Dementia is a common late complication of Parkinson’s disease, but the mechanisms underlying this form of dementia are unclear. Gratwicke et al. consider the development of each core cognitive symptom in turn, and argue that Parkinson’s disease dementia reflects dysfunction in seven distinct brain networks, with implications for therapeutic approaches.
Keywords: Parkinson’s disease, dementia, neural networks, acetylcholine, dopamine
Dementia is a common late complication of Parkinson’s disease, but the mechanisms underlying this form of dementia are unclear. Gratwicke et al. consider the development of each core cognitive symptom in turn, and argue that Parkinson’s disease dementia reflects dysfunction in seven distinct brain networks, with implications for therapeutic approaches.
Abstract
In the long-term, with progression of the illness, Parkinson’s disease dementia affects up to 90% of patients with Parkinson’s disease. With increasing life expectancy in western countries, Parkinson’s disease dementia is set to become even more prevalent in the future. However, current treatments only give modest symptomatic benefit at best. New treatments are slow in development because unlike the pathological processes underlying the motor deficits of Parkinson’s disease, the neural mechanisms underlying the dementing process and its associated cognitive deficits are still poorly understood. Recent insights from neuroscience research have begun to unravel the heterogeneous involvement of several distinct neural networks underlying the cognitive deficits in Parkinson’s disease dementia, and their modulation by both dopaminergic and non-dopaminergic transmitter systems in the brain. In this review we collate emerging evidence regarding these distinct brain networks to give a novel perspective on the pathological mechanisms underlying Parkinson’s disease dementia, and discuss how this may offer new therapeutic opportunities.
Introduction
Parkinson’s disease dementia (PDD) is a late complication of Parkinson’s disease, with a cumulative prevalence of 75–90% of those with a disease duration of 10 years or more (Buter et al., 2008; Hely et al., 2008; Aarsland and Kurz, 2010). It’s development negatively impacts activities of daily living (Rosenthal et al., 2010), and confers significantly increased morbidity and mortality (Reid et al., 1996; Levy et al., 2002b). It is now widely recognized that the clinical phenotype of PDD extends beyond the classical dysexecutive syndrome seen in early Parkinson’s disease to include additional deficits in recognition memory, attention processes and visual perception (Pagonabarraga and Kulisevsky, 2012; Kehagia et al., 2013), as well as visual hallucinations and cognitive fluctuations (Emre, 2003). This constellation of features was recently made explicit in the diagnostic criteria for PDD (Emre et al., 2007). However, in stark contrast to the nigrostriatal pathology underlying the motor aspects of the disorder (Fahn et al., 1971; Hirsch et al., 1988), the pathophysiological mechanisms underlying PDD remain obscure, which hinders the development of new therapies.
One major difficulty in determining the pathological mechanisms contributing to PDD is that the underlying cellular-level pathology is heterogeneous, with Lewy bodies, neurofibrillary tangles, senile plaques, microvascular disease and argyrophilic inclusions all contributing (Irwin et al., 2012; Del Tredici and Braak, 2013; Horvath et al., 2013; Halliday et al., 2014). The anatomical distribution of such pathology varies between different cases (Colosimo et al., 2003; Galvin et al., 2006) and does not always correspond with clinical symptoms. For example, in one large neuropathological series, 55% of Parkinson’s disease cases with Braak stage 5-6 pathology (i.e. limbic and neocortical Lewy bodies) lacked clinical evidence of dementia (Parkkinen et al., 2008). Complicating the picture further, several genes are known to confer an increased risk for development of PDD, including alpha-synuclein (SNCA) and glucocerebrosidase (GBA) mutations, the apolipoprotein ε4 (APOE4) allele and the microtubule-associated protein tau (MAPT) H1 haplotype (reviewed in Halliday et al., 2014), all of which are likely to contribute to cognitive decline via different mechanisms. In illustration of this, a recent study showed that newly diagnosed patients with Parkinson’s disease carrying the APOE4 allele show reduced activity in the medial temporal lobe (MTL) network during memory tasks, whereas MAPT H1 homozygotes instead show reduced activity in the posterior visual network during visuospatial tasks (Nombela et al., 2014).
The complex and varying milieu of neuropathological and genetic factors underlying the development of PDD renders it difficult to provide a generalized pathophysiological mechanism across patients from this perspective to account for the common clinical picture seen. However, diverse molecular and cellular pathologies can give rise to common patterns of dysfunction at the neural systems level, and therefore recent studies have begun to characterize the mechanisms underlying Parkinsonian dementia from this perspective, which provides a more generalizable model. In this review we collate recent evidence from neuropsychological, pharmacological, imaging and electrophysiological approaches to present a novel systems-level perspective on the pathophysiological mechanisms underlying PDD: the syndrome represents variable and interacting dysfunction in a number of diffusely distributed, yet interrelated, neural networks that contribute to distinct cognitive processes, including fronto-striatal, mesocortical, corticopetal cholinergic, fronto-parietal, medial temporal and noradrenergic networks. These are in turn differentially influenced by dopaminergic, cholinergic, and noradrenergic deficits. We propose that viewing the development of PDD from this dysfunctional networks perspective can provide novel insights and opportunities for development of new therapies.
To provide conceptual order to an otherwise anarchic data set we will approach discussion of these networks by addressing in turn each of the major cognitive domains affected by PDD (executive function, attention, memory and visual perceptual ability) and describing the major network dysfunctions underlying deficits in those areas. However, the reader must bear in mind that the division of cognitive ability into these compartmentalized domains is inherently artificial, which in turn renders the assignment of neural networks to the subservience of a constrained domain equally so. The reality is that all these cognitive networks interact and overlap in a complex manner, and the generation of any conceptualized cognitive function such as ‘memory’ is ultimately influenced by many of their individual distributed actions. Nevertheless, evidence suggests that particular neural networks are more strongly implicated in mediating certain cognitive functions than others, which gives validity to approaching the discussion in this manner. Of note the fifth domain, language, is relatively preserved in PDD [the main deficit in this area, impaired verbal fluency, is actually part of the dysexecutive syndrome (impaired self-generated search, Emre, 2003) and consequently is not discussed].
Following discussion of the dysfunctional cognitive networks underlying PDD we go on to address three important issues that arise from taking this perspective. First we consider how this network perspective relates back to the cellular neuropathology of PDD. Second we discuss the relevance of this network viewpoint to current clinical practice, including factors predicting the development of PDD, and the relation to current available therapies. Finally, we discuss how this neural network perspective on PDD may offer new therapeutic opportunities to directly modulate network function, and experimental treatments in this area.
Executive dysfunction: fronto-striatal, mesocortical and noradrenergic networks
‘Executive function’ is an umbrella term encompassing several cognitive abilities, including problem-solving, planning/sequencing, rule-shifting/maintenance, task-switching, manipulation in working memory and response inhibition (Dubois and Pillon, 1997; Kehagia et al., 2010; Parker et al., 2013; see Dirnberger and Jahanshahi, 2013 for review). Some also regard allocation of attention as an executive function (Kehagia et al., 2010), though here we will consider it a separate cognitive domain. Executive dysfunction is often present in Parkinson’s disease from the point of diagnosis (Lees and Smith, 1983; Foltynie et al., 2004a; Muslimovic et al., 2005), and may even be part of a premotor prodromal syndrome (Goldman et al., 2014b). Executive impairment worsens with disease progression (Pagonabarraga and Kulisevsky, 2012; Christopher et al., 2014), and in some series has been found to be predictive of conversion to PDD, though this remains controversial (Levy et al., 2002a; Woods and Tröster, 2003; Janvin et al., 2005; Lee et al., 2013; Biundo et al., 2014). From the patient perspective, progressive difficulties with concentration, retaining information, planning and organizational skills start interfering with social and occupational function (Bronnick et al., 2006). The Montreal Cognitive Assessment is sensitive to the detection of executive deficits in Parkinson’s disease (Zadikoff et al., 2008; Nazem et al., 2009), and consequently is a more sensitive tool for detection of PDD in the clinic than the traditional Mini-Mental State Examination (MMSE) (Hoops et al., 2009; Burdick et al., 2014).
Executive dysfunction is due to disruption of the fronto-striatal dopamine network
The prefrontal cortices are implicated in executive function (Milner, 1982, 1995; Norman and Shallice, 1986; Fuster, 2008), and distinct areas of prefrontal cortex have strong functional connections with the striatum via parallel dopamine-dependent cortico-striatal loops (Alexander et al., 1986; Middleton and Strick, 2000) (Figs 1 and 3). Functional MRI imaging in patients with Parkinson’s disease relates executive impairments on set shifting and working memory tasks to hypo-activation within the fronto-striatal loops connecting dorsolateral and ventrolateral prefrontal cortices, striatum and thalamus (Lewis et al., 2003; Monchi et al., 2004, 2007; Au et al., 2012). However, such hypo-activation was only present during task phases that specifically required co-activation with the striatum in controls, indicating that striatal dysfunction was the determining factor in executive impairment in Parkinson’s disease rather than frontal dysfunction. Both the globus pallidus internus and caudate are heavily affected by dopaminergic degeneration (Taylor et al., 1986), and PET studies have specifically implicated dysfunction of these two structures in interruption of normal processing in the fronto-striatal network; for example, patients with Parkinson’s disease demonstrating executive impairments on tasks involving planning (Owen et al., 1998) or random number generation (Dirnberger et al., 2005) show significantly altered outflow activity from the pallidum to the frontal cortices. In addition, other studies have shown strong correlations between dopamine depletion in the head of the caudate and deficits on executive tasks such as object alternation (Marié et al., 1999) and the Stroop Test (Brück et al., 2001).
Figure 1.
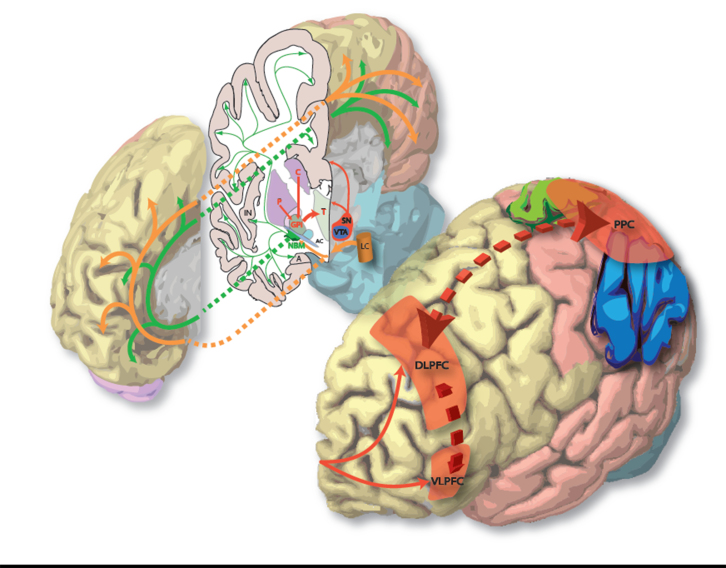
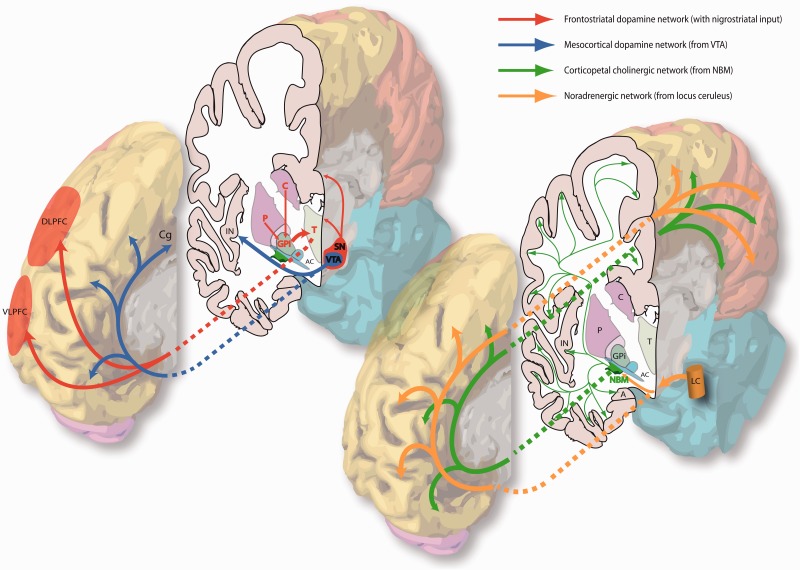
The major subcortical neural networks affected in PDD (according to their dominant neurotransmitters). In this 3D representation the medial surface of the right hemisphere of the human brain is closest to the viewer in both images. A = amygdala; AC = anterior commissure (lateral aspect); C = caudate; Cg = cingulate gyrus; DLPFC = dorsolateral prefrontal cortex; GPi = globus pallidus (internus); IN = insular cortex; LC = locus ceruleus; P = putamen; SN = substantia nigra; T = thalamus; VLPFC = ventrolateral prefrontal cortex; VTA = ventral tegmental area.
Figure 3.
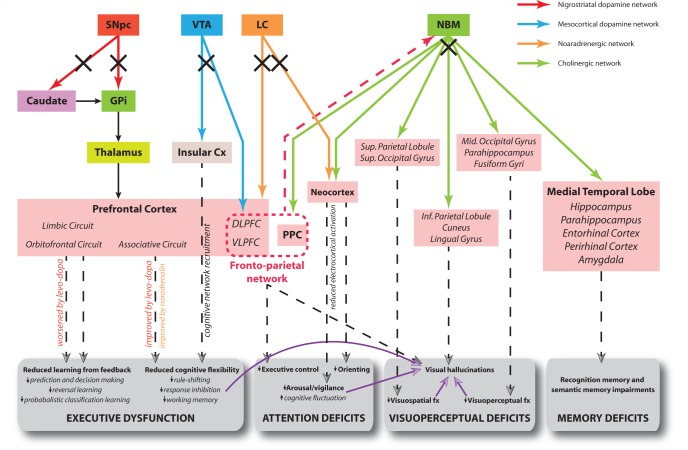
Hypothetical model of the neural networks affected in PDD and corresponding cognitive deficits. Solid arrows correspond to direct neural connections and colours are indicative of the primary neurotransmitter involved as shown in the key. Dashed arrows connect the relevant dysfunctional neural network to its putative cognitive effects. Purple arrows indicate that a deficit in one cognitive domain contributes to the development of impairment in another domain. Black crosses indicate damage to a neural pathway. The red dashed arrow represents direct projections from prefrontal cortex to the NBM, permitting top-down control of attention from the fronto-parietal network via recruitment of this latter structure and its cortical projections. The limbic, orbitofrontal and associative circuits in the prefrontal cortex correspond to the dissociable fronto-striatal loops of Alexander et al. (1986). Note effects of levodopa therapy at improving and worsening executive functions reliant on cognitive flexibility and learning from feedback, respectively. Electrocortical activation refers to cortical EEG desynchonization indicative of the awake/alert state as described in the text, and is driven by corticopetal cholinergic input from the NBM only. Both cholinergic input from NBM and noradrenergic input from the locus ceruleus (LC) modulate processing in sensory cortices to facilitate orienting of attention to stimuli. Cx = cortex; DLPFC = dorsolateral prefrontal cortex; fx = function; GPi = globus pallidus (internus); PPC = posterior parietal cortex; SNpc = substantia nigra pars compacta; VLPFC = ventrolateral prefrontal cortex; VTA = ventral tegmental area.
Therefore, the prevailing view is that executive dysfunction in PDD is due to dopaminergic depletion in the striatum disrupting transmission in the fronto-striatal network (Mortimer et al., 1982; Dubois et al., 1994; Owen et al., 1995; Zgaljardic et al., 2003; Owen, 2004; Pagonabarraga and Kulisevsky, 2012; Kehagia et al., 2013).
Degeneration in the mesocortical dopamine network contributes to executive dysfunction
However, dopamine-dependent neural circuitry underlying executive deficits in Parkinson’s disease may not be limited to the fronto-striatal network alone. The mesocortical dopamine network originates in the midbrain ventral tegmental area (A10) and projects diffusely to neocortical areas, particularly prefrontal, insular and cingulate cortices (Oades and Halliday, 1987) (Figs 1 and 3). Release of dopamine from this network modulates prefrontal D2 receptors and thereby facilitates cognitive flexibility, a core feature of executive processing (Floresco and Magyar, 2006). Insular cortex in particular is considered to mediate such flexibility, acting as a hub to recruit other cognitive circuits such as the fronto-parietal network (Menon and Uddin, 2010). In support of this, insular lesions in human patients have been shown to impair performance on tasks requiring cognitive flexibility (Hodgson et al., 2007).
Post-mortem studies have shown degeneration of the mesocortical network in Patients with Parkinson’s disease (Javoy-Agid and Agid, 1980; Scatton et al., 1983), with a further selective loss of dopaminergic neurons in the lateral ventral tegmental area specific to development of PDD (Hall et al., 2014). In vivo PET imaging studies confirm dopaminergic dysfunction in this network in Parkinson’s disease (Ouchi et al., 1999; Yagi et al., 2010), with a specific reduction of D2 receptor availability in insular cortex occurring in cognitively impaired patients and correlating closely with impairment on executive tests (Christopher et al., 2014). Furthermore, volumetric MRI studies have shown close correlations between atrophy of insular cortex and conversion to PDD (Melzer et al., 2012; Lee et al., 2013). Therefore, substantial evidence implicates a concurrent dysfunction in the mesocortical dopamine network in the pathophysiology of Parkinson’s disease, with specific disruption of projections to insular cortex contributing to worsening executive impairments and PDD, possibly by impairing the ability to recruit other cognitive networks.
Dysexecutive symptoms may emerge when inter-network compensation fails
How dysfunction in the fronto-striatal and mesocortical dopaminergic networks may interact to cause dysexecutive symptoms in PDD is unclear. However the results of Christopher and colleagues (2014) suggest that it is supervening dysfunction in the mesocortical projections to the insular upon existing fronto-striatal network disruption that heralds major executive impairment. Indeed there is limited evidence suggesting some redundancy between the two systems in early Parkinson’s disease: in one study patients performing a set-shifting task did not display behavioural impairment despite fronto-striatal hypoactivation on functional MRI, possibly because they displayed concurrent hyper-activation in the insular and fronto-parietal networks, which was not present in controls (Au et al., 2012). Using functional MRI, Monchi and colleagues (2007) also noted a relative increase in blood-oxygen level-dependent activity within frontal regions in patients during a matching task. Although this evidence is indirect it suggests that the mesocortical network may partially compensate for fronto-striatal dysfunction in early disease, until it too is damaged, compensation is lost, and a full-blown dysexecutive syndrome develops. Such a proposal is compatible with, and extends, the hypothesis proposed by others that deficient interplay between the fronto-striatal and mesocortical dopamine networks underlies the dysexecutive syndrome of Parkinson’s disease (Cools, 2006; Monchi et al., 2004).
Disruptions in non-dopaminergic brain networks contribute to executive dysfunction
Levodopa administration does not improve all executive deficits in PDD (Pillon et al., 1989; Poewe et al., 1991; Jubault et al., 2009), or even in early Parkinson’s disease (Lewis et al., 2005; Muslimovic et al., 2005). In fact the relationship between dopamine replacement and executive performance is complex (Cools, 2006), in that either too high or too low levels of prefrontal dopamine are associated with poor executive performance, and this may relate partly to COMT (catechol O-methyltransferase) genotype (Foltynie et al., 2004b; Williams-Gray et al., 2007b; Nombela et al., 2014). Furthermore, levodopa does not restore dysfunctional cognitive network patterns to normal as it does motor network patterns on either functional MRI (Jubault et al., 2009), or PET (Huang et al., 2007). Therefore, it seems likely that impairments in other brain networks and neurotransmitter systems also contribute to executive dysfunction in PDD (Zgaljardic et al., 2004).
The noradrenergic network projecting from the locus coeruleus to the thalamus, amygdala and cortex (Figs 1 and 3) is also compromised in Parkinson’s disease (Scatton et al., 1983; Bertrand et al., 1997), with the extent of neuronal loss in this system correlating with development of PDD (Cash et al., 1987; Zweig et al., 1993; Del Tredici and Braak, 2013). Noradrenalin release in prefrontal cortex increases the responsiveness of neurons to diverse inputs, thereby facilitating cognitive flexibility (Vazey and Aston-Jones, 2012). Therefore damage to this system in patients with Parkinson’s disease may underlie deficits in executive functions reliant on cognitive flexibility, such as rule-shifting, response inhibition and working memory, and indeed administration of noradrenergic agonists reverses these deficits (Bédard et al., 1998; Riekkinen et al., 1999).
It must also be borne in mind that executive function is interdependent upon other cognitive faculties, such as the ability to maintain an alert and attentive state in order to concentrate on a task. Thus concurrent dysfunction in brain networks mediating these other functions will also contribute to the overall level of executive disability. For example, the nucleus basalis of Meynert (NBM) cholinergic network is strongly implicated in maintenance of an attentive state (discussed below), and degenerates significantly in PDD leading to widespread cortical cholinergic dysfunction (Kuhl et al., 1996; Bohnen et al., 2003; Gratwicke et al., 2013), demonstrated in vivo by a 30% reduction in cholinergic ligand binding on PET across all cortical areas, compared to only 10% in non-demented Parkinson’s disease (Hilker et al., 2005). Close correlations have been demonstrated between this cortical cholinergic dysfunction in PDD and worsening scores on tests of working memory, rule-switching and response inhibition (Bohnen et al., 2006), all of which require a strong attentional component. Therefore this suggests that damage to the NBM attention network indirectly contributes to the executive dysfunction of PDD.
In summary, executive dysfunction in PDD is a complex phenomenon, mediated primarily by dysfunction in fronto-striatal and mesocortical dopaminergic circuitry, but with interacting influences from dysfunctional noradrenergic and cholinergic networks too.
Attention: fronto-parietal, corticopetal cholinergic and noradrenergic networks
Attention is a heterogeneous construct that has been considered to comprise three different subsystems: executive control, orienting and alerting (Posner and Petersen, 1990; Petersen and Posner, 2012). It has been proposed that the executive control subsystem allocates attentional resources to tasks. It is the volitional focusing of attention and considered to depend on ‘top-down’ signals derived from knowledge about task demands (Kastner and Ungerleider, 2000). ‘Orienting’ refers to attention being drawn to an environmental stimulus for focused cognitive processing to the exclusion of other stimuli. It is automatic capture of attention and thought to be driven by ‘bottom-up’ signals from salient stimuli (Desimone and Duncan, 1995). Alerting is a heightened state of arousal and ‘vigilance’ is the maintenance of this aroused state over time (Parasuraman, 1998). Vigilance facilitates faster orienting and reaction time, whereas the opposite state, drowsiness, will impair these functions.
Attention deficits are detectable in Parkinson’s disease from an early stage, particularly on tests sensitive to deficits in executive control such as the digit span, Trail Making Test Part B, Stroop interference test and attentional set-shifting tasks (Muslimovic et al., 2005; Williams-Gray et al., 2008). Non-demented patients also demonstrate impaired orienting of visual and auditory attention (Wright et al., 1990; Sharpe, 1992; Poliakoff et al., 2003). With progression to PDD these deficits worsen, and impaired vigilance also develops with fluctuating levels of alertness (Ballard et al., 2002), which in turn drives fluctuating levels of cognition (Walker, 2000). Attention deficits are the most disabling symptom in PDD, predicting worse activities of daily living and consequent poorer quality of life (Bronnick et al., 2006). Such deficits are easily identifiable in clinic: patients classically lose their train of thought during a sentence, fail to follow the conversation, or display fluctuant alertness.
The complex neural networks that mediate attention functions in the healthy state are subject to ongoing debate (Petersen and Posner, 2012). Nevertheless, experimental evidence suggests that dysfunction in several distinct brain networks underlie the deficits in attentional functions seen in patients with PDD.
Dysfunction in the fronto-parietal network impairs ‘top-down’ executive control
Volitional shifts of attention are thought to depend on ‘top-down’ signals within a fronto-parietal network comprising prefrontal cortical areas and posterior parietal cortices (Figs 2 and 3), wherein prefrontal regions modulate activity in the network according to task demands (Posner and Dehaene, 1994; Kastner and Ungerleider, 2000; Buschman and Miller, 2007).
Figure 2.
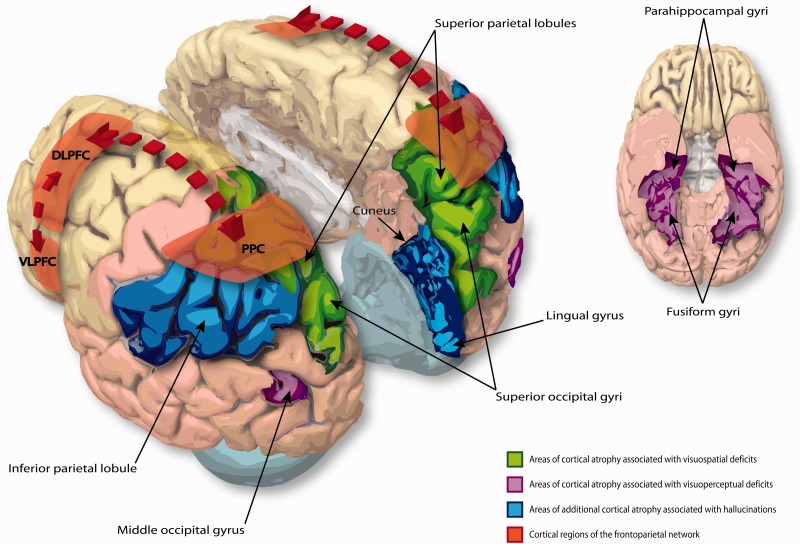
The major cortical neural networks affected in PDD. Areas of cortical atrophy associated with visuospatial and visuoperceptual deficits in PDD (coloured green and purple, respectively) are based on the data presented in Pereira et al. (2009). Areas of cortical atrophy specifically associated with the presence of visual hallucinations in PDD (coloured blue) are based on the data presented in Goldman et al. (2014a). Functional cortical regions comprising the fronto-parietal attention network (highlighted red) are based on the data presented in Williams-Gray et al. (2008). Cortical regions are identified according to the Allen Brain Atlas for the human brain, and manually drawn onto the corresponding 3D brain image. In this representation the same cortical regions are affected symmetrically in both hemispheres, however in the original studies above the extent of atrophy in these regions was not symmetrical between hemispheres, and varied between individual patients. In the inferior view of the cortex the cerebellum has been removed to expose the fusiform gyri more clearly. DLPFC = dorsolateral prefrontal cortex; PPC = posterior parietal cortex; VLPFC = ventrolateral prefrontal cortex.
Imaging studies using fluorodeoxyglucose (FDG)-PET have shown that patients with both Parkinson’s disease with mild cognitive impairment (PD-MCI) and PDD demonstrate extensive hypometabolism in frontal and parietal cortices compared to cognitively normal patients with Parkinson’s disease (Huang et al., 2007, 2008; Yong et al., 2007; Hosokai et al., 2009; Liepelt et al., 2009). In addition, voxel-based morphometric MRI analyses and diffusion tensor imaging (DTI) studies have shown that patients with PDD demonstrate extensive grey matter atrophy and white matter microstructural alterations, respectively within the above cortical regions (Burton et al., 2004; Summerfield et al., 2005; Lee et al., 2010; Song et al., 2011; Hattori et al., 2012; Melzer et al., 2012). To investigate this relationship, one centre co-registered MRI and FDG-PET scans in individual patients with cognitively intact Parkinson’s disease, PD-MCI or PDD, and compared cortical metabolism and atrophy amongst these cognitive groups (González-Redondo et al., 2014). They found that cognitive decline correlated closely with a progressive pattern of sequential hypometabolism followed by atrophy in both frontal and parietal cortices. Furthermore the spatial pattern of fronto-parietal hypometabolism has been shown to correlate closely with deficits on a test of executive control (Trail Making Test Part B), and can be reliably used to predict test scores in other cognitively impaired patients (Huang et al., 2007). Therefore, these studies highlight a progressive degeneration in frontal and parietal cortices in the development of PDD, which correlates closely with deficits in the executive control of attention.
In Alzheimer’s disease it has been shown that atrophy in specific cortical regions damages structural connections and leads to loss of functional connectivity within brain networks (He et al., 2007). Given the extensive atrophy within frontal and parietal cortices seen in PDD then the same may hold true for the fronto-parietal network, and indeed functional imaging evidence supports this. Functional MRI studies show that non-demented Parkinson’s patients activate the fronto-parietal network while performing attentional set-shifting tasks (Williams-Gray et al., 2008); however, activation of the network during such volitional shifts of attention is not as strong as in control subjects due to reduced connectivity within prefrontal cortical regions (Rowe et al., 2002). With progression to PDD there is evidence of a further reduction in connectivity within the network compared to non-demented patients. Investigators in one centre scanned both patients with Parkinson’s disease and PDD with MEG (magnetoencephalography) in the resting state and compared cortical oscillatory activity (Ponsen et al., 2012). They found that patients with PDD demonstrated both a relative decrease in beta oscillatory power in the frontal cortices and reduced functional connectivity across cortical regions in the beta frequency band. In the healthy state it has been shown that an increase in beta-band synchrony within the fronto-parietal network drives the executive control of attention (Buschman and Miller, 2007). Therefore, this relative decrease in fronto-parietal beta-band connectivity in PDD may represent the functional mechanism underlying impairment in this mode of attention.
Therefore, substantial structural and functional evidence exists to support the hypothesis that dysfunction in the fronto-parietal network impairs top-down control of attention in PDD. However, future studies directly exploring the contribution of cortical structural changes to functional connectivity and relating this to attentional impairments in PDD are needed to confirm these observations. Furthermore, the contribution of different neurotransmitters to fronto-parietal network dysfunction remains to be elucidated. Patients with Parkinson’s disease with low activity COMT genotypes (who have higher cortical dopamine levels) appear to under-activate the fronto-parietal network with consequent poorer performance on set-shifting tasks (Williams-Gray et al., 2008), while the pattern of cortical atrophy seen within the network in PDD correlates closely with areas showing cholinergic hypofunction on PET imaging (Hilker et al., 2005).
Dysfunction in cholinergic and noradrenergic networks impairs ‘bottom-up’ orienting of attention
One view of automatic orienting of attention considers it to be mediated by ‘bottom-up’ or stimulus-driven signals from the NBM in the basal forebrain (Sarter et al., 2005). This nucleus consists of 90% cholinergic neurons and its’ widespread projection axons provide the main cholinergic innervation to the entire cortical mantle (‘corticopetal’ innervation) (Mesulam et al., 1983; Mufson et al., 2003; Gratwicke et al., 2013) (Figs 1 and 3). Selective activation of the nucleus basalis of Meynert (NBM) network causes an increase in acetylcholine levels in the cortical target field, which boosts the signal-to-noise ratio for salient stimuli, thereby enhancing the strength of their neural representations (Goard and Dan, 2009; Bentley et al., 2011; Pinto et al., 2013; Soma et al., 2013). In facilitating this process the NBM effectively amplifies detection of salient stimuli by posterior regions of the fronto-parietal network and ensures their attentional significance (Sarter et al., 2006; Buschman and Miller, 2007). Animal experiments have shown that this NBM-driven cortical signal enhancement is responsible for generating event-related potentials (ERPs) on the EEG (Nguyen and Lin, 2014). These can be measured on the human EEG as negative deflections occurring 80–100 ms after an unpredictable stimulus (the N1 ERP), and have long been regarded as the electrophysiological correlate of orienting of attention (Hillyard et al., 1973).
The NBM degenerates in Parkinson’s disease, with human neuropathological series showing 32% cell loss in non-demented patients, rising to 54–70% in PDD, which is closely associated with increasing cortical cholinergic deficits and worsening cognitive impairment (Whitehouse et al., 1983; Gaspar and Gray, 1984; Perry et al., 1985; Hall et al., 2014). This is supported by both volumetric MRI and PET imaging studies, which demonstrate significant NBM atrophy and cortical cholinergic binding reductions, respectively in patients with PDD compared to both cognitively intact Parkinson’s disease patients and control subjects (Hanyu et al., 2002; Hilker et al., 2005; Bohnen et al., 2006; Shimada et al., 2009; Choi et al., 2012). This disruption of NBM cholinergic input to cortex attenuates cortical signal processing (Pinto et al., 2013), which is demonstrated by the fact that patients with PDD performing orienting of attention tasks display increased N1 event-related potential latencies compared to both non-demented patients and controls, which correlate with behavioural errors (Goodin and Aminoff, 1987; Hautecoeur et al., 1991; Stam et al., 1993). Therefore, disruption of bottom-up signal enhancement in the NBM network appears to underlie the deficits in orienting seen in PDD.
Interestingly, direct prefrontal cortical projections to the NBM may modulate activity of its cholinergic inputs to sensory cortices and has been suggested to represent a component of the top-down fronto-parietal attention network (Sarter et al., 2005). Thus depending on the type of stimulus and task characteristics, activity in the NBM network may reflect the combined effects of top-down and bottom-up modes of attention (Bentley et al., 2004; Sarter et al., 2006), meaning that degeneration in this network in PDD may play a key role not only in orienting deficits but in deficits in executive control of attention as well (Fig. 3).
Finally, the ascending noradrenergic network is also implicated in orienting of attention (Aston-Jones et al., 1999) and, as described above, this network degenerates progressively in PDD. Administration of the selective alpha-1 noradrenergic agonist naphtoxazine to patients with PD-MCI improves performance on an orienting of attention task accompanied by improved lateralization of the N1 event-related potential (Bédard et al., 1998). This suggests that lack of bottom-up noradrenergic input from the locus coeruleus may also play a role in orienting deficits in PDD; however, its interaction with the cholinergic system and their relative contributions remain unclear.
Slowed cortical rhythms on the EEG reflect impaired vigilance and underlie cognitive fluctuation
As mentioned above the onset of impaired vigilance and fluctuating attention/cognition is particularly characteristic of progression to PDD (Emre et al., 2007). In tandem with its role in enhancing processing of salient stimuli, the NBM cholinergic network also plays a key role in the ascending arousal network. The NBM receives noradrenergic afferents from the locus coeruleus (Fig. 1) and glutamatergic afferents from the reticular formation and acts as an extra-thalamic relay to the cortex and limbic system (Szymusiak, 1995; Jones, 2004). Its cholinergic projections can directly desynchronize the neocortical EEG, replacing slow synchronized delta waves (0.5–4 Hz, indicative of the non-aroused state) with fast beta and gamma waves (13–30 and 30+ Hz, respectively, indicative of arousal) (Metherate et al., 1992; Lee et al., 2005; Kalmbach et al., 2012).
Awake EEG studies in patients with PDD have consistently shown an increase in slow delta wave activity across the cortex, with a progressive gradient of increasing delta wave activity seen when comparing cognitively intact patients with Parkinson’s disease, patients with PD-MCI and PDD (Soikkeli et al., 1991; Neufeld et al., 1994; Caviness et al., 2007). In agreement with this resting state, MEG studies have also shown a relative increase in cortical delta oscillatory power in patients with PDD compared to non-demented Parkinson’s disease, alongside a relative decrease in faster beta and gamma activity (Bosboom et al., 2006; Ponsen et al., 2012). Administration of the acetylcholinesterase inhibitor (AChEI) rivastigmine to patients with PDD undergoing MEG returns these slowed cortical rhythms to normal (Bosboom et al., 2009). This therefore supports the hypothesis that dysfunction in the NBM cholinergic network underlies the electrocortical depression characteristic of PDD. Rodents with NBM lesions have similar slow delta activity on the EEG and concurrently display reduced arousal or coma (Buzsaki et al., 1988; Fuller et al., 2011). Therefore, NBM cholinergic dysfunction leading to progressive electrocortical depression in PDD may represent the pathophysiological correlate of impaired vigilance (Fig. 3).
In addition, Bonanni et al. (2008) have shown that patients with PDD with significant cognitive fluctuations (measured by the Clinician Assessment of Fluctuation Scale) demonstrate pseudocyclic patterns of slow wave activity on the EEG in the delta-theta-pre-alpha range (1–7.9 Hz), whereas patients with PDD without fluctuations do not (Bonanni et al., 2008). This therefore implies that development of slow EEG rhythms cycling between relatively greater and lesser states of cortical arousal may represent the pathophysiological basis of cognitive fluctuation in PDD. However, further work is needed to establish the mechanisms underlying generation of such rhythms, and why some patients with PDD develop them while others do not.
Memory: medial temporal lobe and corticopetal cholinergic networks
Memory is an all-encompassing term for the cognitive processes involved in the encoding, storage and retrieval of information. As with the other cognitive domains it is not a pure process, and is interdependent upon a person being able to orient attention to a stimulus (to allow encoding), and use executive processes to allow retrieval in a particular context. As discussed above, patients with Parkinson’s disease and PDD exhibit deficits in each of these latter processes, which means that apparent memory impairments have a multifactorial basis here. For example, patients with Parkinson’s disease exhibit impaired free recall (spontaneous retrieval) but benefit substantially from cueing, demonstrating that externally triggered retrieval is intact (Lees and Smith, 1983; Costa et al., 2014). Recognition memory is also intact at this stage (Lees and Smith, 1983; Taylor et al., 1986) although there is some debate about this (Whittington et al., 2000). Overall, this indicates that memories are encoded and stored, but not independently retrieved. Performance on free recall in this group is significantly predicted by scores on executive tests, indicating that executive dysfunction contributes to retrieval failure (deficient internal search strategies), and is responsible for the apparent mnemonic deficit rather than a dysfunction of storage (Pillon et al., 1993; Costa et al., 2014). This contrasts with Alzheimer’s disease where both recall and recognition are equally impaired from early on, implicating a temporal-limbic storage deficit (Helkala et al., 1988; Pillon et al., 1993).
With progression from Parkinson’s disease to PDD, however, both a cross-sectional study and a meta-analysis have shown that difficulties with recognition memory also become apparent, implicating a supervening dysfunction of temporal lobe storage mechanisms upon pre-existing executive retrieval deficits when patients convert to dementia (Whittington et al., 2000, 2006). This is supported by data showing that patients with PDD exhibit significant impairments with confrontation naming (a test of visual recognition memory) and greater deficits in semantic than phonemic verbal fluency (both require efficient executive retrieval but the former has a greater dependence on temporal lobe storage) (Henry and Crawford, 2004). Both confrontation naming and semantic verbal fluency are dependent on semantic information (previously learnt general factual information) (Tulving, 1972) and therefore these tests are relatively resistant to attentional impairments since encoding of such information would have taken place in the pre-morbid state. Therefore, it seems likely that a true mnemonic storage deficit is present in PDD in addition to the problems with deficient attention/encoding and poor executive retrieval that manifest earlier in Parkinson’s disease.
In the clinic, problems with memory are one of the most frequent non-motor symptoms reported by both patients and carers (Breen and Drutyte, 2013). However, the differentiation between apparent memory deficits due to attentional or executive impairments, and ‘true’ temporal-limbic storage deficits is not evident from the patients’ self-reported memory complaints, and this requires detailed questioning or cognitive testing to delineate.
Atrophy within the medial temporal lobe network correlates with progression to Parkinson’s disease dementia
Medial temporal lobe structures (hippocampus, parahippocampus, entorhinal and perirhinal cortices and amygdala) (Fig. 3) are involved in memory storage and retrieval (Squire et al., 2004; Lech and Suchan, 2013), and patients with Parkinson’s disease demonstrate hypoactivation of these structures during visual memory tasks from the point of diagnosis (although mnemonic deficits are subclinical at this time) (Nombela et al., 2014). However, previous volumetric MRI studies have provided conflicting results as to whether significant MTL atrophy occurs in PDD (Camicioli et al., 2003; Junqué et al., 2005; Tam et al., 2005; Ibarretxe-Bilbao et al., 2008). These discrepancies are likely due to the differing criteria for dementia used, and the fact that results were not co-varied by motor scores to determine atrophy specific to cognitive decline. To address these issues, a recent study used the MDS (Movement Disorders Society) Task Force Criteria for PDD (Emre et al., 2007) and recent criteria for PD-MCI (Dalrymple-Alford et al., 2011) to select representative patient groups for voxel-based morphometry MRI analysis (Melzer et al., 2012). Having adjusted results by individual UPDRS (Unified Parkinson’s Disease Rating Scale, part III) motor scores they showed that cognitive progression from Parkinson’s disease to PD-MCI to PDD specifically correlated with increasing grey matter atrophy in MTL structures including the hippocampi, parahippocampi and amygdalae. A recent meta-analysis of six voxel-based morphometry MRI studies involving a total of 105 patients with PDD and 131 control subjects confirms this (Pan et al., 2013).
Although these data confirm that progression to PDD is associated with worsening MTL atrophy, further studies are needed to specifically demonstrate a link between damage to this network and worsening memory storage deficits (assessed by decline on tests of recognition or semantic memory). At present we can only hypothesize that this is the case based on the known functional anatomy of the MTL network (Squire et al., 2004).
Dysfunction of the nucleus basalis of Meynert cholinergic network impairs encoding of memories
Aside from its role in orienting of attention, the NBM cholinergic network has also been implicated in memory encoding. The release of acetylcholine from its end terminals has been shown to induce plastic reorganization of cortical receptive field maps, representing the putative encoding of a ‘physiological memory’ (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998; McLin et al., 2002). Simultaneously, as described above, this transmitter release directly desynchronizes the neocortical EEG by inducing fast gamma, beta and theta oscillations (Lee et al., 2005; Kalmbach et al., 2012), and evidence suggests that phase-coupling of these oscillations between cortical and MTL regions is necessary for memory encoding in humans (Huerta and Lisman, 1993; Fell and Axmacher, 2011; Lee et al., 2013). Conversely NBM lesions in animals have been shown to block this electrocortical activation (Buzsaki et al., 1988; Fuller et al., 2011), and cause impairments of learning and memory (Bartus et al., 1985; Mandel et al., 1989; Butt and Hodge, 1995; Leanza et al., 1996), as well as impairments in orienting of attention (Voytko et al., 1994; Voytko, 1996).
As described in the previous section, the NBM cholinergic network degenerates significantly in PDD with up to 70% cell loss (Whitehouse et al., 1983), which correlates with progressive electrocortical depression on MEG (Bosboom et al., 2006; Ponsen et al., 2012). Therefore, we hypothesize that dysfunction in this network impairs both orienting of attention to a stimulus and (in conjunction with dysfunction in the MTL network) the induction of electrocortical synchrony necessary for the successful encoding of that stimulus into memory (Fig. 3). Further electrophysiological studies in patients with PDD are needed to investigate this further; however, it is not surprising that dysfunction of the NBM cholinergic network is implicated in both attention and memory deficits as neuroimaging and computational studies in healthy humans suggest that cholinergic enhancement of cortical signal detection (orienting of attention) facilitates formation of novel input associations (memory encoding) (Hasselmo and McGaughy, 2004; Bentley et al., 2009). Thus these cognitive functions are interrelated and are actually part of a continuous process for recording salient environmental stimuli into memory (Sarter et al., 2003).
Visual perceptual dysfunction and hallucinations: multiple network involvement
Patients with Parkinson’s disease exhibit both subtle visuospatial deficits (difficulties with the perception of extra-personal space) (Lee et al., 1998; Levin et al., 1991; Montse et al., 2001), and visuoperceptive deficits (difficulties recognizing objects based on their form) (Villardita et al., 1982; Laatu et al., 2004; Kida et al., 2007), in some patients from early in the disease (Foltynie et al., 2004a). These deficits become more marked and more common with disease progression (Levin et al., 1991) and show high sensitivity in detecting the transition to PDD (Zgaljardic et al., 2004; Kehagia et al., 2010; Biundo et al., 2014). Indeed impairment on the Pentagon Copying Test from the MMSE at baseline has been shown to be predictive of PDD at 5-year follow-up (Williams-Gray et al., 2009).
Visual hallucinations are also well-recognized in Parkinson’s disease and are typically complex, consisting of well-formed people, animals or objects (Barnes and David, 2001). Although they can be induced by anti-parkinsonian drugs, correlations between use of these agents and presence of hallucinations are actually relatively weak, and instead cognitive impairment has been shown to be the major risk factor, indicating that they are a core symptom of the dementing process (Fénelon et al., 2000; Williams and Lees, 2005). Visual hallucinations generally occur in the latter stages of the disease course with a progressive nature (Goetz et al., 2001; Williams and Lees, 2005). Their presence is a strong predictor of PDD (Galvin et al., 2006; Santangelo et al., 2007) and indeed the prevalence of hallucinations in PDD is 70% (Fénelon et al., 2000). Although insight is initially maintained in patients with PDD, 81% will lose insight over 3 years (Fénelon et al., 2000; Goetz et al., 2006), which severely affects quality of life for both patients and caregivers (Goetz and Stebbins, 1993; Aarsland et al., 2000).
Visual perceptual dysfunction correlates with atrophy in posterior visual cortices
Only one study has specifically looked at in vivo neuroanatomical correlates of visual perceptual dysfunction in PDD. Using voxel-based morphometry MRI analysis, Pereira and colleagues (2009) showed that PD-MCI patients have greater grey matter atrophy in both occipito-temporal and dorsal parietal cortices compared to controls, and that these patterns correlated with impairments on tests of visuoperceptual and visuospatial abilities, respectively (Pereira et al., 2009) (Fig. 2). These correlations agree with the dual-stream hypothesis of visual processing, wherein the dorsal stream from the occipital to the parietal lobe processes spatial location while the ventral stream from occipital lobe to temporal and limbic structures processes object recognition (Ungerleider and Mishkin, 1982). Indeed functional imaging in patients with Parkinson’s disease performing visuospatial tasks shows reduced parietal activation which correlates with increasing errors (Nombela et al., 2014). The above patterns of cortical atrophy show spatial congruence with areas showing significant hypometabolism and cholinergic deficits in patients with PDD, in-line with deafferentation from the NBM network (Hilker et al., 2005; Klein et al., 2010). This is supported by DTI studies which demonstrate significant white matter microstructural alterations in bilateral posterior cingulate bundles in patients with PDD compared to non-demented Parkinson’s disease (Matsui et al., 2007), the same fibre tracts through which cholinergic projections from NBM to visual cortices travel (Gratwicke et al., 2013). As bottom-up NBM cholinergic input is known to enhance visual cortical responses and thereby improve visual discrimination ability (Bhattacharyya et al., 2013; Pinto et al., 2013; Soma et al., 2013), dysfunction in this network due to NBM degeneration may underlie the visual perceptual dysfunction seen in PDD (Fig. 3).
Independent dysfunction in posterior visual processing networks underlies visual hallucinations
The mechanism underlying the generation of visual hallucinations in PDD is more complex and likely represents interacting dysfunction between several different brain networks. Since the presence of hallucinations is closely correlated with visuospatial and visuoperceptual deficits in PDD (Ramírez-Ruiz et al., 2006; Sinforiani et al., 2006), dysfunction in associative visual cortices within the dorsal and ventral processing streams has long been implicated in their generation. This is supported by neuropathological studies which have demonstrated strong correlations between Lewy body burden in parietal and temporal lobes (particularly limbic structures) and the presence of hallucinations in PDD (Harding et al., 2002; Papapetropoulos et al., 2006; Kalaitzakis et al., 2009; Gallagher et al., 2011). Nevertheless, MRI studies comparing brain atrophy patterns between patients with Parkinson’s disease with and without visual hallucinations have not consistently supported these pathological associations, differentially implicating medial temporal (Ibarretxe-Bilbao et al., 2008, 2010; Shin et al., 2012), insular (Shine et al., 2014), pedunculopontine nucleus (Janzen et al., 2012) and frontal atrophy (Ibarretxe-Bilbao et al., 2010; Sanchez-Castaneda et al., 2010). All may play a part in generation of hallucinations; however, the degree of cognitive impairment between patients with and without hallucinations was not controlled for in these studies, meaning that atrophy patterns may have related to cognitive differences rather than the presence of hallucinations per se. In addition, the use of differing classification criteria for PDD [including MMSE <24, Diagnostic Statistical Manual (DSM) IV-TR or Movement Disorders Society Task Force criteria] further complicates interpretation of these results.
A recent study overcame these problems, by using Movement Disorders Society criteria to select patients with PDD with and without visual hallucinations and ensured they were matched for antiparkinsonian medications, global cognitive decline and scores on all cognitive subdomains, including visuoperceptual impairments (Goldman et al., 2014a). Structural MRI scans from both groups were analysed using voxel-based morphometry, then compared. PDD hallucinators exhibited significant grey matter atrophy in the cuneus, lingual and fusiform gyri, middle occipital lobe and inferior parietal lobule compared to non-hallucinators (Figs 2 and 3). These results seem to confirm that discrete areas of atrophy in the posterior visual processing networks specifically underlie the generation of hallucinations in PDD, and thereby provide an in vivo correlate to neuropathological data. Of note, these atrophy patterns were independent of visuoperceptual impairments, suggesting that generation of visual hallucinations in PDD does not merely represent a progression of such impairments but is instead dependent on different mechanisms.
Functional neuroimaging studies provide further evidence that dysfunction in posterior visual processing networks underlies generation of visual hallucinations in PDD. Resting state SPECT (single-positron emission computed tomography) and FDG-PET studies have shown decreased perfusion and metabolic rates, respectively in posterior visual cortices in patients with Parkinson’s disease with hallucinations compared to those without (Oishi et al., 2005; Matsui et al., 2006; Boecker et al., 2007). Furthermore, functional MRI studies during visual stimulation paradigms have demonstrated hypoactivation of posterior visual areas in patients with Parkinson’s disease with hallucinations in comparison to those without (Stebbins et al., 2004; Meppelink et al., 2009).
Thus recent structural and functional neuroimaging evidence supports earlier neuropathological data and indicates that specific damage to posterior visual processing networks in PDD contributes to the generation of hallucinations. The exact pathophysiological process responsible remains to be shown definitively. However, these dysfunctional visual regions again show significant congruence with areas of cholinergic deafferentation as described above, indicating that loss of cortical input from the NBM network in PDD could underlie aberrant processing in visual cortices and thereby contribute to generation of hallucinations. This is supported by clinical trial data showing that treatment of patients with PDD with the mixed AChEI/nicotinic acetylcholine receptor agonist galantamine can markedly reduce hallucinations (Litvinenko et al., 2008). Because NBM activation alters cortical acetylcholine levels and thereby enhances neuronal signal-to-noise ratios (Goard and Dan, 2009; Pinto et al., 2013; Soma et al., 2013) then damage to this network in PDD could decrease the signal-to-noise ratio of salient stimuli, thereby allowing irrelevant intrinsic and sensory information that would normally be suppressed to enter perceptual awareness in the form of hallucinations (Perry and Perry, 1995).
Concomitant dysfunction in frontal and arousal networks contributes to generation of visual hallucinations
Overlapping dysfunctions in a number of other cognitive networks are also likely to contribute to the generation of visual hallucinations in PDD. For example, several functional MRI studies comparing patients with Parkinson’s disease with hallucinations to those without during performance of visual paradigms have demonstrated not only dysfunction in visual cortical areas in the former, but also simultaneous disruption of activity in frontal areas (Stebbins et al., 2004; Meppelink et al., 2009; Shine et al., 2014). The presence of hallucinations in PDD is closely associated with worsening impairments on tests of attentional control (Meppelink et al., 2008; Bronnick et al., 2011), as well as impairments on tests of inhibitory control such as the Stroop Test and Go/No-Go Task (Barnes and Boubert, 2008), deficits that might in part be attributable to dysfunctions in the fronto-parietal and noradrenergic networks respectively (as described above). This therefore suggests that breakdown in these frontal networks may play a contributory role in the generation of visual hallucinations in PDD, perhaps by reducing attentional and inhibitory control of perceptual errors arising from dysfunction in posterior visual cortices, allowing them to enter conscious perception as hallucinations (Shine et al., 2011) (Fig. 3).
In addition, disrupted sleep-wake cycling and REM (rapid eye movement) sleep behavioural disorder are also strongly associated with the presence of visual hallucinations in Parkinson’s disease (Nomura et al., 2003; Whitehead et al., 2008), and intrusion of episodes of REM sleep during wakefulness is proposed to contribute to generation of hallucinations (Diederich et al., 2005). Control of both arousal and REM sleep appears to be regulated by the NBM (Lee et al., 2005, and as discussed above) and therefore dysfunction in this network may contribute to generation of visual hallucinations in PDD not only by disrupting visual perception as above, but also by deregulating arousal mechanisms.
Overall, therefore, concomitant dysfunction in a number of brain networks involved in visual perception, inhibitory control and arousal may all play a role in the generation of visual hallucinations in PDD, which is supported by clinical data indicating that the strongest determinants of hallucinations in Parkinson’s disease are impairments of visuoperceptual and frontal functions combined with the presence of REM sleep behavioural disorder (Gallagher et al., 2011). However, the relative contributions of these network dysfunctions and how they interact to produce visual hallucinations remains unclear, and further studies are needed to examine this.
Relation of the neural network perspective to the neuropathology of Parkinson’s disease dementia
At the neuropathological level the consensus from most studies to date is that the amount of Lewy-related pathology (including Lewy bodies and Lewy neurites) in neocortical and limbic areas is the most important factor in the development of PDD (Hurtig et al., 2000; Apaydin et al., 2002; Sabbagh et al., 2009; Kempster et al., 2010; Irwin et al., 2012; see Halliday et al., 2014 for review). However, the significance of Lewy-related pathology occurrence in particular cortical areas is debated, for example one retrospective autopsy study found that severity of cognitive decline in PDD correlated with Lewy-related pathology in the frontal and cingulate gyri (Mattila et al., 2000), while another found no significant correlations in these regions but did find one in relation to temporal lobe Lewy-related pathology (Harding and Halliday, 2001). Meanwhile some patients with Parkinson’s disease with cortical Lewy-related pathology do not develop dementia at all (Colosimo et al., 2003; Kempster et al., 2010; Irwin et al., 2012). The significance of concurrent Alzheimer-type pathologies (senile plaques and neurofibrillary tangles) is hotly debated (Mattila et al., 1998; Apaydin et al., 2002; Hely et al., 2008; Sabbagh et al., 2009), although a recent study quantitatively assessing cortical Lewy-related pathology and Alzheimer-type pathologies found that a combination of both correlated most robustly with development of PDD (Compta et al., 2011). The relative contributions of other pathologies including microvascular disease, cerebral amyloid angiopathy, argyrophilic grains and TARDBP (previously known as TDP-43) remain unclear (Del Tredici and Braak, 2013; Halliday et al., 2014).
Despite the heterogeneity described above, specific elements of the neuropathology of PDD do bear a direct relationship to the dysfunctional neural networks perspective we describe. For example, the well-documented alpha-synuclein pathology affecting substantia nigra pars compacta neurons projecting to the striatum (Gibb and Lees, 1991; Braak et al., 2003) not only underlies dopamineric loss leading to the movement symptoms of Parkinson’s disease but also dopaminergic loss in the frontostriatal network leading to dysexecutive symptoms (as discussed above). There is also well-documented evidence of early Lewy-related pathology in the midbrain ventral tegmental area and locus coeruleus (Braak et al., 2003), which underlie the extensive degeneration in the mesocortical dopaminergic and noradrenergic networks specific to PDD, respectively (Cash et al., 1987; Del Tredici and Braak, 2013; Hall et al., 2014), thereby contributing to deficits in executive function and orienting of attention. Meanwhile, Lewy-related pathology has been shown to develop in the NBM from the early stages of Parkinson’s disease (Braak stage 3, Braak et al., 2003), and increasing alpha-synuclein burden in this structure correlates with increasing cell loss and the development of PDD (Hall et al., 2014), thereby underlying the cholinergic dysfunction which impacts across all cognitive domains as detailed above.
Several neuropathological series have shown significantly higher densities of Lewy-related pathology and amyloid-β senile plaques in the hippocampi of patients with PDD compared to non-demented Parkinson’s disease (Irwin et al., 2012; Hall et al., 2014), which potentially drives the atrophy of MTL regions specific to PDD, and may consequently underlie mnemonic deficits. Interestingly one study also demonstrated a significant reduction of cholinergic innervation to the hippocampus specific to PDD (Hall et al., 2014), indicating one way in which combined dysfunction in both the NBM and MTL networks may interact to cause memory impairments.
Finally, as mentioned above, strong correlations have been demonstrated between Lewy-related pathology burden in frontal, parietal and temporo-limbic cortices and the presence of visual hallucinations in PDD (Harding et al., 2002; Papapetropoulos et al., 2006; Kalaitzakis et al., 2009; Gallagher et al., 2011). These patterns of Lewy-related pathology deposition correspond to the areas of frontal hypofunction and parietal and limbic cortical atrophy in PDD described above, and therefore represent the potential neuropathological basis for deficits in the executive control of attention and visual perception, respectively, which in combination contribute to generation of visual hallucinations.
Thus, although there is variation in the overall distribution and type of cellular neuropathology underlying PDD, pathological changes at key cognitive nodes display a relative consistency, and support the neural network model of PDD. Our model is therefore complementary to the established neuropathological basis of PDD, and indeed builds upon it by providing a functional mechanism.
Implications of the neural network perspective of Parkinson’s disease dementia for clinical practice
Prognostic factors for development of Parkinson’s disease dementia
In the clinic the diagnosis of PDD is based upon the Movement Disorders Society Task Force criteria (Dubois et al., 2007; Emre et al., 2007), which incorporates detection of the cognitive features described above. However, it is recognized that early identification of patients at risk of developing PDD is useful in order to monitor them more closely so that therapeutic and supportive strategies can be implemented at a stage of the disease when they are likely to have greatest efficacy. Detailed neuropsychological testing, although able to detect early subclinical deficits, is not widely available, and therefore identification of clinical features with high predictive value for PDD has pragmatic value for clinicians. Large longitudinal cohort studies have demonstrated that inability to copy the intersecting pentagons figure on the MMSE, impairments of semantic verbal fluency and recognition memory, and development of a postural instability and gait difficulty (PIGD) motor phenotype (whether at baseline or later) are significant predictors for PDD (Levy et al., 2002a; Alves et al., 2006; Burn, 2006; Williams-Gray et al., 2007a, 2013; Hely et al., 2008). Errors on the Pill Questionnaire (in which patients are asked to describe their medication regime and its time schedule) and presence of REM sleep behavioural disorder are also associated with later development of PDD, although their positive predictive values are lower (Postuma et al., 2012; Martinez-Martin, 2013).
It is interesting to note that all of these predictive clinical features have a strong putative cholinergic basis according to the neural network model described above; deficits on pentagon copying are due to visual perceptive dysfunction while impairments in semantic verbal fluency and recognition memory are due to deficits in memory encoding and temporo-limbic storage, and dysfunction in the NBM network contributes to all of these. The Pill Questionnaire requires cued recall that probes semantic memory, while REM sleep behavioural disorder is caused by deregulation of brainstem arousal networks including the NBM. Meanwhile motor symptoms of postural instability and gait difficulty in Parkinson’s disease are attributed to dysfunction in a brainstem cholinergic nucleus, the pedunculopontine nucleus (Fling et al., 2013). The pedunculopontine nucleus has a strong anatomical connection with the NBM (Pahapill and Lozano, 2000; Gratwicke et al., 2013), and possibly a functional connection since deep brain stimulation of the pedunculopontine nucleus appeared to improve attention, memory and visuospatial abilities in a patient with PDD (Ricciardi et al., 2014). Therefore development of postural instability and gait difficulty might represent a marker of underlying cholinergic dysfunction that will affect both circuits. Overall, the predominance of underlying cholinergic dysfunction in the clinical predictors of PDD highlights the relative importance of damage to this network above others in its pathogenesis, a theory that is supported by our proposed neural network model because the NBM system is implicated across all cognitive impairments of PDD. This agrees with the ‘dual-syndrome hypothesis’, which proposes that while cognitive deficits in early Parkinson’s disease are mainly mediated by dysfunction in the fronto-striatal dopamine network, the onset of dementia is characterized by superimposition of additional dysfunction in cholinergic networks (Kehagia et al., 2013).
Management of Parkinson’s disease dementia
The relative importance of cholinergic network dysfunction in PDD is reflected in current treatment strategies, which focus on the use of AChEIs, such as rivastigmine, donepezil and galantamine, to boost cholinergic function. Two large placebo-controlled trials have shown that rivastigmine significantly improves deficits in orienting of attention, vigilance and cognitive fluctuation in patients with PDD (Emre et al., 2004; Wesnes et al., 2005b), and indeed patients with more severe attentional deficits appear to respond best (Wesnes et al., 2005a). These results serve to reinforce the cholinergic basis of attention deficits in PDD according to the neural network model. Benefits from AChEIs have also been demonstrated for executive deficits (action sequencing, response inhibition and verbal fluency) visuospatial tasks (the Clock Drawing Test) and hallucinations (Emre et al., 2004; Litvinenko et al., 2008). Whether these improvements are due to amelioration of the cholinergic network deficits underlying these cognitive processes, or are secondary to an overall improvement in attention assisting other overlapping cognitive functions, or a combination of both, is unknown.
In general, the cognitive benefits seen with the use of AChEIs in PDD translate into overall improvements in global cognition and activities of daily living (Ravina et al., 2005; Rolinski et al., 2012), which are actually larger than those seen with use of AChEIs in Alzheimer’s disease (Weintraub et al., 2011). This is most likely due to the fact that NBM degeneration and resultant cholinergic network dysfunction is more severe in PDD than Alzheimer’s disease (Bohnen et al., 2003; Gratwicke et al., 2013). However, clinical experience indicates that there is actually substantial variation in the beneficial response seen with AChEIs amongst patients with PDD, the reasons for which are likely multifactorial. Differences in pharmacokinetics and absorption between individuals, varying sensitivity to the systemic side effects of AChEIs (which can be detrimental to their subjective perceived benefit), and the relative balance of different network dysfunctions amongst individual patients likely all play a part. With regard to the latter, one would expect greater impact from AChEIs in patients with PDD with predominant cholinergic network dysfunction, but less of an impact in patients where catecholaminergic network dysfunctions are similar or equal to cholinergic ones. If so, then it may be possible to predict which patients will respond better to AChEIs by characterizing their cognitive symptomatology according to the neural network model above.
Several other medications also provide limited benefits to cognitive symptoms in PDD. Levodopa administration improves executive functions requiring cognitive flexibility (as above), which are mediated by the associative fronto-striatal circuit (which is dopamine depleted early), as well as the insular. However, it simultaneously worsens executive functions which involve learning from feedback (prediction and decision making, reversal and probabilistic classification learning) through ‘overdosing’ the relatively more intact limbic and orbitofrontal circuits in early Parkinson’s disease (Gotham et al., 1988; Swainson et al., 2000; Cools et al., 2001; Jahanshahi et al., 2010; see Dirnberger and Jahanshahi, 2013 for review). Thus clinicians titrating levodopa therapy to address motor symptoms in PDD also need to be aware of the concurrent impact this can have on the dysexecutive syndrome. Furthermore, differences in COMT activity have additional impact on optimal dopamine replacement strategies, and whether patients should be genotyped for this purpose requires further study (Foltynie et al., 2004b).
The mixed NMDA (N-methyl D-aspartate) and nicotinic acetylcholine receptor antagonist memantine showed modest benefit on a test of executive control of attention in PDD in one randomized study (Aarsland et al., 2009), but significant benefits were not confirmed in another larger trial (Emre et al., 2010). There is also preliminary evidence from several small clinical trials that the noradrenalin re-uptake inhibitor atomoxetine may increase arousal levels, vigilance and deficits in response inhibition in Parkinson’s disease (Marsh et al., 2009; Weintraub et al., 2010; Kehagia et al., 2014), in-line with the aforementioned roles of the noradrenergic network in mediating attention and cognitive flexibility, respectively. However, the patients in these trials were not demented, and therefore further studies in the PDD population are needed to fully ascertain potential therapeutic benefits.
Finally, there is also growing recognition of the importance of cognitive rehabilitation therapies for managing dementias such as PDD. Several small controlled studies have shown that sessions of cognitive remediation training or regular completion of puzzles requiring a high cognitive load (e.g. Sudoku exercises) leads to broad sustained improvements in cognitive performance in PD-MCI patients, particularly on tests of executive functions (Sinforiani et al., 2004; Sammer et al., 2006; Nombela et al., 2011; París et al., 2011), which is paralleled by return of frontal cortical activation patterns to normal on functional MRI (Nombela et al., 2011). Although the improvements seen were modest, this result suggests that cognitive training benefits PD-MCI patients, possibly by reinforcing cognitive strategies, or improving cognitive reserve, which could be due to plastic effects on the underlying neural networks. However, the efficacy of this type of intervention has not yet been trialled in patients with PDD, and the potential benefits may be limited in this patient group who find it hard to engage in complex cognitive exercises on a regular basis. The effects of physical rehabilitation and non-invasive brain stimulation on cognitive performance have also been assessed in patients with Parkinson’s disease with varying, sometimes conflicting, results (see Hindle et al., 2013 for review), and so far no evidence for use of these in PDD exists.
Future directions and treatment strategies
As this review has shown, the dysfunctional neural networks underlying the cognitive symptoms of PDD are diverse and distributed throughout the brain. There is overlap between network functions, each of which depend on differing primary neurotransmitters. In addition, evidence suggests that neurotransmitters can modulate the functional effects of one another (Calabresi et al., 2006), and thereby damage to one network during the pathogenesis of PDD may in turn influence dysfunction in another (Srinivasan and Schmidt, 2003; Rommelfanger and Weinshenker, 2007). Furthermore, as discussed in detail above, the cellular-level pathology causing damage to these networks in PDD is heterogeneous, while the effects of different genes on the pathophysiology of the disorder is only now being slowly unravelled.
Given this complex milieu of pathological changes, transmitter interactions and genetic influences underlying PDD, it is perhaps not surprising that attempting to treat the dementia syndrome with drugs targeting single neurotransmitter systems with generalized mechanisms of action have thus far shown only modest results. We propose that it is time to refocus the therapeutic drive in PDD to address cognitive deficits through targeted intervention at the network level. This approach has distinct advantages over the traditional model of single-ligand-targeted drug therapy. First, to compensate for deficits in all the neurotransmitter systems involved in the pathophysiology of PDD using replacement pharmacotherapy would necessitate polypharmacy for patients, with the associated risks of multiple side effects. Second, the heterogeneity of the underlying molecular pathology means that pharmacologic agents aiming to reduce aggregation of abnormal proteins, such as alpha-synuclein, may be either inappropriate or insufficiently effective in a substantial number of patients. Novel network-targeted therapies can avoid these difficulties by attempting to modulate the disease process downstream at a systems-level to restore normal neural processing patterns and thereby relieve symptoms.
Such network-modulating therapies are already under development. One potential route is using deep brain stimulation. Deep brain stimulation has proven efficacy in ameliorating the movement symptoms of Parkinson’s disease by altering processing of motor signals at the neural network level (Deuschl et al., 2006; Williams et al., 2010; McConnell et al., 2012). Emerging evidence suggests that it achieves this by altering brain functional and structural connectivity via neural plastic mechanisms to return dysfunctional motor network processing back to its natural state (Fenoy et al., 2014; Kahan et al., 2014; van Hartevelt et al., 2014). This same approach is now being employed for modulation of cognitive networks in PDD, using the NBM as the target structure since the cholinergic network is involved in all aspects of cognitive impairment (Freund et al., 2009; Barnikol et al., 2010). The fact that it is a discrete anatomical structure also makes it easier to target compared to more diffuse cognitive networks, such as the fronto-parietal network, where the optimum site of network modulation is currently unclear. However, caution should be exercised as the outcome of NBM DBS in Alzheimer’s disease has been variable, with some patients experiencing slowing of cognitive decline while others did not (Kuhn et al., 2015). On the other hand, as mentioned above, cholinergic deficits in PDD are greater than in Alzheimer’s disease, which might predict a larger response in PDD, similar to that seen with AChEI therapy. Nevertheless, the ability of patients with PDD to give valid informed consent for surgery must be carefully considered, and the elevated risks of invasive neurosurgery in demented patients must be borne in mind (Foltynie and Hariz, 2010). It should also be remembered that patients with PDD usually have advanced motor symptoms requiring concurrent therapy, but that they are ineligible for subthalamic nucleus deep brain stimulation for these motor symptoms due to a risk of worsening verbal fluency and inhibitory control deficits seen with this target (Witt et al., 2008). However, the NBM is located directly below the globus pallidus internus (Gratwicke et al., 2013), a deep brain stimulation target for alleviation of motor symptoms without detrimental effects on executive function. This means that a single pair of electrodes could be placed to span both structures, allowing therapeutic modulation of both motor and cognitive networks simultaneously, and clinical trials of this approach in patients with PDD are currently ongoing (www.clinicaltrials.gov).
An alternative approach is to try to prevent neurodegeneration in specific cognitive networks in PDD. Intrahippocampal transplantation of human stem cells engineered either to differentiate into NBM-like cholinergic cells (Liu et al., 2013) or produce nerve growth factor (Lee et al., 2012) has been shown to rescue learning deficits in rodents, and may hold potential to treat mnemonic deficits in PDD by countering MTL atrophy. However, results from rodent studies do not often translate easily to human research, and the previous mixed results from instrastriatal transplantation of dopamine-rich foetal stem cells to treat the dopaminergic deficit in patients with Parkinson’s disease must be borne in mind (Hagell et al., 1999). Meanwhile, the development of encapsulated cell bio-delivery systems and demonstration of their safe implantation into the NBM of dementia patients provides a platform for targeted long-term delivery of neurotrophic factors to prevent degeneration in specific cognitive networks (Wahlberg et al., 2012; Emerich et al., 2014).
However, to enable further development of such therapies for PDD a number of issues still need to be addressed. The division of cognitive ability into separate domains is in itself largely artificial and how processes involved in executive, mnemonic, attention and visual perceptual functions overlap with one another to produce the conceptualized ‘dementia syndrome’ is far from clear. Studies tend to have focused on neurochemical correlates of cognitive decline, but studies examining electrophysiological correlates of cognitive processing both in health and disease are relatively lacking. Finally, clinical trials of treatments for PDD to date have evaluated outcomes using a variety of neuropsychological tests, often validated in non-Parkinson’s disease populations. Consensus on a standardized testing battery for PDD research is needed to allow comparisons between different interventions (Burn et al., 2014). Moreover, given the fluctuating nature of cognition in PDD, trials need to incorporate measures of cognition-related functional abilities to provide a more comprehensive evaluation of treatment effects.
Conclusion
Although the pathology underlying the motor symptoms of Parkinson’s disease is now well understood and effective treatments are available, understanding the dysfunctional neural processes underlying parkinsonian dementia remains a formidable challenge, and available treatments are inadequate. The frequency and severity of PDD and its implications for the quality of life of both patient and carer emphasize the need for greater attention in this area. We hope that the synthesis of novel insights from across the spectrum of neuroscience and neurology research that we present in this manuscript helps shed new light on this important issue, and provides a framework for new avenues of research into understanding and potentially treating PDD at the network level.
Funding
J.G. is supported by a grant from the Brain Research Trust awarded to T.F. The Unit of Functional Neurosurgery is partly funded by the UK Department of Health’s National Institute of Health Research Biomedical Research Centres funding scheme, and is also supported by the Monument Trust and the Parkinson’s Appeal.
Glossary
Abbreviations
- AChEI
acetylcholinesterase inhibitor
- MTL
medial temporal lobe
- NBM
nucleus basalis of Meynert
- PDD
Parkinson’s disease dementia
- PD-MCI
Parkinson’s disease with mild cognitive impairment
References
- Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8:613–18. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48:938–42. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21:1123–30. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59:102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–20. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Au WL, Zhou J, Palmes P, Sitoh Y-Y, Tan LC, Rajapakse JC. Levodopa and the feedback process on set-shifting in Parkinson’s disease. Hum Brain Mapp. 2012;33:27–39. doi: 10.1002/hbm.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–24. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard CG, Aarsland D, McKeith I, O’Brien J, Gray A, Cormack F, et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology. 2002;59:1714–20. doi: 10.1212/01.wnl.0000036908.39696.fd. [DOI] [PubMed] [Google Scholar]
- Barnes J, Boubert L. Executive functions are impaired in patients with Parkinson’s disease with visual hallucinations. J Neurol Neurosurg Psychiatry. 2008;79:190–2. doi: 10.1136/jnnp.2007.116202. [DOI] [PubMed] [Google Scholar]
- Barnes J, David AS. Visual hallucinations in Parkinson’s disease: a review and phenomenological survey. J Neurol Neurosurg Psychiatry. 2001;70:727–33. doi: 10.1136/jnnp.70.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnikol TT, Pawelczyk NBA, Barnikol UB, Kuhn J, Lenartz D, Sturm V, et al. Changes in apraxia after deep brain stimulation of the nucleus basalis Meynert in a patient with Parkinson dementia syndrome. Mov Disord. 2010;25:1519–20. doi: 10.1002/mds.23141. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Flicker C, Dean RL, Pontecorvo M, Figueiredo JC, Fisher SK. Selective memory loss following nucleus basalis lesions: long term behavioral recovery despite persistent cholinergic deficiencies. Pharmacol Biochem Behav. 1985;23:125–35. doi: 10.1016/0091-3057(85)90139-x. [DOI] [PubMed] [Google Scholar]
- Bédard MA, el Massioui F, Malapani C, Dubois B, Pillon B, Renault B, et al. Attentional deficits in Parkinson’s disease: partial reversibility with naphtoxazine (SDZ NVI-085), a selective noradrenergic alpha 1 agonist. Clin Neuropharmacol. 1998;21:108–17. [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Modulation of fusiform cortex activity by cholinesterase inhibition predicts effects on subsequent memory. Brain. 2009;132:2356–71. doi: 10.1093/brain/awp176. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol. 2011;94:360–88. doi: 10.1016/j.pneurobio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–82. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Lechowicz W, Szpak GM, Dymecki J. Qualitative and quantitative analysis of locus coeruleus neurons in Parkinson’s disease. Folia Neuropathol. 1997;35:80–86. [PubMed] [Google Scholar]
- Bhattacharyya A, Veit J, Kretz R, Bondar I, Rainer G. Basal forebrain activation controls contrast sensitivity in primary visual cortex. BMC Neurosci. 2013;14:55. doi: 10.1186/1471-2202-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biundo R, Weis L, Facchini S, Formento-Dojot P, Vallelunga A, Pilleri M, et al. Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism Relat Disord. 2014;20:394–9. doi: 10.1016/j.parkreldis.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Boecker H, Ceballos-Baumann AO, Volk D, Conrad B, Forstl H, Haussermann P. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol. 2007;64:984–8. doi: 10.1001/archneur.64.7.984. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol. 2006;253:242–7. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, et al. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60:1745–8. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson's disease with dementia patients with a 2-year follow-up. Brain. 2008;131:690–705. doi: 10.1093/brain/awm322. [DOI] [PubMed] [Google Scholar]
- Bosboom JLW, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, et al. Resting state oscillatory brain dynamics in Parkinson’s disease: an MEG study. Clin Neurophysiol. 2006;117:2521–31. doi: 10.1016/j.clinph.2006.06.720. [DOI] [PubMed] [Google Scholar]
- Bosboom JLW, Stoffers D, Wolters EC, Stam CJ, Berendse HW. MEG resting state functional connectivity in Parkinson’s disease related dementia. J Neural Transm. 2009;116:193–202. doi: 10.1007/s00702-008-0132-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos R a I, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Breen KC, Drutyte G. Non-motor symptoms of Parkinson’s disease: the patient's perspective. J Neural Transm. 2013;120:531–5. doi: 10.1007/s00702-012-0928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnick K, Ehrt U, Emre M, De Deyn PP, Wesnes K, Tekin S, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1136–42. doi: 10.1136/jnnp.2006.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnick K, Emre M, Tekin S, Haugen SB, Aarsland D. Cognitive correlates of visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2011;26:824–9. doi: 10.1002/mds.23525. [DOI] [PubMed] [Google Scholar]
- Brück A, Portin R, Lindell A, Laihinen A, Bergman J, Haaparanta M, et al. Positron emission tomography shows that impaired frontal lobe functioning in Parkinson’s disease is related to dopaminergic hypofunction in the caudate nucleus. Neurosci Lett. 2001;311:81–4. doi: 10.1016/s0304-3940(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Burdick DJ, Cholerton B, Watson GS, Siderowf A, Trojanowski JQ, Weintraub D, et al. People with Parkinson’s disease and normal MMSE score have a broad range of cognitive performance. Mov Disord. 2014;29:1258–64. doi: 10.1002/mds.25924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn DJ. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77:585–9. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn D, Weintraub D, Ravina B, Litvan I. Cognition in movement disorders: where can we hope to be in ten years? Mov Disord. 2014;29:704–11. doi: 10.1002/mds.25850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–2. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70:1017–22. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- Butt AE, Hodge GK. Acquisition, retention, and extinction of operant discriminations in rats with nucleus basalis magnocellularis lesions. Behav Neurosci. 1995;109:699–713. doi: 10.1037//0735-7044.109.4.699. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–26. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Parnetti L, Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006;5:974–83. doi: 10.1016/S1474-4422(06)70600-7. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord. 2003;18:784–90. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- Cash R, Dennis T, L’Heureux R, Raisman R, Javoy-Agid F, Scatton B. Parkinson’s disease and dementia: norepinephrine and dopamine in locus ceruleus. Neurology. 1987;37:42. doi: 10.1212/wnl.37.1.42. [DOI] [PubMed] [Google Scholar]
- Caviness JN, Hentz JG, Evidente VG, Driver-Dunckley E, Samanta J, Mahant P, et al. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:348–54. doi: 10.1016/j.parkreldis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Choi SH, Jung TM, Lee JE, Lee S-K, Sohn YH, Lee PH. Volumetric analysis of the substantia innominata in patients with Parkinson’s disease according to cognitive status. Neurobiol Aging. 2012;33:1265–72. doi: 10.1016/j.neurobiolaging.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Christopher L, Marras C, Duff-Canning S, Koshimori Y, Chen R, Boileau I, et al. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain. 2014;137:565–75. doi: 10.1093/brain/awt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:852–6. doi: 10.1136/jnnp.74.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, Collins C, et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Costa A, Monaco M, Zabberoni S, Peppe A, Perri R, Fadda L, et al. Free and cued recall memory in Parkinson’s disease associated with amnestic mild cognitive impairment. PLoS One. 2014;9:e86233. doi: 10.1371/journal.pone.0086233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Livingston L, MacAskill MR, Graham C, Melzer TR, Porter RJ, et al. Characterizing mild cognitive impairment in Parkinson’s disease. Mov Disord. 2011;26:629–36. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J Neurol Neurosurg Psychiatry. 2013;84:774–83. doi: 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord. 2005;20:130–40. doi: 10.1002/mds.20308. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Frith CD, Jahanshahi M. Executive dysfunction in Parkinson’s disease is associated with altered pallidal-frontal processing. Neuroimage. 2005;25:588–99. doi: 10.1016/j.neuroimage.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–24. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- Dubois B, Malapani C, Verin M, Rogelet P, Deweer B, Pillon B. [Cognitive functions and the basal ganglia: the model of Parkinson disease] Rev Neurol (Paris) 1994;150:763–70. [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Orive G, Thanos C, Tornoe J, Wahlberg LU. Encapsulated cell therapy for neurodegenerative diseases: from promise to product. Adv Drug Deliv Rev. 2014;67-68:131–41. doi: 10.1016/j.addr.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Emre M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003;2:229–37. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351:2509–18. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707; quiz 1837. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:969–77. doi: 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- Fahn S, Libsch LR, Cutler RW. Monoamines in the human neostriatum: topographic distribution in normals and in Parkinson’s disease and their role in akinesia, rigidity, chorea, and tremor. J Neurol Sci. 1971;14:427–55. doi: 10.1016/0022-510x(71)90178-x. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–18. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123 (Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- Fenoy AJ, Goetz L, Chabardès S, Xia Y. Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci Ther. 2014;20:191–201. doi: 10.1111/cns.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136:2405–18. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CEG, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain. 2004a;127:550–60. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Goldberg TE, Lewis SGJ, Blackwell AD, Kolachana BS, Weinberger DR, et al. Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Mov Disord. 2004b;19:885–91. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Hariz MI. Surgical management of Parkinson’s disease. Expert Rev Neurother. 2010;10:903–14. doi: 10.1586/ern.10.68. [DOI] [PubMed] [Google Scholar]
- Freund H-J, Kuhn J, Lenartz D, Mai JK, Schnell T, Klosterkoetter J, et al. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009;66:781–5. doi: 10.1001/archneurol.2009.102. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–56. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. 4th edn. London: Elsevier; 2008. The prefrontal cortex. [Google Scholar]
- Gallagher DA, Parkkinen L, O’Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain. 2011;134:3299–309. doi: 10.1093/brain/awr225. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67:1605–11. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Gray F. Dementia in idiopathic Parkinson’s disease. A neuropathological study of 32 cases. Acta Neuropathol. 1984;64:43–52. doi: 10.1007/BF00695605. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–9. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of ‘benign hallucinations’ in Parkinson disease. Arch Neurol. 2006;63:713–16. doi: 10.1001/archneur.63.5.713. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson’s disease. Neurology. 2001;57:2078–82. doi: 10.1212/wnl.57.11.2078. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology. 1993;43:2227–9. doi: 10.1212/wnl.43.11.2227. [DOI] [PubMed] [Google Scholar]
- Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, Detoledo-Morrell L, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain. 2014a;137 (Pt 3):849–59. doi: 10.1093/brain/awt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Williams-Gray C, Barker RA, Duda JE, Galvin JE. The spectrum of cognitive impairment in Lewy body diseases. Mov Disord. 2014b;29:608–21. doi: 10.1002/mds.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Redondo R, García-García D, Clavero P, Gasca-Salas C, García-Eulate R, Zubieta JL, et al. Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: a two-step process. Brain. 2014;137 (Pt 8):2356–67. doi: 10.1093/brain/awu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin DS, Aminoff MJ. Electrophysiological differences between demented and nondemented patients with Parkinson’s disease. Ann Neurol. 1987;21:90–4. doi: 10.1002/ana.410210116. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111 (Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Gratwicke J, Kahan J, Zrinzo L, Hariz M, Limousin P, Foltynie T, et al. The nucleus basalis of Meynert: a new target for deep brain stimulation in dementia? Neurosci Biobehav Rev. 2013;37:2676–88. doi: 10.1016/j.neubiorev.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain. 1999;122 (Pt 6):1121–32. doi: 10.1093/brain/122.6.1121. [DOI] [PubMed] [Google Scholar]
- Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, et al. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain. 2014;137 (Pt 9):2493–508. doi: 10.1093/brain/awu193. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord. 2014;29:634–50. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Asano T, Sakurai H, Tanaka Y, Takasaki M, Abe K. MR analysis of the substantia innominata in normal aging, Alzheimer disease, and other types of dementia. Am J Neuroradiol. 2002;23:27–32. [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–63. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–31. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hattori T, Orimo S, Aoki S, Ito K, Abe O, Amano A, et al. Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum Brain Mapp. 2012;33:727–39. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautecoeur P, Gallois P, Forzy G, Chatelet P, Choteau P, Dereux JF. [Late auditory evoked potentials in subcortical cognitive deterioration] Rev Neurol (Paris) 1991;147:293–9. [PubMed] [Google Scholar]
- He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Helkala EL, Laulumaa V, Soininen H, Riekkinen PJ. Recall and recognition memory in patients with Alzheimer’s and Parkinson's diseases. Ann Neurol. 1988;24:214–17. doi: 10.1002/ana.410240207. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2004;10:608–22. doi: 10.1017/S1355617704104141. [DOI] [PubMed] [Google Scholar]
- Hilker R, Thomas a V, Klein JC, Weisenbach S, Kalbe E, Burghaus L, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology. 2005;65:1716–22. doi: 10.1212/01.wnl.0000191154.78131.f6. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–80. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson’s disease: a systematic review. Mov Disord. 2013;28:1034–49. doi: 10.1002/mds.25377. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334:345–8. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, et al. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007;130:1525–37. doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kövari E. Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:864–8; discussion 864. doi: 10.1016/j.parkreldis.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson’s disease with and without mild cognitive impairment. Mov Disord. 2009;24:854–62. doi: 10.1002/mds.22444. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70:1470–7. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34:714–23. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–5. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–21. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Bargallo N, et al. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry. 2010;81:650–7. doi: 10.1136/jnnp.2009.179655. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Ramírez-Ruiz B, Tolosa E, Martí MJ, Valldeoriola F, Bargalló N, et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol. 2008;255:1324–31. doi: 10.1007/s00415-008-0885-8. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–98. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Wilkinson L, Gahir H, Dharminda A, Lagnado DA. Neuropsychologia Medication impairs probabilistic classification learning in Parkinson’s disease. 2010;48:1096–103. doi: 10.1016/j.neuropsychologia.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18:149–54. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- Janzen J, van’t Ent D, Lemstra AW, Berendse HW, Barkhof F, Foncke EMJ. The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J Neurol. 2012;259:147–54. doi: 10.1007/s00415-011-6149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson disease? Neurology. 1980;30:1326. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–69. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Jubault T, Monetta L, Strafella AP, Lafontaine A-L, Monchi O. L-dopa medication in Parkinson’s disease restores activity in the motor cortico-striatal loop but does not modify the cognitive network. PLoS One. 2009;4:e6154. doi: 10.1371/journal.pone.0006154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqué C, Ramírez-Ruiz B, Tolosa E, Summerfield C, Martí M-J, Pastor P, et al. Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord. 2005;20:540–4. doi: 10.1002/mds.20371. [DOI] [PubMed] [Google Scholar]
- Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, et al. Resting state functional MRI in Parkinson’s disease: the impact of deep brain stimulation on ‘effective’ connectivity. Brain. 2014:137: 1130–1144. doi: 10.1093/brain/awu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RKB, Gentleman SM. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:196–204. doi: 10.1016/j.parkreldis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Kalmbach A, Hedrick T, Waters J. Selective optogenetic stimulation of cholinergic axons in neocortex. J Neurophysiol. 2012;107:2008–19. doi: 10.1152/jn.00870.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–13. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis. 2013;11:79–92. doi: 10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Housden CR, Regenthal R, Barker RA, Muller U, Rowe J, et al. Targeting impulsivity in Parkinson’s disease using atomoxetine. Brain. 2014;137:1986–1997. doi: 10.1093/brain/awu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133:1755–62. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- Kida Y, Tachibana H, Takeda M, Yoshikawa H, Okita T. Recognition memory for unfamiliar faces in Parkinson’s disease: behavioral and electrophysiologic measures. Parkinsonism Relat Disord. 2007;13:157–64. doi: 10.1016/j.parkreldis.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–18. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–92. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson's disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol Psychiatry. 2015;20:353–60. doi: 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- Laatu S, Revonsuo A, Pihko L, Portin R, Rinne JO. Visual object recognition deficits in early Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:227–33. doi: 10.1016/j.parkreldis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Leanza G, Muir J, Nilsson OG, Wiley RG, Dunnett SB, Bjorklund A. Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur J Neurosci. 1996;8:1535–44. doi: 10.1111/j.1460-9568.1996.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Lech RK, Suchan B. The medial temporal lobe: memory and beyond. Behav Brain Res. 2013;254:45–9. doi: 10.1016/j.bbr.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Calvert JE. Impairments of mental rotation in Parkinson’s disease. Neuropsychologia. 1998;36:109–114. doi: 10.1016/s0028-3932(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Lee H, Fell J, Axmacher N. Electrical engram: how deep brain stimulation affects memory. Trends Cogn Sci. 2013;17:574–84. doi: 10.1016/j.tics.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lim IJ, Park SW, Kim YB, Ko Y, Kim SU. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012;21:2487–96. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- Lee JE, Cho KH, Song SK, Kim HJ, Lee HS, Sohn YH, et al. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014;85:7–16. doi: 10.1136/jnnp-2013-305062. [DOI] [PubMed] [Google Scholar]
- Lee JE, Park H-J, Park B, Song SK, Sohn YH, Lee JD, et al. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson’s disease dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2010;81:320–6. doi: 10.1136/jnnp.2009.184747. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–9. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–70. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Levin BE, Llabre MM, Reisman S, Weiner WJ, Sanchez-Ramos J, Singer C, et al. Visuospatial impairment in Parkinson’s disease. Neurology. 1991;41:365. doi: 10.1212/wnl.41.3.365. [DOI] [PubMed] [Google Scholar]
- Levy G, Jacobs DM, Tang M-X, Côté LJ, Louis ED, Alfaro B, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Mov Disord. 2002a;17:1221–6. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- Levy G, Tang M-X, Louis ED, Cote LJ, Alfaro B, Mejia H, et al. The association of incident dementia with mortality in PD. Neurology. 2002b;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23:6351–6. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–32. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Liepelt I, Reimold M, Maetzler W, Godau J, Reischl G, Gaenslen A, et al. Cortical hypometabolism assessed by a metabolic ratio in Parkinson’s disease primarily reflects cognitive deterioration-[18F]FDG-PET. Mov Disord. 2009;24:1504–11. doi: 10.1002/mds.22662. [DOI] [PubMed] [Google Scholar]
- Litvinenko IV, Odinak MM, Mogil’naya VI, Emelin AY. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial) Neurosci Behav Physiol. 2008;38:937–45. doi: 10.1007/s11055-008-9077-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, et al. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol. 2013;31:440–7. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel RJ, Gage FH, Thal LJ. Spatial learning in rats: correlation with cortical choline acetyltransferase and improvement with NGF following NBM damage. Exp Neurol. 1989;104:208–17. doi: 10.1016/0014-4886(89)90031-9. [DOI] [PubMed] [Google Scholar]
- Marié RM, Barré L, Dupuy B, Viader F, Defer G, Baron JC. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson’s disease. Neurosci Lett. 1999;260:77–80. doi: 10.1016/s0304-3940(98)00928-8. [DOI] [PubMed] [Google Scholar]
- Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: a pilot open-label study. Mov Disord. 2009;24:277–82. doi: 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin P. Dementia in Parkinson’s disease: usefulness of the pill questionnaire. Mov Disord. 2013;28:1832–7. doi: 10.1002/mds.25649. [DOI] [PubMed] [Google Scholar]
- Matsui H, Nishinaka K, Oda M, Hara N, Komatsu K, Kubori T, et al. Hypoperfusion of the visual pathway in parkinsonian patients with visual hallucinations. Mov Disord. 2006;21:2140–4. doi: 10.1002/mds.21140. [DOI] [PubMed] [Google Scholar]
- Matsui H, Nishinaka K, Oda M, Niikawa H, Kubori T, Udaka F. Dementia in Parkinson’s disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:177–81. doi: 10.1111/j.1600-0404.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- Mattila PM, Rinne JO, Helenius H, Dickson DW, Röyttä M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000;100:285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- Mattila PM, Röyttä M, Torikka H, Dickson DW, Rinne JO. Cortical Lewy bodies and Alzheimer-type changes in patients with Parkinson’s disease. Acta Neuropathol. 1998;95:576–82. doi: 10.1007/s004010050843. [DOI] [PubMed] [Google Scholar]
- McConnell GC, So RQ, Hilliard JD, Lopomo P, Grill WM. Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J Neurosci. 2012;32:15657–68. doi: 10.1523/JNEUROSCI.2824-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 2002;99:4002–7. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:188–94. doi: 10.1136/jnnp-2011-300828. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meppelink AM, de Jong BM, Renken R, Leenders KL, Cornelissen FW, van Laar T. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain. 2009;132:2980–93. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- Meppelink AM, Koerts J, Borg M, Leenders KL, van Laar T. Visual object recognition and attention in Parkinson’s disease patients with visual hallucinations. Mov Disord. 2008;23:1906–12. doi: 10.1002/mds.22270. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–97. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–11. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res Rev. 2000;31:236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Milner B. Some Cognitive Effects of Frontal-Lobe Lesions in Man. Philos Trans R Soc B Biol Sci. 1982;298:211–26. doi: 10.1098/rstb.1982.0083. [DOI] [PubMed] [Google Scholar]
- Milner B. Aspects of human frontal lobe function. Adv Neurol. 1995;66:67–81; discussion 81–4. [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004;24:702–10. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130:233–44. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montse A, Pere V, Carme J, Francesc V, Eduardo T. Visuospatial deficits in Parkinson’s disease assessed by judgment of line orientation test: error analyses and practice effects. J Clin Exp Neuropsychol. 2001;23:592–8. doi: 10.1076/jcen.23.5.592.1248. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Pirozzolo FJ, Hansch EC, Webster DD. Relationship of motor symptoms to intellectual deficits in Parkinson disease. Neurology. 1982;32:133. doi: 10.1212/wnl.32.2.133. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–42. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Nazem S, Siderowf AD, Duda JE, Have TT, Colcher A, Horn SS, et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with ‘normal’ global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57:304–8. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld MY, Blumen S, Aitkin I, Parmet Y, Korczyn AD. EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia. 1994;5:23–8. doi: 10.1159/000106690. [DOI] [PubMed] [Google Scholar]
- Nguyen DP, Lin S-C. A frontal cortex event-related potential driven by the basal forebrain. Elife. 2014;3:e02148. doi: 10.7554/eLife.02148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela C, Bustillo PJ, Castell PF, Sanchez L, Medina V, Herrero MT. Cognitive rehabilitation in Parkinson’s disease: evidence from neuroimaging. Front Neurol. 2011 doi: 10.3389/fneur.2011.00082. DEC: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela C, Rowe JB, Winder-Rhodes SE, Hampshire A, Owen AM, Breen DP, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain. 2014;137:2743–58. doi: 10.1093/brain/awu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Inoue Y, Mitani H, Kawahara R, Miyake M, Nakashima K. Visual hallucinations as REM sleep behavior disorders in patients with Parkinson’s disease. Mov Disord. 2003;18:812–7. doi: 10.1002/mds.10439. [DOI] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: willed and automatic control of behaviour. In: Davidson R, Schwartz G, Shapiro D, editors. Consciousness and self-regulation. Vol. 4. New York: Plenum; 1986. pp. 1–18. [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–15. doi: 10.1212/01.wnl.0000187116.13370.e0. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Kanno T, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S, et al. Presynaptic and postsynaptic dopaminergic binding densities in the nigrostriatal and mesocortical systems in early Parkinson’s disease: a double-tracer positron emission tomography study. Ann Neurol. 1999;46:723–31. doi: 10.1002/1531-8249(199911)46:5<723::aid-ana7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain. 1998;121 (Pt 5):949–65. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian B, Hodges JR, Summers BA, Polkey CE, Robbins TW. Dopamine-dependent frontostriatal planning deficits in early Parkinson’s disease. Neuropsychology. 1995;9:126–140. [Google Scholar]
- Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson’s disease. Neurobiol Dis. 2012;46:590–6. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123 (Pt 9):1767–83. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Pan PL, Shi HC, Zhong JG, Xiao PR, Shen Y, Wu LJ, et al. Gray matter atrophy in Parkinson’s disease with dementia: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34:613–19. doi: 10.1007/s10072-012-1250-3. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, McCorquodale DS, Gonzalez J, Jean-Gilles L, Mash DC. Cortical and amygdalar Lewy body burden in Parkinson’s disease patients with visual hallucinations. Parkinsonism Relat Disord. 2006;12:253–6. doi: 10.1016/j.parkreldis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. Brain systems of vigilance. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- París AP, Saleta HG, de la Cruz Crespo Maraver M, Silvestre E, Freixa MG, Torrellas CP, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord. 2011;26:1251–58. doi: 10.1002/mds.23688. [DOI] [PubMed] [Google Scholar]
- Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson’s disease and timing deficits. Front Integr Neurosci. 2013;7:75. doi: 10.3389/fnint.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen L, Pirttilä T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Junqué C, Martí M-J, Ramirez-Ruiz B, Bargalló N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Mov Disord. 2009;24:1193–9. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, et al. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1985;48:413–421. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Perry RH. Acetylcholine and hallucinations: disease-related compared to drug-induced alterations in human consciousness. Brain Cogn. 1995;28:240–58. doi: 10.1006/brcg.1995.1255. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer’s, Huntington's, and Parkinson's diseases. Arch Neurol. 1993;50:374–9. doi: 10.1001/archneur.1993.00540040036010. [DOI] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Bonnet A-M, Esteguy M, Guimaraes J, Vigouret J-M, et al. Cognitive slowing in Parkinson’s disease fails to respond to levodopa treatment: the 15-objects test. Neurology. 1989;39:762. doi: 10.1212/wnl.39.6.762. [DOI] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee S-H, et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–63. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Berger W, Benke T, Schelosky L. High-speed memory scanning in Parkinson’s disease: adverse effects of levodopa. Ann Neurol. 1991;29:670–3. doi: 10.1002/ana.410290616. [DOI] [PubMed] [Google Scholar]
- Poliakoff E, O’Boyle DJ, Moore AP, McGlone FP, Cody FWJ, Spence C. Orienting of attention and Parkinson’s disease: tactile inhibition of return and response inhibition. Brain. 2003;126:2081–92. doi: 10.1093/brain/awg210. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Stam CJ, Bosboom JLW, Berendse HW, Hillebrand A. A three dimensional anatomical view of oscillatory resting-state activity and functional connectivity in Parkinson’s disease related dementia: an MEG study using atlas-based beamforming. NeuroImage Clin. 2012;2:95–102. doi: 10.1016/j.nicl.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–9. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Bertrand J-A, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study. Mov Disord. 2012;27:720–6. doi: 10.1002/mds.24939. [DOI] [PubMed] [Google Scholar]
- Ramírez-Ruiz B, Junqué C, Martí M-J, Valldeoriola F, Tolosa E. Neuropsychological deficits in Parkinson’s disease patients with visual hallucinations. Mov Disord. 2006;21:1483–7. doi: 10.1002/mds.20965. [DOI] [PubMed] [Google Scholar]
- Ravina B, Putt M, Siderowf A, Farrar JT, Gillespie M, Crawley A, et al. Donepezil for dementia in Parkinson’s disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatry. 2005;76:934–9. doi: 10.1136/jnnp.2004.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WG, Hely MA, Morris JG, Broe GA, Adena M, Sullivan DJ, et al. A longitudinal of Parkinson’s disease: clinical and neuropsychological correlates of dementia. J Clin Neurosci. 1996;3:327–33. doi: 10.1016/s0967-5868(96)90028-4. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Piano C, Rita Bentivoglio A, Fasano A. Pedunculopontine nucleus stimulation in Parkinson’s disease dementia. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.027. pii: S0006-3223(14)00590-3. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Jäkälä P, Kejonen K, Riekkinen P. The alpha2 agonist, clonidine, improves spatial working performance in Parkinson’s disease. Neuroscience. 1999;92:983–9. doi: 10.1016/s0306-4522(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson's disease. Cochrane database Syst Rev. 2012;3:CD006504. doi: 10.1002/14651858.CD006504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson’s disease. Biochem Pharmacol. 2007;74:177–90. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, Weintraub D, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25:1170–6. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson’s disease: impaired effective connectivity among frontal cortical regions. Brain. 2002;125:276–89. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–7. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson’s disease. J Neurol Sci. 2006;248:115–19. doi: 10.1016/j.jns.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25:615–22. doi: 10.1002/mds.22873. [DOI] [PubMed] [Google Scholar]
- Santangelo G, Trojano L, Vitale C, Ianniciello M, Amboni M, Grossi D, et al. A neuropsychological longitudinal study in Parkinson’s patients with and without hallucinations. Mov Disord. 2007;22:2418–25. doi: 10.1002/mds.21746. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–56. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–60. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Sharpe MH. Auditory attention in early Parkinson’s disease: an impairment in focused attention. Neuropsychologia. 1992;30:101–6. doi: 10.1016/0028-3932(92)90019-i. [DOI] [PubMed] [Google Scholar]
- Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73:273–8. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- Shin S, Lee JE, Hong JY, Sunwoo M-K, Sohn YH, Lee PH. Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:1155–61. doi: 10.1136/jnnp-2012-303391. [DOI] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum Brain Mapp. 2014;35:2206–19. doi: 10.1002/hbm.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Naismith SL, Lewis SJG. Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov Disord. 2011;26:2154–9. doi: 10.1002/mds.23896. [DOI] [PubMed] [Google Scholar]
- Sinforiani E, Banchieri L, Zucchella C, Pacchetti C, Sandrini G. Cognitive rehabilitation in Parkinson’s disease. Arch Gerontol Geriatr Suppl. 2004:387–91. doi: 10.1016/j.archger.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Sinforiani E, Zangaglia R, Manni R, Cristina S, Marchioni E, Nappi G, et al. REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson’s disease. Mov Disord. 2006;21:462–6. doi: 10.1002/mds.20719. [DOI] [PubMed] [Google Scholar]
- Soikkeli R, Partanen J, Soininen H, Pääkkönen A, Riekkinen P. Slowing of EEG in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1991;79:159–65. doi: 10.1016/0013-4694(91)90134-p. [DOI] [PubMed] [Google Scholar]
- Soma S, Shimegi S, Suematsu N, Tamura H, Sato H. Modulation-specific and laminar-dependent effects of acetylcholine on visual responses in the rat primary visual cortex. PLoS One. 2013;8:e68430. doi: 10.1371/journal.pone.0068430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Lee JE, Park H-J, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson’s disease according to cognitive status. Mov Disord. 2011;26:289–96. doi: 10.1002/mds.23477. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci. 2003;17:2586–92. doi: 10.1046/j.1460-9568.2003.02684.x. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Visser SL, Op de Coul AA, De Sonneville LM, Schellens RL, Brunia CH, et al. Disturbed frontal regulation of attention in Parkinson’s disease. Brain. 1993;116 (Pt 5):1139–58. doi: 10.1093/brain/116.5.1139. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Goetz CG, Carrillo MC, Bangen KJ, Turner DA, Glover GH, et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology. 2004;63:1409–16. doi: 10.1212/01.wnl.0000141853.27081.bd. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Junqué C, Tolosa E, Salgado-Pineda P, Gómez-Ansón B, Martí MJ, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62:281–5. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- Tam CWC, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64:861–5. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109 (Pt 5):845–83. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic; 1972. pp. 381–403. [Google Scholar]
- Ungerleider LG, Mishkin M. Cambridge, MA: MIT Press; 1982. Two cortical visual systems. [Google Scholar]
- van Hartevelt TJ, Cabral J, Deco G, Møller A, Green AL, Aziz TZ, et al. Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. PLoS One. 2014;9:e86496. doi: 10.1371/journal.pone.0086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G. The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front Behav Neurosci. 2012;6:48. doi: 10.3389/fnbeh.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villardita C, Smirni P, le Pira F, Zappala G, Nicoletti F. Mental deterioration, visuoperceptive disabilities and constructional apraxia in Parkinson’s disease. Acta Neurol Scand. 1982;66:112–20. doi: 10.1111/j.1600-0404.1982.tb03135.x. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: memory or attention? Behav Brain Res. 1996;75:13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–86. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg LU, Lind G, Almqvist PM, Kusk P, Tornøe J, Juliusson B, et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: a technology platform for restorative neurosurgery. J Neurosurg. 2012;117:340–7. doi: 10.3171/2012.2.JNS11714. [DOI] [PubMed] [Google Scholar]
- Walker MP. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale: Two methods to assess fluctuating confusion in dementia. Br J Psychiatry. 2000;177:252–6. doi: 10.1192/bjp.177.3.252. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Mavandadi S, Mamikonyan E, Siderowf AD, Duda JE, Hurtig HI, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75:448–55. doi: 10.1212/WNL.0b013e3181ebdd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Somogyi M, Meng X. Rivastigmine in Alzheimer’s disease and Parkinson's disease dementia: an ADAS-cog factor analysis. Am J Alzheimers Dis Other Demen. 2011;26:443–9. doi: 10.1177/1533317511424892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes KA, Edgar C, Thomas J. Rivastigmine reduces the attentional deficit in dementia associated with Parkinson’s disease and treatment response is dependent on the initial severity of the deficits. Int Psychogeriatr. 2005a;17:263–4. [Google Scholar]
- Wesnes KA, McKeith I, Edgar C, Emre M, Lane R. Benefits of rivastigmine on attention in dementia associated with Parkinson disease. Neurology. 2005b;65:1654–6. doi: 10.1212/01.wnl.0000184517.69816.e9. [DOI] [PubMed] [Google Scholar]
- Whitehead DL, Davies ADM, Playfer JR, Turnbull CJ. Circadian rest-activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov Disord. 2008;23:1137–45. doi: 10.1002/mds.22057. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Hedreen JC, White CL, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol. 1983;13:243–8. doi: 10.1002/ana.410130304. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Podd J, Kan MM. Recognition memory impairment in Parkinson’s disease: power and meta-analyses. Neuropsychology. 2000;14:233–46. doi: 10.1037//0894-4105.14.2.233. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Podd J, Stewart-Williams S. Memory deficits in Parkinson’s disease. J Clin Exp Neuropsychol. 2006;28:738–54. doi: 10.1080/13803390590954236. [DOI] [PubMed] [Google Scholar]
- Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9:581–91. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 2005;4:605–10. doi: 10.1016/S1474-4422(05)70146-0. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–69. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007a;130:1787–98. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val 158 met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. J Neurosci. 2007b;27:4832–8. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84:1258–64. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–14. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- Woods SP, Tröster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson’s disease. J Int Neuropsychol Soc. 2003;9:17–24. doi: 10.1017/s1355617703910022. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Burns RJ, Geffen GM, Geffen LB. Covert orientation of visual attention in Parkinson’s disease: an impairment in the maintenance of attention. Neuropsychologia. 1990;28:151–9. doi: 10.1016/0028-3932(90)90097-8. [DOI] [PubMed] [Google Scholar]
- Yagi S, Yoshikawa E, Futatsubashi M, Yokokura M, Yoshihara Y, Torizuka T, et al. Progression from unilateral to bilateral parkinsonism in early Parkinson disease: implication of mesocortical dopamine dysfunction by PET. J Nucl Med. 2010;51:1250–7. doi: 10.2967/jnumed.110.076802. [DOI] [PubMed] [Google Scholar]
- Yong SW, Yoon JK, An YS, Lee PH. A comparison of cerebral glucose metabolism in Parkinson’s disease, Parkinson's disease dementia and dementia with Lewy bodies. Eur J Neurol. 2007;14:1357–62. doi: 10.1111/j.1468-1331.2007.01977.x. [DOI] [PubMed] [Google Scholar]
- Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RMA, Wadia P, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23:297–9. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Foldi NS, Borod JC. Cognitive and behavioral dysfunction in Parkinson’s disease: neurochemical and clinicopathological contributions. J Neural Transm. 2004;111:1287–301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]
- Zweig RM, Cardillo JE, Cohen M, Giere S, Hedreen JC. The locus ceruleus and dementia in Parkinson’s disease. Neurology. 1993;43:986. doi: 10.1212/wnl.43.5.986. [DOI] [PubMed] [Google Scholar]