Abstract
Fibromyalgia syndrome (FMS) is a chronic, idiopathic condition of widespread musculoskeletal pain, affecting primarily women. It is clinically characterized by chronic, nonarticular pain and a heightened response to pressure along with sleep disturbances, fatigue, bowel and bladder abnormalities, and cognitive dysfunction. The diagnostic criteria have changed repeatedly, and there is neither a definitive pathogenesis nor reliable diagnostic or prognostic biomarkers. Clinical and laboratory studies have provided evidence of altered central pain pathways. Recent evidence suggests the involvement of neuroinflammation with stress peptides triggering the release of neurosenzitizing mediators. The management of FMS requires a multidimensional approach including patient education, behavioral therapy, exercise, and pain management. Here we review recent data on the pathogenesis and propose new directions for research and treatment.
Introduction
Fibromyalgia syndrome (FMS) is a chronic, idiopathic condition of widespread musculoskeletal pain that is clinically characterized by aches, soft tissue tenderness, stiffness, general fatigue, and sleep disturbances (Clauw et al., 2011; Schmidt-Wilcke and Clauw, 2011; Clauw, 2014) as well as cognitive dysfunction (Theoharides et al., 2015b). FMS is estimated to affect 2%–8% of the adult population and is considered to be the most common cause of generalized, musculoskeletal pain in women between the ages of 20 and 55 years (Branco et al., 2010). Diagnosis of FMS has changed over the last 10 years, but there are still no objective criteria (McBeth and Mulvey, 2012; Wolfe and Walitt, 2013). FMS belongs to a family of overlapping conditions that involve diffuse pain; they are called central sensitivity syndromes and may occur concomitantly (Table 1). These include chronic fatigue syndrome, irritable bowel syndrome, functional dyspepsia, myogenic temporomandibular disorder, tension headache, myofacial pain syndrome, restless leg syndrome, interstitial cystitis/bladder pain syndrome, posttraumatic stress disorder (PTSD), and Gulf War syndrome (Table 1) (Yunus, 2007; Theoharides, 2013a).
TABLE 1.
Central sensitivity syndromes often comorbid with FMS
| Chronic fatigue syndrome (CFS) |
| Functional dyspepsia |
| Gulf War syndrome |
| Interstitial cystitis/bladder pain syndrome (IC/BPS) |
| Irritable bowel syndrome (IBS) |
| Myogenic temporomandibular disorder (TMD) |
| Myofacial pain syndrome |
| Posttraumatic stress disorder (PTSD) |
| Restless leg syndrome |
| Tension headache |
Here we review recent data on the pathogenesis of FMS, especially with respect to the involvement of stress peptides triggering the release of inflammatory and neurosenzitizing mediators. We also propose new directions for research and treatment.
Diagnosis
In the absence of any objective biomarker, the diagnosis of FMS is based on the chief complaint of pain and associated symptoms of fatigue, sleep disturbance, cognitive decline, and mood changes. In the past, diagnosis was principally based on the presence of widespread pain for ≥3 months in at least 11 of 18 “tender points.” In 2010, the American College of Rheumatology (ACR) proposed preliminary diagnostic criteria for FMS that placed increased emphasis on patient symptoms (Wolfe et al., 2011). A later modification of the ACR 2010 criteria used a self-report questionnaire (the Fibromyalgia Survey Questionnaire) to assess patient symptoms (Ferrari and Russell, 2013) with a score of ≥12 having 93.1% sensitivity and 91.7% specificity, as compared with 90.2% and 89.5%, respectively, of the modified ACR criteria (Clauw, 2014).
A stepwise diagnostic work-up of patients with chronic widespread pain in primary care is recommended, with referral to specialists in cases of mental disorders (Clauw, 2014). The absence of distinct pathogenesis and objective markers hinders the development of effective treatment (Boomershine and Crofford, 2009; Clauw, 2010).
Pathogenesis
Investigations on the possible mechanisms involved in the etiology and pathogenesis of FMS have focused on dysfunction of the autonomic and central nervous systems, abnormalities in brain functional and neuroimaging studies, as well as genetic and environmental factors (Table 2). These include physical trauma (McLean et al., 2011), viral infections (hepatitis C, Epstein-Barr, human papillomavirus, human immunodeficiency virus, parvovirus, coxsackie B), and Lyme disease (Buskila et al., 2008).
TABLE 2.
Pathogenetic mechanisms in FMS
| Genetic factors |
| Linkage to the chromosome 17p11.2-q11.2 region |
| Linkage to serotonin receptor 2A region of chromosome 13 |
| Linkage to HLA region of chromosome 6 |
| Polymorphisms associated with the serotonin transporter (5-HTT) gene regulatory region |
| Linkage to catecholamine methyltransferase (COMT) genes |
| Negative association with the COMT val158met polymorphism |
| Association with dopamine-D-3 receptor (DRD3) Ser9Gly polymorphism |
| Single nucleotide polymorphisms (SNPs) involving gamma-aminobutyric acid receptor subunit beta 3 (GABRB3), trace amine receptors (TAAR1), and guanylate binding protein 1 (GBP1) |
| Neural processes |
| Altered heat and cold thresholds |
| Reduced tolerance for pain and nociceptive reflex threshold |
| Smaller than normal brain gray matter volumes |
| Less connectivity within the brain’s pain inhibitory network |
| Chiari malformation |
| Neuroinflammation |
| High serum IL-6 |
| High serum TNF |
| High plasma monocyte chemoattractant protein-1 (MCP-1/CCL2) and eotaxin (CCL) |
| High serum and CSF levels of IL-8 (CXCL8) |
| Increased plasma levels of IL-17A |
| Increased CSF levels of SP and nerve growth factor |
| Increased skin mast cells |
| Oxidative stress |
| Lower total nitrite levels |
| Higher serum prolidase activity, |
| Higher total oxidative status (TOS) |
| Reduced level of coenzyme Q10 (CoQ10) |
| High level of reactive oxygen species (ROS) |
A number of studies have linked FMS to early sexual abuse (Paras et al., 2009). In addition, emotional or psychologic stress, especially associated with deployment to war, may also trigger FMS (Eisen et al., 2005) (Figure 1).
Fig. 1.
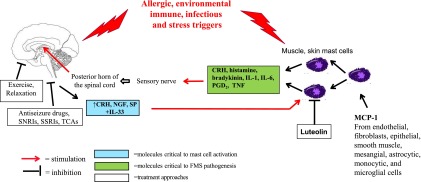
Diagram representation of the proposed steps involved in the pathogenesis of FMS and targets for treatment. Stress peptides (CRH, nerve growth factor, neurotensin, SP) are released from the spinal cord in peripheral tissues (blood vessels, muscles, skin) in response to allergic, environmental, immune, infectious, and stress triggers (blue box). There, they act synergistically with IL-33 to stimulate mast cells, which secrete inflammatory and neurosensitizing molecules such asCRH, histamine, bradykinin, IL-1, IL-6, prostaglandin D2, and TNF (green box). These molecules can either activate peripheral sensory nerves directly or reach the brain through the systemic circulation, thus creating a self-sustaining pain circuit. Treatment approaches (white box) include exercise and relaxation to reduce stress (specific norepinephrine reuptake inhibitors (SNRIs); specific serotonin reuptake inhibitors (SSRIs); tricyclic antidepressants (TCAs) to reduce anxiety and depression; as well as TCAs and antiseizure medications to provide analgesia. Finally, luteolin and related compounds can inhibit the release of MC mediators.
Genetic Factors.
About one-third of patients with FMS have a close relative, usually a female, who is similarly affected (Russell and Larson, 2009). One study performed genomewide linkage analysis in members of 116 families from the Fibromyalgia Family Study with 341 microsatellite markers and showed an estimated sibling recurrence risk ratio for FMS of 13.6 (95% confidence interval, 10.0–18.5), based on a reported population prevalence of 2% (Arnold et al., 2013). This was also one of the first reports of genomewide suggestive linkage of FMS to the chromosome 17p11.2-q11.2 region (Arnold et al., 2013).
Functional polymorphisms have linked FMS to serotonin receptor 2A region of chromosome 13 and the HLA region of chromosome 6 (Dudek et al., 2003). A significantly higher frequency of a polymorphism of the serotonin transporter (5-HTT) gene regulatory region was found in FMS patients (31%) compared with healthy controls (16%) (Offenbaecher et al., 1999). The serotonin transporter (5-HTT) gene was also found to be more frequent in patients affected by FMS who also had anxiety traits (Cohen et al., 2002).
Other genetic factors may also account for the decrease of pain thresholds in FMS patients (Buskila et al., 2007). Catecholamine methyltransferase (COMT) genes that have been implicated in predisposition to both pain and depression have also been invoked in FMS. There was an association between FMS and the COMT val158met polymorphism, with the COMT met allele appearing to confer “protection” to nonaffected relatives from developing full-blown FMS symptomatology (Cohen et al., 2009). There is also increased evidence that COMT is associated with increased psychologic vulnerability (Finan et al., 2011). Another study reported that a dopamine-D-3 receptor Ser9Gly polymorphism influenced diffuse noxious inhibitory control efficacy and pain tolerance in FMS patients (Potvin et al., 2009). There has also been evidence for the association of FMS with various adrenergic receptor gene polymorphisms (Vargas-Alarcon et al., 2009).
A large candidate gene association study examined over 350 genes in 496 FMS patients and 348 chronic-pain-free controls; three unsuspected genes [gamma-aminobutyric acid receptor subunit-β3 (GABRB3), trace amine receptors (TAAR1) and guanylate binding protein 1 (GBP1)] harbored single-nucleotide polymorphisms that differed in frequency between FMS patients and healthy controls (Smith et al., 2012).
Neural Processes.
Nociception is composed of two opposed components: pronociception and antinociception. FMS patients exhibit increased pronociception and decreased antinociception, resulting in chronic allodynia (Russell and Larson, 2009). Central sensitization is the main mechanism involved in the development and maintenance of chronic pain (Woodman, 2013), and it is characterized by allodynia and hyperalgesia (Staud et al., 2001). Allodynia is defined as the perception of pain resulting from a stimulus that would not normally be painful, whereas hyperalgesia occurs when an actual painful stimulus is perceived as more painful than it should be (Woolf, 2011). In FMS, allodynia is evidenced by pain at “tender points” with a stimulus (pressure ≤4 kg) that is not normally painful to healthy normal controls (Russell and Larson, 2009).
A quantitative sensory testing study in 85 FMS patients and 40 matched controls found that FMS patients had altered heat and cold thresholds; these patients also exhibited a reduced tolerance for pain as well as a reduced nociceptive reflex threshold, a measure of central excitability (Desmeules et al., 2003).
Functional brain imaging studies have provided compelling evidence for abnormal pain processing in FMS, including brain activity that correlated with patients’ pain sensitivity (hyperalgesia/allodynia), temporal summation of pain, and prolonged pain after noxious sensations (Staud, 2011). Voxel morphometric examination of brain MRI images of FMS patients showed significantly smaller than normal brain gray matter volumes, with this loss rapidly progressing with time compared with healthy controls (Kuchinad et al., 2007).
A number of studies have provided further evidence of dysfunctional connectivity of the pain network in FMS (Jensen et al., 2012; Flodin et al., 2014). One study found that FMS patients exhibited higher sensitivity to pain provocation than controls and that they required less pressure to evoke equal pain, but they failed to respond to pain provocation in the primary link in the descending pain-regulating system (the rostral anterior cingulate cortex) (Flodin et al., 2014). Another study comparing the functional connectivity of the descending pain inhibitory network in age-matched FMS patients and healthy controls found that patients displayed less connectivity within the brain’s pain inhibitory network during calibrated pressure pain compared with healthy controls (Jensen et al., 2012).
Some studies have suggested that FMS patients may suffer from compressive cervical myelopathy (Holman, 2012), possibly secondary to Chiari malformation (Heffez et al., 2004), which may be correctable surgically.
Psychologic Stress and Trauma.
Stress seems to increase the risk of developing FMS (Geenen et al., 2002). Psychologic factors have been shown to influence pain severity in FMS, and they may modulate the severity of perceived distress (Bote et al., 2012, 2013). The normal circadian rhythm for plasma cortisol level is disrupted in FMS patients as evidenced by elevated plasma concentrations in the evening (Crofford et al., 2004). Corticotropin-releasing hormone (CRH), the principal central nervous system mediator of the stress response, was elevated in the cerebrospinal fluid (CSF) of FMS patients and was associated with pain but not fatigue symptoms (McLean et al., 2006).
FMS is quite common in patients with systemic mastocytosis (Theoharides et al., 2015b), a disorder characterized by a higher number and reactivity of mast cells (MCs) (Theoharides et al., 2015c). Emotional stress is the most common trigger of symptoms in mastocytosis patients and is correlated with elevated serum levels of CRH, a receptor of which was expressed on bone marrow MCs (Theoharides et al., 2014). In fact, CRH can trigger selective release of vascular endothelial growth factor (VEGF) from human MCs (Cao et al., 2005) and also acts synergistically with the neuropeptide neurotensin to augment VEGF release, increasing vascular permeability (Donelan et al., 2006). CRH also leads to blood-brain barrier disruption (Theoharides and Konstantinidou, 2007) through brain MC activation (Esposito et al., 2002).
Recent studies have reported that estradiol augments immune (Kovats, 2015) and allergic (Hox et al., 2015) reactions. We showed that MCs express estrogen receptors (Pang et al., 1995) and that 17β-estradiol augmented substance P (SP)-induced MC activation (Theoharides et al., 1993). These findings may explain the higher prevalence of FMS in women. Levels of SP are increased in the CSF of FMS patients (Russell, 1998), and SP can stimulate MCs (Theoharides et al., 2010, 2012a). In fact, SP-induced MCs express CRH receptor-1 (Scholzen et al., 2001), and the SP receptor neurokinin-1 has been implicated in the pathophysiology of pain (Greenwood-Van Meerveld et al., 2014).
Nerve growth factor is elevated in the CSF of patients with FMS (Giovengo et al., 1999) and has been considered as a target for analgesic therapy (Lewin et al., 2014). Serum, plasma, and CSF levels of brain-derived neurotrophic factor are elevated in FMS (Nugraha et al., 2012), but it is not clear whether this is secondary to dysfunction of its receptor.
Neuroinflammation.
It has been suggested that MCs may be involved in FMS (Lucas et al., 2006; Pollack, 2015) as well as other comorbid conditions (Theoharides, 2013a). MCs have been increasingly associated with inflammation (Galli et al., 2008; Theoharides et al., 2012a) and pain (Heron and Dubayle, 2013; Chatterjea and Martinov, 2015). The number of MCs was significantly increased in the papillary dermis of FMS patients (Blanco et al., 2010). Moreover, chronic urticaria, which involves MCs, is more common in FMS (Torresani et al., 2009).
Monocyte chemoattractant protein-1 (MCP-1/CCL2) and eotaxin (CCL) have both been reported to be elevated in plasma of FMS patients (Zhang et al., 2008). MCP-1 also plays a pivotal role in inflammatory myopathies; myoblasts treated with MCP-1 or eotaxin secrete significant amounts of interleukin-1β (IL-1β) (Zhang et al., 2008). In addition, a study using a rat model to evaluate the involvement of MCP-1 in stress-induced chronic pain showed that MCP-1 induces long-lasting muscle hyperalgesia and a state of latent chronic sensitization to other allogenic substances through activation of its high-affinity receptor, CCR2, located on the peripheral terminals of IB4+ nociceptors (Alvarez et al., 2014). MCP-1 is a strong MC chemoattractant (Conti et al., 1998) and also triggers MCs (Conti and Theoharides, 1994).
MCs develop from bone marrow progenitors in response to stem cell factor, the ligand of the transmembrane tyrosine kinase Kit receptor, which regulates growth, migration, survival, and effector functions of MCs (Galli et al., 2011). These progenitors migrate from the blood into all tissues, including the brain, lung, mucosal interfaces, muscles, and skin where they mature in close proximity to blood vessels and nerve endings (Theoharides et al., 2015c).
MCs are major effector cells stimulated by allergens crosslinking specific IgE bound to their high-affinity surface receptors (Rivera et al., 2008). MCs also express Toll-like receptors, which can be activated by bacterial and viral antigens (Abraham and St John, 2010). Once stimulated, MCs secrete numerous vasoactive and proinflammatory mediators, leading to multiple symptoms (Theoharides et al., 2012a). MC activation can be enhanced by IL-33 (Fux et al., 2014), which synergizes with SP to induce VEGF release (Theoharides et al., 2010), acting as a “sensor of cell injury” (Enoksson et al., 2011; Theoharides et al., 2015a).
Preformed molecules stored in MC secretory granules include histamine, serotonin, bradykinin, proteases (chymase, carboxypeptidase, tryptase), and tumor necrosis factor (TNF) (Olszewski et al., 2007), which also participates in T-cell activation (Nakae et al., 2005; Kempuraj et al., 2008). MCs also release other proinflammatory and neurosensitizing molecules such as newly synthesized leukotrienes, prostaglandins, and platelet-activating factor as well as many cytokines (IL-6, IL-9, IL-13, TNF) and chemokines (CXCL8, CCL2, CCL5) (Theoharides et al., 2015c). MCs can release various cytokines, such as IL-6, selectively, without degranulation (Theoharides et al., 2007), which permits them to participate in many diverse functions. In addition, MCs secrete mitochondrial DNA (mtDNA), which has autocrine and paracrine stimulatory actions (Zhang et al., 2012), as well as exosomes that could deliver regulatory molecules such as microRNAs (Tsilioni et al., 2014; Kawikova and Askenase, 2015). MCs are now considered important in innate immunity (Galli et al., 2011), autoimmunity (Rottem and Mekori, 2005), and neuroinflammation (Theoharides et al., 2012a).
Chemokines act as modulators of nociception, enhancing sensitivity to pain by direct action on chemokine receptors throughout the pain pathway (Abbadie, 2005; Charo and Ransohoff, 2006). Several studies have shown elevated levels of the proinflammatory chemokine IL-8 (CXCL8) in both serum and CSF of patients with FMS (Ross et al., 2010; Kadetoff et al., 2012; Rodriguez-Pintó et al., 2014). However, exercise induced a decrease in the systemic concentration of IL-8 as compared with an exercise-induced increase in healthy women (Bote et al., 2012); this finding may explain the beneficial effect of mild exercise in FMS.
Sudden changes in the inflammatory cytokine profile may influence the severity of symptoms (Carvalho et al., 2008; Nugraha et al., 2013) and an imbalance between pro- and anti-inflammatory cytokine levels could explain, at least in part, the induction and maintenance of symptoms in FMS patients (Bazzichi et al., 2007). There appears to be an increase in cytokines early in the course of the disease that may sensitize peripheral and central nociceptors.
A number of studies have reported disturbances in cytokine levels in the blood and CSF of FMS patients, but the results vary considerably depending on whether they were measured in the plasma, serum, or from activated peripheral blood mononuclear cells (PBMCs), as well as the type of assay used. Moreover, measuring many of these mediators in biologic fluids may be tricky as they may be released episodically and are broken down quickly; so they may be best measured in 24-hour urine collected and stored cold as done for methylhistamine and prostaglandin F2α (Branco et al., 2010).
For instance, in one study, IL-6 was increased in the serum of FMS patients (Behm et al., 2012) and correlated with FMS severity (Uceyler et al., 2011). A recent study of plasma cytokines/chemokines in patients with chronic fatigue syndrome reported slightly elevated IL-1RA, IL-4, IL-13, but only during the short and not the long duration of the disease (Hornig et al., 2015). It is of interest that acute restraint stress of mice led to increased serum IL-6 that was entirely MC dependent (Geiss et al., 2012).
Other mediator levels were uniformly low, but were characterized by bursts of secretion with an increased ratio of IL-10 to that of IL-1β, IL-8, and TNF (Togo et al., 2009). A recent study using a Multiplex assay reported that the cytokine/chemokine release (IL-6, IL-8, MIP-1α and MIP-1β) from PBMCs in FMS patients was lower than in controls, as well as lower than in rheumatoid arthritis and systemic lupus erythematosus patients (Wallace et al., 2015). IFN-γ, IL-5, IL-6, IL-8, IL-10, MIP-1β, MCP-1, and MIP-1α released from stimulated PBMCs were also reported to be lower in FMS patients compared with healthy controls, and there was no difference in plasma levels (Behm et al., 2012).
One study showed increased plasma levels of IL-17A in patients with FMS and correlated their levels with increased levels of TNF (Pernambuco et al., 2013). CSF and serum IL-17 also positively correlated with indices of pain (Meng et al., 2013), depression, and anxiety (Liu et al., 2012), which are symptoms frequently reported by patients with FMS. TNF and IL-17 seem to act together in perpetuating the inflammatory process (Romero-Sanchez et al., 2011; Griffin et al., 2012). MC-derived IL-6 and transforming growth factor-β induce the development of TH17 cells through dendritic cell maturation (Dudeck et al., 2011); moreover, MCs themselves can secrete IL-17 (Kenna and Brown, 2013).
Increased levels of IL-4 and IL-10 may suggest a possible attempt to regulate the overproduction of IL-17 and other inflammatory cytokines (Wang et al., 1995). Both IL-4 and IL-10 seem to be necessary for regulatory T-cell–mediated suppression of the Tr17 response (McGeachy et al., 2007).
Oxidative Stress.
FMS patients have higher oxidative stress index and lower total nitrite levels than healthy controls (Neyal et al., 2013). In particular, patients with FMS demonstrated higher serum prolidase activity, total oxidative status, and oxidative stress index than healthy controls, and serum prolidase activity positively correlated with pain and fatigue scores (Bozkurt et al., 2014). Moreover, PBMCs from FMS patients showed reduced levels of coenzyme Q10 (CoQ10) and mtDNA contents, but high levels of mt reactive oxygen species and serum TNF (Cordero et al., 2013). Oxidative stress is present during tissue inflammation and also triggers MCs (Frossi et al., 2003). These findings may be relevant to our reports that MCs secrete extracellularly mtDNA, which has proinflammatory actions (Zhang et al., 2012), and that the mt uncoupling protein 2 (UCP2) inhibits MC secretion (Tagen et al., 2009).
Coenzyme Q10 (CoQ10) is an essential electron carrier in the mt respiratory chain and a strong antioxidant. Low CoQ10 levels have been detected in patients with FMS (Iqbal et al., 2011). One study showed decreased CoQ10, catalase, and ATP levels with increased level of lactoperoxidase in blood mononuclear cells from FMS patients as compared with normal controls; there was a significant negative correlation between these levels and headaches in FMS (Cordero et al., 2012a). Interestingly, CoQ10 deficiency has also been detected in depression and chronic fatigue, two common symptoms found in FMS patients, and both symptoms were markedly improved after CoQ10 supplementation (Maes et al., 2009).
Treatment
Unfortunately, there are no effective treatments of FMS presently available, but a number of drugs have been shown to reduce pain to variable extents. Management of FMS patients should integrate pharmacologic and nonpharmacologic approaches, while engaging patients as active participants in the process (Table 3) (Russell, 2008). However, a recent meta-analysis showed that multicomponent treatment is effective in the short term for improving key symptoms of FMS including pain, fatigue, depression, and quality of life, but disappointingly there is no evidence for a continued effect other than maintenance of physical fitness (Hauser et al., 2009a) (Figure 1).
TABLE 3.
Treatment options
| Nonpharmacologic |
| Cognitive behavioral therapy |
| Exercise |
| Qigong, tai-chi, yoga |
| Education |
| Pharmacologic |
| Amitriptyline |
| Cyclobenzaprine |
| Duloxetine |
| Gabapentin |
| Pregabalin |
| Complementary |
| CoQ10 |
| l-carnitine |
| Luteolin |
Nonpharmacologic Treatment.
One study found that patients receiving educational intervention had significantly more improvement than controls and that the beneficial effects continued for 3 to 12 months after the sessions had ended (Goldenberg et al., 2004). A meta-analysis of randomized clinical trials using cognitive behavioral therapy showed it could reduce fear of pain and fear of activity (Bernardy et al., 2010); however, it provided little benefit as single modality except possibly in juvenile FMS (Bennett and Nelson, 2006). Comprehensive reviews of Chinese stress reduction exercise programs, such as tai-chi and qigong, reported improvement of symptoms in FMS patients but with little difference when compared with controls (Mist et al., 2013; Sawynok and Lynch, 2014).
Pharmacologic Treatment.
The anticonvulsant drug pregabalin (PGB) has been approved by the U.S. Food and Drug Administration for the treatment of FMS (Hauser et al., 2011). PGB hyperpolarizes neurons and thus possibly lowers the firing threshold of sensory neurons, leading to reduced pain sensation. PGB is believed to reduce the magnitude of the enhanced pronociception process in FMS (Crofford et al., 2005; Arnold et al., 2008; Mease et al., 2008). Imaging studies using fMRI confirmed that PGB connects neuronal connectivity and biochemical aspects of pain in FMS (Harris et al., 2013; Kim et al., 2013).
A meta-analysis of five placebo-controlled randomized trials (four with PGB and one with gabapentin) consisting of 2918 patients with FMS showed that they significantly reduced pain and improved sleep and quality of life compared with placebo (Hauser et al., 2009c). However, a 2014 Cochrane review concluded that gabapentin (1200 mg or more per day) reduced pain intensity by 50% in only 37% of patients as compared with 21% on placebo (Moore et al., 2014).
A 2009 meta-analysis of 18 randomized trials using a variety of agents reported that antidepressants significantly improved pain, fatigue, depressed mood, sleep disturbance, and health-related quality of life (Hauser et al., 2009b). A 2014 systematic meta-analysis of six randomized trials involving 2249 patients using duloxetine (60 mg daily), a serotonin-norepinephrine reuptake inhibitor (SNRI), concluded that it was significantly more likely than placebo to reduce pain by at least 50% at 12 and 28 weeks (Lunn et al., 2014). The efficacy of duloxetine in patients with FMS was initially demonstrated in two multicenter trials of 12-week duration. In one trial, pain was reduced by at least 30% in a significantly greater proportion of patients receiving duloxetine (60 mg once or twice daily) compared with those taking placebo (Arnold et al., 2005). A longer-term benefit was demonstrated in a subsequent 6-month, multicenter, randomized, double-blind, placebo-controlled trial of 520 patients who were assigned to a single daily dose of either 60 mg or 120 mg of duloxetine or to placebo; duloxetine significantly reduced pain severity and improved mental fatigue in patients receiving duloxetine at 3 and 6 months (Russell et al., 2008).
Cyclobenzaprine is a muscle relaxant used to relieve skeletal muscle spasms and associated pain in acute musculoskeletal conditions. A randomized 8-week trial conducted with 36 patients showed that use of very low-dose cyclobenzaprine (1 to 4 mg at bedtime) significantly improved the symptoms of FMS, including pain, fatigue, and depression, compared with symptoms at baseline and with use of placebo (Moldofsky et al., 2011).
Amitriptyline (25–50 mg/day), a tricyclic antidepressant (TCA), was compared with duloxetine and milnacipran and was shown to be effective in reducing pain, sleep disturbance, and fatigue (Hauser et al., 2011). The apparent beneficial action of amitriptyline may be related to its ability to inhibit MC activation (Clemons et al., 2011).
Oxycodone was not found to be useful in patients with FMS (Gaskell et al., 2014). Nonsteroidal anti-inflammatory drugs (NSAIDs) may have a synergistic beneficial effect on pain when combined with antidepressants or anticonvulsants (Abrams et al., 2002). However, NSAIDs decrease the antidepressant action of specific serotonin reuptake inhibitors (SSRIs), but not TCAs (Theoharides et al., 2011).
Complementary and Emerging Treatments.
Nutritional supplementation is often used in FMS (Porter et al., 2010; Arranz et al., 2012), but the objective findings are limited. CoQ10 supplementation improved clinical symptoms in some FMS patients (Cordero et al., 2012a,b). One multicenter, double-blind, trial investigated the effect of 1000 mg oral and 500 mg of l-carnitine for 20 days and showed statistically significant benefits up to 10 weeks (Rossini et al., 2007).
Nutraceutical formulations containing the natural flavonoids quercetin and luteolin hold promise because they have anti-inflammatory, antioxidant, antiallergic, and antimicrobial actions (Middleton et al., 2000; Cazarolli et al., 2008). Flavonoids may exert anti-inflammatory actions via their ability to inhibit reactive oxygen or nitrogen compounds (Izzi et al., 2012), inhibiting MC activation (Kimata et al., 2000). The flavone luteolin can inhibit MCs (Asadi and Theoharides, 2012) and keratinocytes (Weng et al., 2014b). The luteolin structural analog tetramethoxyluteolin is more potent than luteolin (Weng et al., 2014a).
Flavonoids are safe (Kawanishi et al., 2005; Harwood et al., 2007). A recent clinical trial reported statistically significant benefits of a luteolin-containing dietary supplement in children with autism (Taliou et al., 2013), many of whom have “allergic-like” symptoms (Theoharides, 2013b), which implicates MC activation (Theoharides et al., 2012b). In fact, flavonoids have recently been discussed as a possible treatment of central nervous system disorders (Jager and Saaby, 2011; Grosso et al., 2013).
Conclusions and Future Directions
FMS is a complex disorder that is difficult to diagnose, and it needs a multimodal treatment approach (Garcia-Campayo et al., 2008). Many patients can achieve moderate symptom control, but pharmacologic treatments should be initiated in low doses with gradual titration upward to minimize side effects.
(Figure 1) Research should focus on the potential synergistic pathogenetic effect of neuropeptides such as CRH, nerve growth factor, and SP, and the possible role of episodic release of neurosensitizing molecules such as histamine, prostaglandin D2, IL-6, MCP-1, and TNF. Other potentially useful target molecules include IL-33, which has been considered an “alarmin” of tissue damage (Chan et al., 2012; Bessa et al., 2014). Available biologics, such as the TNF blockers etanercept and adalimumab, which are currently approved by the Food and Drug Administration for rheumatoid and psoriatic arthritis, should also be considered for FMS. CRH receptor-1, neurokinin-1, and TrKA antagonists should be considered, and luteolin and related flavonoids should be explored further (Figure 1).
Abbreviations
- ACR
American College of Rheumatology
- COMT
catecholamine methyltransferase
- CoQ10
coenzyme Q10
- CSF
cerebrospinal fluid
- CRH
corticotropin-releasing hormone
- CXCL8
proinflammatory chemokine IL-8
- FMS
fibromyalgia syndrome
- IL
interleukin
- MC
mast cell
- MCP-1/CCL2
monocyte chemoattractant protein-1
- mtDNA
mitochondrial DNA
- NSAID
nonsteroidal anti-inflammatory drug
- PBMCs
peripheral blood mononuclear cells
- PGB
pregabalin
- SP
substance P
- TCA
tricyclic antidepressant
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Theoharides, Tsilioni, Arbetman, Panagiotidou, Stewart, Gleason, Russell.
Footnotes
This work was supported in part by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS38326] and National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant AR47652], as well as the Michael and Katherine Johnson Family Fund (to T.C.T.).
References
- Abbadie C. (2005) Chemokines, chemokine receptors and pain. Trends Immunol 26:529–534. [DOI] [PubMed] [Google Scholar]
- Abraham SN, St John AL. (2010) Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10:440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 187:116–126. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Green PG, Levine JD. (2014) Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain 155:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, Olson JM, Iyengar SK. (2013) The fibromyalgia family study: a genome-wide linkage scan study. Arthritis Rheum 65:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LM, Rosen A, Pritchett YL, D’Souza DN, Goldstein DJ, Iyengar S, Wernicke JF. (2005) A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 119:5–15. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Russell IJ, Diri EW, Duan WR, Young JP, Jr, Sharma U, Martin SA, Barrett JA, Haig G. (2008) A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 9:792–805. [DOI] [PubMed] [Google Scholar]
- Arranz LI, Canela MA, Rafecas M. (2012) Dietary aspects in fibromyalgia patients: results of a survey on food awareness, allergies, and nutritional supplementation. Rheumatol Int 32:2615–2621. [DOI] [PubMed] [Google Scholar]
- Asadi S, Theoharides TC. (2012) Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin. J Neuroinflammation 9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzichi L, Rossi A, Massimetti G, Giannaccini G, Giuliano T, De Feo F, Ciapparelli A, Dell’Osso L, Bombardieri S. (2007) Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol 25:225–230. [PubMed] [Google Scholar]
- Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, Gashkoff PA, Gillis BS. (2012) Unique immunologic patterns in fibromyalgia. BMC Clin Pathol 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R, Nelson D. (2006) Cognitive behavioral therapy for fibromyalgia. Nat Clin Pract Rheumatol 2:416–424. [DOI] [PubMed] [Google Scholar]
- Bernardy K, Füber N, Köllner V, Häuser W. (2010) Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 37:1991–2005. [DOI] [PubMed] [Google Scholar]
- Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, Cote-Sierra J. (2014) Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun 55:33–41. [DOI] [PubMed] [Google Scholar]
- Blanco I, Béritze N, Argüelles M, Cárcaba V, Fernández F, Janciauskiene S, Oikonomopoulou K, de Serres FJ, Fernández-Bustillo E, Hollenberg MD. (2010) Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol 29:1403–1412. [DOI] [PubMed] [Google Scholar]
- Boomershine CS, Crofford LJ. (2009) A symptom-based approach to pharmacologic management of fibromyalgia. Nat Rev Rheumatol 5:191–199. [DOI] [PubMed] [Google Scholar]
- Bote ME, García JJ, Hinchado MD, Ortega E. (2012) Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation 19:343–351. [DOI] [PubMed] [Google Scholar]
- Bote ME, Garcia JJ, Hinchado MD, Ortega E. (2013) Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS One 8:e74524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt M, Caglayan M, Oktayoglu P, Em S, Batmaz I, Sariyildiz MA, Nas K, Ucar D, Yüksel H, Sarac AJ. (2014) Serum prolidase enzyme activity and oxidative status in patients with fibromyalgia. Redox Rep 19:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, Saraiva F, Nacci F, Thomas E, Caubère JP, et al. (2010) Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum 39:448–453. [DOI] [PubMed] [Google Scholar]
- Buskila D, Atzeni F, Sarzi-Puttini P. (2008) Etiology of fibromyalgia: the possible role of infection and vaccination. Autoimmun Rev 8:41–43. [DOI] [PubMed] [Google Scholar]
- Buskila D, Sarzi-Puttini P, Ablin JN. (2007) The genetics of fibromyalgia syndrome. Pharmacogenomics 8:67–74. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. (2005) Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol 174:7665–7675. [DOI] [PubMed] [Google Scholar]
- Carvalho LS, Correa H, Silva GC, Campos FS, Baião FR, Ribeiro LS, Faria AM, d’Avila Reis D. (2008) May genetic factors in fibromyalgia help to identify patients with differentially altered frequencies of immune cells? Clin Exp Immunol 154:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, Pizzolatti MG, Silva FR. (2008) Flavonoids: prospective drug candidates. Mini Rev Med Chem 8:1429–1440. [DOI] [PubMed] [Google Scholar]
- Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. (2012) Alarmins: awaiting a clinical response. J Clin Invest 122:2711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621. [DOI] [PubMed] [Google Scholar]
- Chatterjea D, Martinov T. (2015) Mast cells: versatile gatekeepers of pain. Mol Immunol 63:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ. (2010) Pain management: fibromyalgia drugs are ‘as good as it gets’ in chronic pain. Nat Rev Rheumatol 6:439–440. [DOI] [PubMed] [Google Scholar]
- Clauw DJ. (2014) Fibromyalgia: a clinical review. JAMA 311:1547–1555. [DOI] [PubMed] [Google Scholar]
- Clauw DJ, Arnold LM, McCarberg BH, FibroCollaborative (2011) The science of fibromyalgia. Mayo Clin Proc 86:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons A, Vasiadi M, Kempuraj D, Kourelis T, Vandoros G, Theoharides TC. (2011) Amitriptyline and prochlorperazine inhibit proinflammatory mediator release from human mast cells: possible relevance to chronic fatigue syndrome. J Clin Psychopharmacol 31:385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Buskila D, Neumann L, Ebstein RP. (2002) Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 46:845–847. [DOI] [PubMed] [Google Scholar]
- Cohen H, Neumann L, Glazer Y, Ebstein RP, Buskila D. (2009) The relationship between a common catechol-O-methyltransferase (COMT) polymorphism val(158) met and fibromyalgia. Clin Exp Rheumatol 27(5, Suppl 56)S51–S56. [PubMed] [Google Scholar]
- Conti P, Reale M, Barbacane RC, Letourneau R, Theoharides TC. (1998) Intramuscular injection of hrRANTES causes mast cell recruitment and increased transcription of histidine decarboxylase in mice: lack of effects in genetically mast cell-deficient W/WV mice. FASEB J 12:1693–1700. [DOI] [PubMed] [Google Scholar]
- Conti P, Theoharides TC. (1994) Monocyte chemotactic protein-1 (MCP-1) is active on mast cells and causes clump formation. Int J Immunopathol Pharmacol 7:149–151. [Google Scholar]
- Cordero MD, Díaz-Parrado E, Carrión AM, Alfonsi S, Sánchez-Alcazar JA, Bullón P, Battino M, de Miguel M. (2013) Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxid Redox Signal 18:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MD, Cano-García FJ, Alcocer-Gómez E, De Miguel M, Sánchez-Alcázar JA. (2012a) Oxidative stress correlates with headache symptoms in fibromyalgia: coenzyme Q₁₀ effect on clinical improvement. PLoS One 7:e35677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero MD, Cotán D, del-Pozo-Martín Y, Carrión AM, de Miguel M, Bullón P, Sánchez-Alcazar JA. (2012b) Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 28:1200–1203. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, Young JP, Jr, LaMoreaux LK, Martin SA, Sharma U, Pregabalin 1008-105 Study Group (2005) Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 52:1264–1273. [DOI] [PubMed] [Google Scholar]
- Crofford LJ, Young EA, Engleberg NC, Korszun A, Brucksch CB, McClure LA, Brown MB, Demitrack MA. (2004) Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav Immun 18:314–325. [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. (2003) Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 48:1420–1429. [DOI] [PubMed] [Google Scholar]
- Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, Theoharides TC. (2006) Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA 103:7759–7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck A, Suender CA, Kostka SL, von Stebut E, Maurer M. (2011) Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol 41:1883–1893. [DOI] [PubMed] [Google Scholar]
- Dudek DM, Arnold LM, Lyengar SK. (2003) Genetic linkage of fibromyalgia ot the serotonin receptor 2A region of chromosome 13 and the HLA region on chromosome 6. Am J Hum Genet 75:468. [Google Scholar]
- Eisen SA, Kang HK, Murphy FM, Blanchard MS, Reda DJ, Henderson WG, Toomey R, Jackson LW, Alpern R, Parks BJ, et al. Gulf War Study Participating Investigators (2005) Gulf War veterans’ health: medical evaluation of a U.S. cohort. Ann Intern Med 142:881–890. [DOI] [PubMed] [Google Scholar]
- Enoksson M, Lyberg K, Möller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. (2011) Mast cells as sensors of cell injury through IL-33 recognition. J Immunol 186:2523–2528. [DOI] [PubMed] [Google Scholar]
- Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. (2002) Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther 303:1061–1066. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Russell AS. (2013) A questionnaire using the modified 2010 American College of Rheumatology criteria for fibromyalgia: specificity and sensitivity in clinical practice. J Rheumatol 40:1590–1595. [DOI] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. (2011) COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain 152:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodin P, Martinsen S, Löfgren M, Bileviciute-Ljungar I, Kosek E, Fransson P. (2014) Fibromyalgia is associated with decreased connectivity between pain- and sensorimotor brain areas. Brain Connect 4:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C. (2003) Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol 33:2168–2177. [DOI] [PubMed] [Google Scholar]
- Fux M, Pecaric-Petkovic T, Odermatt A, Hausmann OV, Lorentz A, Bischoff SC, Virchow JC, Dahinden CA. (2014) IL-33 is a mediator rather than a trigger of the acute allergic response in humans. Allergy 69:216–222. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA. (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12:1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. (2008) The development of allergic inflammation. Nature 454:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Campayo J, Magdalena J, Magallón R, Fernández-García E, Salas M, Andrés E. (2008) A meta-analysis of the efficacy of fibromyalgia treatment according to level of care. Arthritis Res Ther 10:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell H, Moore RA, Derry S, Stannard C. (2014) Oxycodone for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 6:CD010692. [DOI] [PubMed] [Google Scholar]
- Geenen R, Jacobs JW, Bijlsma JW. (2002) Evaluation and management of endocrine dysfunction in fibromyalgia. Rheum Dis Clin North Am 28:389–404. [DOI] [PubMed] [Google Scholar]
- Geiss A, Rohleder N, Anton F. (2012) Evidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology 37:671–684. [DOI] [PubMed] [Google Scholar]
- Giovengo SL, Russell IJ, Larson AA. (1999) Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol 26:1564–1569. [PubMed] [Google Scholar]
- Goldenberg DL, Burckhardt C, Crofford L. (2004) Management of fibromyalgia syndrome. JAMA 292:2388–2395. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Mohammadi E, Tyler K, Pietra C, Bee LA, Dickenson A. (2014) Synergistic effect of 5-hydroxytryptamine 3 and neurokinin 1 receptor antagonism in rodent models of somatic and visceral pain. J Pharmacol Exp Ther 351:146–152. [DOI] [PubMed] [Google Scholar]
- Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, et al. (2012) IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188:6287–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso C, Valentão P, Ferreres F, Andrade PB. (2013) The use of flavonoids in central nervous system disorders. Curr Med Chem 20:4694–4719. [DOI] [PubMed] [Google Scholar]
- Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, et al. (2013) Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 119:1453–1464. [DOI] [PubMed] [Google Scholar]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 45:2179–2205. [DOI] [PubMed] [Google Scholar]
- Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M. (2009a) Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum 61:216–224. [DOI] [PubMed] [Google Scholar]
- Häuser W, Bernardy K, Uçeyler N, Sommer C. (2009b) Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA 301:198–209. [DOI] [PubMed] [Google Scholar]
- Häuser W, Bernardy K, Uçeyler N, Sommer C. (2009c) Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain 145:69–81. [DOI] [PubMed] [Google Scholar]
- Häuser W, Petzke F, Üçeyler N, Sommer C. (2011) Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology (Oxford) 50:532–543. [DOI] [PubMed] [Google Scholar]
- Heffez DS, Ross RE, Shade-Zeldow Y, Kostas K, Shah S, Gottschalk R, Elias DA, Shepard A, Leurgans SE, Moore CG. (2004) Clinical evidence for cervical myelopathy due to Chiari malformation and spinal stenosis in a non-randomized group of patients with the diagnosis of fibromyalgia. Eur Spine J 13:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héron A, Dubayle D. (2013) A focus on mast cells and pain. J Neuroimmunol 264:1–7. [DOI] [PubMed] [Google Scholar]
- Holman AJ. (2012) Using dynamic MRI to diagnose neck pain: the import of positional cervical cord compression (PC3). Pract Pain Manag 12:51–55. [Google Scholar]
- Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, Peterson DL, Gottschalk CG, Schultz AF, Che X, et al. (2015) Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv 1:e1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox V, Desai A, Bandara G, Gilfillan AM, Metcalfe DD, Olivera A. (2015) Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J Allergy Clin Immunol 135:729–36.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal R, Mughal MS, Arshad N, Arshad M. (2011) Pathophysiology and antioxidant status of patients with fibromyalgia. Rheumatol Int 31:149–152. [DOI] [PubMed] [Google Scholar]
- Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, Bei R. (2012) The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front Biosci (Landmark Ed) 17:2396–2418. [DOI] [PubMed] [Google Scholar]
- Jäger AK, Saaby L. (2011) Flavonoids and the CNS. Molecules 16:1471–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, et al. (2012) Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. (2012) Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 242:33–38. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Oikawa S, Murata M. (2005) Evaluation for safety of antioxidant chemopreventive agents. Antioxid Redox Signal 7:1728–1739. [DOI] [PubMed] [Google Scholar]
- Kawikova I, Askenase PW. (2015) Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res 1617:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolfberg A, Theoharides TC. (2008) Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br J Pharmacol 155:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna TJ, Brown MA. (2013) The role of IL-17-secreting mast cells in inflammatory joint disease. Nat Rev Rheumatol 9:375–379. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Lee S, Mun CW. (2013) Evaluation of the effectiveness of pregabalin in alleviating pain associated with fibromyalgia: using functional magnetic resonance imaging study. PLoS One 8:e74099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. (2000) Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy 30:501–508. [DOI] [PubMed] [Google Scholar]
- Kovats S. (2015) Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. (2007) Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci 27:4004–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Lechner SG, Smith ES. (2014) Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. Handbook Exp Pharmacol 220:251–282. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. (2012) The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis 15:183–187. [DOI] [PubMed] [Google Scholar]
- Lucas HJ, Brauch CM, Settas L, Theoharides TC. (2006) Fibromyalgia—new concepts of pathogenesis and treatment. Int J Immunopathol Pharmacol 19:5–10. [PubMed] [Google Scholar]
- Lunn MP, Hughes RA, Wiffen PJ. (2014) Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev 1:CD007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. (2009) Lower plasma coenzyme Q10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuroendocrinol Lett 30:462–469. [PubMed] [Google Scholar]
- McBeth J, Mulvey MR. (2012) Fibromyalgia: mechanisms and potential impact of the ACR 2010 classification criteria. Nat Rev Rheumatol 8:108–116. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8:1390–1397. [DOI] [PubMed] [Google Scholar]
- McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, et al. (2011) Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain 12:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G, Park KM, Liberzon I, Sen A, Gracely RH, et al. (2006) Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology 31:2776–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Russell IJ, Arnold LM, Florian H, Young JP, Jr, Martin SA, Sharma U. (2008) A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol 35:502–514. [PubMed] [Google Scholar]
- Meng X, Zhang Y, Lao L, Saito R, Li A, Bäckman CM, Berman BM, Ren K, Wei PK, Zhang RX. (2013) Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 154:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751. [PubMed] [Google Scholar]
- Mist SD, Firestone KA, Jones KD. (2013) Complementary and alternative exercise for fibromyalgia: a meta-analysis. J Pain Res 6:247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldofsky H, Harris HW, Archambault WT, Kwong T, Lederman S. (2011) Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol 38:2653–2663. [DOI] [PubMed] [Google Scholar]
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. (2014) Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 4:CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. (2005) Mast cells enhance T cell activation: importance of mast cell-derived TNF. Proc Natl Acad Sci USA 102:6467–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyal M, Yimenicioglu F, Aydeniz A, Taskin A, Saglam S, Cekmen M, Neyal A, Gursoy S, Erel O, Balat A. (2013) Plasma nitrite levels, total antioxidant status, total oxidant status, and oxidative stress index in patients with tension-type headache and fibromyalgia. Clin Neurol Neurosurg 115:736–740. [DOI] [PubMed] [Google Scholar]
- Nugraha B, Karst M, Engeli S, Gutenbrunner C. (2012) Brain-derived neurotrophic factor and exercise in fibromyalgia syndrome patients: a mini review. Rheumatol Int 32:2593–2599. [DOI] [PubMed] [Google Scholar]
- Nugraha B, Korallus C, Kielstein H, Gutenbrunner C. (2013) CD3+CD56+ natural killer T cells in fibromyalgia syndrome patients: association with the intensity of depression. Clin Exp Rheumatol 31;(Suppl 79):S9–S15. [PubMed] [Google Scholar]
- Offenbaecher M, Bondy B, de Jonge S, Glatzeder K, Krüger M, Schoeps P, Ackenheil M. (1999) Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheum 42:2482–2488. [DOI] [PubMed] [Google Scholar]
- Olszewski MB, Groot AJ, Dastych J, Knol EF. (2007) TNF trafficking to human mast cell granules: mature chain-dependent endocytosis. J Immunol 178:5701–5709. [DOI] [PubMed] [Google Scholar]
- Pang X, Cotreau-Bibbo MM, Sant GR, Theoharides TC. (1995) Bladder mast cell expression of high affinity oestrogen receptors in patients with interstitial cystitis. Br J Urol 75:154–161. [DOI] [PubMed] [Google Scholar]
- Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, Elamin MB, Seime RJ, Prokop LJ, Zirakzadeh A. (2009) Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA 302:550–561. [DOI] [PubMed] [Google Scholar]
- Pernambuco AP, Schetino LP, Alvim CC, Murad CM, Viana RS, Carvalho LS, Reis DA. (2013) Increased levels of IL-17A in patients with fibromyalgia. Clin Exp Rheumatol 31(6, Suppl 79)S60–S63. [PubMed] [Google Scholar]
- Pollack S. (2015) Mast cells in fibromyalgia. Clin Exp Rheumatol 33;(Suppl 88):S140. [PubMed] [Google Scholar]
- Porter NS, Jason LA, Boulton A, Bothne N, Coleman B. (2010) Alternative medical interventions used in the treatment and management of myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia. J Altern Complement Med 16:235–249. [DOI] [PubMed] [Google Scholar]
- Potvin S, Larouche A, Normand E, de Souza JB, Gaumond I, Grignon S, Marchand S. (2009) DRD3 Ser9Gly polymorphism is related to thermal pain perception and modulation in chronic widespread pain patients and healthy controls. J Pain 10:969–975. [DOI] [PubMed] [Google Scholar]
- Rivera J, Fierro NA, Olivera A, Suzuki R. (2008) New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol 98:85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pintó I, Agmon-Levin N, Howard A, Shoenfeld Y. (2014) Fibromyalgia and cytokines. Immunol Lett 161:200–203. [DOI] [PubMed] [Google Scholar]
- Romero-Sanchez C, Jaimes DA, Londoño J, De Avila J, Castellanos JE, Bello JM, Bautista W, Valle-Oñate R. (2011) Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin Exp Rheumatol 29:828–834. [PubMed] [Google Scholar]
- Ross RL, Jones KD, Bennett RM, Ward RL, Druker BJ, Wood LJ. (2010) Preliminary evidence of increased pain and elevated cytokines in fibromyalgia patients with defective growth hormone response to exercise. Open Immunol J 3:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini M, Di MO, Valentini G, Bianchi G, Biasi G, Cacace E, Malesci D, La MG, Viapiana O, Adami S. (2007) Double-blind, multicenter trial comparing acetyl l-carnitine with placebo in the treatment of fibromyalgia patients. Clin Exp Rheumatol 25:182–188. [PubMed] [Google Scholar]
- Rottem M, Mekori YA. (2005) Mast cells and autoimmunity. Autoimmun Rev 4:21–27. [DOI] [PubMed] [Google Scholar]
- Russell IJ. (1998) Advances in fibromyalgia: possible role for central neurochemicals. Am J Med Sci 315:377–384. [DOI] [PubMed] [Google Scholar]
- Russell IJ. (2008) Fibromyalgia syndrome: approach to management. CNS Spectr 13(3, Suppl 5)27–33. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Larson AA. (2009) Neurophysiopathogenesis of fibromyalgia syndrome: a unified hypothesis. Rheum Dis Clin North Am 35:421–435. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Mease PJ, Smith TR, Kajdasz DK, Wohlreich MM, Detke MJ, Walker DJ, Chappell AS, Arnold LM. (2008) Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 136:432–444. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Lynch M. (2014) Qigong and fibromyalgia: randomized controlled trials and beyond. Evid Based Complement Alternat Med 2014:379715 DOI: 10.1155/2014/379715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Clauw DJ. (2011) Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol 7:518–527. [DOI] [PubMed] [Google Scholar]
- Scholzen TE, Steinhoff M, Bonaccorsi P, Klein R, Amadesi S, Geppetti P, Lu B, Gerard NP, Olerud JE, Luger TA, et al. (2001) Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J Immunol 166:1285–1291. [DOI] [PubMed] [Google Scholar]
- Smith SB, Maixner DW, Fillingim RB, Slade G, Gracely RH, Ambrose K, Zaykin DV, Hyde C, John S, Tan K, et al. (2012) Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum 64:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R. (2011) Brain imaging in fibromyalgia syndrome. Clin Exp Rheumatol 29(6, Suppl 69)S109–S117. [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. (2001) Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 91:165–175. [DOI] [PubMed] [Google Scholar]
- Tagen M, Elorza A, Kempuraj D, Boucher W, Kepley CL, Shirihai OS, Theoharides TC. (2009) Mitochondrial uncoupling protein 2 inhibits mast cell activation and reduces histamine content. J Immunol 183:6313–6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliou A, Zintzaras E, Lykouras L, Francis K. (2013) An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther 35:592–602. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. (2013a) Atopic conditions in search of pathogenesis and therapy. Clin Ther 35:544–547. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. (2013b) Is a subtype of autism an allergy of the brain? Clin Ther 35:584–591. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, et al. (2012a) Mast cells and inflammation. Biochim Biophys Acta 1822:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, Toniato E, Kalogeromitros D. (2012b) Mast cell activation and autism. Biochim Biophys Acta 1822:34–41. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Asadi S, Weng Z, Zhang B. (2011) Serotonin-selective reuptake inhibitors and nonsteroidal anti-inflammatory drugs—important considerations of adverse interactions especially for the treatment of myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Psychopharmacol 31:403–405. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Dimitriadou V, Letourneau R, Rozniecki JJ, Vliagoftis H, Boucher W. (1993) Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain myelin changes resembling early stages of demyelination. Neuroscience 57:861–871. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. (2007) Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev 217:65–78. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Konstantinidou AD. (2007) Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci 12:1615–1628. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Petra AI, Stewart JM, Tsilioni I, Panagiotidou S, Akin C. (2014) High serum corticotropin-releasing hormone (CRH) and bone marrow mast cell CRH receptor expression in a mastocytosis patient. J Allergy Clin Immunol 134:1197–1199. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P. (2015a) The importance of IL-33 in allergic inflammation. J Pharmacol Exp Ther 354:24–31 [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Stewart JM, Hatziagelaki E, Kolaitis G. (2015b) Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Front Neurosci 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Valent P, Akin C. (2015c) Mast cells, mastocytosis and related diseases. N Engl J Med 373:163–172. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, et al. (2010) IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA 107:4448–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo F, Natelson BH, Adler GK, Ottenweller JE, Goldenberg DL, Struzik ZR, Yamamoto Y. (2009) Plasma cytokine fluctuations over time in healthy controls and patients with fibromyalgia. Exp Biol Med (Maywood) 234:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torresani C, Bellafiore S, De Panfilis G. (2009) Chronic urticaria is usually associated with fibromyalgia syndrome. Acta Derm Venereol 89:389–392. [DOI] [PubMed] [Google Scholar]
- Tsilioni I, Panagiotidou S, Theoharides TC. (2014) Exosomes in neurologic and psychiatric disorders. Clin Ther 36:882–888. [DOI] [PubMed] [Google Scholar]
- Uçeyler N, Häuser W, Sommer C. (2011) Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord 12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Alarcón G, Fragoso JM, Cruz-Robles D, Vargas A, Martinez A, Lao-Villadóniga JI, García-Fructuoso F, Vallejo M, Martínez-Lavín M. (2009) Association of adrenergic receptor gene polymorphisms with different fibromyalgia syndrome domains. Arthritis Rheum 60:2169–2173. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Gavin IM, Karpenko O, Barkhordar F, Gillis BS. (2015) Cytokine and chemokine profiles in fibromyalgia, rheumatoid arthritis and systemic lupus erythematosus: a potentially useful tool in differential diagnosis. Rheumatol Int 35:991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wu P, Siegel MI, Egan RW, Billah MM. (1995) Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem 270:9558–9563. [DOI] [PubMed] [Google Scholar]
- Weng Z, Patel A, Panagiotidou S, Theoharides TC. (2014a) The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol 14:01574–01577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Z, Patel AB, Vasiadi M, Therianou A, Theoharides TC. (2014b) Luteolin inhibits human keratinocyte activation and decreases NF-κB induction that is increased in psoriatic skin. PLoS One 9:e90739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38:1113–1122. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Walitt B. (2013) Culture, science and the changing nature of fibromyalgia. Nat Rev Rheumatol 9:751–755. [DOI] [PubMed] [Google Scholar]
- Woodman I. (2013) Fibromyalgia: fibromyalgia-all in the brain? Nat Rev Rheumatol 9:565. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3, Suppl)S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus MB. (2007) Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 36:339–356. [DOI] [PubMed] [Google Scholar]
- Zhang B, Asadi S, Weng Z, Sismanopoulos N, Theoharides TC. (2012) Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS One 7:e49767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cherryholmes G, Mao A, Marek C, Longmate J, Kalos M, Amand RP, Shively JE. (2008) High plasma levels of MCP-1 and eotaxin provide evidence for an immunological basis of fibromyalgia. Exp Biol Med (Maywood) 233:1171–1180. [DOI] [PubMed] [Google Scholar]


