Abstract
Humans do not generally walk at constant speed, except perhaps on a treadmill. Normal walking involves starting, stopping and changing speeds, in addition to roughly steady locomotion. Here, we measure the metabolic energy cost of walking when changing speed. Subjects (healthy adults) walked with oscillating speeds on a constant-speed treadmill, alternating between walking slower and faster than the treadmill belt, moving back and forth in the laboratory frame. The metabolic rate for oscillating-speed walking was significantly higher than that for constant-speed walking (6–20% cost increase for ±0.13–0.27 m s−1 speed fluctuations). The metabolic rate increase was correlated with two models: a model based on kinetic energy fluctuations and an inverted pendulum walking model, optimized for oscillating-speed constraints. The cost of changing speeds may have behavioural implications: we predicted that the energy-optimal walking speed is lower for shorter distances. We measured preferred human walking speeds for different walking distances and found people preferred lower walking speeds for shorter distances as predicted. Further, analysing published daily walking-bout distributions, we estimate that the cost of changing speeds is 4–8% of daily walking energy budget.
Keywords: legged locomotion, walking, acceleration, preferred speeds, metabolic cost, energy optimality
1. Introduction
Walking in typical human life requires changing speeds. Most daily walking appears to happen in short bouts [1], starting and ending at rest. Here, to better understand such behaviour, we measure the metabolic cost of changing walking speeds. Although much is known about constant-speed walking [2,3], the cost of changing speeds has not been measured without non-inertial treadmill speed changes or step-frequency control [4]. Here, we show that the cost of changing speed is significant and an appreciable fraction of daily walking energy budget. This cost may have behavioural implications: we predict lower optimal walking speeds for short distances; we then measured and found that our subjects prefer lower speeds for shorter distances.
2. Material and methods
(a). Experiment: metabolic cost of oscillating-speed walking
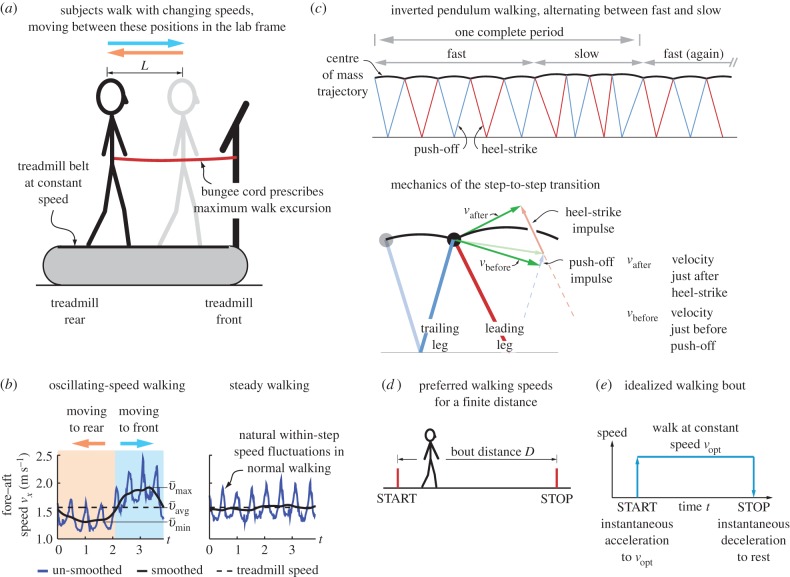
Subjects (N = 16, 12 males and 4 females, 23.25 ± 2.1 years, height 177.08 ± 7.4 cm, mass 75.99 ± 12.94 kg, mean ± s.d.) performed both ‘steady' (constant-speed) and ‘oscillating-speed' walking trials. Oscillating walking speeds were achieved on a constant-speed treadmill by alternately walking faster and slower than the belt (figure 1a). Two distinct audible tones of durations Tfwd and Tbck alternated in a loop indicating whether the subjects should move towards the treadmill front or rear. We used three (Tfwd,Tbck) combinations, (1.9,1.9) s, (2.8,2.8) s and (1.9,2.8) s, obtaining different speed fluctuations. We instructed subjects to walk between fixed positions on the treadmill (0.48 m apart) giving mean excursion length L = 0.41 ± 0.08 m (figure 1a). The subjects obeyed the imposed back-and-forth time period constraints: mean periods differed from prescribed periods by 0.97 ± 0.24%. While humans do not usually walk with oscillating speeds, this protocol was designed to isolate the cost of changing speed.
Figure 1.
Experimental protocols and theoretical models. (a) Subject walking with oscillating speeds on a constant-speed treadmill, walking faster and slower than the belt, moving between two prescribed positions. A longitudinal bungee cord (never to be made taut) constrains the rear-most position and a bungee cord perpendicular to sagittal plane (not shown, never to be touched) constrains the forward-most position. (b) Sacral marker fore–aft velocities, original and smoothed. (c) A nine-step periodic inverted pendulum walking motion, with five steps faster and four steps slower than the mean speed. Initial and final stance-leg directions are shown for each step (red and blue). Details of one step-to-step transition are shown; downward velocity at the end of one step is redirected by push-off and heel-strike impulses. (d) Measuring preferred walking speeds as a function of bout distance D; subjects start and stop at rest. (e) An idealized bout: human travels the whole distance D at single speed vopt, starting and stopping instantaneously.
Oscillating-speed trials were at one or both constant treadmill speeds 1.12 and 1.56 m s−1 (equal to the mean speeds): 10 subjects at both speeds, four subjects at 1.12 m s−1 only and two at 1.56 m s−1 only, with random speed order. Steady walking trials were performed at speeds ranging from 0.89 to 1.78 m s−1, including 1.12 and 1.56 m s−1.
Metabolic rate per unit mass (W kg−1) was estimated using respirometry (Oxycon Mobile), approximated as 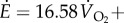
 (
(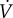 in ml kg−1 s−1), denoted
in ml kg−1 s−1), denoted 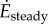 and
and 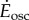 for steady- and oscillating-speed trials, respectively. Trials lasted 7 min: 4 min to reach metabolic steady state and 3 min to estimate the mean metabolic rate. The speed oscillation periods (3.8–5.6 s) are much smaller than typical metabolic time-constants (30 s), so our metabolic steady state is nominally constant. A sacral marker's motion was measured with marker-based motion capture.
for steady- and oscillating-speed trials, respectively. Trials lasted 7 min: 4 min to reach metabolic steady state and 3 min to estimate the mean metabolic rate. The speed oscillation periods (3.8–5.6 s) are much smaller than typical metabolic time-constants (30 s), so our metabolic steady state is nominally constant. A sacral marker's motion was measured with marker-based motion capture.
(b). Experiment: preferred walking speed
Subjects (N = 10) were asked to walk ten distances (D = 0.5, 1, 2, 4, 6, 8, 10, 12, 14 and 89 m) at a comfortable speed, starting and ending at rest (figure 1d). We had three trials per distance, all trials in random order, but performed 0.5–1 m trials separately.
(c). Model 1: kinetic energy fluctuations
In this model, we attribute the metabolic cost increase for oscillating-speed walking over steady walking to fore–aft kinetic energy fluctuations beyond what happens within each step in constant-speed walking. Figure 1b shows fore–aft velocity vx(t) of the sacral marker for oscillating-speed and steady walking, approximating centre of mass motion. Smoothing vx(t) with an averaging window equal to step period gives 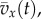 removing within-step speed fluctuations (figure 1b). The mass-normalized metabolic cost increase for oscillating-speed walking over steady walking due to the kinetic energy fluctuations for each cycle is modelled as
removing within-step speed fluctuations (figure 1b). The mass-normalized metabolic cost increase for oscillating-speed walking over steady walking due to the kinetic energy fluctuations for each cycle is modelled as 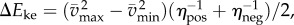 where
where 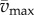 and
and 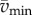 are maximum and minimum smoothed fore–aft speeds for that cycle and ηpos = 0.25 and ηneg = 1.2 are typical positive and negative muscle work efficiencies [5]. The model-predicted metabolic rate increase
are maximum and minimum smoothed fore–aft speeds for that cycle and ηpos = 0.25 and ηneg = 1.2 are typical positive and negative muscle work efficiencies [5]. The model-predicted metabolic rate increase 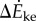 for each oscillating-speed trial was the median ΔEke/Tperiod over all cycles.
for each oscillating-speed trial was the median ΔEke/Tperiod over all cycles.
(d). Model 2: inverted pendulum walking
We consider inverted pendulum walking of a point-mass biped, for which the total walking metabolic cost is the sum of (i) a step-to-step transition cost (described below) and (ii) a leg-swing cost [6]. Using numerical optimization, we found the multi-step-periodic inverted pendulum walking motion (e.g. figure 1c) satisfying our oscillating-speed experimental constraints and minimizing this metabolic cost. Here, we derived and used expressions for step-to-step transition cost for non-constant-speed walking, generalizing previous constant-speed expressions (see electronic supplementary information). This step-to-step cost accounts for the push-off and heel-strike work to redirect the centre of mass velocity during the step-to-step transition (figure 1c), and depends on leg-angles and centre of mass velocities. The model prediction 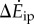 is the difference between the optimal oscillating-speed and constant-speed costs at the same mean speed. A metabolic cost term proportional to the integral of leg forces contributed almost equally to oscillating-speed and constant-speed walking costs and did not contribute to their difference (see the electronic supplementary material for details).
is the difference between the optimal oscillating-speed and constant-speed costs at the same mean speed. A metabolic cost term proportional to the integral of leg forces contributed almost equally to oscillating-speed and constant-speed walking costs and did not contribute to their difference (see the electronic supplementary material for details).
3. Results
(a). Metabolic rate of oscillating-speed walking
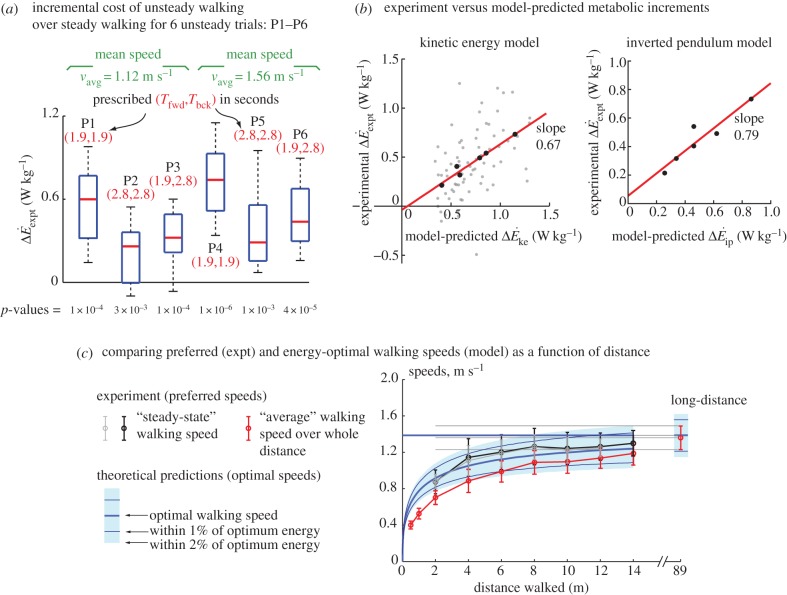
Metabolic rates for all six oscillating-speed trials (P1–P6, figure 2a) were significantly higher than the corresponding steady-state costs. Metabolic rate increment over constant-speed walking 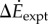 was significantly greater than zero for all trials (one-sample t-test, for all p < 2 × 10−3, figure 2a). Oscillating-speed trials with higher speed fluctuations had higher metabolic rates with one exception (P1 > P3 > P2, P4 > P6 and P4 > P5, all p < 0.02).
was significantly greater than zero for all trials (one-sample t-test, for all p < 2 × 10−3, figure 2a). Oscillating-speed trials with higher speed fluctuations had higher metabolic rates with one exception (P1 > P3 > P2, P4 > P6 and P4 > P5, all p < 0.02).
Figure 2.
(a) Difference 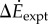 between oscillating- and constant-speed walking metabolic rates for six oscillating-speed trials (P1–P6): three (Tfwd,Tbck) combinations and two mean speeds. Box plot shows median (red bar), 25–75th percentile (box), and 10–90th percentile (whiskers); p-values use one-sided t-tests for the alternative hypothesis that metabolic rate differences are from a distribution with greater-than-zero mean. (b)
between oscillating- and constant-speed walking metabolic rates for six oscillating-speed trials (P1–P6): three (Tfwd,Tbck) combinations and two mean speeds. Box plot shows median (red bar), 25–75th percentile (box), and 10–90th percentile (whiskers); p-values use one-sided t-tests for the alternative hypothesis that metabolic rate differences are from a distribution with greater-than-zero mean. (b) 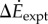 compared with kinetic energy model
compared with kinetic energy model 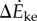 and inverted pendulum model
and inverted pendulum model 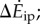 we show experimental and model means (black filled circles), the best-fit line (red, solid) and all subjects' trials (scatter plot, grey dots); no scatter plot for inverted pendulum model as it produces only one prediction per trial. (c) Distance-dependence of the model-based energy-optimal walking speeds (blue, solid) and experimentally measured preferred speeds (red and black error bars). Ranges of model-based energy-optimal speeds within 1% (blue line, thin) and 2% (blue band) of optimal energy cost are shown. We show whole-bout ‘average' speeds (red) and ‘steady-state' speeds over middle 1.42 m (black, thick), indistinguishable from over middle 0.75 m (grey, thin). Average preferred speeds for 0.5–14 m trials were significantly lower than that for the 89 m trial (paired t-test, p < 0.01); similarly, the ‘steady-state' speeds for 2–6 m were significantly lower than that for 89 m (paired t-test, p < 0.04).
we show experimental and model means (black filled circles), the best-fit line (red, solid) and all subjects' trials (scatter plot, grey dots); no scatter plot for inverted pendulum model as it produces only one prediction per trial. (c) Distance-dependence of the model-based energy-optimal walking speeds (blue, solid) and experimentally measured preferred speeds (red and black error bars). Ranges of model-based energy-optimal speeds within 1% (blue line, thin) and 2% (blue band) of optimal energy cost are shown. We show whole-bout ‘average' speeds (red) and ‘steady-state' speeds over middle 1.42 m (black, thick), indistinguishable from over middle 0.75 m (grey, thin). Average preferred speeds for 0.5–14 m trials were significantly lower than that for the 89 m trial (paired t-test, p < 0.01); similarly, the ‘steady-state' speeds for 2–6 m were significantly lower than that for 89 m (paired t-test, p < 0.04).
(b). Model predictions
Both the kinetic energy fluctuation model 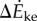 and the inverted pendulum model
and the inverted pendulum model 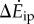 were correlated with measured metabolic rate increments
were correlated with measured metabolic rate increments 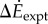 (figure 2b). The kinetic energy model and experimental costs are best-fitted by the line:
(figure 2b). The kinetic energy model and experimental costs are best-fitted by the line: 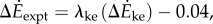 with λke = 0.67 whether we use trial means (R2 = 0.96, 95% CI of λke = 0.48–0.86) or all data (R2 = 0.24, 95% CI of λke = 0.39–0.95). Similarly, the inverted pendulum model and experimental costs are best-fitted by
with λke = 0.67 whether we use trial means (R2 = 0.96, 95% CI of λke = 0.48–0.86) or all data (R2 = 0.24, 95% CI of λke = 0.39–0.95). Similarly, the inverted pendulum model and experimental costs are best-fitted by 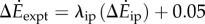 with λip = 0.79 (R2 = 0.88, 95% CI of λip = 0.39–1.19).
with λip = 0.79 (R2 = 0.88, 95% CI of λip = 0.39–1.19).
(c). Daily energy budget for starting and stopping
Humans mostly walk in short bouts [1]. For simplicity, we idealize a bout of distance D and mean speed v as instantaneously accelerating from rest to speed v, walking at constant speed v and stopping instantaneously at time D/v (figure 1e). The total metabolic energy per unit mass Ebout(D,v) for this idealized bout has two components: (i) a starting and stopping cost, extrapolating from the kinetic energy model, 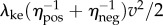 and (ii) a cost for steady-state walking at speed v, given by
and (ii) a cost for steady-state walking at speed v, given by 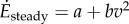 with a = 2.22 W kg−1 and b = 1.15 W kg−1 (m s–1)−2 [2], so that
with a = 2.22 W kg−1 and b = 1.15 W kg−1 (m s–1)−2 [2], so that 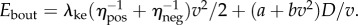 Applying this model to data in [1] with
Applying this model to data in [1] with 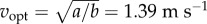 and the 95% CI of λke suggests that starting–stopping costs are 4–8% of daily walking energy expenditure (electronic supplementary material); this cost fraction (4–8%) may apply primarily to the subject population of [1], adults working in offices, but could be estimated for other populations given the distribution of their daily walking bout lengths.
and the 95% CI of λke suggests that starting–stopping costs are 4–8% of daily walking energy expenditure (electronic supplementary material); this cost fraction (4–8%) may apply primarily to the subject population of [1], adults working in offices, but could be estimated for other populations given the distribution of their daily walking bout lengths.
(d). Optimal and preferred walking speeds are lower for shorter distances
For the idealized bout of distance D (figure 1e), the energy-optimal walking speed vopt that minimizes Ebout(D,v) is given by the implicit function: 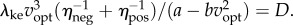 This metabolically optimal speed increases with distance D, approaching
This metabolically optimal speed increases with distance D, approaching 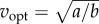 for large distances (figure 2c).
for large distances (figure 2c).
As predicted by the distance-dependence of optimal walking speeds, preferred human walking speeds in our experiment, both ‘average' and ‘steady-state' speeds, increased with distance (figure 2c). ‘Average’ preferred speed is the mean speed over the whole bout; a proxy for the ‘steady-state' preferred speed is the mean over the bout's middle 0.75 m (indistinguishable from averaging the middle 1.4 m). Model-predicted optimal speeds have a 0.96 correlation coefficient (Pearson's) with experimental steady-state preferred speeds, which were within 1–2% optimal cost. Our subjects could accelerate to higher mean or steady-state speeds, but they preferred not to. Therefore, the time taken to accelerate–decelerate cannot explain lower speeds for shorter distances.
4. Discussion
We have shown that oscillating-speed walking costs more than constant-speed walking. These cost-increments are correlated with kinetic energy fluctuation and inverted pendulum model predictions; inverted pendulum model predictions were closer to experimental values (regression slope closer to 1), perhaps because the kinetic energy model ignores walking mechanics. The cost of changing speeds implies lower energy-optimal speeds for shorter distances, reflected in our preferred speed experiments here and previous amputee data [7].
Preferred walking speeds are used to quantify mobility and rehabilitation [8], so bout distances should be chosen to avoid artificially lowering speeds. Using the cost of changing speeds may improve daily activity tracking, energy balance estimations for obesity, and metabolic estimations during sports (e.g. soccer [9]).
A previous experiment [4] considered walking with greater speed fluctuations (±0.15 to ±0.56 m s−1) than our study (±0.13 to ±0.27 m s−1) and similar kinetic energy fluctuations (electronic supplementary material), and found significant cost increase over steady walking for their highest speed fluctuations. However, this study [4] required walking on an oscillating-speed treadmill belt or controlling step durations in overground walking (derived from oscillating-speed treadmill trials). An oscillating-speed treadmill, being a non-inertial frame (in contrast to a constant-speed treadmill), can perform mechanical work, and is not mechanically equivalent to overground oscillating-speed walking (as noted in [4]). Further, prescribing step durations to control overground speed fluctuations is different from prescribing speed fluctuations directly [10].
Future work could involve overground experiments (say by having subjects follow a laser projection [11]), detailed biped and metabolic cost models (including muscle force and history dependence), using different speed fluctuations and measuring metabolic cost while subjects alternate between walking, stopping and starting (being directly applicable to walking bouts, relying less on extrapolation).
Supplementary Material
Ethics
The Ohio State University's IRB approved the experiments. Subjects gave informed consent.
Data accessibility
Data available through Dryad (http://dx.doi.org/10.5061/dryad.15v26).
Authors' contributions
N.S. performed all experiments, analysis and modelling, partly in discussion with M.S. M.S. performed some analyses. N.S. and M.S. wrote the paper.
Competing interests
We declare we have no competing interest.
Funding
This work was supported by NSF grant no. 1254842.
References
- 1.Orendurff MS, Schoen JA, Bernatz GC, Segal AD, Klute GK. 2008. How humans walk: bout duration, steps per bout, and rest duration. J. Rehabil. Res. Dev. 45, 1077–1089. ( 10.1682/JRRD.2007.11.0197) [DOI] [PubMed] [Google Scholar]
- 2.Bobbert AC. 1960. Energy expenditure in level and grade walking. J. Appl. Physiol. 15, 1015–1021. [Google Scholar]
- 3.Srinivasan M. 2009. Optimal speeds for walking and running, and walking on a moving walkway. Chaos 19, 026112 ( 10.1063/1.3141428) [DOI] [PubMed] [Google Scholar]
- 4.Minetti AE, Ardigò LP, Capodaglio EM, Saibene F. 2001. Energetics and mechanics of human walking at oscillating speeds. Am. Zool. 41, 205–210. ( 10.1093/icb/41.2.205) [DOI] [Google Scholar]
- 5.Margaria R. 1976. Biomechanics and energetics of muscular exercise. Oxford, UK: Clarendon Press. [Google Scholar]
- 6.Srinivasan M. 2011. Fifteen observations on the structure of energy-minimizing gaits in many simple biped models. J. R. Soc. Interface 8, 74–98. ( 10.1098/rsif.2009.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klute GK, Berge JS, Orendurff MS, Williams RM, Czerniecki JM. 2006. Prosthetic intervention effects on activity of lower-extremity amputees. Arch. Phys. Med. Rehabil. 87, 717–722. ( 10.1016/j.apmr.2006.02.007) [DOI] [PubMed] [Google Scholar]
- 8.Lamontagne A, Fung J. 2004. Faster is better: implications for speed-intensive gait training after stroke. Stroke 35, 2543–2548. ( 10.1161/01.STR.0000144685.88760.d7) [DOI] [PubMed] [Google Scholar]
- 9.Osgnach C, Poser S, Bernardini R, Rinaldo R, Di Prampero PE. 2010. Energy cost and metabolic power in elite soccer: a new match analysis approach. Med. Sci. Sports Exerc. 42, 170–178. ( 10.1249/MSS.0b013e3181ae5cfd) [DOI] [PubMed] [Google Scholar]
- 10.Bertram JEA, Ruina A. 2001. Multiple walking speed-frequency relations are predicted by constrained optimization. J. Theor. Biol. 209, 445–453. ( 10.1006/jtbi.2001.2279) [DOI] [PubMed] [Google Scholar]
- 11.Minetti AE, Gaudino P, Seminati E, Cazzola D. 2012. The cost of transport of human running is not affected, as in walking, by wide acceleration/deceleration cycles. J. Appl. Physiol. 114, 498–503. ( 10.1152/japplphysiol.00959.2012) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available through Dryad (http://dx.doi.org/10.5061/dryad.15v26).