesis in APC(min/+) mice by SHetA2 (NSC721689) without toxicity. Cancer Prev Res (Phila) 2013; 6:908-16; PMID:23852423; http://dx.doi.org/ 10.1158/1940-6207.CAPR-13-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, Wang Y, Gao Z, Qu X. The comparative study of acetyl-11-keto-beta-boswellic acid (AKBA) and aspirin in the prevention of intestinal adenomatous polyposis in APC(Min/+) mice. Drug Discov Ther 2014; 8:25-32; PMID:24647155; http://dx.doi.org/ 10.5582/ddt.8.25 [DOI] [PubMed] [Google Scholar]
- 24.Jongbloed F, de Bruin RW, Pennings JL, Payan-Gomez C, van den Engel S, van Oostrom CT, de Bruin A, Hoeijmakers JH, van Steeg H, JN IJ, et al.. Preoperative fasting protects against renal ischemia-reperfusion injury in aged and overweight mice. PLoS One 2014; 9:e100853; PMID:24959849; http://dx.doi.org/ 10.1371/journal.pone.0100853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al.. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 2012; 4:124ra27; PMID:22323820; http://dx.doi.org/ 10.1126/scitranslmed.3003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene 2001; 20:3021-7; PMID:11420717; http://dx.doi.org/ 10.1038/sj.onc.1204326 [DOI] [PubMed] [Google Scholar]
- 27.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A.. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 1999; 96:5522-7; PMID:10318916; http://dx.doi.org/ 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Z, Wei W, Dunaway S, Darnowski JW, Calabresi P, Sedivy J, Hendrickson EA, Balan KV, Pantazis P, Wyche JH.. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem 2002; 277:17154-60; PMID:11877436; http://dx.doi.org/ 10.1074/jbc.M112401200 [DOI] [PubMed] [Google Scholar]
- 29.Taylor WR, Stark GR.. Regulation of the G2/M transition by p53. Oncogene 2001; 20:1803-15; PMID:11313928; http://dx.doi.org/ 10.1038/sj.onc.1204252 [DOI] [PubMed] [Google Scholar]
- 30.Guerin E, Raffelsberger W, Pencreach E, Maier A, Neuville A, Schneider A, Bachellier P, Rohr S, Petitprez A, Poch O, et al.. In vivo topoisomerase I inhibition attenuates the expression of hypoxia-inducible factor 1alpha target genes and decreases tumor angiogenesis. Mol Med 2012; 18:83-94; PMID:22033674; http://dx.doi.org/ 10.2119/molmed.2011.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janakiram NB, Mohammed A, Qian L, Choi CI, Steele VE, Rao CV. Chemopreventive effects of RXR-selective rexinoid bexarotene on intestinal neoplasia of Apc(Min/+) mice. Neoplasia 2012; 14:159-68; PMID:22431924; http://dx.doi.org/ 10.1593/neo.111440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahowesso C, Piccolo E, Li XM, Dulong S, Hossard V, La Sorda R, Filipski E, Tinari N, Delaunay F, Iacobelli S, et al.. Relations between strain and gender dependencies of irinotecan toxicity and UGT1A1, CES2 and TOP1 expressions in mice. Toxicol Lett 2010; 192:395-401; PMID:19931604; http://dx.doi.org/ 10.1016/j.toxlet.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 33.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD.. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY) 2009; 1:988-1007; PMID:20157582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick LR, Small JS, Greene WH, Karpa KD, Keller D.. Bacillus Coagulans GBI-30 (BC30) improves indices of Clostridium difficile-Induced colitis in mice. Gut Pathog 2011; 3:16; PMID:22014083; http://dx.doi.org/ 10.1186/1757-4749-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]


