Detection of Parkinson’s disease pathology is currently limited to lesions that occur late in the disease process. Roberts et al. present a new method for the direct detection of alpha-synuclein oligomers – the alpha-synuclein proximity ligation assay – and reveal previously unrecognised early-stage pathology in Parkinson’s disease post-mortem tissue.
Keywords: alpha-synuclein, Parkinson’s disease, oligomers, pathology
Detection of Parkinson’s disease pathology is currently limited to lesions that occur late in the disease process. Roberts et al. present a new method for the direct detection of alpha-synuclein oligomers – the alpha-synuclein proximity ligation assay – and reveal previously unrecognised early-stage pathology in Parkinson’s disease post-mortem tissue.
Abstract
Oligomeric forms of alpha-synuclein are emerging as key mediators of pathogenesis in Parkinson’s disease. Our understanding of the exact contribution of alpha-synuclein oligomers to disease is limited by the lack of a technique for their specific detection. We describe a novel method, the alpha-synuclein proximity ligation assay, which specifically recognizes alpha-synuclein oligomers. In a blinded study with post-mortem brain tissue from patients with Parkinson’s disease (n = 8, age range 73–92 years, four males and four females) and age- and sex-matched controls (n = 8), we show that the alpha-synuclein proximity ligation assay reveals previously unrecognized pathology in the form of extensive diffuse deposition of alpha-synuclein oligomers. These oligomers are often localized, in the absence of Lewy bodies, to neuroanatomical regions mildly affected in Parkinson’s disease. Diffuse alpha-synuclein proximity ligation assay signal is significantly more abundant in patients compared to controls in regions including the cingulate cortex (1.6-fold increase) and the reticular formation of the medulla (6.5-fold increase). In addition, the alpha-synuclein proximity ligation assay labels very early perikaryal aggregates in morphologically intact neurons that may precede the development of classical Parkinson’s disease lesions, such as pale bodies or Lewy bodies. Furthermore, the alpha-synuclein proximity ligation assay preferentially detects early-stage, loosely compacted lesions such as pale bodies in patient tissue, whereas Lewy bodies, considered heavily compacted late lesions are only very exceptionally stained. The alpha-synuclein proximity ligation assay preferentially labels alpha-synuclein oligomers produced in vitro compared to monomers and fibrils, while stained oligomers in human brain display a distinct intermediate proteinase K resistance, suggesting the detection of a conformer that is different from both physiological, presynaptic alpha-synuclein (proteinase K-sensitive) and highly aggregated alpha-synuclein within Lewy bodies (proteinase K-resistant). These disease-associated conformers represent previously undetected Parkinson’s disease pathology uncovered by the alpha-synuclein proximity ligation assay.
Introduction
Parkinson’s disease is the second most common neurodegenerative disorder and is characterized by progressive neuronal loss in nuclei throughout the brain, notably the heavy loss of dopaminergic neurons in the substantia nigra pars compacta. Although the precise mechanism underlying cell death in Parkinson’s disease is not yet understood, accumulating evidence suggests that small aggregates of alpha-synuclein, known as oligomers, play a key role in neuronal toxicity (Chen et al., 2007; Danzer et al., 2007; Tetzlaff et al., 2008; Nasstrom et al., 2011; Winner et al., 2011) by impairing a variety of cellular processes (Gosavi et al., 2002; Lashuel et al., 2002a, b; Kim et al., 2009; Emmanouilidou et al., 2010; Colla et al., 2012; Choi et al., 2013).
The primary event underlying the formation of alpha-synuclein oligomers is thought to be the initiation of abnormal alpha-synuclein self-interaction, beginning with alpha-synuclein dimers, whose formation has been shown to be the rate-limiting step for alpha-synuclein aggregation (Krishnan et al., 2003; Roostaee et al., 2013). The subsequent variety of transient, structurally diverse oligomeric species formed are thought to drive the pathogenesis of Parkinson’s disease and may be associated with the spread of the disease between different structures in the brain (Desplats et al., 2009; Hansen et al., 2011; Luk et al., 2012a). Further aggregation and compaction of alpha-synuclein oligomers leads to the formation of the pathological hallmark of Parkinson’s disease, Lewy bodies, which are thought to form as an attempt by the cell to sequester toxic oligomeric species away from the cellular machinery (Bucciantini et al., 2002; Muchowski, 2002; Soto and Estrada, 2008). Fibrillar alpha-synuclein with the distinctive cross-beta sheet structure of amyloid is a major component of Lewy bodies (Spillantini et al., 1997; Serpell et al., 2000). Using existing techniques, detection of Parkinson’s disease pathology is largely limited to lesions that occur late in the disease, including Lewy bodies, in degenerating neuronal populations.
As the self-interaction of alpha-synuclein is thought to be intimately involved in the initiation of pathogenic processes of Parkinson’s disease (Caughey and Lansbury, 2003), we hypothesized that a method to detect self-interacting alpha-synuclein molecules in human tissue could reveal earlier pathology. Many previous studies are heavily reliant on in vitro formed oligomers that may not be relevant to the disease-causative species. If we are to understand the exact role of oligomers in disease, it is of paramount importance to detect the endogenous pathogenic species in patient tissue. Here we report the development and validation of a novel method for the detection of alpha-synuclein oligomers, the alpha-synuclein proximity ligation assay (AS-PLA, Supplementary Fig. 1). Proximity ligation assays (PLA) enable the sensitive and specific detection of endogenous protein interactions and remove the need for tagging proteins to investigate interactions, a requirement for current techniques such as fluorescence resonance energy transfer (FRET) and bimolecular complementation (Soderberg et al., 2006; Weibrecht et al., 2010). PLA has previously been used to sensitively detect disease-relevant protein interactions, such as those between the various components of the transcription factor activator protein 1 (AP-1) in breast cancer (Baan et al., 2010) and between GRP78 (now known as HSPA5) and AKT1 during endoplasmic reticulum stress (Yung et al., 2011). Larger structures such as prostasomes, associated with prostate cancer (Tavoosidana et al., 2011) can be detected with PLA, as well as viruses, including avian influenza viruses (Schlingemann et al., 2010), and foot and mouth virus (Nordengrahn et al., 2008). Amyloid-β protofibrils have also been previously detected in homogenized brain from transgenic mice using PLA (Kamali-Moghaddam et al., 2010). We describe here the AS-PLA, the first PLA-based assay to detect alpha-synuclein oligomers in human tissues.
Using AS-PLA we have been able to specifically detect oligomeric species of alpha-synuclein. Furthermore, we show in post-mortem Parkinson’s disease brain tissue that AS-PLA detects early stage Parkinson’s disease lesions, such as pale bodies, and previously unreported oligomeric pathology. AS-PLA will be an excellent tool to further understand the role of alpha-synuclein oligomers in early Parkinson’s disease pathology.
Material and methods
Cell culture
HEK293 cells were maintained in high glucose Dulbecco’s modified Eagle’s medium (PAA) supplemented with 10% foetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine (all obtained from Life Technologies). BE(2)M17 cells were maintained in Opti-MEM® supplemented with 10% foetal bovine serum and 1% penicillin/streptomycin (all obtained from Life Technologies). All cells were grown at 37°C with 5% CO2, except where indicated.
Transfection
Cells were seeded on poly-l-lysine coated coverslips in 24-well plates at a density of 2 × 105 cells per well and transfected 24 h later. A suitable amount of DNA per well was transfected in serum-free media (Opti-MEM®) using Lipofectamine® 2000 and Plus reagents (Life Technologies). The medium was changed 4 h following transfection.
Alpha-synuclein bimolecular fluorescence complementation assay
Professor Tiago Outeiro (University of Göttingen) kindly provided us with the alpha-synuclein bimolecular fluorescence complementation constructs (Outeiro et al., 2008). For assays in 24-well plates, 0.25 μg of each construct per well was transfected into HEK293 cells. Following incubation at 37°C for 4 h after transfection, the cells were transferred to 30°C in 25 mM HEPES to allow the GFP chromophore to mature. Cells were imaged, fixed for immunofluorescence and AS-PLA or harvested for western blotting 48 h after transfection. Experiments were carried out in triplicate.
Generation of FKBP-alpha-synuclein and FRB-alpha-synuclein constructs
FK506 binding protein (FKBP)-alpha-synuclein and FK506 rapamycin binding (FRB)-alpha-synuclein constructs were generated by PCR using primers 5′TTTTTTCTTAAGGTTGAGGGTGGTGGTACTGGTGTTGCCACCATGGGCG- CCGGCGGCGCCGGCGGCGGCGCCGGAGTGCAGGTGGAAACC3′ and 5′TTTTTT-CCGCGGTTAACTCGAGCCGCCGGCGCCGCCGGCGCCGCCGCCAGGCGCGCCTTCCA-GTTTTAGAAGCTCCACATC3′ for FKBP; 5′TTTTTTCTTAAGGTTGAGGGTGGT- GGTACTGGTGTTGCCACCATGGGCGCCGGCGGCGCCGGCGGCGGCGCCATGTGGCATGAAGGCCTGG3′ and 5′TTTTTTCCGCGGTTAACTCGAGCCGCCGGCGCCGCC- GGCGCCGCCGCCAGGCGCGCCCTTTGAGATTCGTCGGAACAC3′ for FRB; and 5′T- TTTTTCTTAAGGGCGCGCCCTCGAGCGCCACCATGGATGTATTCATGAAAGGAC3′ and 5′TTTTTTCCGCGGACACCAGTACCAGCCTTAGGCTTCAGGTTCGTAGTCTTG3′ for alpha-synuclein. FKBP (FKBP1A gene) and FRB (MTOR gene) were obtained from plasmids kindly provided by Professor Carolyn Bertozzi (University of California, Berkeley; Addgene plasmids 20 211 and 20 228) (Czlapinski et al., 2008) and alpha-synuclein from the bimolecular fluorescence complementation constructs. The PCR fragments were digested, cloned into pGEM®-T (Promega), and verified by DNA sequencing. Alpha-synuclein and FKBP or FRB were subcloned from pGEM®-T into pcDNA4/TO/myc-HisB (Life Technologies). FRB-alpha-synuclein and FKBP- alpha-synuclein were both subcloned as a XhoI/SacII fragment.
FKBP-FRB-rapamycin assay
FKBP-alpha-synuclein and FRB-alpha-synuclein constructs (25 ng each) were transfected into HEK293 cells, as described above. After 4 h at 37°C after transfection, the transfection reagents were replaced with normal HEK293 media supplemented with 400 nM rapamycin for the +rapamycin condition. Cells were fixed for AS-PLA analysis 1 h after transfection. Experiments were carried out in triplicate.
Protein extraction
Cells were washed and then scraped in PBS. Cells were pelleted by centrifugation for 10 min at 2000 rpm at 4°C. For denaturing protein extraction, the pellet was snap frozen then resuspended and lysed in RIPA buffer (1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 150 mM NaCl, 50 mM Tris pH 8) with one protease inhibitor cocktail tablet (Roche) added per 50 ml, followed by sonication. The lysate was centrifuged at 3000 rpm for 10 min at 4°C and the supernatant was retained and stored at −80°C. For native protein extraction, the pellet was snap frozen then resuspended in 35 µl solubilization buffer (50 mM NaCl, 50 mM imidazole, 2 mM 6-aminohexanoic acid, 1 mM EDTA, pH 7), and solubilized with 8 µl digitonin (20% stock in water). After 15 min centrifugation at 100 000 g, the supernatant was retained and stored at −80°C (Wittig and Schagger, 2005). Protein content was quantified by BCA assay according to the manufacturer’s instructions (Sigma).
Alpha-synuclein sequential extraction
Sequential extraction was performed as previously described with minor modifications (Tofaris et al., 2003). Briefly, 100 mg of tissue was homogenized in three volumes of TBS+ [50 mM Tris HCl pH 7.4, 175 mM NaCl, 5 mM EDTA, plus complete protease inhibitor tablet (Roche)]. The homogenate was centrifuged for 5 min at 1000 g at 4°C then the supernatant was ultracentrifuged for 30 min at 120 000 g at 4°C. The resulting supernatant was the TBS+ soluble fraction. The pellet was rinsed twice in TBS+ then resuspended in TBS+ with 1% Triton™ X-100 and centrifuged for 20 min at 120 000 g at 4°C. The resulting supernatant was the Triton soluble fraction. The pellet was resuspended in RIPA [50 mM Tris-HCl pH 7.4, 175 mM NaCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS plus complete protease inhibitor tablet (Roche)] and centrifuged for 20 min at 120 000 g at 4°C. The pellet was washed three times with TBS+, then resuspended in 8 M urea/5% SDS for the urea soluble fraction.
Protein separation and western blotting
Ten micrograms of protein from cell lysates or 0.1 μg of recombinant protein was suspended in Laemmli buffer and heated to 95°C for 10 min for SDS-PAGE, or suspended in SDS-free sample buffer for non-denaturing PAGE. Proteins were separated on 10% SDS-polyacrylamide or non-denaturing polyacrylamide gels, except in Fig. 3A where proteins were separated on a pre-cast Bio-Rad Criterion™ TGX™ 4–15% gradient gel. After transfer of proteins to polyvinylidene difluoride (PVDF) membranes (Millipore) and blocking in 3% (w/v) powdered skimmed milk in Tris-buffered saline/0.1% Tween 20 (TBS-T), membranes were incubated overnight at 4°C in primary antibody [mouse anti-alpha synuclein 211 (Abcam) or mouse anti-amyloid β 4G8 (Covance)] diluted in blocking solution. After washing three times in TBS-T, the membrane was incubated with secondary antibody for 1 h at room temperature. The membrane was washed again in TBS-T and the signal visualized with ECL reagent (Millipore) and exposure in the Bio-Rad Gel Doc™.
Figure 3.
In vitro formed oligomers are specifically detected by AS-PLA. (A) Western blot analysis of alpha-synuclein oligomers, alpha-synuclein fibrils and amyloid-β (Aβ) oligomers. (i) Unmodified recombinant alpha-synuclein (monomer), alpha-synuclein oligomers produced by incubating recombinant protein with the aldehyde HNE for 18 h and alpha-synuclein fibrils, produced by shaking recombinant alpha-synuclein at 37°C for 5 days were resolved by SDS-PAGE and visualized with syn211. (ii) Recombinant amyloid-β (monomer) and amyloid-β oligomers produced by incubating recombinant protein with the aldehyde HNE for 18 h were visualized with the 4G8 antibody. (Bi) Electron microscopy analysis of monomeric alpha-synuclein, HNE-induced alpha-synuclein oligomers, alpha-synuclein fibrils and HNE-induced amyloid-β oligomers, at ×23 000 magnification. Scale bars = 50 nm. (Bii and iii) Alpha-synuclein immunofluorescence (AS-IF) and AS-PLA analysis of monomeric alpha-synuclein, HNE-induced alpha-synuclein oligomers and alpha-synuclein fibrils. Amyloid-β-IF and AS-PLA analysis of HNE-induced amyloid-β oligomers. Representative images of three independent experiments are shown. Negative control images are shown inset of each image. AS = alpha-synuclein. Scale bars = 10 µm. (C) Analysis of (i) IF and (ii) AS-PLA signal; au = arbitrary units. Quantitation of three independent experiments is shown. Error bars are +SEM. *P < 0.05, **P < 0.01. One-way ANOVA with Tukey’s multiple comparisons test.
Proximity ligation assay
Alpha-synuclein proximity ligation assay experiments were carried out using Duolink kits supplied by Olink Bioscience according to the manufacturer’s instructions. We chose an alpha-synuclein antibody for the AS-PLA probes that has previously been shown to display blocking activity (syn211; El-Agnaf et al., 2006). Briefly, the conjugates were prepared using the Duolink® Probemaker kit by incubating 20 μg of antibody (mouse anti-alpha-synuclein 211, 1 mg/ml, no BSA or azide, Abcam) with the Probemaker activated oligonucleotide (Minus or Plus) and conjugation buffer and leaving overnight at room temperature. The conjugates were incubated with Probemaker stop solution for 30 min at room temperature, and then suspended in Probemaker storage buffer. Cells in culture were fixed in 4% paraformaldehyde in preparation for fluorescent PLA. Paraffin embedded tissue was prepared for brightfield PLA by dewaxing in xylene, rehydrating via graded alcohols, blocking endogenous peroxidases with hydrogen peroxide for 1 h at room temperature and antigen retrieval with citrate buffer, pH 6, and microwave heating for a total of 10 min. All samples were incubated in Duolink® block solution at 37°C for 1 h, followed by the conjugates diluted in Duolink® PLA diluent (1:750 for fluorescent PLA experiments and 1:100 for brightfield PLA experiments) for 1 h at 37°C, then overnight at 4°C. After washing in TBS + 0.05% Tween 20, samples were incubated with Duolink® ligation solutions and ligase for 1 h at 37°C, before washing and incubation with Duolink® amplification reagents and polymerase for 2.5 h at 37°C. For fluorescent PLA experiments, samples were then washed in the dark and counterstained and mounted as for immunofluorescence. For tissue sections, samples were washed and then incubated with a Duolink® detection solution for 1 h at room temperature followed by a Duolink® substrate solution for 20 min at room temperature. The tissue sections were then counterstained with haematoxylin and dehydrated in graded alcohols and xylene, before mounting with DPX mounting reagent.
Immunofluorescence
Cells cultured in 24-well plates on poly-l-lysine coated glass coverslips were fixed in 4% paraformaldehyde for 15 min at room temperature and permeabilized with IF block solution (0.1% Triton™ X-100, 10% normal goat serum in TBS) for 1 h at room temperature. Coverslips were washed in TBS + 0.1% Triton™ X-100 and primary antibodies (mouse anti-alpha synuclein 211, Abcam: 1:1000) were diluted in IF block solution and incubated overnight at 4°C with gentle shaking. Cells were then washed four times in IF wash solution (0.1% Triton™ X-100 in TBS) and appropriate Alexa Fluor® IgG secondary antibodies (Invitrogen) were applied for 1 h at room temperature with gentle rocking and protected from light. Cells were counterstained with the fluorescent nuclear stain diluted in 4,6-diamidino-2-phenylindole (DAPI) diluted 1:2000. Coverslips were mounted using FluorSave™ (Merck) and imaged using fluorescent microscopy (Nikon Eclipse TE200-U).
Immunohistochemistry
Paraffin embedded tissue was dewaxed in xylene and rehydrated in graded alcohols then blocked in 10% H2O2 for 1 h at room temperature in the dark to quench endogenous peroxidases. Antigens were retrieved by microwave heating with citrate buffer, pH 6, for a total of 10 min. Tissue was then blocked in 10% normal goat serum in TBS + 0.1% Triton™ X-100 for 1 h at room temperature. Primary antibodies (mouse anti-alpha-synuclein 211, Abcam; 1:2000) were incubated with the tissue overnight at 4°C, followed by washing and incubation with biotinylated goat anti-mouse IgG secondary antibody (Jackson Immunoresearch) for 1 h at room temperature. After washing, VectaStain ABC reagent (Vector Labs) was added for 1 h at room temperature, then sections were incubated with 3,3′-Diaminobenzidine (DAB, Sigma) substrate for 3 min. The tissue sections were then counterstained with haematoxylin and dehydrated in graded alcohols and xylene, before mounting with DPX mounting reagent.
Preparation and analysis of alpha-synuclein oligomers, amyloid-β oligomers and alpha-synuclein fibrils
Oligomers were produced by incubating recombinant human alpha-synuclein (r-Peptide) or amyloid-β (Tocris Bioscience) at 1 mg/ml (70 μM and 220 μM, respectively) with a 30:1 M excess of 4-hydroxy-2-nonenal (HNE) at 37°C for 18 h. After incubation, unbound aldehyde was removed with an Amicon 3 kDa cut-off ultra-centrifugal unit (Millipore).
To produce alpha-synuclein fibrils, 2 mg/ml (140 μM) recombinant human alpha-synuclein (r-Peptide) in assembly buffer (50 mM Tris pH 7.4, 100 mM NaCl) was shaken at 37°C with constant agitation (1000 rpm) for 5 days.
Western blot analysis was carried out as described above. For immunofluorescence analysis, 20 μg of protein was serially diluted and spotted onto poly-d-lysine coated coverslips and fixed for 30 min at room temperature in 4% paraformaldehyde. Immunofluorescence and fluorescent PLA protocols were then carried out as described above. Experiments were carried out in triplicate.
Electron microscopy
Ten microlitres of 50 μM protein sample was applied to carbon formvar copper grids, which had been glow discharged immediately before use, and incubated for 2 min. Grids were blotted using filter paper and transferred to a droplet of 2% aqueous uranyl acetate for 60 s, then blotted and transferred to a droplet of filtered milli-Q water for 60 s, blotted and dried. Grids were imaged in a FEI Tecnai 12 transmission electron microscope operated at 120 kV and digital images were captured using a Gatan US1000 ccd camera.
Human tissue
Tissue samples from patients with Parkinson’s disease and control subjects and associated clinical and neuropathological data were supplied by the Parkinson’s UK Tissue Bank, funded by Parkinson’s UK, a charity registered in England and Wales (258 197) and in Scotland (SC037554). Sections from eight patients and eight age and gender matched control subjects were paraffin embedded and supplied at 5 -μm thick. We studied sections at the level of the third oculomotor nerve in the midbrain, of the inferior olive in the medulla and the anterior cingulate cortex.
Tissue samples from patients with dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy and Alzheimer’s disease were supplied by the Oxford Brain Bank. The regions studied were: anterior cingulate cortex for dementia with Lewy bodies sections, substantia nigra for multiple system atrophy and progressive supranuclear palsy sections and hippocampus for Alzheimer’s disease sections. Sections from two patients per disease were paraffin embedded and supplied at 5 -μm thick.
Neuropathological analysis
For the quantification of AS-PLA staining, two independent assessors analysed the blinded slides at ×20 magnification. Pre-defined neuroanatomical areas in each region were studied (Table 1).
Table 1.
Neuroanatomical regions studied for the quantification of AS-PLA.
| Regions included |
||||||
|---|---|---|---|---|---|---|
| Neuroanatomical area | Area 1 | Area 2 | Area 3 | Area 4 | Area 5 | Area 6 |
| Medulla | Pyramid (corticospinal tract) | Inferior olivary nucleus | Raphe | Reticular formation | Intermediate reticular zone | Remaining tegmentum |
| Midbrain | Crus cerebri | Substantia nigra | Red nucleus | Reticular formation | Superior colliculi | Periaqueductal grey |
| Cingulate cortex | Cingulate cortex | White matter | Corpus callosum | |||
Within each area, the heaviness of staining in blinded sections was scored semi-quantitatively on a scale between 0 and 5 against predefined scoring standard plates (Supplementary Fig. 5) and the highest diffuse score that filled a whole field of view in each area was recorded. The average of two assessors’ scores was taken; no significant difference between the assessors’ scores was found. The number of Parkinson’s disease lesions stained was also counted.
Proteinase K assay
After antigen retrieval, sections were treated with 50 µg/ml proteinase K in 10 mM Tris HCl pH 7.8, 100 mM NaCl and 0.1% NP-40 (all from Sigma) at 37°C for the indicated times and then washed in tap water for 5 min.
Results
Specific detection of alpha-synuclein oligomers using AS-PLA
AS-PLA signal is conditionally produced by the dual recognition of interacting molecules of alpha-synuclein by the AS-PLA probes (Supplementary Fig. 1). To ensure that no spurious signals were produced by more than one AS-PLA probe binding to a molecule of alpha-synuclein, we used the same alpha-synuclein antibody (syn211) to make both probes. Syn211 has previously been shown to display blocking activity on the epitope (El-Agnaf et al., 2006) and thus should prevent more than one probe binding per alpha-synuclein molecule, allowing AS-PLA to recognize only alpha-synuclein molecules that are interacting (i.e. oligomers) and not monomers. To demonstrate the recognition of alpha-synuclein oligomers by AS-PLA we used several in vitro alpha-synuclein oligomerization systems. Firstly, we used a bimolecular fluorescence complementation assay, where alpha-synuclein is fused to a split GFP reporter. When alpha-synuclein self-interacts the non-fluorescent halves of GFP-fold together, allowing maturation of the fluorophore [Fig. 1A(i)]. In HEK293 cells expressing both constructs, we observed green fluorescence indicative of alpha-synuclein self-interaction [Fig. 1A(ii) and C(i)]. We showed with SDS-PAGE and non-denaturing PAGE that oligomeric species ranging from dimers to higher molecular weight species were formed, consistent with previous observations [Fig. 1B(i and ii); Outeiro et al., 2008]. These oligomeric species are recognized by AS-PLA, demonstrated by the co-localization between punctate AS-PLA signal and bimolecular fluorescence complementation signal in transfected HEK cells [Fig. 1C(ii)]. Furthermore, AS-PLA signal was identified solely in cells fluorescing green and not in untransfected cells [Fig. 1C(ii)].
Figure 1.
AS-PLA specifically recognizes spontaneous bimolecular fluorescence complementation formed alpha-synuclein oligomers. (Ai) To visualize the formation of alpha-synuclein oligomers, non-fluorescent halves of GFP were fused to alpha-synuclein. When alpha-synuclein monomers interact, the fluorophore of GFP is reconstituted and fluorescence is observed, as in (Aii). Scale bar = 50 µm. (B) Lysates from transfected HEK293 cells were ultracentrifuged and proteins resolved on non-reducing denaturing PAGE (i) or non-denaturing PAGE (ii), before transfer to PVDF membrane that was probed with anti-alpha-synuclein antibody 211. A variety of oligomeric species was observed, as indicated on blots. Lanes for all blots: U = untransfected; N = GFP N-terminal-alpha-synuclein; C = alpha-syn-GFP C-terminal; N+C = GN-alpha-syn + alpha-syn-GC. Asterisk indicates non-specific bands. (C) HEK293 cells were transfected with alpha-synuclein bimolecular fluorescence complementation (BiFC) constructs. GFP fluorescence indicates that alpha-synuclein oligomerization has occurred (left, arrows). Each fluorescent red dot of AS-PLA signal represents a single oligomeric event (middle). Arrowheads indicate non-transfected cells that act as an internal negative control for PLA. (D) BE(2)M17 cells, which endogenously express robust levels of alpha-synuclein as demonstrated by immunofluorescence (IF, left), do not contain AS-PLA-detectable alpha-synuclein oligomers. Scale bars = 10 µm. All experiments were carried out in triplicate.
To demonstrate that AS-PLA selectively detects oligomeric forms of alpha-synuclein, we stained BE(2)M17 neuroblastoma cells that endogenously express alpha-synuclein (Ryan et al., 2013). Although the BE(2)M17 cells expressed robust levels of alpha-synuclein, as demonstrated by immunofluorescence [Fig. 1D(i)], no AS-PLA signal was observed [Fig. 1D(ii)]. This suggests that the generation of AS-PLA signal is dependent on the presence of oligomers and not simply the non-pathological expression of alpha-synuclein.
Next, we developed a system for the inducible formation of alpha-synuclein oligomers by exploiting the ternary complex formed between FKBP, the FRB domain of the mammalian target of rapamycin, and rapamycin (Muthuswamy et al., 1999; Gruber et al., 2006). We generated fusion proteins of alpha-synuclein and FKBP or FRB that allowed us to conditionally induce interactions between alpha-synuclein monomers in the presence of rapamycin (Fig. 2A). Because of alpha-synuclein’s tendency to aggregate when it is over-expressed (Zhang et al., 2008), we carried out the inducible oligomerization experiments under conditions of low expression of the exogenous proteins. In cells positive for expression of alpha-synuclein as indicated by immunofluorescence, we observed a 4-fold increase in AS-PLA signal in those that had been exposed to rapamycin for 1 h post-transfection (Fig. 2B and C), which was statistically significantly higher both in terms of number of puncta and their area (Fig. 2C(ii + iii)].
Figure 2.
Inducible alpha-synuclein oligomers but not monomers are detected by AS-PLA. (A) Interactions between alpha-synuclein molecules fused to FKBP or FRB are induced in the presence of rapamycin through the formation of a ternary complex. (B and C) AS-PLA signal increases in the presence of rapamycin, suggesting AS-PLA detects oligomers formed inducibly in this system. (B) Representative images. Scale bars = 10 µm. (C) Quantification of signal by number of AS-PLA puncta or total area of AS-PLA signal. Error bars are +SEM. *P < 0.05. Student’s t-test. All experiments were carried out in triplicate.
We generated recombinant oligomers and fibrils in vitro to: (i) ensure that the generation of AS-PLA signal was not affected by the tags on alpha-synuclein in the bimolecular fluorescence complementation and FKBP-FRB constructs; (ii) investigate which alpha-synuclein species were recognized by AS-PLA; and (iii) assess the specificity of AS-PLA for alpha-synuclein oligomers rather than oligomers of other proteins. We incubated recombinant alpha-synuclein and recombinant amyloid-β with the aldehyde 4-hydroxynonenal (HNE) to generate untagged alpha-synuclein and amyloid-β oligomers, respectively, and incubated recombinant alpha-synuclein with shaking to produce fibrils. All preparations had assembled into supramolecular structures, much larger than monomers, as demonstrated by western blot (Fig. 3A) and electron microscopy [Fig. 3B(i)]. Electron microscopy analysis revealed alpha-synuclein oligomers as small round structures arranged in chains of variable lengths that migrated as species of different sizes by western blot, whereas amyloid-β oligomers were not so regularly organized and migrated at a much higher molecular weight on western blot. Alpha-synuclein monomers showed an amorphous ultrastructure and as expected alpha-synuclein fibrils showed a fibrillar helical ultrastructure. We spotted equal amounts of monomeric alpha-synuclein, alpha-synuclein oligomers, alpha-synuclein fibrils and amyloid-β oligomers onto coverslips (Lee and Lee, 2002; Kramer and Schulz-Schaeffer, 2007) and probed sequentially with immunofluorescence (alpha-synuclein or amyloid-β) and AS-PLA [Fig. 3B(ii) and 3B(iii)]. AS-PLA signal was approximately 4-fold higher in alpha-synuclein oligomers over a minimal background signal in the monomeric samples and >2-fold higher in alpha-synuclein oligomers than in alpha-synuclein fibrils, despite very similar levels of immunofluorescence signal and therefore alpha-synuclein protein (Fig. 3C). The fibril preparations were not statistically differently labelled by AS-PLA than monomeric alpha-synuclein, whereas no AS-PLA signal was obtained with amyloid-β oligomers.
Finally, when either of the probes was omitted or when the ligase or polymerase enzymes were omitted from their respective steps, no AS-PLA signal was observed in FKBP-FRB transfected cells treated with rapamycin, or from alpha-synuclein in vitro produced oligomers suggesting the signal we observed was not background (Supplementary Fig. 2).
Cumulatively, these data definitively demonstrate that AS-PLA detects alpha-synuclein oligomers specifically, whereas it does not detect monomeric alpha-synuclein.
Early Parkinson’s disease lesions are selectively recognized by AS-PLA
To examine alpha-synuclein oligomeric pathology and its relationship to Lewy body pathology we stained post-mortem brain tissue from eight patients with Parkinson’s disease and eight age and sex-matched controls with AS-PLA (Supplementary Table 1). We chose three neuroanatomical areas relevant to Parkinson’s disease that according to the Braak hypothesis are progressively affected by alpha-synuclein pathology: medulla, midbrain and cingulate cortex (Braak et al., 2003).
Pale bodies and other early lesions in the brainstem displayed prominent AS-PLA staining (medulla and midbrain, Fig. 4A and B, arrows). However, brainstem Lewy bodies (Fig. 4A and B, arrowheads), which are considered late-stage lesions, were only very exceptionally stained, in contrast to the widespread staining with alpha-synuclein immunohistochemistry (AS-IHC). For example, a low magnification image of consecutive sections of the dorsal motor nucleus of the vagus reveals extensive staining of Lewy bodies with AS-IHC (Supplementary Fig. 3A), whereas AS-PLA is much more selective, staining mainly pale bodies and extrasomal Lewy bodies (Supplementary Fig. 3B, arrows); quantification of the number of lesions detected by both technique supports this (Supplementary Fig. 4A and B). AS-PLA detected significantly more pale bodies than AS-IHC, suggesting greater sensitivity to earlier lesions. AS-IHC detected more lesions overall, suggesting the majority of the pathology is Lewy bodies, which is unsurprising as all patients were at least Braak stage III when the medulla and midbrain are already heavily affected.
Figure 4.
AS-PLA stains alpha-synuclein oligomers in early Parkinson’s disease lesions. Tissue from eight patients and eight age and gender matched controls was stained with alpha-synuclein PLA. In the medulla (A) and midbrain (B), AS-PLA selectively stained pale bodies and extrasomal Lewy bodies (arrows in A and B). If any staining was associated with Lewy bodies, it was at their periphery (Av and Bi and iii). We also observed staining in cells where no obvious pale body or Lewy body was present, suggesting even earlier aggregation centres were detected (Aiv). In the cingulate cortex (C), cortical Lewy bodies were detected (Ci–iii). No lesions were detected in control tissue (Avi, Bvi and Civ). In all regions studied, there were significantly more Parkinson’s disease lesions detected in patients (Avii, Bvii and Cv). Scale bars = 10 µm. Error bars are +SEM. *P < 0.05; **P < 0.01; ****P < 0.0001. Student’s t-test.
Lesions detected by AS-PLA were present in nuclei associated with Parkinson’s disease pathology, and where pathology was also found using AS-IHC. These include the dorsal motor nucleus of the vagus, intermediate reticular zone, raphe and reticular formation in the medulla; in the midbrain the reticular formation, raphe, periaqueductal grey and oculomotor nucleus were affected. Many lesions were also detected in the substantia nigra pars compacta (Fig. 4B). Similarly to the dorsal motor nucleus of the vagus, pale bodies and extrasomal Lewy bodies were strongly detected here (Fig. 4A and B), whereas intracellular Lewy bodies were unstained [Fig. 4A(ii) and B(i and ii)]. Occasionally, a crown of AS-PLA stained oligomers surrounded Lewy bodies [Fig. 4A(v) and B(iii)]. We demonstrated that the lack of Lewy body staining by AS-PLA is not due to an inability of the antibody or the AS-PLA probes to access such structures, as the unmodified antibody and both AS-PLA probes strongly labelled Lewy bodies when used as a primary antibody for immunohistochemistry (Supplementary Fig. 5).
Most strikingly, we found AS-PLA reveals diffuse cytoplasmic staining in neurons that contained neither pale bodies nor Lewy bodies [Fig. 4A(iv)]. This suggests that AS-PLA is able to detect alpha-synuclein oligomerization in cells very early in the aggregation process, even before the larger aggregates forming pale bodies appear. The majority of surviving neurons that lacked pale bodies or Lewy bodies did not have any AS-PLA staining [indicated in Fig. 4A(iv) adjacent to a neuron harbouring diffuse cytoplasmic alpha-synuclein oligomers], suggesting that alpha-synuclein self-interaction is not a physiological event occurring constitutively in all cells. In addition, no diffuse cytoplasmic AS-PLA signal was observed in controls, indicating that this type of staining is strictly pathological and may represent early aggregates that precede currently recognized pathology.
In contrast to the brainstem, Lewy bodies in the cingulate cortex were strongly detected by AS-PLA (Fig. 4C). Interestingly, there was a strong correlation between the number of lesions detected with AS-PLA and AS-IHC (P < 0.001), with a trend towards the detection of more lesions with AS-PLA compared to AS-IHC (Supplementary Fig. 4C), suggesting that not only are cortical Lewy bodies detected by both AS-PLA and AS-IHC, but that AS-PLA displays more sensitivity for their detection.
Quantification of pathological lesions detected by AS-PLA in each region showed that no Parkinson’s disease lesions were detected in tissue from control cases confirming their association with Parkinson’s disease [Fig. 4A(vii), B(vii) and C(v)].
We next investigated if pathological lesions found in other neurodegenerative diseases were stained by AS-PLA. We stained cingulate cortex from patients with dementia with Lewy bodies, substantia nigra from multiple system atrophy and progressive supranuclear palsy cases and hippocampus from Alzheimer’s disease cases. First we confirmed that the selected regions were affected by pathological lesions of each disease using IHC (Fig. 5A, B and C). Lesions from patients with dementia with Lewy bodies and multiple system atrophy, known as alpha-synucleinopathies, were stained by AS-PLA. In tissue from patients with dementia with Lewy bodies, cortical Lewy bodies were detected by AS-PLA (Fig. 5A), similarly to Lewy bodies in the affected cortex of patients with Parkinson’s disease. In multiple system atrophy cases, diseased glial cells harbouring glial cytoplasmic inclusions showed cytoplasmic labelling with AS-PLA at the periphery of glial cytoplasmic inclusions similar to brainstem Lewy bodies in Parkinson’s (arrows, Fig. 5B). In contrast, tau inclusions in tissue from patients with progressive supranuclear palsy and tau neurofibrillary tangles and amyloid-β plaques from patients with Alzheimer’s disease were unstained (Fig. 5C and D).
Figure 5.
AS-PLA stains aggregates in dementia with Lewy bodies and multiple system atrophy but not Alzheimer’s disease or progressive supranuclear palsy. Tissue from patients with dementia with Lewy bodies (A) and multiple system atrophy (B) were stained with AS-PLA and AS-IHC. Glial cytoplasmic inclusions stained on their periphery by AS-PLA in (B) are indicated by arrows. Alzheimer’s disease tissue (C) was stained with 4G8 (i) for amyloid-β IHC or AT8 (ii) for tau IHC and AS-PLA. AT8 IHC was also used to stain progressive supranuclear palsy tissue. Scale bars: A and B = 12.5 μm; C and D = 50 μm.
Taken together, these data suggest that AS-PLA has a preference for early lesions and detects earlier disease processes than techniques previously described.
AS-PLA reveals previously unrecognized diffuse alpha-synuclein oligomeric pathology
Further to the staining of early Parkinson’s disease lesions, AS-PLA revealed a qualitatively distinct diffuse type of staining localized to specific nuclei including areas usually mildly affected by classical Parkinson’s disease pathology. This second type of AS-PLA staining was diffuse and located in the neuropil, clumping around neurons in the most severely affected areas, or less frequently in the white matter (Fig. 6).
Figure 6.
Previously unrecognized oligomeric pathology is revealed by AS-PLA. Striking diffuse AS-PLA staining was revealed in post-mortem brain sections. The heaviness of diffuse staining in blinded sections was scored semi-quantitatively by two independent assessors against predefined standards. In the medulla, staining was most prominent in patients in the reticular formation (Aii), and intermediate reticular zone (IRZ, Aiii) in the patients, and contrasted with AS-IHC staining, where only presynaptic staining or Lewy bodies were stained (bottom). A significant difference between the heaviness of AS-PLA diffuse staining in patients and controls was scored in these regions (C). In the midbrain, there was no difference in the amount of staining in patients and controls (D). In the cingulate, strong staining was observed in the grey of patients (B) compared to the controls but no difference in AS-PLA signal was observed in the white matter and corpus callosum (E). Similar levels of AS-IHC synaptic staining were present in patients and controls (B). Scale bars = 10 µm. *P < 0.05 one-way ANOVA with Dunn’s multiple comparisons test. Regions included in analyses in the medulla were the tegmentum (teg), intermediate reticular zone (IRZ), reticular formation, raphe, inferior olivary nucleus (olive) and corticospinal tract (CS tract). In the midbrain, the periaqueductal grey (PAG), superior colliculus (SC), reticular formation, red nucleus (RN), substantia nigra pars compacta (SNc) and corticospinal tract (CS tract) were analysed.
To quantify the diffuse type of AS-PLA staining and evaluate its potential association with Parkinson’s disease, we scored its heaviness in blinded sections (Supplementary Fig. 6). Patients with Parkinson’s disease specifically showed intense diffuse AS-PLA staining in the reticular formation [Fig. 5A(ii)] and intermediate reticular zone [Fig. 6A(iii)] of the medulla, where the mean scores were 6.5 and 4.5-fold higher, respectively, than controls (Fig. 6A and C), whereas in the cingulate cortex patients had a mean score 1.6-fold higher than controls (Fig. 6B and E). Staining in the raphe of the medulla was 2.75-fold higher in patients on average, although this was not statistically significant. In the midbrain, no region had a higher deposition of oligomers in patients compared to controls. Several white matter tracts, including the corticospinal tract, the subcortical white matter of the cingulate gyrus and the corpus callosum showed a weak diffuse AS-PLA staining but not a pathological deposition of oligomers above the level of controls.
Next, we compared diffuse AS-PLA staining to AS-IHC staining in consecutive sections. We found that the diffuse oligomeric pathology detected by AS-PLA did not correspond to any type of pathological staining that could be detected with AS-IHC including Lewy bodies and pale bodies (Fig. 6A). Furthermore, the diffuse neuropil oligomeric pathology did not correspond to physiological alpha-synuclein presynaptic staining (Fig. 6B).
Western blot analysis of sequentially extracted alpha-synuclein from human brain tissue corroborated an accumulation of high molecular alpha-synuclein species in Parkinson’s disease compared to control subjects, in keeping with our findings with AS-PLA (Supplementary Fig. 7). We show, by western blot, an accumulation of a ∼60 kDa oligomer and higher molecular species in the urea fractions containing aggregated proteins in the midbrain and cingulate cortex of patients with Parkinson’s disease, whereas in the medulla only accumulation of the ∼60 kDa oligomer was observed. The ∼60 kDa oligomer also variably accumulated in the TBS soluble and Triton soluble fractions, which correspond to cytoplasmic and membrane-associated proteins, respectively, in the midbrain and medulla of patients with Parkinson’s disease.
The alpha-synuclein oligomeric species revealed by AS-PLA have a distinct intermediate sensitivity to proteinase K
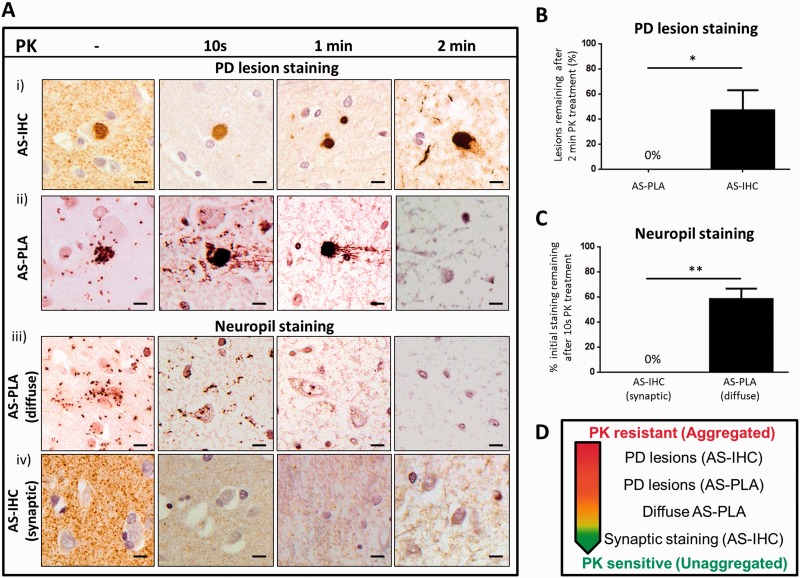
To further understand the structural properties of the alpha-synuclein oligomeric species revealed by AS-PLA, we assessed their proteinase K sensitivity. Proteinase K resistance is a property of highly aggregated proteins that usually display high beta-sheet content and is associated with amyloid-like misfolded proteins (Forloni, 1996; Neumann et al., 2004). We treated sections of cingulate cortex tissue from patients and controls with proteinase K for 10 s, 1 min or 2 min, as high levels of both cortical Lewy bodies and diffuse neuropil AS-PLA staining were found in this region. We first studied the proteinase K sensitivity of the alpha-synuclein protein species detected by either AS-IHC or AS-PLA within cortical Lewy bodies of patients with Parkinson’s disease. As expected, a large proportion (∼50%) of cortical Lewy bodies stained by AS-IHC showed proteinase K resistance and were still present after harsh proteinase K treatment [2 min, Fig. 7A(i) and 7B]. Occasional dystrophic neurites were also stained by AS-IHC after 2 min of proteinase K treatment (Fig. 7Ai). In contrast, the protein species stained by AS-PLA within cortical Lewy bodies were consistently more sensitive to proteinase K treatment, and no staining was present after 2 min of proteinase K digestion [Fig. 7A(ii)], which quantification (Fig. 7B) showed was significantly different from the response of AS-IHC stained cortical Lewy bodies to proteinase K.
Figure 7.
AS-PLA displays a different profile of proteinase K sensitivity to synaptic and pathological staining detected by AS-IHC. Tissue from the cingulate cortex of three patients and three controls was exposed to proteinase K (PK) for the indicated times and stained with AS-PLA or AS-IHC. Cortical Lewy bodies in patients were proteinase K resistant and still remained after 2 min of proteinase K treatment (Ai and B). In contrast, AS-PLA detection of cortical Lewy bodies was abolished after 2 min of proteinase K treatment (Aii and B). AS-PLA diffuse staining was no longer present after 1 min of proteinase K treatment (Aiii and C). AS-IHC synaptic staining was removed after 10 s of proteinase K treatment (Aiv and C). Therefore, oligomers detected by AS-PLA have a distinct intermediate resistance to proteinase K (D). *P < 0.05, **P < 0.01. Student’s t-test. Scale bars = 10 µm. PD = Parkinson’s disease.
We next studied the proteinase K sensitivity of the alpha-synuclein species detected by AS-PLA and AS-IHC in the neuropil. The diffuse alpha-synuclein oligomeric species stained by AS-PLA were moderately sensitive to proteinase K treatment and an intermediate proteinase K treatment [1 min, Fig. 7A(iii) and 7C] abolished diffuse AS-PLA signal. Physiological AS-IHC presynaptic staining in both patients and controls was proteinase K sensitive and removed after only 10-s exposure to proteinase K [Fig. 7A(iv) and 7C]. This suggests that AS-PLA stained oligomers are in an early stage of aggregation due to their intermediate proteinase K resistance (Fig. 7D).
Discussion
Here we have presented AS-PLA, a new method that for the first time allows the histological detection of alpha-synuclein oligomers in human tissue. Our findings provide the first direct evidence that alpha-synuclein self-interaction is a key pathological marker in diseased brain.
The association of alpha-synuclein self-interaction with pathogenesis is supported by the identification of extensive diffuse oligomeric pathology in specific neuroanatomical regions in patients using AS-PLA that had no AS-IHC counterpart in the form of Parkinson’s disease lesions or in physiological synaptic AS-IHC staining. Alpha-synuclein oligomeric pathology cannot be specifically recognized using AS-IHC. The PET-blot, a modification of AS-IHC, was developed to detect earlier pathology than traditional AS-IHC (Kramer and Schulz-Schaeffer, 2007), but the extremely proteinase K-resistant species revealed are likely to represent aggregates with high beta-sheet content that occur late in the misfolding process (Miake et al., 2002). In contrast, we demonstrated that the oligomeric species detected by AS-PLA show an intermediate proteinase K sensitivity, suggesting they are oligomers in a very early stage of aggregation, clearly folded differently than physiological presynaptic alpha-synuclein but which have not yet acquired a highly compact structure resistant to prolonged digestion by proteinase K.
There are several lines of evidence suggesting that oligomers with intermediate beta-sheet content are responsible for the spread and toxicity of alpha-synuclein (Volles et al., 2001; Apetri et al., 2006; Celej et al., 2012; Cremades et al., 2012) and it is possible that the species detected by AS-PLA have similar properties. Indeed, several recent studies in mice have shown that aggregates of alpha-synuclein, but not monomers, injected stereotaxically can spread to neuroanatomically connected regions and initiate de novo aggregation (Luk et al., 2012a, b; Masuda-Suzukake et al., 2013). These observations are consistent with the hypothesis of prion-like spread of pathology in Parkinson’s disease according to which alpha-synuclein molecules must directly self-interact to allow the misfolded species to transfer their pathogenic folding to newly recruited units (Brundin et al., 2010). Therefore, interacting contiguous alpha-synuclein molecules, which we demonstrate are not only present in human brain tissues but strongly associated with disease, would be a necessary although by no means a sufficient step for disease propagation according to this hypothesis. Our data suggest, nevertheless, that alpha-synuclein self-interaction is an early and more widespread phenomenon than previously thought.
Dysfunction at synapses and altered axonal transport has been postulated to be some of the earliest pathological events in Parkinson’s disease (Schulz-Schaeffer, 2010; Kim-Han et al., 2011; Nakata et al., 2012). Therefore, the striking deposition of alpha-synuclein oligomers in the neuropil may represent aberrant oligomerization at the synapse or abnormal transport of pathological species along axons to the cell body. Consistent with these pathological features, we also found extensive diffuse AS-PLA signal in patients in white matter tracts such as those in the medullary reticular formation.
Intriguingly, a small amount of AS-PLA-stained oligomers were also found in specific white matter tracts including the corticospinal tract, the subcortical white matter of the cingulate gyrus and the corpus callosum in some controls. Although it is possible that AS-PLA is detecting a range of alpha-synuclein oligomeric species, it is unlikely that AS-PLA detects the recently proposed physiological tetrameric form of alpha-synuclein (Bartels et al., 2011; Wang et al., 2011) for a number of reasons. Firstly, AS-PLA signal was not present in BE(2)M17 cells nor untransfected HEK293 cells, which were both shown to contain tetrameric physiological forms of alpha-synuclein (Bartels et al., 2011); in our hands, BE(2)M17 cells expressed high levels of monomeric alpha-synuclein. Second, although alpha-synuclein is highly and widely expressed in the CNS (Jakes et al., 1994; Iwai et al., 1995), the low levels of alpha-synuclein oligomers detected by AS-PLA in controls had a restricted neuroanatomical distribution. Third, the diffuse oligomers of both patient and controls share an intermediate sensitivity to proteinase K, suggesting that they contain a proportion of beta-sheet content unlike tetrameric alpha-synuclein, which is formed by alpha helices (Bartels et al., 2011; Wang et al., 2011). Interestingly, the white matter tracts containing AS-PLA-stained alpha-synuclein oligomers in some controls are either composed of long projection axons, or adjacent or projecting to nuclei where we found striking accumulations of oligomers exclusively in patients, suggesting oligomer accumulation in white matter tracts may represent very early subclinical pathology.
Diffuse AS-PLA oligomeric pathology is likely to be a precursor to recognized neuritic and perikaryal pathology. Diffuse cytoplasmic alpha-synuclein oligomers were also detected by AS-PLA in neurons of otherwise normal appearance. Their detection is indicative of the capacity of AS-PLA to detect very early alpha-synuclein oligomerization in neurons, even before the appearance of pale bodies or Lewy bodies. These accumulations may be related to punctate alpha-synuclein aggregates or perikaryal threads previously described (Arima et al., 1998; Kuusisto et al., 2003), which are hypothesized to progressively coalesce into pale bodies, in which alpha-synuclein and other proteins are further compacted to become Lewy bodies (Dale et al., 1992; Gomez-Tortosa et al., 2000; Kuusisto et al., 2003; Kanazawa et al., 2012).
AS-PLA selectively and more sensitively detected pale bodies compared to AS-IHC, further supporting that AS-PLA detects earlier pathology. Brainstem Lewy bodies were rarely stained, which is likely due to their composition of fibrillar alpha-synuclein as in vitro AS-PLA specifically recognized alpha-synuclein oligomers over fibrils. Fibrils were not statistically differently labelled than the background signal of monomers and the mild residual labelling of fibrils by AS-PLA is probably due to contaminating oligomers, as shown by western blot and electron microscopy. Staining on the periphery of brainstem Lewy bodies was more frequent, which may represent the incorporation of oligomers from the cytoplasm into the fibrillar structure of Lewy bodies, a potential protective mechanism to sequester toxic species away from the cellular machinery (Bucciantini et al., 2002; Muchowski, 2002; Soto and Estrada, 2008). Conversely, AS-PLA staining on the periphery of brainstem Lewy bodies could be oligomers disassociating from Lewy bodies, as a dynamic equilibrium has been proposed to exist between fibrils in Lewy bodies and alpha-synuclein oligomers (Bosco et al., 2011; Cremades et al., 2012). The strong staining of cortical Lewy bodies, which do not have the classical core-halo structure of brainstem Lewy bodies and are believed to contain less compact aggregates (Kosaka, 1978; Katsuse et al., 2003), and pale bodies suggests that AS-PLA detects oligomers in the process of being compacted and not those that are already extensively fibrillar in structure as in brainstem Lewy bodies.
Cortical Lewy bodies in dementia with Lewy bodies were positively stained by AS-PLA similarly to Parkinson’s, suggesting that Parkinson’s disease and dementia with Lewy bodies belong to the same disease spectrum, as previously suggested (McKeith, 2000; Zarranz et al., 2004). Although neurofibrillary tangles and amyloid plaques in Alzheimer’s and tau inclusions in progressive supranuclear palsy were negatively stained by AS-PLA, positive cytoplasmic staining around glial cytoplasmic inclusions was found in multiple system atrophy. This suggests that similar protein species are formed in three alpha-synucleinopathies (Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy), which warrants future investigation.
In conclusion, AS-PLA detects alpha-synuclein oligomeric pathology in neuroanatomical areas ahead of AS-IHC, providing an extra level of detail and resolution of alpha-synuclein pathology and potentially a new spatio-temporal window to detect and follow early pathological changes.
Supplementary Material
Acknowledgements
We thank the brain banks that provided us with samples. Tissue samples from Parkinson’s patients and controls and associated clinical and neuropathological data were supplied by the Parkinson’s UK Tissue Bank, funded by Parkinson’s UK, a charity registered in England and Wales (258 197) and in Scotland (SC037554). We acknowledge the Oxford Brain Bank, supported by the Medical Research Council (MRC), Brains for Dementia Research (BDR) and the NIHR Oxford Biomedical Research Centre for providing tissue samples from additional diseases. We thank Dr Errin Johnson (Imaging Services, Dunn School of Pathology, University of Oxford) for the electron microscopy imaging, Professor Tiago Outeiro (University of Göttingen) for the alpha-synuclein bimolecular fluorescence complementation constructs and Professor Ola Soderberg (University of Uppsala) for his technical expertise. This paper is dedicated to the memory of Paul Robertson, an exceptional neuroscientist and a dear friend.
Glossary
Abbreviations
- AS-PLA
alpha-synuclein proximity ligation assay
- AS-IHC
alpha-synuclein immunohistochemistry
- FKBP
FK506 binding protein
- FRB
FK506 rapamycin binding
- HNE
4-hydroxy-2-nonenal
Funding
This work was supported by a Cure Parkinson’s Trust Project Grant, a Parkinson’s UK Innovation Grant and the Monument Trust Discovery Award from Parkinson’s UK.
Supplementary material
Supplementary material is available at Brain online.
References
- Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE. Secondary structure of alpha-synuclein oligomers: characterization by raman and atomic force microscopy. J Mol Biol. 2006;355:63–71. doi: 10.1016/j.jmb.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Arima K, Ueda K, Sunohara N, Hirai S, Izumiyama Y, Tonozuka-Uehara H, et al. Immunoelectron-microscopic demonstration of NACP/alpha-synuclein-epitopes on the filamentous component of Lewy bodies in Parkinson’s disease and in dementia with Lewy bodies. Brain Res. 1998;808:93–100. doi: 10.1016/s0006-8993(98)00734-3. [DOI] [PubMed] [Google Scholar]
- Baan B, Pardali E, ten Dijke P, van Dam H. In situ proximity ligation detection of c-Jun/AP-1 dimers reveals increased levels of c-Jun/Fra1 complexes in aggressive breast cancer cell lines in vitro and in vivo. Mol Cell Proteomics. 2010;9:1982–90. doi: 10.1074/mcp.M110.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ. Alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–10. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Lavoie MJ, Petsko GA, Ringe D. Proteostasis and movement disorders: Parkinson’s disease and amyotrophic lateral sclerosis. Cold Spring Harb Perspect Biol. 2011;3:a007500. doi: 10.1101/cshperspect.a007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–11. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–98. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Celej MS, Sarroukh R, Goormaghtigh E, Fidelio GD, Ruysschaert JM, Raussens V. Toxic prefibrillar alpha-synuclein amyloid oligomers adopt a distinctive antiparallel beta-sheet structure. Biochem J. 2012;443:719–26. doi: 10.1042/BJ20111924. [DOI] [PubMed] [Google Scholar]
- Chen L, Jin J, Davis J, Zhou Y, Wang Y, Liu J, et al. Oligomeric alpha-synuclein inhibits tubulin polymerization. Biochem Biophys Res Commun. 2007;356:548–53. doi: 10.1016/j.bbrc.2007.02.163. [DOI] [PubMed] [Google Scholar]
- Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, et al. Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013;110:4087–92. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK. Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J Neurosci. 2012;32:3301–5. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, et al. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149:1048–59. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlapinski JL, Schelle MW, Miller LW, Laughlin ST, Kohler JJ, Cornish VW, et al. Conditional glycosylation in eukaryotic cells using a biocompatible chemical inducer of dimerization. J Am Chem Soc. 2008;130:13186–7. doi: 10.1021/ja8037728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN. Relationships between lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol. 1992;83:525–9. doi: 10.1007/BF00310030. [DOI] [PubMed] [Google Scholar]
- Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–32. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20:419–25. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol Aging. 2010;31:953–68. doi: 10.1016/j.neurobiolaging.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Forloni G. Neurotoxicity of beta-amyloid and prion peptides. Curr Opin Neurol. 1996;9:492–500. doi: 10.1097/00019052-199612000-00017. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Newell K, Irizarry MC, Sanders JL, Hyman BT. Alpha-synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol. 2000;99:352–7. doi: 10.1007/s004010051135. [DOI] [PubMed] [Google Scholar]
- Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ. Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J Biol Chem. 2002;277:48984–92. doi: 10.1074/jbc.M208194200. [DOI] [PubMed] [Google Scholar]
- Gruber S, Arumugam P, Katou Y, Kuglitsch D, Helmhart W, Shirahige K, et al. Evidence that loading of cohesin onto chromosomes involves opening of its SMC hinge. Cell. 2006;127:523–37. doi: 10.1016/j.cell.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Kamali-Moghaddam M, Pettersson FE, Wu D, Englund H, Darmanis S, Lord A, et al. Sensitive detection of Abeta protofibrils by proximity ligation–relevance for Alzheimer’s disease. BMC Neurosci. 2010;11:124. doi: 10.1186/1471-2202-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Adachi E, Orimo S, Nakamura A, Mizusawa H, Uchihara T. Pale neurites, premature alpha-synuclein aggregates with centripetal extension from axon collaterals. Brain Pathol. 2012;22:67–78. doi: 10.1111/j.1750-3639.2011.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuse O, Iseki E, Marui W, Kosaka K. Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies. J Neurol Sci. 2003;211:29–35. doi: 10.1016/s0022-510x(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Kim-Han JS, Antenor-Dorsey JA, O’Malley KL. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31:7212–21. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Cho MK, Kumar A, Maier E, Siebenhaar C, Becker S, et al. Structural properties of pore-forming oligomers of alpha-synuclein. J Am Chem Soc. 2009;131:17482–9. doi: 10.1021/ja9077599. [DOI] [PubMed] [Google Scholar]
- Kosaka K. Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol. 1978;42:127–34. doi: 10.1007/BF00690978. [DOI] [PubMed] [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–10. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Chi EY, Wood SJ, Kendrick BS, Li C, Garzon-Rodriguez W, et al. Oxidative dimer formation is the critical rate-limiting step for Parkinson’s disease alpha-synuclein fibrillogenesis. Biochemistry. 2003;42:829–37. doi: 10.1021/bi026528t. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Parkkinen L, Alafuzoff I. Morphogenesis of lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol. 2003;62:1241–53. doi: 10.1093/jnen/62.12.1241. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002a;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, et al. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002b;322:1089–102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee SJ. Characterization of cytoplasmic alpha-synuclein aggregates. Fibril formation is tightly linked to the inclusion-forming process in cells. J Biol Chem. 2002;277:48976–83. doi: 10.1074/jbc.M208192200. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012b;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136(Pt 4):1128–38. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol Clin. 2000;18:865–902. doi: 10.1016/s0733-8619(05)70230-9. [DOI] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277:19213–9. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. doi: 10.1016/s0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19:6845–57. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y, Yasuda T, Fukaya M, Yamamori S, Itakura M, Nihira T, et al. Accumulation of alpha-synuclein triggered by presynaptic dysfunction. J Neurosci. 2012;32:17186–96. doi: 10.1523/JNEUROSCI.2220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasstrom T, Fagerqvist T, Barbu M, Karlsson M, Nikolajeff F, Kasrayan A, et al. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of alpha-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic Biol Med. 2011;50:428–37. doi: 10.1016/j.freeradbiomed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Neumann M, Muller V, Kretzschmar HA, Haass C, Kahle PJ. Regional distribution of proteinase K-resistant alpha-synuclein correlates with Lewy body disease stage. J Neuropathol Exp Neurol. 2004;63:1225–35. doi: 10.1093/jnen/63.12.1225. [DOI] [PubMed] [Google Scholar]
- Nordengrahn A, Gustafsdottir SM, Ebert K, Reid SM, King DP, Ferris NP, et al. Evaluation of a novel proximity ligation assay for the sensitive and rapid detection of foot-and-mouth disease virus. Vet Microbiol. 2008;127:227–36. doi: 10.1016/j.vetmic.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, et al. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One. 2008;3:e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostaee A, Beaudoin S, Staskevicius A, Roucou X. Aggregation and neurotoxicity of recombinant alpha-synuclein aggregates initiated by dimerization. Mol Neurodegener. 2013;8:5. doi: 10.1186/1750-1326-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BJ, Lourenco-Venda L, Crabtree MJ, Hale AB, Channon KM, Wade-Martins R. Alpha-synuclein and mitochondrial bioenergetics regulate tetrahydrobiopterin levels in a human dopaminergic model of Parkinson disease. Free Radic Biol Med. 2013;67:58–68. doi: 10.1016/j.freeradbiomed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann J, Leijon M, Yacoub A, Schlingemann H, Zohari S, Matyi-Toth A, et al. Novel means of viral antigen identification: improved detection of avian influenza viruses by proximity ligation. J Virol Methods. 2010;163:116–22. doi: 10.1016/j.jviromet.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–43. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci USA. 2000;97:4897–902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–9. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Tavoosidana G, Ronquist G, Darmanis S, Yan J, Carlsson L, Wu D, et al. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci USA. 2011;108:8809–14. doi: 10.1073/pnas.1019330108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff JE, Putcha P, Outeiro TF, Ivanov A, Berezovska O, Hyman BT, et al. CHIP targets toxic alpha-Synuclein oligomers for degradation. J Biol Chem. 2008;283:17962–8. doi: 10.1074/jbc.M802283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278:44405–11. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–9. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, et al. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA. 2011;108:17797–802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibrecht I, Leuchowius KJ, Clausson CM, Conze T, Jarvius M, Howell WM, et al. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev Proteomics. 2010;7:401–9. doi: 10.1586/epr.10.10. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–9. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig I, Schagger H. Advantages and limitations of clear-native PAGE. Proteomics. 2005;5:4338–46. doi: 10.1002/pmic.200500081. [DOI] [PubMed] [Google Scholar]
- Yung HW, Charnock-Jones DS, Burton GJ. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One. 2011;6:e17894. doi: 10.1371/journal.pone.0017894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang NY, Tang Z, Liu CW. alpha-Synuclein protofibrils inhibit 26 S proteasome-mediated protein degradation: understanding the cytotoxicity of protein protofibrils in neurodegenerative disease pathogenesis. J Biol Chem. 2008;283:20288–98. doi: 10.1074/jbc.M710560200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.