Abstract
There is increasing evidence that a low vitamin D status may be an important and hitherto neglected factor of cardiovascular disease. This review is an overview of the current body of literature, and presents evidence of the mechanisms through which vitamin D deficiency affects the cardiovascular system in general and the heart in particular. Available data indicate that the majority of congestive heart failure patients have 25-hydroxyvitamin D deficiency. Furthermore, the low serum 25-hydroxyvitamin D level has a higher impact on hypertension, coronary artery disease an on the occurrence of relevant cardiac events. A serum 25-hydroxyvitamin D level below 75 nmol/l (30 ng/l) is generally regarded as vitamin D insufficiency in both adults and children, while a level below 50 nmol/l (20 ng/l) is considered deficiency. Levels below 50 nmol/l (20 ng/l) are linked independently to cardiovascular morbidity and mortality.
Keywords: Vitamin D deficiency, Congestive heart failure, Coronary artery disease, Cardiovascular outcomes
Abbreviations
- 25(OH) D
25-hydroxyvitamin D
- CAD
coronary artery disease
- HTN
hypertension
- CHF
congestive heart failure
- VDR
vitamin D receptor
- RAAS
renin-angiotensin-aldosterone system
- IL
interleukin
- TNF
tumor necrosis factor
- NYHA
New York Heart Association
- NT pro-BNP
N-terminal of the prohormone brain natriuretic peptide
- NT-proANP
N-terminal of the prohormone atrial natriuretic peptide
- LVEF
left ventricle ejection fraction
- LURIC
Ludwigshafen Risk and Cardiovascular Health (LURIC) Study
- HR
hazard ratio
- OR
Odds Ratio
- RR
Relative Ratio
Introduction
Vitamin D deficiency is widely prevalent in the United States and worldwide [1]. Low levels of 25-hydroxy vitamin D [25(OH) D], the principal circulating storage form of vitamin D, are present in one third to one half of otherwise healthy middle-aged to elderly adults [1–3]. Although most consequences of vitamin D deficiency involve the musculoskeletal system, there is a growing body of evidence suggesting that low levels of vitamin D may adversely affect the cardiovascular system [4]. A serum 25-hydroxyvitamin D level below 75 nmol/l (30 ng/l) is generally regarded as vitamin D insufficiency in both adults and children, while a level below 50 nmol/l (20 ng/l) is considered deficiency in both populations [5,6]. Vitamin D deficiency, which is affected by multiple factors (Table 1), appears to have an association with diverse cardiac diseases starting with its direct effect on the cardiac cell, its association with coronary artery disease (CAD), and its risk factors such as diabetes and hypertension (HTN); ending at last and probably not least in its relation with congestive heart failure (CHF). Similarly, there is some evidence that links vitamin deficiency to increased risk of stroke.
Table 1.
Examples of some causes of vitamin D deficiency or resistance.
| Causes of vitamin D deficiency or resistance | |
| Deficient intake or absorption |
|
| Defective 25-hydroxylation |
|
| Loss of vitamin D binding protein |
|
| Defective 1-alpha 25-hydroxylation |
|
| Defective target organ response to calcitriol |
|
This article is an overview of the current data describing the effect of vitamin D on the cardiac cell and the relationship of its deficiency with HTN, CAD, and heart failure. We compare vitamin D to its clinical and laboratory markers as well as to clinical outcomes and cardiac events. Finally, we explore the impact of vitamin D deficiency on cardiovascular outcomes including death, cardiac death, stroke, and myocardial infarction.
Methods
Relevant studies were identified through electronic searches of MEDLINE and the databases of the Cochrane Central Register of Controlled Trials. The search used the terms “cardiovascular disorders,” “cardiovascular disease,” “hypertension,” “coronary artery disease,” or “congestive heart failure” paired with “vitamin D deficiency” and “calcitriol”. In addition, we searched bibliographies of relevant studies, reviews and editorial letters between 1990 and 2015 for articles in English. We reviewed the references to identify the studies addressing the role of vitamin D hemostasis in the pathogenesis of cardiovascular disease, and the impact of vitamin D deficiency and supplementation on HTN, CAD, CHF, and cardiovascular outcomes. Although human studies were of interest in regards to outcomes, many animal model studies were included for the purpose of the pathogenesis of vitamin D deficiency role in cardiovascular disease. Out of 3026 articles, 88 were identified as meeting our criteria and were reviewed. Of those 88, 43 were used in the final version of this article.
Vitamin D deficiency and the pathogenesis of cardiovascular disease
The contractile properties of cardiac cells are mainly controlled by the direct interaction between calcium, the contractile proteins, actin and myosin, and the intracellular handling of calcium. The extracellular calcium homeostasis affected by vitamin D levels impact the intracellular calcium and can indirectly influence cardiac cell contractility [7].
Calcitriol, the active vitamin D form (1,25-dihydroxyvitamin D), impacts the function of almost all body cells including cardiac cells, endothelial cells, and vascular smooth muscle cells through the cytosolic vitamin D receptor (VDR). This impact varies among these cells but is crucial in cardiac cells as they depend solely on the circulating blood concentration of calcitriol secondary to the absence of an enzymatically active 25-hydroxyvitamin D-1 [alpha]-hydroxylase system [8,9]. The absence of VDR receptors, therefore, has many adverse effects on cardiac cells. Isolated cardiac cells from VDR knockout mice are noted to have accelerated rates of contraction and relaxation that are not affected by calcitriol levels when compared to control mice [10,11]. Furthermore, cellular hypertrophy of heart myofibrils resulting in cardiomegaly was noticed in VDR knockout mice [12]. This can be explained by the fact that tissue inhibitors of matrix metalloproteinases were significantly under-expressed in VDR knockout mice compared to control mice [12]. Without this inhibition of matrix metalloproteinases, the extracellular matrix remodeling mediated by matrix metalloproteinases leads to progressive left ventricular remodeling, dilation, and heart failure.
Calcitriol, through VDR receptors, has significant impact on the morphology, proliferation, and growth of cardiac cells. Treatment with calcitriol increases the expression of the cardiac muscle protein, myotrophin, reduces expression of atrial natriuretic peptide, and increases expression and nuclear localization of the VDR in these cells [11,13]. Vitamin D3 deficiency in rats was associated with significant hypocalcaemia, an increase in plasma parathyroid hormone, a reduction in the size of myofibrils, and accumulation of collagen fibers, resulting in interstitial fibrosis [14]. However, calcitriol supplementation may prevent the development of cardiac hypertrophy. In rats with spontaneously hypertensive heart failure, calcitriol treatment resulted in lower heart weight, myocardial collagen levels, left ventricular diameters, and cardiac output compared with untreated rats [11].
VDR receptors have different genotypes. A recent study evaluated the association between VDR gene variants and ischemic stroke in an Asian Indian population. Among the three single nucleotide polymorphisms of the VDR gene that were genotyped using PCR-RFLP method, and studied for the association (Fok I, Apa I, and Taq I), both Apa I and Taq I polymorphisms were not found to be associated with ischemic stroke. However, presence of ff genotype of Fok I was found to confer 2.97-fold risk of ischemic stroke (95% CI = 1.16–7.63, p = 0.02) as compared to FF genotype of Fok I. This association was found to be independent of various demographic and important biochemical covariates including age, gender, smoking, alcohol intake, BMI, and serum glucose, lipid profile, insulin, 25-hydroxyvitamin D, and plasma nitric oxide levels [OR = 2.27, 95% CI = 1.25–4.09, p = 0.01] [15].
Vitamin D deficiency and hypertension
HTN is associated with inappropriate stimulation of the renin-angiotensin-aldosterone system (RAAS). Calcitriol is known to be one of the negative endocrine regulators of RAAS [16,17]. In VDR receptor knockout mice, HTN was more prevalent, probably secondary to the stimulation of RAAS as it was corrected by angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists [16,17]. In several trials, calcitriol supplementation was shown to reduce plasma renin activity, angiotensin II levels, blood pressure, and myocardial hypertrophy [16,18]. It has been demonstrated that regular exposure to ultraviolet B radiation increases circulating 25(OH) D above a level of 100 nmol/l and significantly reduces blood pressure by approximately 6 mmHg in hypertensive patients with initial 25(OH) D levels of 26 nmol/l within an intervention period of six weeks [19]. In another study, vitamin D deficient elderly women who were supplemented with calcium and 20 μg vitamin D3 daily had an increase in serum 25(OH) D of 20 nmol/l (p < 0.01), a decrease in serum parathyroid hormone of 17% (p < 0.05), a decrease in systolic blood pressure of 9.3% (p < 0.025), and a decrease in heart rate of 5.4% (p < 0.025) compared to those who were supplemented with calcium alone [20]. Therefore, a normal level of circulating calcitriol is crucial not only for calcium homeostasis, but also for the homeostasis of electrolytes, volume, and blood pressure.
Vitamin D deficiency and coronary artery disease
Many studies show an association of vitamin D deficiency with well-established atherosclerosis risk factors (Fig. 1), including obesity, glucose intolerance, metabolic syndrome, HTN, and hyperlipidemia [21–23]. The Third National Health and Nutrition Examination Survey, a cross-sectional representative sample of the U.S. population, also showed a significant increase in the prevalence of HTN [OR = 1.30 (1.13–1.49); p < 0.001], diabetes mellitus [OR = 1.73 (1.38–2.16); p < 0.001], obesity [OR = 2.29 (1.99–2.63); p < 0.001], and hypertriglyceridemia [OR = 1.47 (1.30–1.65); p < 0.001] in patients with vitamin D levels of less than 21 ng/mL compared to those with levels higher than 37 ng/mL (p < 0.001).[23] That association was further supported by a recent study from the University of Utah that linked vitamin D deficiency to an increased risk of hyperlipidemia (HR = 1.12, p = 0.002), diabetes (HR = 1.33, p < 0.0001), and HTN (HR = 1.26, p < 0.0001) in vitamin D deficient individuals [22].
Figure 1.
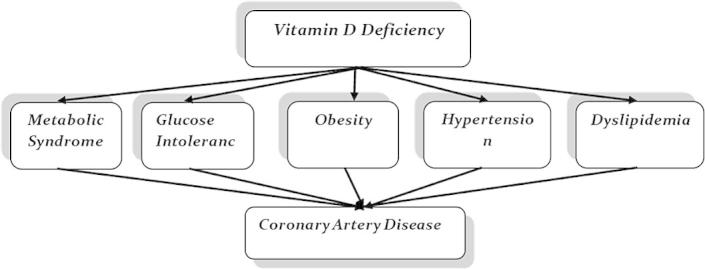
The relationship between vitamin D and CAD risk factors.
The presumed role of vitamin D in the pathogenesis of CAD is not necessarily linked only to atherosclerosis, but may involve vascular calcification. The basis of this presumption comes from patients with end-stage renal disease. As a consequence of reduced renal calcitriol synthesis, in addition to other factors, secondary hyperparathyroidism emerges in the early stages of chronic kidney disease [24]. In patients with end-stage renal disease, secondary hyperparathyroidism is considered an important risk factor in the pathogenesis of CAD leading to vascular calcification [25]. In hemodialysis patients, the use of active vitamin D and synthetic vitamin D analogs has been shown to reduce the risk of death from cardiovascular disease [26]. In the general population, the presence of vascular calcification is a predictor of poorer five-year survival rates [27]. Interestingly, the use of calcitriol was found to be inversely correlated with the extent of vascular calcification, independently from other risk factors for ischemic heart disease [28].
However, whether enough evidence exists to consider vitamin D deficiency as an independent risk factor for cardiovascular diseases is debatable. It looks like other confounding factors may impact this association, such as HTN and gender.
In a nonrandomized prospective study of 1739 Framingham Offspring Study participants without prior cardiovascular disease, individuals with low 25(OH) D levels and defined as: <37.5 nmol/l had a higher incident of a composite of myocardial infarction, coronary insufficiency, and heart failure [adjusted hazard ratio (HR) = 1.62; (1.11–2.36); p = 0.01] compared with those with normal levels. However, this effect was evident only in hypertensive participants [HR = 2.13; (1.30–3.48)] and not in the normotensive ones [HR = 1.04, (0.55–1.96)] [29].
Similarly, gender seems to play a role on the impact that vitamin D deficiency has on CAD. While men with low 25(OH) D levels (⩽37.5 nmol/l) had a higher risk [relative risk RR = 2.09 (1.24–3.54)] of myocardial infarction compared to men with sufficient levels (⩽75 nmol/l) after adjustment for various lifestyle and other risk factors, this trend was not the same for women [30].
Nonetheless, a recent study showed that vitamin D levels could be independently linked to the risk of cardiovascular disease. In a cohort of 14,641 men and women living in Norfolk, United Kingdom, aged 42–82, during the period between 1997 and 2000, and who were followed up to 2012, an increase of 20-nmol/L 25(OH) D levels was associated with HR of 0.92 ([0.88–0.96]; p < 0.001) for total mortality and 0.96 ([0.93–0.99]; p = 0.014) for prevalence of cardiovascular disease, even after adjustment for multiple variants in both men and women [31].
Vitamin D deficiency and heart failure
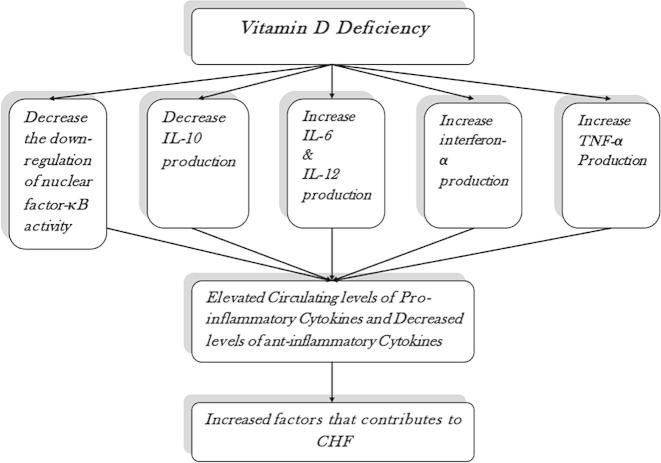
There is increasing evidence that vitamin D deficiency may be an important factor in the pathogenesis of heart failure [17]. Vitamin D deficiency seems to be linked to various mechanisms that play a significant role in the pathogenesis of CHF [32]. These mechanisms include the activation of RAAS [16,33], the presence of oxidative stress in different tissues including the skin, skeletal muscles, heart, and peripheral blood mononuclear cells [34], and activation of pro-inflammatory cytokines as interleukin (IL)-8 and tumor necrosis factor (TNF)-alpha [35].
Vitamin D suppresses the pro-inflammatory state by downregulating nuclear factor-κB activity and decreasing IL-6, IL-12, interferon-γ, and TNF-α production [36]. At the same time, it increases anti-inflammatory cytokines [36]. Vitamin D deficiency therefore leads to a reduction in the downregulation of nuclear factor-κB activity, a decrease in IL-10 production, and an increase in IL-6, IL-12, interferon-γ, and TNF-α production, all of which lead to the pro-inflammatory state of CHF (Fig. 2) [36].
Figure 2.
Vitamin D deficiency role in the pathogenesis of congestive heart failure.
The level of vitamin D is linked to many of the clinical and laboratory parameters of CHF, including the New York Heart Association (NYHA) functional classifications, NT pro-BNP (N-terminal of the prohormone brain natriuretic peptide), NT-proANP (N-terminal of the prohormone atrial natriuretic peptide) and even LVEF (left ventricle ejection fraction); (Fig. 3). In the Ludwigshafen Risk and Cardiovascular Health (LURIC) prospective cohort study of 3299 consecutive male and female patients scheduled for coronary angiography, the levels of NT-pro-BNP was significantly inversely correlated with those of 25(OH) D (correlation coefficient R = −0.190; p < 0.001) and calcitriol (R = −0.253; p < 0.001) [37]. Even after multivariable adjustments for multiple potential confounding factors, the inverse correlation of NTpro-BNP with 25(OH) D and calcitriol levels was still observed (β coefficient = −0.082; p < 0.001) and (β coefficient = −0.180; p < 0.001) respectively [37,38]. Similarly, in CHF patients, the levels of 25(OH) D and calcitriol were also inversely correlated with NT-proANP (r2 = 0.16; p < 0.001 and r2 = 0.12; p < 0.01, respectively) [39].
Figure 3.
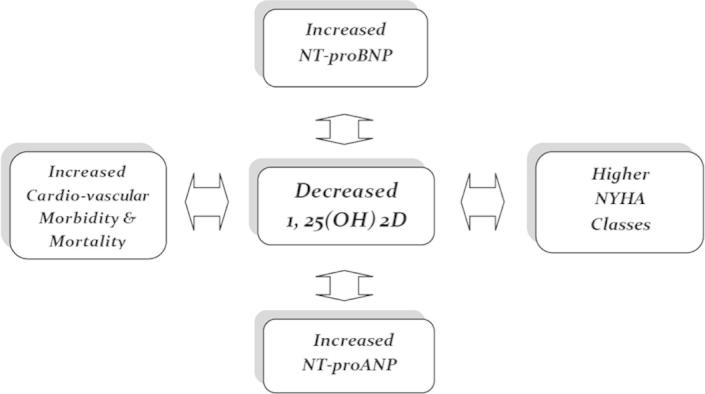
The relationship between vitamin D levels and the clinical and laboratory parameters of congestive heart failure.
Further, vitamin D level was linked to left ventricle function. 25(OH) D and calcitriol levels were inversely correlated with impaired left ventricle function (p < 0.001 for both), and this association remained significant after multivariable adjustments (p < 0.001 for both) [37]. Similarly, higher NYHA classes were also associated with lower levels of 25(OH) D and calcitriol (p < 0.001 for both) [37].
On the other hand, a recent study that followed 3731 men aged 60–79 years prospectively with no prevalent heart failure and followed up for a mean period of 13 years showed that the risk of developing heart failure was linked to Parathyroid Hormone (PTH) level rather than those of 25(OH) D. Elevated PTH (⩾55.6 pg/mL) was associated with significantly higher risk of incident HF after adjustment for lifestyle characteristics and comorbidities (HR = 1.66; [1.30–2.13]). In contrast, the correlation between 25(OH) D levels and incident HF was not significant [40].
Vitamin D deficiency and stroke
As mentioned earlier, vitamin D deficiency is linked to many of the well-established risk factors for atherosclerosis and ischemic stroke, including diabetes, HTN, and hyperlipidemia [21–23]. Furthermore, a recent study linked some genotypes of VDR to increased risk of stroke [15]. Vitamin D deficiency also appears to be associated with increased risk of chronic cerebral small vessel disease [41]. A recent study of 59 consecutive patients with acute ischemic stroke or transient ischemic attack who underwent magnetic resonance imaging of the brain showed that 25(OH) D levels below 25 nmol/L was associated with lacunes (regression coefficient, 0.5; 95% CI, 0.04–0.95), severe white matter hyperintensity (OR = 2.74; [1.31–6.45]), and deep cerebral microbleeds (OR = 1.68; [1.03–2.78]) [41].
The impact of vitamin D level on cardiovascular outcomes
Vitamin D deficiency was linked to increased risk of death and cardiovascular death in multiple studies. Follow-up of the 3258 participants in the LURIC study over a median period of 7.7 years showed that patients with severe and moderate vitamin D deficiency (median levels of 25(OH) D of 19.0 and 33.3 nmol/l) had higher incidence of death [HR = 2.08, (1.60–2.70) and HR = 1.53 (1.17–2.01), respectively, and cardiovascular death [HR = 2.22; (1.57–3.13) and HR = 1.82 (1.29–2.58), respectively, when compared to patients with normal levels (median levels of 71.0 nmol/l). Similar results were obtained for patients in the lowest calcitriol quartile [38,42].
The follow-up of 1739 Framingham Offspring Study participants without prior cardiovascular disease with vitamin D deficiency over a mean of 5.4 years showed an increased risk of and a considerably high cardiovascular event (composite of death, myocardial infarction, stroke and CHF) rate of 6.9%. Lower levels of 25(OH) D of 15 ng/mL were associated with higher risk of cardiovascular events [HR = 2.04 (1.42–2.940; p < 0.001) compared to the rest of the cohort [29]. Similarly, the University of Utah study showed an increased risk of cardiac events including death (HR = 1.77, p < 0.0001), coronary artery disease (HR = 1.45, p < 0.0001), heart failure (HR = 2.01, p < 0.0001), and stroke (HR = 1.78, p = 0.004) in vitamin D deficient individuals [22].
The Heart and Soul Study followed 946 participants with stable cardiovascular disease in San Francisco, California for a median of eight years and recently reported that levels less than 20 ng/mL remained independently associated with cardiovascular events (HR = 1.30 [1.01–1.67]) even after adjustment for socio-demographic factors, season of blood measurement, health behaviors, and comorbid conditions [43].
Conclusion
There is increasing evidence that vitamin D deficiency may be an important and hitherto neglected factor in the pathogenesis of cardiovascular disease. Vitamin D deficiency is related to a broad spectrum of cardiovascular disease and its risk factors. Furthermore, it is associated with increased morbidity and mortality. Vitamin D supplementation may play a role in decreasing morbidity and mortality of cardiovascular disease. Further studies on that role may be warranted.
Disclosure: Authors have nothing to disclose with regard to commercial support.
References
- 1.von der Recke P., Hansen M.A., Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106(3):273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi K., Ross P.D., Orloff J., Davis J.W., Katagiri H., Wasnich R.D. Low bone density is not associated with aortic calcification. Calcif Tissue Int. 2001;69(1):20–24. doi: 10.1007/s002230020003. [DOI] [PubMed] [Google Scholar]
- 3.Samelson E.J., Kiel D.P., Broe K.E., Zhang Y., Cupples L.A., Hannan M.T. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159(6):589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 4.Rajasree S., Rajpal K., Kartha C.C., Sarma P.S., Kutty V.R., Iyer C.S. Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease. Eur J Epidemiol. 2001;17(6):567–571. doi: 10.1023/a:1014559600042. [DOI] [PubMed] [Google Scholar]
- 5.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Contreras J.J., Hiestand B., O’Neill J.C., Schwartz R., Nadkarni M. Vitamin D deficiency in children with fractures. Pediatr Emerg Care. 2014;30(11):777–781. doi: 10.1097/PEC.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 7.Weber K.T., Simpson R.U., Carbone L.D. Vitamin D and calcium dyshomeostasis-associated heart failure. Heart. 2008;94(5):540–541. doi: 10.1136/hrt.2007.126359. [DOI] [PubMed] [Google Scholar]
- 8.Zittermann A., Koerfer R. Vitamin D in the prevention and treatment of coronary heart disease. Curr Opin Clin Nutr Metab Care. 2008;11(6):752–757. doi: 10.1097/MCO.0b013e328312c33f. [DOI] [PubMed] [Google Scholar]
- 9.Hewison M., Zehnder D., Chakraverty R., Adams J.S. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215(1–2):31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Tishkoff D.X., Nibbelink K.A., Holmberg K.H., Dandu L., Simpson R.U. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149(2):558–564. doi: 10.1210/en.2007-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancuso P., Rahman A., Hershey S.D., Dandu L., Nibbelink K.A., Simpson R.U. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008;51(6):559–564. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- 12.Rahman A., Hershey S., Ahmed S., Nibbelink K., Simpson R.U. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103(3–5):416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 13.Nibbelink K.A., Tishkoff D.X., Hershey S.D., Rahman A., Simpson R.U. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103(3–5):533–537. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weishaar R.E., Kim S.N., Saunders D.E., Simpson R.U. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258 (1 Pt 1):E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 15.Prabhakar P., Majumdar V., Kulkarni G.B., Christopher R. Genetic variants of vitamin D receptor and susceptibility to ischemic stroke. Biochem Biophys Res Commun. 2015;456(2):631–636. doi: 10.1016/j.bbrc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Li Y.C. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88(2):327–331. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 17.Zittermann A., Schleithoff S.S., Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev. 2006;11(1):25–33. doi: 10.1007/s10741-006-9190-8. [DOI] [PubMed] [Google Scholar]
- 18.Park C.W., Oh Y.S., Shin Y.S., Kim C.M., Kim Y.S., Kim S.Y. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33(1):73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- 19.Krause R., Bühring M., Hopfenmüller W., Holick M.F., Sharma A.M. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer M., Begerow B., Minne H.W., Nachtigall D., Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 21.Michos E.D., Melamed M.L. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11(1):7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J.L., May H.T., Horne B.D., Bair T.L., Hall N.L., Carlquist J.F. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106(7):963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Martins D., Wolf M., Pan D., Zadshir A., Tareen N., Thadhani R. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 24.Drüeke T.B., McCarron D.A. Paricalcitol as compared with calcitriol in patients undergoing hemodialysis. N Engl J Med. 2003;349(5):496–499. doi: 10.1056/NEJMe038104. [DOI] [PubMed] [Google Scholar]
- 25.Rostand S.G., Drüeke T.B. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56(2):383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 26.Shoji T., Shinohara K., Kimoto E., Emoto M., Tahara H., Koyama H. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19(1):179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 27.Margolis J.R., Chen J.T., Kong Y., Peter R.H., Behar V.S., Kisslo J.A. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. 1980;137(3):609–616. doi: 10.1148/radiology.137.3.7444045. [DOI] [PubMed] [Google Scholar]
- 28.Watson K.E., Abrolat M.L., Malone L.L., Hoeg J.M., Doherty T., Detrano R. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 29.Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannucci E., Liu Y., Hollis B.W., Rimm E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaw K.T., Luben R., Wareham N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. Am J Clin Nutr. 2014;100(5):1361–1370. doi: 10.3945/ajcn.114.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laguardia S.P., Dockery B.K., Bhattacharya S.K., Nelson M.D., Carbone L.D., Weber K.T. Secondary hyperparathyroidism and hypovitaminosis D in African-Americans with decompensated heart failure. Am J Med Sci. 2006;332(3):112–118. doi: 10.1097/00000441-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Swedberg K., Eneroth P., Kjekshus J., Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82(5):1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- 34.Cesselli D., Jakoniuk I., Barlucchi L., Beltrami A.P., Hintze T.H., Nadal-Ginard B. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res. 2001;89(3):279–286. doi: 10.1161/hh1501.094115. [DOI] [PubMed] [Google Scholar]
- 35.Damås J.K., Gullestad L., Aass H., Simonsen S., Fjeld J.G., Wikeby L. Enhanced gene expression of chemokines and their corresponding receptors in mononuclear blood cells in chronic heart failure – modulatory effect of intravenous immunoglobulin. J Am Coll Cardiol. 2001;38(1):187–193. doi: 10.1016/s0735-1097(01)01335-3. [DOI] [PubMed] [Google Scholar]
- 36.Schleithoff S.S., Zittermann A., Tenderich G., Berthold H.K., Stehle P., Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 37.Pilz S., März W., Wellnitz B., Seelhorst U., Fahrleitner-Pammer A., Dimai H.P. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93(10):3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 38.Murr C., Pilz S., Grammer T.B., Kleber M.E., Meinitzer A., Boehm B.O. Vitamin D deficiency parallels inflammation and immune activation, the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chem Lab Med. 2012;50(12):2205–2212. doi: 10.1515/cclm-2012-0157. [DOI] [PubMed] [Google Scholar]
- 39.Zittermann A., Schleithoff S.S., Tenderich G., Berthold H.K., Körfer R., Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(1):105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 40.Wannamethee S.G., Welsh P., Papacosta O., Lennon L., Whincup P.H., Sattar N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circ Heart Fail. 2014;7(5):732–739. doi: 10.1161/CIRCHEARTFAILURE.114.001272. [DOI] [PubMed] [Google Scholar]
- 41.Chung P.W., Park K.Y., Kim J.M., Shin D.W., Park M.S., Chung Y.J. 25-hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke. 2015;46(1):248–251. doi: 10.1161/STROKEAHA.114.007706. [DOI] [PubMed] [Google Scholar]
- 42.Dobnig H., Pilz S., Scharnagl H., Renner W., Seelhorst U., Wellnitz B. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 43.Welles C.C., Whooley M.A., Karumanchi S.A., Hod T., Thadhani R., Berg A.H. Vitamin D deficiency and cardiovascular events in patients with coronary heart disease: data from the Heart and Soul Study. Am J Epidemiol. 2014;179(11):1279–1287. doi: 10.1093/aje/kwu059. [DOI] [PMC free article] [PubMed] [Google Scholar]