Abstract
Chitin deacetylase, the enzyme that catalyzes the hydrolysis of acetamido groups of N-acetylglucosamine in chitin, has been purified to homogeneity from mycelial extracts of the fungus Mucor rouxii and further characterized. The enzyme exhibits a low pI (approximately 3). Its apparent molecular mass was determined to be approximately 75 kDa by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and approximately 80 kDa by size-exclusion chromatography, suggesting that the enzyme exists as a monomer. Carbohydrate analysis of purified chitin deacetylase revealed that the enzyme is a high-mannose glycoprotein and that its carbohydrate content is approximately 30% by weight. Chitin deacetylase is active on several chitinous substrates and chitin derivatives. The enzyme requires at least four N-acetylglucosamine residues (chitotetraose) for catalysis, and it is inhibited by carboxylic acids, particularly acetic acid. When glycol chitin (a water-soluble chitin derivative) was used as substrate, the optimum temperature for enzyme activity was determined to be approximately 50 degrees C and the optimum pH was approximately 4.5.
Full text
PDF
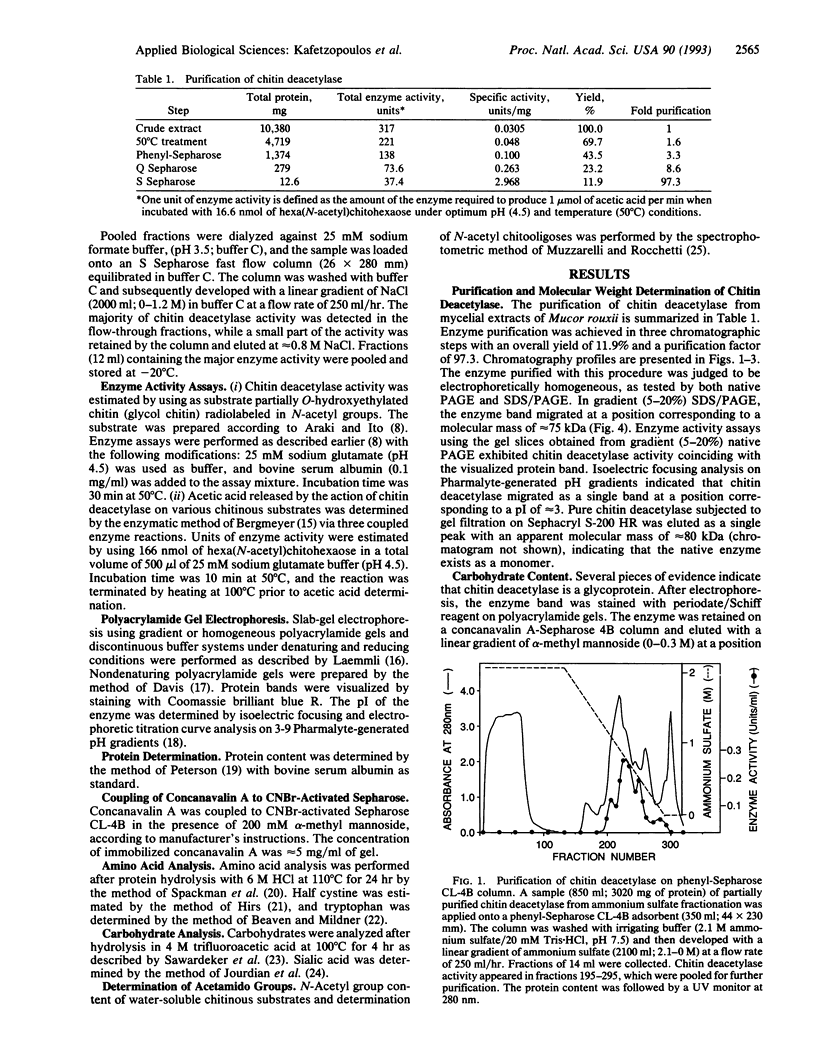
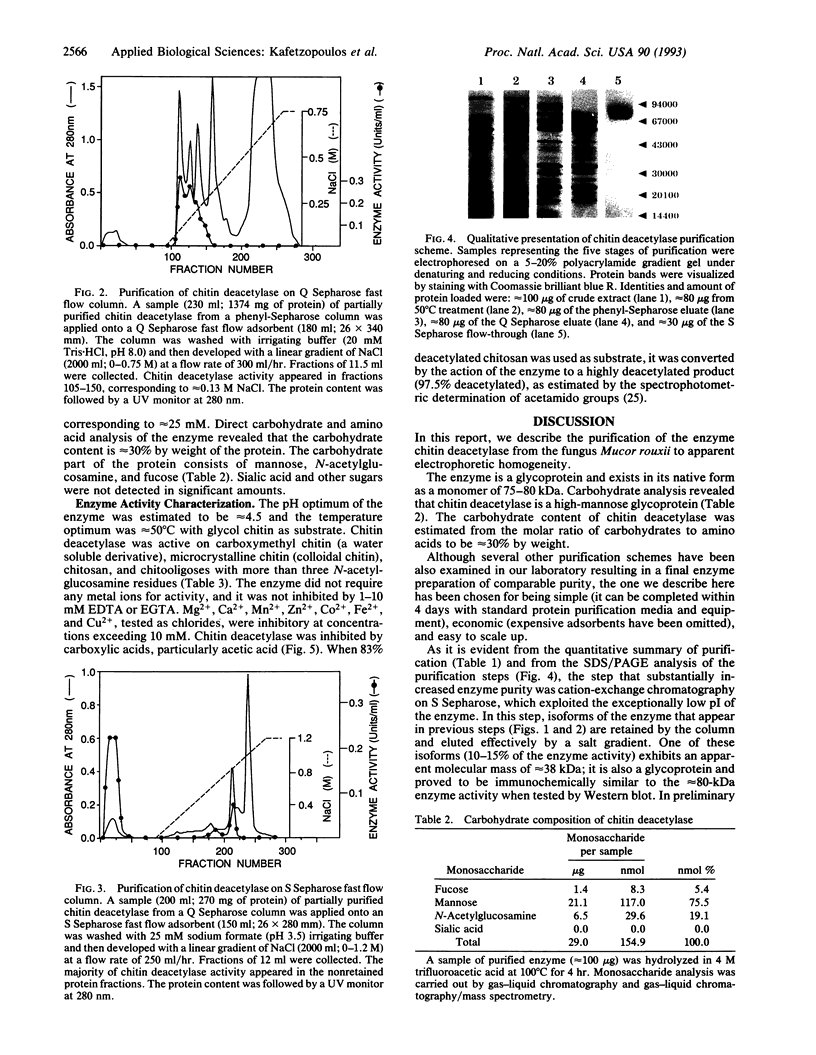
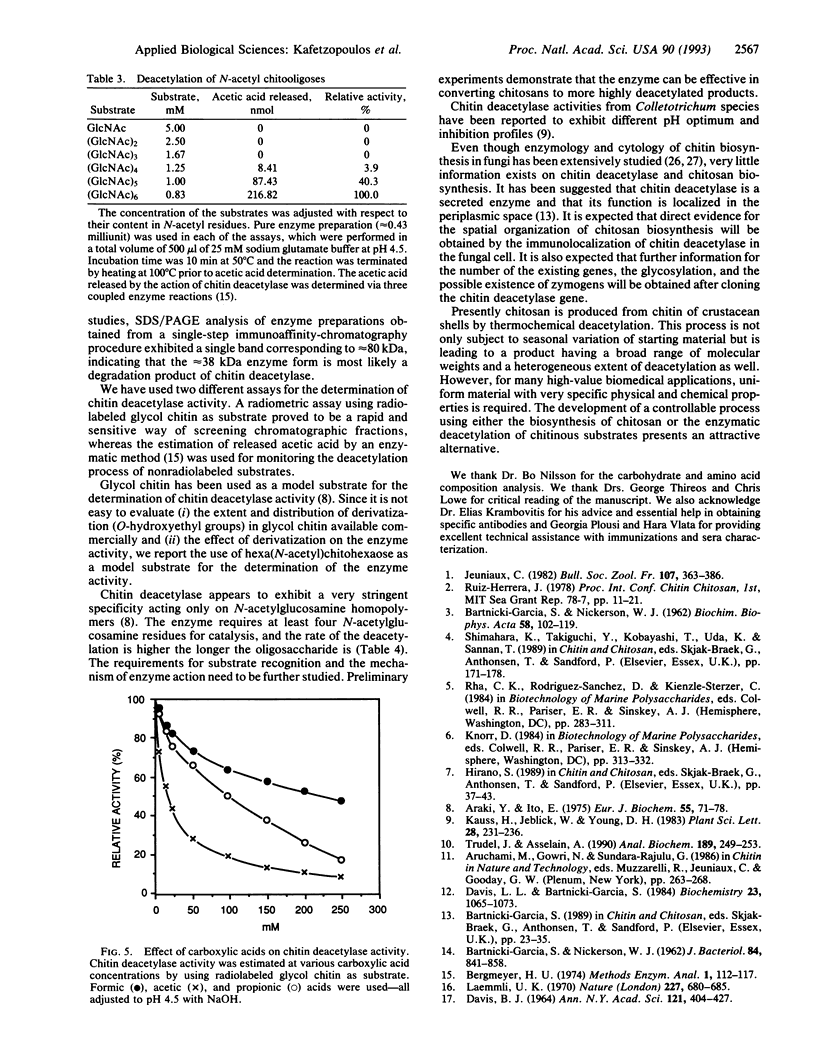

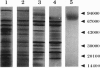
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Ito E. A pathway of chitosan formation in Mucor rouxii. Enzymatic deacetylation of chitin. Eur J Biochem. 1975 Jun 16;55(1):71–78. doi: 10.1111/j.1432-1033.1975.tb02139.x. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Cabib E. The synthesis and degradation of chitin. Adv Enzymol Relat Areas Mol Biol. 1987;59:59–101. doi: 10.1002/9780470123058.ch2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gianazza E., Gelfi C., Righetti P. G. Isoelectric focusing following by electrophoresis of proteins for visualizing their titration curves by zymogram and immunofixation. J Biochem Biophys Methods. 1980 Aug;3(2):65–75. doi: 10.1016/0165-022x(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Trudel J., Asselin A. Detection of chitin deacetylase activity after polyacrylamide gel electrophoresis. Anal Biochem. 1990 Sep;189(2):249–253. doi: 10.1016/0003-2697(90)90116-q. [DOI] [PubMed] [Google Scholar]