Abstract
Nuclei undergo dynamic shape changes during plant development, but the mechanism is unclear. In Arabidopsis, Sad1/UNC-84 (SUN) proteins, WPP domain-interacting proteins (WIPs), WPP domain-interacting tail-anchored proteins (WITs), myosin XI-i, and CROWDED NUCLEI 1 (CRWN1) have been shown to be essential for nuclear elongation in various epidermal cell types. It has been proposed that WITs serve as adaptors linking myosin XI-i to the SUN-WIP complex at the nuclear envelope (NE). Recently, an interaction between Arabidopsis SUN1 and SUN2 proteins and CRWN1, a plant analog of lamins, has been reported. Therefore, the CRWN1-SUN-WIP-WIT-myosin XI-i interaction may form a linker of the nucleoskeleton to the cytoskeleton complex. In this study, we investigate this proposed mechanism in detail for nuclei of Arabidopsis root hairs and trichomes. We show that WIT2, but not WIT1, plays an essential role in nuclear shape determination by recruiting myosin XI-i to the SUN-WIP NE bridges. Compared with SUN2, SUN1 plays a predominant role in nuclear shape. The NE localization of SUN1, SUN2, WIP1, and a truncated WIT2 does not depend on CRWN1. While crwn1 mutant nuclei are smooth, the nuclei of sun or wit mutants are invaginated, similar to the reported myosin XI-i mutant phenotype. Together, this indicates that the roles of the respective WIT and SUN paralogs have diverged in trichomes and root hairs, and that the SUN-WIP-WIT2-myosin XI-i complex and CRWN1 independently determine elongated nuclear shape. This supports a model of nuclei being shaped both by cytoplasmic forces transferred to the NE and by nucleoplasmic filaments formed under the NE.
Keywords: Arabidopsis, CRWN, KASH, LINC, nuclear shape, nuclear envelope, SUN
Abbreviations
- CDS
coding sequence
- CRWN1
CROWDED NUCLEI 1
- KASH
Klarsicht/ANC-1/Syne-1 Homology
- LINC
linker of the nucleoskeleton to the cytoskeleton
- NE
nuclear envelope
- NLI
nuclear envelope localization index
- SUN
Sad1/UNC-84
- sun1-KO sun2-KD
sun1-knockout sun2-knockdown
- WIP
WPP domain-interacting protein
- WIT
WPP domain-interacting tail-anchored protein
- XI-iC642
myosin XI-i C-terminal 642 amino acids.
Introduction
Nuclear morphology changes are associated with cell polarization and migration, which are critical for cell proliferation and tissue development in eukaryotes.1-4 LINC complexes are essential players in nuclear shape determination and cell migration. Formed by the outer nuclear membrane Klarsicht/ANC-1/Syne-1 Homology (KASH) proteins and the inner nuclear membrane Sad1/UNC-84 (SUN) proteins at the nuclear envelope (NE), LINC complexes connect lamins, the chief component of the nucleoskeleton, to the cytoskeleton. Mutations in these proteins lead to nuclear shape abnormalities and human diseases.5-7
Nuclear shape undergoes changes during plant development,8,9 but the mechanism is not fully understood. In Arabidopsis root hairs, the nuclei can reach ∼40 μm in length, which is more dramatic than in other cell types.10 Elongated nuclei have also been observed in Arabidopsis leaf epidermal cells and trichomes.10,11 Recently, constituent proteins of the LINC complexes have been reported to be essential for the elongated nuclear shape in Arabidopsis.10-12 Arabidopsis WPP domain-interacting protein 1 (WIP1), WIP2, and WIP3 are plant-specific KASH proteins that interact with SUN1 and SUN2 at the NE.10 The wip1-1 wip2-1 wip3-1 triple null mutant and SUN mutants including the sun1-knockout sun2-knockdown (sun1-KO sun2-KD) mutant harbor round nuclei in root hairs, epidermal cells, and trichomes.10,12 A similar phenotype has been observed in the wit1-1 wit2-1 double null mutant and in myosin XI-i mutants.11 WIT1 and WIT2 encode WPP domain-interacting tail-anchored proteins.13 WIT1 is a NE protein that interacts with WIP1, WIP2, and myosin XI-i. 11,13 It is proposed that the force from myosin XI-i is transferred to the SUN-WIP complex through WITs, which leads to nuclear elongation.11
At the nucleoplasmic side, lamins are critical for nuclear shape in mammals.14 However, no lamin homologs have been found in plant proteomes.15 Instead, Nuclear Matrix Constituent Proteins (NMCP)16-18 and their Arabidopsis homologs, CROWDED NUCLEI (CRWN, also known as LITTLE NUCLEI) proteins, were identified as putative analogs of mammalian lamins in plants.15 NMCP/CRWN proteins are coiled-coil proteins isolated from plant nuclear fractions and are proposed to share structural similarity to lamins.15,16,19 The Arabidopsis genome encodes 4 CRWN genes, but only CRWN1 and CRWN4 are localized to the nuclear periphery.19,20 CRWN1 and CRWN4 single mutants have small, round nuclei but show no developmental phenotypes.19-21 A recent study provided evidence for an interaction of CRWN1 with SUN1 and SUN2 at the NE, and therefore, CRWN1 may be part of a plant LINC complex.22
In this study, using Arabidopsis root hair and trichome nuclei, we investigated the nuclear shape determined by the putative SUN-WIP-WIT-myosin XI-i complex and CRWN1. We discovered that WIT2, but not WIT1, is essential for recruiting myosin XI-i to the plant NE and for maintaining the elongated nuclear shape. The nuclei of the wit2-1 null mutant were invaginated and round, similar to the nuclei of sun1-KO sun2-KD and myosin XI-i mutants. A truncated WIT2 protein (designated as WIT2*) was able to partially rescue the phenotype, and was shown to interact with WIP1, WIP2, WIP3, and the C-terminus of myosin XI-i. In sun1-KO sun2-KD and wit2-1, the NE localization of the C-terminus of myosin XI-i is also impaired, supporting the existence of LINC complexes comprised of SUN, WIP, WIT2 and myosin XI-i. SUN1, SUN2, WIP1, and WIT2* are still properly localized at the NE in the crwn1-1 null mutant, and the round nuclei of crwn1-1 are smooth without invaginations. These data suggest that plant nuclear shape is determined independently by the LINC complexes and CRWN1.
Results
WIT2, but not WIT1, mediates the elongated nuclear shape in Arabidopsis root hairs and trichomes
Arabidopsis root hair and trichome nuclei, unlike the nuclei of other cell types, are nearly uniformly elongated, which ensures accurate assessments of nuclear shape. Therefore, to identify the key factor that affects the elongated nuclear shape in Arabidopsis, we used root hair and trichome nuclei as models. As shown in Figure 1A, in contrast to the elongated nuclear shape in wild type (Columbia ecotype, unless otherwise indicated; please see Materials and Methods for details), the nuclear shape of wit2-1 in both cell types is round and is similar to wit1-1 wit2-1. Because the wild-type root hair nuclei are very elongated with additional hyper-elongated, irregularly shaped “tails," the length of the root hair nuclei was used as an index.10 For trichome nuclei, which exhibit regular spindle-like or round shape, the ratio of the width and length of the maximal cross section of a nucleus was used as a circularity index.10 As shown in Figure 1 B and C, the circularity indices of wit2-1 in both cell types are significantly different from wild type (P < 0.01, 2-tailed t-test) and are similar to those of wit1-1 wit2-1 (P > 0.05, 2-tailed t-test). The length of wit1-1 root hair nuclei is slightly reduced compared to wild type (0.05 > P > 0.01, 2-tailed t-test), and the index of wit1-1 trichome nuclei is indistinguishable from that of wild type (Fig. 1, P > 0.05, 2-tailed t-test).
Figure 1.
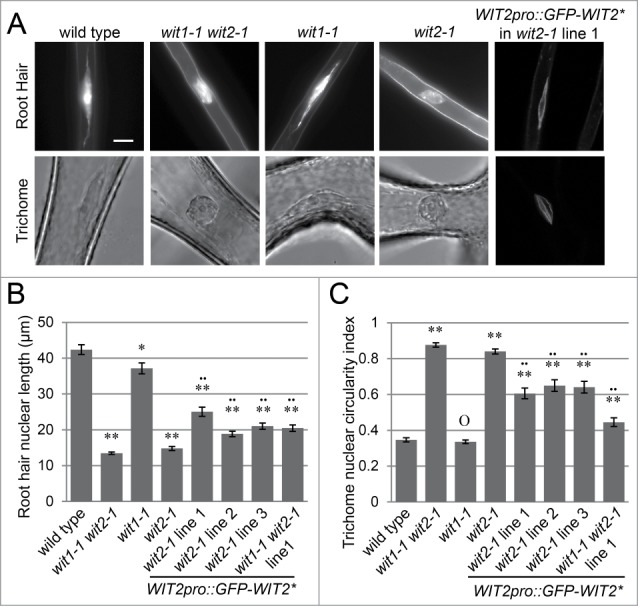
WIT2 is essential for the elongated nuclear shape in root hairs and trichomes. (A) Root hair and trichome nuclear morphology of wild type, wit1-1 wit2-1, wit1-1, wit2-1, and WIT2pro::GFP-WIT2*-transformed wit2-1. Nuclei of WIT2pro::GFP-WIT2*-transformed wit2-1 were viewed using confocal microscopy. For all others, fluorescence microscopy was used to image Hoechst 33342-stained nuclei, and the trichome nuclei were imaged directly using bright field microscopy. Scale bar equals 10 μm. Images are at the same magnification scale. (B) Root hair nuclear shape quantified by the length of the nuclei. Single asterisk, 0.05 > P > 0.01 when compared to wild type. Double asterisks, P < 0.01 when compared to wild type. Double dots, P < 0.01 when compared to wit1-1 wit2-1. Two-tailed t-test was used and n = 80. Error bars represent SE. (C) Nuclear circularity index of trichome nuclei. The ratio of width and length of the maximum nuclear cross section was used as the index. Double asterisks, P < 0.01, and “O,” P > 0.05, when compared to wild type. Two-tailed t-test was used and n = 50.Double dots, P < 0.01 when compared to wit1-1 wit2-1. Error bars represent SE.
To confirm that the nuclear shape phenotype of wit2-1 was caused by a mutation in the WIT2 gene, a truncated WIT2 cDNA was cloned. WIT2 was constantly truncated in Escherichia coli during cloning (at least 3 independent cloning trials), resulting in a sequence encoding the WIT2 protein model At1G68910.3 (total protein length: 582aa) with the G62-L188 fragment deleted. WIT2 is a long coiled-coil protein in which the G62-L188 encodes a small part of the coiled-coil domain at the N-terminus. The resulting gene was designated as WIT2*. GFP-WIT2* driven by the WIT2 promoter (WIT2pro::GFP-WIT2*) was transformed to wit2-1 and wit1-1 wit2-1. Three wit2-1 transgenic lines and one wit1-1 wit2-1 transgenic line were analyzed. As shown in Figure 1 A-C, GFP-WIT2* was located at the NE and was able to partially complement the nuclear shape phenotype (compared to wit2-1 and wit1-1 wit2-1, P < 0.01, 2-tailed t-test). We then performed similar complementation experiments using wit1-1 wit2-1 transformed with GFP-WIT1 driven by the WIT1 promoter (WIT1pro::GFP-WIT1). Two independent transgenic lines, lines 1 and 3 were examined. As shown in Figure S1 A and B, WIT1pro::GFP-WIT1 failed to rescue the nuclear shape phenotype of wit1-1 wit2-1. However, a previously reported pollen vegetative nuclear migration phenotype was rescued in these lines, showing that WIT1pro::GFP-WIT1 is functional.23 These data suggest that WIT2, but not WIT1, is critical for the elongated nuclear shape in Arabidopsis root hairs and trichomes.
SUN1 plays a predominant role in maintenance of elongated root hair and trichome nuclear shape
Since SUN and WIP are necessary for WIT NE localization and maintaining elongated nuclear shape in root hairs and trichomes,10,12 we examined whether each WIP and SUN gene contributes equally to the nuclear shape phenotype observed in wit mutants. As shown in Figure S2, wip1-1 wip2-1, wip2-1 wip3-1, and wip1-1 wip3-1 double null mutants exhibited a similar increase in circularity index in trichome nuclei, suggesting that each WIP gene contributes equally to nuclear elongation. Trichome nuclei in the sun1-1, sun1-KO, and sun1-KO sun2-KD mutant lines have a similar circularity index and are statistically significantly different from wild type (Fig. 2, P < 0.01, 2-tailed t-test). Similarly, in root hairs, both sun1-1 and sun1-KO display less elongated root hair nuclei than wild type (P < 0.01, 2-tailed t-test), though not as severe as sun1-KO sun2-KD (Fig. 2). sun2-1 is in the Wassilewskija (Ws-4) background, which also has elongated root and trichome nuclei, and the sun2-1 mutation does not reduce the elongated nuclear shape in both cell types (Fig. 2). These data suggest that SUN1 plays a predominant role in nuclear shape determination.
Figure 2.
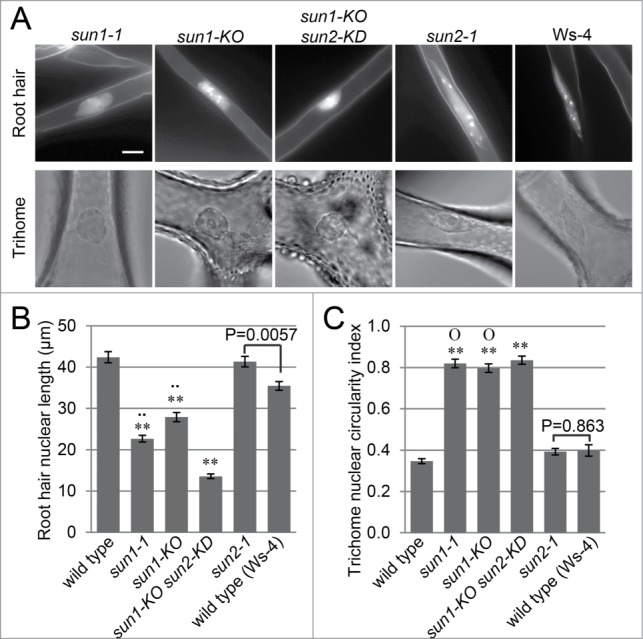
SUN1 plays a predominant role in nuclear shape determination. (A) Root hair and trichome nuclear morphology of sun-1-1, sun1-KO, sun2-1, and sun1-KO sun2-KD. Fluorescence microscopy was used to image Hoechst 33342-stained nuclei, and the trichome nuclei were imaged using bright field microscopy. Scale bar equals 10 μm. Images are at the same magnification scale. (B) Root hair nuclear shape quantified by the length of the nuclei. Double asterisks, P < 0.01, when compared to wild type. Double dots, P < 0.01 when compared to sun1-KO sun2-KD. The P value of the t-test between sun2-1 and Ws-4 is indicated in the figure. Two-tailed t-test was used and n = 80. Error bars represent SE. (C) Nuclear circularity index of trichome nuclei. The ratio of width and length of the maximum nucleus cross section was used as the index. Double asterisks, P < 0.01, when compared to wild type. “O,” P > 0.05 when compared to sun1-KO sun2-KD. The P value of the t-test between sun2-1 and Ws-4 is indicated in the figure. Two-tailed t-test was used and n = 50. Error bars represent SE.
WIT2 bridges myosin XI-i to WIPs
Since WIT1 interacts with WIP1 and WIP2, the ability of WIT2* to bind WIPs was examined. GFP-WIT2* was transiently co-expressed with Myc-WIP1, Myc-WIP2, and Myc-WIP3, respectively, in Nicotiana benthamiana leaves. GFP-NLS-GFP-NES was co-expressed with Myc-WIP3 to serve as a GFP-fusion protein control. As shown in Fig. 3A, GFP-WIT2* co-immunoprecipitated Myc-tagged WIP1, 2 and 3, while GFP-NLS-GFP-NES did not co-immunoprecipitate Myc-WIP3.
Figure 3.
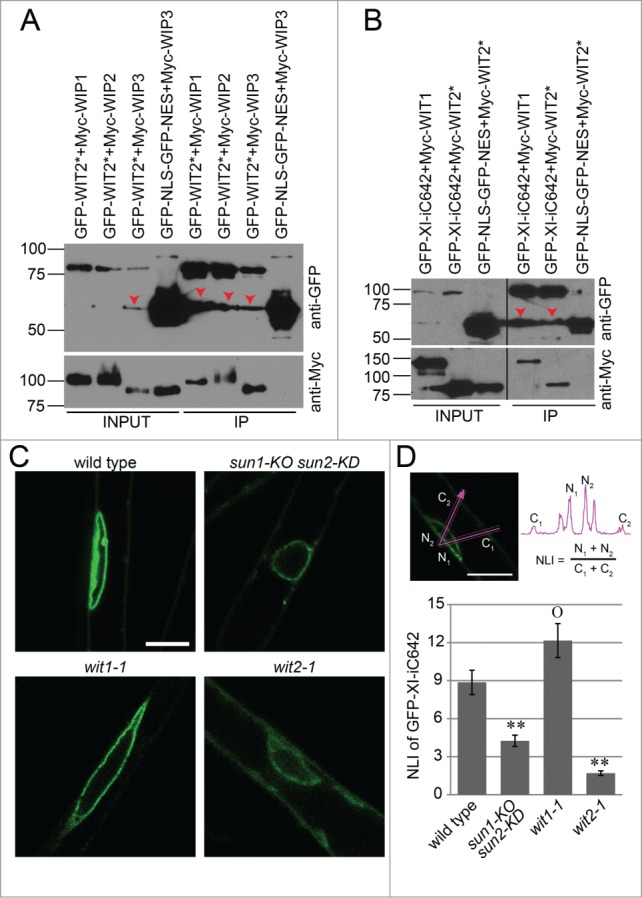
WIT2 connects myosin XI-i to the SUN-WIP NE bridges.(A) WIT2* interacts with WIP1, WIP2, and WIP3. GFP-tagged proteins were immunoprecipitated and detected with anti-GFP antibody. Myc-tagged proteins were detected with an anti-Myc antibody. Input/IP ratio was 1:9. Numbers on the left indicate molecular mass in kilodaltons. Red arrow heads indicate possible truncated GFP-fusion proteins. (B) WIT1 and WIT2* interacts with XI-iC642. GFP-tagged proteins were immunoprecipitated and detected with anti-GFP antibody. Myc-tagged proteins were detected with an anti-Myc antibody. Input/IP ratio was 1:18. Numbers on the left indicate molecular mass in kilodaltons. Red arrow heads indicate possible truncated GFP-fusion proteins. Vertical back lines represent intervening lanes removed for display purposes. (C) Localization of GFP-XI-iC642 in wild type, sun1-KO sun2-KD, wit1-1, and wit2-1. Scale bar equals 10 μm. All images are single optical sections at the same magnification. (D) NLI of GFP-XI-iC642 in wild type, sun1-KO sun2-KD, wit1-1, and wit2-1. The sum of the 2 maximum NE intensities divided by the sum of the 2 maximum cytoplasmic intensities was used as an NLI (illustrated in the top panel). Double asterisks, P < 0.01, and “O,” P > 0.05, when compared to wild type. Two-tailed t-test was used. For each genotype, 3 transgenic lines and 10 nuclei from each line were analyzed (total n = 30). Error bars represent SE.
Myosin XI-i is involved in maintaining elongated nuclear shape in Arabidopsis.11 Its C-terminal 642 amino acid region (XI-iC642, containing the coiled coil domain and the dilute domain, but lacking the motor domain) interacts with WIT1 and is localized at the plant NE. 11,24 Therefore, we examined the interaction between myosin XI-iC642 and WIT2*. GFP-XI-iC642 was co-expressed with Myc-WIT1 and with Myc-WIT2* in N. benthamiana leaves. GFP-NLS-GFP-NES was also co-expressed with Myc-WIT2* to serve as a negative control. As previously reported, Myc-WIT1 was co-immunoprecipitated by GFP-XI-iC642 (Fig. 3B). More importantly, Myc-WIT2* was co-immunoprecipitated by GFP-XI-iC642 but not by GFP-NLS-GFP-NES (Fig. 3B). These data suggest that WIT2* is able to link myosin XI-i to the SUN-WIP LINC complex.
To further investigate whether WIT2 and SUN proteins are necessary for recruiting myosin XI-i to the NE, GFP-XI-iC642 was expressed under the cauliflower mosaic virus 35S promoter in wild type, sun1-KO sun2-KD, wit1-1, and wit2-1, respectively. As shown in Figure 3C, GFP-XI-iC642 was properly localized at the NE in wild type and wit1-1 root hairs, but a decrease in NE enrichment was observed in sun1-KO sun2-KD and wit2-1. NE enrichment was quantified using the nuclear localization index (NLI).10 In short, a line was drawn across a cell and through the NE, and the ratio of the sum of the two maximum NE intensities and the sum of the two maximum cytoplasmic intensities was calculated. The NLI of GFP-XI-iC642 in wit2-1 and sun1-KO sun2-KD (Fig. 3D) is significantly decreased when compared to wild type (P < 0.01, 2-tailed t-test). The NLI of sun1-KO sun2-KD is slightly larger than wit2-1, which can be explained by the residual SUN2 protein in this mutant. In contrast, wit1-1, which has relatively normal nuclear shape, has a NLI similar to wild type (P > 0.05, 2-tailed t-test). These data suggest that WIT2 bridges myosin XI-i to the SUN-WIP complex and that a LINC complex is formed that involves SUN, WIP, WIT2, and myosin XI-i.
The NE localization of SUN1, SUN2, WIP1, and WIT2* does not depend on CRWN1
Mutations in CRWN1 or CRWN4 lead to small, round nuclei, and CRWN1 plays a more predominant role in this phenotype than CRWN4.21 In addition, a recent study provided evidence suggesting the interaction between CRWN1 and SUN proteins at the NE.22 Therefore, CRWN1 may be essential for the NE localization of the SUN-WIP-WIT2-myosin XI-i complex. To address this question, the NE localization of SUN1, SUN2, WIP1, and WIT2* was examined in root hairs of crwn1-1. The NE localization of these proteins was quantified using the NLI illustrated in Figure 3D. As shown in Figure 4, SUN1 and SUN2 were properly localized at the NE in crwn1-1 when compared with wild type (P > 0.05). The NE localization of WIP1 was slighted decreased (p = 0.035), but the NE localization of WIT2* was not affected (P > 0.05). Since CRWN1 is co-localized with DNA during mitosis,19 and plays a role in chromatin organization during interphase,21 we examined the expression level of SUN1, SUN2, WIP1, and WIT2 in crwn1-1. As shown in Fig. S3, no significant difference in the expression level was observed when compared with wild type. These data suggest that loss of CRWN1 does not impact the SUN-WIP-WIT2 complex at the NE. We further noticed that the NE morphology of crwn1-1 is smooth, which is different from the invaginated nuclei of sun1-KO sun2-KD and wit2-1 (Fig. 5A). This observation was confirmed by quantification (Fig. 5B). It has been reported that the NE of myosin XI-i mutants is also invaginated,11 supporting the hypothesis that myosin XI-i acts in the same pathway as SUN proteins and WIT2.
Figure 4.
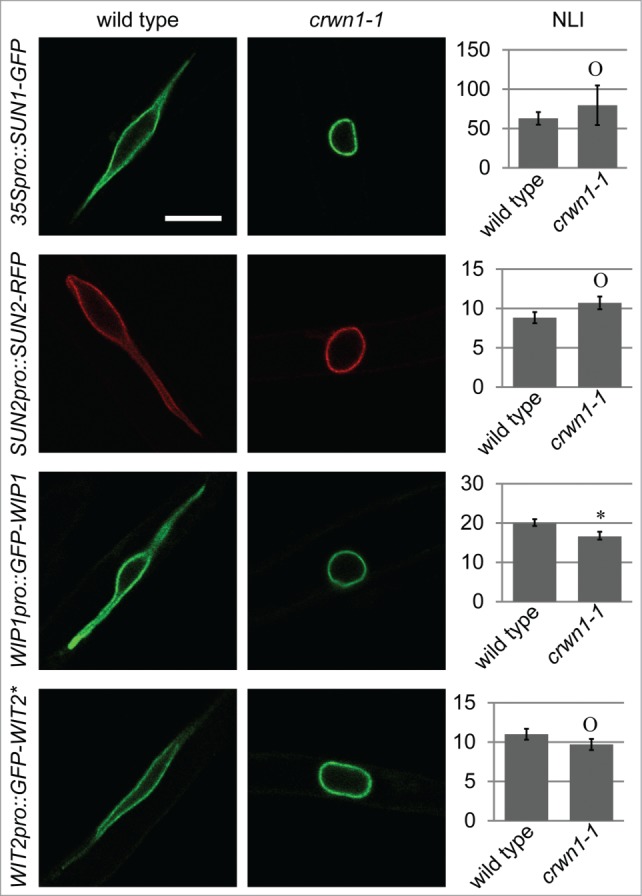
CRWN1 is not required for the NE-association of the SUN-WIP-WIT2 complex. The localization of SUN1-GFP, SUN2-RFP, GFP-WIP1, and GFP-WIT2* in wild type (left column) and crwn1-1 (middle column) are shown. Scale bar equals 10 μm, and all images are at the same magnification. The NLI are shown in the right column. Asterisk, p = 0.035, and “O,” P > 0.05, when compared to wild type. For each genotype, 3 transgenic lines and 30 nuclei from each line were measured (total n = 90). Error bars represent SE.
Figure 5.
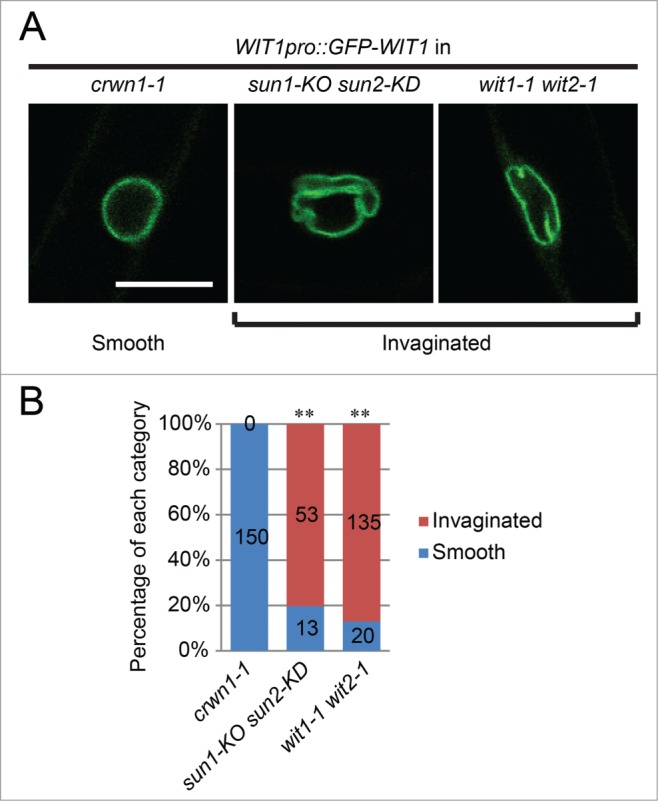
Root hair nuclear morphology of crwn1-1 is different from sun1-KO sun2-KD and wit1-1 wit2-1. (A) NE morphology illustrated by GFP-WIT1.The nuclei of crwn1-1 are smooth, while the nuclei of sun1-KO sun2-KD and wit1-1 wit2-1 are predominantly invaginated. Scale bar equals 10 μm. All images are at the same magnification. (B) Quantification of smooth nuclei and invaginated nuclei in crwn1-1, sun1-KO sun2-KD, and wit1-1 wit2-1. Double asterisks, P < 0.01, when compared with crwn1-1, 2-tailed Fisher′s exact test. The n of each category is indicated in the figure.
In summary, our data suggest a model of nuclear shape determined independently by the SUN-WIP-WIT2-myosin XI-i complex and CRWN1 (Fig. 6). We propose that the SUN-WIP-WIT2-myosin XI-i complex transfers the cytoplasmic motor forces to the NE, while CRWN1 regulates nuclear shape by forming lamina-like structures at the nucleoplasmic side.
Figure 6.
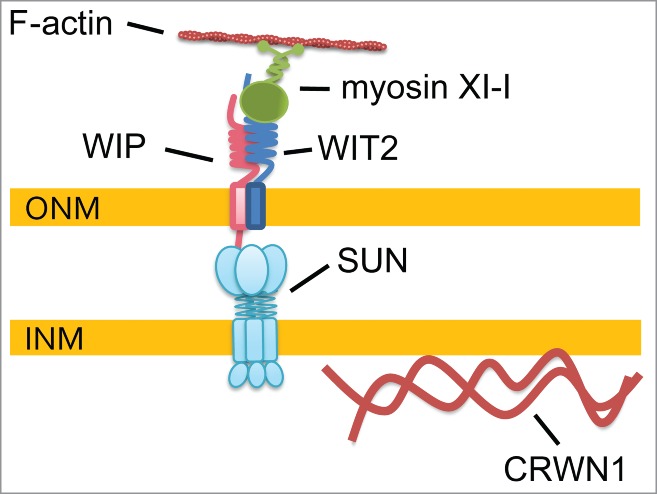
Model of nuclear shape determination. Myosin XI-i is anchored to the SUN-WIP NE bridges by WIT2. This SUN-WIP-WIT2-myosin XI-i complex transfers cytoplasmic forces to the NE. In the nucleoplasm, CRWN1 acts independently on nuclear shape determination by forming lamina-like structures. Other nucleoplasmic proteins that affect nuclear shape—CRWN4, KAKU4, and NUP136—and the CRWN1-SUN interaction of unknown function are not drawn.
Discussion
Functional divergence of protein homologs
WIT1 and WIT2 have a high percentage of amino acid sequence identity (34%) and similarity (56%), and similar expression patterns.13 A recent study has shown that both WIT1 and WIT2 are involved in male fertility.23 Here, we reported that only WIT2 is required for the elongated nuclear shape in root hairs and trichomes (Fig. 1 and Fig. S1). Similarly, SUN1 and SUN2 are closely related (their protein sequences are 62% identical and 72% similar),25 but SUN1 is predominant in nuclear shape determination. This functional divergence among protein homologs is also found in the newly identified KASH proteins SINE1 and SINE2. SINE1 is associated with F-actin and plays a role in guard cell central nuclear anchorage, while SINE2 contributes to innate immunity against an oomycete pathogen.26 This suggests that closely related paralogs of plant nuclear envelope-associated proteins have adapted to specific functions, which should be considered when addressing their biological roles.
Model for nuclear shape determination
In this study, we provide evidence that myosin XI-i is anchored to the SUN-WIP complex at the NE through WIT2. As has been proposed, the myosin XI-i motor force can thus be transferred to the NE and cause an elongated nuclear shape.11 Disrupting the SUN-WIP-WIT2-myosin XI-i complex leads to invaginated, round nuclei, which is different from the smooth, round nuclei of crwn1-1. Our data also show that loss of CRWN1 does not affect localization of the SUN-WIP-WIT2 complex, suggesting that CRWN1 acts independently. Other NE proteins that play a role in nuclear shape include KAKU4, CRWN4, and NUP136. KAKU4 is a newly identified inner nuclear membrane-associated protein that interacts with CRWN1.27 KAKU4 mutants have spherical epidermal nuclei. 27 Overexpressing KAKU4 caused nuclear membrane deformation, and co-overexpressing KAKU4 with CRWN1 enhanced this deformation.27 Therefore, KAKU4 has been proposed to be a putative component of plant lamina-like structures.27 CRWN4 is a homolog of CRWN1 that is also localized at the NE. 19 CRWN4 mutants have similar nuclear shape change to CRWN1 mutants, except that CRWN4 mutants still have tail-like projections on their spherical nuclei.19-21 Overexpressing CRWN4 in leaf epidermal cells caused abnormally elongated nuclei,19 a phenotype that is similar to NUP136-overexpressing plants.28 Nucleoporin NUP136 is considered a functional homolog of animal NUP153, and its mutants also have spherical nuclei.28 Since NUP153 physically interacts with lamins at the nucleoplasmic side, it has been proposed that plant NUP136 also interacts with plant lamin-like proteins.15,27,29 These pieces of evidence indicate that the plant nuclear shape is orchestrated by the cytoplasmic motor forces transferred through the LINC complexes and by nucleoplasmic lamina-like structures formed by CRWN1, CRWN4, KAKU4, and NUP136.
The relationship between CRWN1 and LINC complexes
In mammalian cells, the NE localization of SUN2 and the KASH protein, Nesprin-2, depends on lamin A/C.30-32 In Drosophila, the NE localization of the SUN protein Klaroid, and the KASH protein Klarsicht, depends on the lamin encoded by Dm0.33,34 In Arabidopsis, our data show that the NE localization of SUN1, SUN2, or WIT2* does not depend on CRWN1, and the NE localization of WIP1 is only slightly affected by the loss of CRWN1 (Fig. 4). It is possible that CRWN4 might serve as a redundant NE anchor of SUN proteins. It is also possible that the NE localization of CRWN1 depends on SUN proteins, because a recent study has shown that 1) expressing SUN proteins affects the NE localization and the mobility of CRWN1, and 2) CRWN1 and SUN protein interact with each other as determined by fluorescence resonance energy transfer.22
Potential functions of an elongated nuclear shape
Although the epidermal nuclear shape is changed in sun1-KO sun2-KD, wit2-1, CRWN1 mutants, and myosin XI-i mutants, these mutations do not appear to affect plant development and physiology. One phenotype associated with the nuclear shape change is altered nuclear movement. Impaired root nuclear movement has been observed in wit1-1 wit2-1 and myosin XI-i mutants.11 Dark-induced nuclear movement in leaf mesophyll cells is also impaired in wit1-1 wit2-1 and myosin XI-i mutants, but light-induced nuclear movement is not disrupted.11 Light-induced nuclear movement defects have not been observed in either CRWN1 or CRWN4 mutants.19 Lack of plant growth phenotypes under standard laboratory conditions in these mutants currently leaves the biological role of the elongated nuclear shape a puzzle. One possible function of elongated nuclear shape in root hairs and epidermal cells is to reduce physical resistance during nuclear migration. Nuclear migration has also been shown to be associated with plant-microbe interactions.35,36 Upon microbe invasion or symbiotic interaction, quick nuclear migration to the microbe contacting site is one of the early plant responses. Since a single root hair cell can reach a length visible to the naked eye, an elongated nuclear shape might be suitable for agile nuclear migration over a long distance. Studying genes that determine nuclear shape could thus help to uncover the function of the nucleus in plant-microbial interactions.
Materials and Methods
Plant materials
Arabidopsis thaliana were grown at 25°C in soil under 16-h light and 8-h dark or on Murashige and Skoog (Caisson laboratories, MSP01-50LT) medium with 1% sucrose under constant light. wip1-1, wip2-1, wip3-1, wit1-1, and wit2-1 have been reported previously.13,37 sun1-KO and sun1-KO sun2-KD were gifts from Dr. Susan Armstrong and K. Osman (University of Birmingham, UK) and have been reported previously.10 sun1-1 (WiscDsLox330H03) has been reported previously12 and was obtained from the Arabidopsis Biological Resource Center. sun2-1 (FLAG_625B02) is in Ws-4 background with a T-DNA inserted in the second exon (confirmed by sequencing) and was obtained from the Versailles Arabidopsis Stock Center. For genotyping sun2-1, the following primers were used. LP: 5′-CCGTCACGACGACCAAACATGGGTT-3′, RP: 5′-TGAGTTAAATGCTAAAAGACGGGTTCATAGCAAAGCGGTT-3′; and RB4: 5′-TCACGGGTTGGGGTTTCTACAGGAC-3′. As shown in Fig. S3, sun2-1 is a null mutant with no full SUN2 length transcripts made. crwn1-1 (SALK_025347) has been reported previously20 and was obtained from the Arabidopsis Biological Resource Center. All wip, wit, sun1, and crwn1 mutants used in this study are in Columbia background, and the Columbia ecotype was used as “wild type.” For sun2-1, Ws-4 was used as “wild type.” N. benthamiana plants were grown at 28°C in soil under constant light.
Constructs
The WIT2 promoter (∼2.2kb upstream the start codon of WIT2) was amplified by PCR from Arabidopsis DNA using 5′-GGCCCGGCGCGCCACTGATGAATCATTCACCAAGAGTGGT-3′ and 5′-CTCGCCCTTGCTCACCATTGACTCCACAAAAAAATCTATC-3′, and cloned into the PmlI-digested pHOAG 26 by In-fusion (Clontech, 639684) recombination. After confirmation by sequencing, the pHWIT2proAG vector was obtained. WIT2 CDS was cloned from Arabidopsis whole seedling cDNA using 5′-GGGGACAAGTTTGTACAAAAAA GCAGGCTTTATGGAGGAAATCATTAGGGAGGAC-3′ and 5′-GGGGACCACTTTGTACAAGAA AGCTGGGTATTAATAAGTCACACCAAAGAATGAA-3′, and cloned into pDONR221 (Life Technologies, 12536-017) by a BP reaction (Life Technologies, 11789-020). After sequencing, we found that the T182-C562 fragment of the WIT2 CDS was constantly (3 independent cloning trials) lost in E. coli, resulting in a sequence encoding the WIT2 gene model At1G68910.3 with the protein sequence G62-L188 deleted. This truncated WIT2 was named WIT2*. WIT2* CDS was then moved from pDONR221 to pHWIT2proAG by an LR reaction (Life Technologies, 11791-020) to obtain the WIT2pro::GFP-WIT2* construct. WIT2* CDS in pDONR221 was also cloned into pH7WGF2 38 and pGWB21 39 by an LR reaction to obtain 35S promoter-driven GFP-WIT2* and Myc-WIT2*, respectively. WIP1, WIP2, WIP3, and WIT1 CDS cloned in pENTR/D-TOPO were reported previously,37,13 and were moved to pGWB21 by LR reactions to obtain 35S promoter-driven Myc-WIP1, Myc-WIP2, and Myc-WIP3, respectively.
pH7RWG238 was digested with XbaI and SpeI and the fragment containing the Gateway cassette was ligated to XbaI-digested pPZP-Hyg-RCS2 vector26 to obtain the pHAR vector. SUN2 CDS without the stop codon was amplified by PCR using 5′-ATTCTGTAGTTCAAAATTGAAGATCATGTCGGCGTCAACGGTGTCAATCA-3′ and 5′-AGCATGAGCAACAGAGACTGAGTCTAG-3′. SUN2 promoter (∼2kb upstream of the ATG) was amplified by PCR using 5′-CACCTATCGAGTTTGCCCGAGGAGATGCCAATTC-3′ and 5′-GGTGATTGACACCG TTGACGCCGACATGATCTTCAATTTTGAACTACAGA-3′. The DNA fragment containing SUN2 promoter and CDS was then amplified by overlapping PCR using the above 2 PCR products as templates and the following primers: 5′-CACCTATCGAGTTTGCCCGAGGAGATGCCAATTC-3′ and 5′-AGCATGAGCAACAGAGACTGAGTCTAG-3′. The SUN2 promoter-CDS fragment was then cloned into pENTR/D-TOPO, confirmed by sequencing, and then moved to pHAR by LR reactions to obtain the SUN2pro:SUN2-RFP construct. SUN1 CDS was amplified by PCR using 5′-CACCATGTCGGCATCAACGGTGTCG-3′ and 5′-TTCACTTTCAGGTGAAGAGTCCTG-3′. The PCR product was cloned into pENTR/D-TOPO, confirmed by sequencing. The CDS was then moved to pB7FWG2 38 to obtain the 35Spro:SUN1-GFP construct.
Agrobacterium transformation
Agrobacterium tumefaciens strain ABI was transformed with the corresponding constructs by triparental mating.40 In brief, the E. coli carrying the constructs of interest were co-incubated overnight at 30°C on lysogeny broth agar (1.5%) plates with Agrobacterium ABI and the E. coli helper strain containing the vector pRK2013. Then, the bacterial mixture was streaked on lysogeny broth agar (1.5%) plates with proper antibiotics to select transformed Agrobacterium which was confirmed by PCR.
N. benthamiana transient transformation
Agrobacterium cultures containing plasmids expressing the proteins of interest were co-infiltrated transiently into N. benthamiana leaves as described previously.24 In brief, Agrobacterium cultures were collected by centrifuging and resuspended to OD600 = 1.0 in the infiltration buffer containing 10 mM MgCl2, 10 mM MES, pH 5.4, and 100 μM acetosyringone (Sigma-Aldrich, D134406-1G). The Agrobacterium suspension was pressure infiltrated into N. benthamiana leaves with a plastic syringe. Plants were grown for 2-3 d before being collected for the subsequent experiments.
Arabidopsis stable transformation
Transgenic Arabidopsis plants were obtained by Agrobacterium-mediated floral dip.41 Agrobacterium strains carrying the constructs of interest were inoculated in lysogeny broth liquid medium and grown overnight at 30°C. The bacteria were collected by centrifuging and resuspended in transformation solution containing 5% sucrose and 300 μl/L silwet L-77 (Lehle Seeds, VIS-01) to OD600 = 0.8. The inflorescence of Arabidopsis was dipped in the bacterial suspension. After being kept moist in the dark overnight at room temperature, the plants were moved to a growth chamber and allowed to set seeds. The transgenic plants were selected on Murashige and Skoog agar (0.8%) plates containing proper antibiotics.
Co-immunoprecipitation experiments
N. benthamiana leaves were collected, ground in liquid nitrogen into powders, and Co-IP experiments were performed at 4°C. One ml NP-40 buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM EDTA, 3 mM DTT, 1 mM PMSF, and 1% protease inhibitor cocktail [Sigma-Aldrich, P9599-5ML]) was used to extract 500 μl plant tissue. One-tenth of each protein extract was used as an input sample, and the rest was used for IP using protein A-sepharose beads (GE Healthcare) pre-coated with an anti-GFP antibody (Abcam Cambridge, ab290). After three washes using NP-40 buffer, the immunoprecipitates and the input samples were separated by 8% SDS-PAGE, transferred to PVDF membranes (Bio-Rad, 162-0177), and detected with an anti-GFP (1:2000, Clontech, 632569,) or anti-Myc (1:1000, Sigma-Aldrich, M5546) antibody.
Confocal microscopy
Six- to 8-day old Arabidopsis seedlings were imaged using a Nikon Eclipse C90i confocal microscope with small or medium pinhole and gain setting range of 7.0 to 7.5. The 488nm laser was set at 30–50% power for imaging 35Spro:GFP-XI-iC642 transgenic plants whose transgene expression was low in all lines, while the other transgenic lines and N. benthamiana leaves were imaged using 10%–30% laser power. All images were taken at room temperature using Murashige and Skoog medium as medium with Nikon Plan Apo VC 60x H lens (numerical aperture 1.4). The transmitted light detector was turned on to collect transmitted light signal simultaneously. Images were exported to PNG format by Nikon NIS-Elements software and organized in Adobe Photoshop and Illustrator. Intensity readings were performed in Nikon NIS-Elements software.
Hoechst 33342 staining
Six- to 8-d old Arabidopsis seedlings grown on Murashige and Skoog plates were used. Samples were stained for 20 min in a phosphate buffered saline (pH 7.4) solution containing 4 μM Hoechst 33342 (Thermo Scientific, 28491-52-3) and 4% paraformaldehyde (Sigma-Aldrich, 76240). Root hair nuclei images were taken by a Nikon DS-Qi1Mc digital camera. The length of the nuclei was measured using the Nikon NIS-Elements software.
RT-PCR analysis
Leaves of 20-day-old Arabidopsis plants were ground in liquid nitrogen and total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, 74903). First strand cDNA was synthesized using the SuperScript® III First-Strand Synthesis System (Life Technologies, 18080-051) and oligo dT as primer. Primers used for PCR were listed in Table S1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
We thank the National Science Foundation for financial support (NSF-MCB 1243844).
References
- 1. Gladfelter A, Berman J. Dancing genomes: fungal nuclear positioning. Nat Rev Microbiol 2009; 7:875-86; PMID:19898490; http://dx.doi.org/ 10.1038/nrmicro2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 2010; 26:421-44; PMID:20507227; http://dx.doi.org/ 10.1146/annurev-cellbio-100109-104037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gundersen GG, Worman HJ. Nuclear positioning. Cell 2013; 152:1376-89; PMID:23498944; http://dx.doi.org/ 10.1016/j.cell.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Razafsky D, Wirtz D, Hodzic D. Nuclear Envelope in Nuclear Positioning and Cell Migration. In: Schirmer EC, de las Heras JI, eds. Cancer Biology and the Nuclear Envelope: Springer; New York, 2014:471-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mejat A, Misteli T. LINC complexes in health and disease. Nucleus 2010; 1:40-52; PMID:21327104; http://dx.doi.org/ 10.4161/nucl.1.1.10530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stroud MJ, Banerjee I, Veevers J, Chen J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ Res 2014; 114:538-48; PMID:24481844; http://dx.doi.org/ 10.1161/CIRCRESAHA.114.301236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol 2013; 23:R1113-21; PMID:24355792; http://dx.doi.org/ 10.1016/j.cub.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamura K, Hara-Nishimura I. Involvement of the nuclear pore complex in morphology of the plant nucleus. Nucleus 2011; 2:168-72; PMID:21818409; http://dx.doi.org/ 10.4161/nucl.2.3.16175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chytilova E, Macas J, Sliwinska E, Rafelski SM, Lambert GM, Galbraith DW. Nuclear dynamics in Arabidopsis thaliana. Mol Biol Cell 2000; 11:2733-41; PMID:10930466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou X, Graumann K, Evans DE, Meier I. Novel plant SUN-KASH bridges are involved in RanGAP anchoring and nuclear shape determination. J Cell Biol 2012; 196:203-11; PMID:22270916; http://dx.doi.org/ 10.1083/jcb.201108098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tamura K, Iwabuchi K, Fukao Y, Kondo M, Okamoto K, Ueda H, Nishimura M, Hara-Nishimura I. Myosin XI-i links the nuclear membrane to the cytoskeleton to control nuclear movement and shape in Arabidopsis. Curr Biol 2013; 23:1776-81; PMID:23973298; http://dx.doi.org/ 10.1016/j.cub.2013.07.035 [DOI] [PubMed] [Google Scholar]
- 12. Oda Y, Fukuda H. Dynamics of Arabidopsis SUN proteins during mitosis and their involvement in nuclear shaping. Plant J 2011; 66:629-41; PMID:21294795 [DOI] [PubMed] [Google Scholar]
- 13. Zhao Q, Brkljacic J, Meier I. Two distinct interacting classes of nuclear envelope-associated coiled-coil proteins are required for the tissue-specific nuclear envelope targeting of Arabidopsis RanGAP. Plant Cell 2008; 20:1639-51; PMID:18591351; http://dx.doi.org/ 10.1105/tpc.108.059220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Houben F, Ramaekers FC, Snoeckx LH, Broers JL. Role of nuclear lamina-cytoskeleton interactions in the maintenance of cellular strength. Biochim Biophys Acta 2007; 1773:675-86; PMID:17050008; http://dx.doi.org/ 10.1016/j.bbamcr.2006.09.018 [DOI] [PubMed] [Google Scholar]
- 15. Ciska M, Moreno Diaz de la Espina S. The intriguing plant nuclear lamina. Front Plant Sci 2014; 5:166; PMID:24808902; http://dx.doi.org/ 10.3389/fpls.2014.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masuda K, Xu ZJ, Takahashi S, Ito A, Ono M, Nomura K, Inoue M. Peripheral framework of carrot cell nucleus contains a novel protein predicted to exhibit a long alpha-helical domain. Exp Cell Res 1997; 232:173-81; PMID:9141634; http://dx.doi.org/ 10.1006/excr.1997.3531 [DOI] [PubMed] [Google Scholar]
- 17. Masuda K, Haruyama S, Fujino K. Assembly and disassembly of the peripheral architecture of the plant cell nucleus during mitosis. Planta 1999; 210:165-7; PMID:10592045; http://dx.doi.org/ 10.1007/s004250050666 [DOI] [PubMed] [Google Scholar]
- 18. Ciska M, Masuda K, Moreno Diaz de la Espina S. Lamin-like analogues in plants: the characterization of NMCP1 in Allium cepa. J Exp Bot 2013; 64:1553-64; PMID:23378381; http://dx.doi.org/ 10.1093/jxb/ert020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakamoto Y, Takagi S. LITTLE NUCLEI 1 and 4 regulate nuclear morphology in Arabidopsis thaliana. Plant Cell Physiol 2013; 54:622-33; PMID:23396599; http://dx.doi.org/ 10.1093/pcp/pct031 [DOI] [PubMed] [Google Scholar]
- 20. Dittmer TA, Stacey NJ, Sugimoto-Shirasu K, Richards EJ. LITTLE NUCLEI genes affecting nuclear morphology in Arabidopsis thaliana. Plant Cell 2007; 19:2793-803; PMID:17873096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Dittmer TA, Richards EJ. Arabidopsis CROWDED NUCLEI (CRWN) proteins are required for nuclear size control and heterochromatin organization. BMC Plant Biol 2013; 13:200; PMID:24308514; http://dx.doi.org/ 10.1186/1471-2229-13-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graumann K. Evidence for LINC1-SUN associations at the plant nuclear periphery. PloS One, 2014:e93406; PMID: 24667841; http://dx.doi.org/ 10.1371/journal.pone.0093406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X, Meier I. Efficient plant male fertility depends on vegetative nuclear movement mediated by two families of plant outer nuclear membrane proteins. Proc Natl Acad Sci USA 2014; 111:11900-5; PMID:25074908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avisar D, Abu-Abied M, Belausov E, Sadot E, Hawes C, Sparkes IA. A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol 2009; 150:700-9; PMID:19369591; http://dx.doi.org/ 10.1104/pp.109.136853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graumann K, Runions J, Evans DE. Characterization of SUN-domain proteins at the higher plant nuclear envelope. Plant J 2010; 61:134-44; PMID:19807882; http://dx.doi.org/ 10.1111/j.1365-313X.2009.04038.x [DOI] [PubMed] [Google Scholar]
- 26. Zhou X, Graumann K, Wirthmueller L, Jones JDG, Meier I. Identification of unique SUN-interacting nuclear envelope proteins with diverse functions in plants. J Cell Bio 2014; 205:677-92; PMID:24891605; http://dx.doi.org/ 10.1083/jcb.201401138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto C, Tamura K, Fukao Y, Shimada T, Hara-Nishimura I. The Novel Nuclear Envelope Protein KAKU4 Modulates Nuclear Morphology in Arabidopsis. Plant Cell 2014; 26:2143-55; PMID:24824484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I. Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell 2010; 22:4084-97; PMID:21189294; http://dx.doi.org/ 10.1105/tpc.110.079947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamura K, Hara-Nishimura I. The molecular architecture of the plant nuclear pore complex. J Exp Bot 2013; 64:823-32; PMID:22987840; http://dx.doi.org/ 10.1093/jxb/ers258 [DOI] [PubMed] [Google Scholar]
- 30. Libotte T, Zaim H, Abraham S, Padmakumar VC, Schneider M, Lu W, Munck M, Hutchison C, Wehnert M, Fahrenkrog B, et al. . Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol Biol Cell 2005; 16:3411-24; PMID:15843432; http://dx.doi.org/ 10.1091/mbc.E04-11-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol 2006; 26:3738-51; PMID:16648470; http://dx.doi.org/ 10.1128/MCB.26.10.3738-3751.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem 2010; 285:3487-98; PMID:19933576; http://dx.doi.org/ 10.1074/jbc.M109.071910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kracklauer MP, Banks SML, Xie XH, Wu YN, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly 2007; 1:75-85; PMID:18820457 [DOI] [PubMed] [Google Scholar]
- 34. Patterson K, Molofsky AB, Robinson C, Acosta S, Cater C, Fischer JA. The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol Biol Cell 2004; 15:600-10; PMID:14617811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Griffis AH, Groves NR, Zhou X, Meier I. Nuclei in motion: movement and positioning of plant nuclei in development, signaling, symbiosis, and disease. Front Plant Sci 2014; 5:129; PMID:24772115; http://dx.doi.org/ 10.3389/fpls.2014.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takagi S, Islam MS, Iwabuchi K. Chapter four - Dynamic Behavior of Double-Membrane-Bounded Organelles in Plant Cells. In: Kwang WJ, ed. Int Rev Cell Mol Biol: Academic Press, 2011:181-222. [DOI] [PubMed] [Google Scholar]
- 37. Xu XM, Meulia T, Meier I. Anchorage of plant RanGAP to the nuclear envelope involves novel nuclear-pore-associated proteins. Curr Biol 2007; 17:1157-63; PMID:17600715; http://dx.doi.org/ 10.1016/j.cub.2007.05.076 [DOI] [PubMed] [Google Scholar]
- 38. Karimi M, Inze D, Depicker A. GATEWAYTM vectors for Agrobacterium -mediated plant transformation. Trends Plant Sci 2002; 7:193-5; PMID:11992820 [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 2007; 104:34-41; PMID:17697981; http://dx.doi.org/ 10.1263/jbb.104.34 [DOI] [PubMed] [Google Scholar]
- 40. Wise AA, Liu Z, Binns AN. Three methods for the introduction of foreign DNA into Agrobacterium. Methods Mol Biol 2006; 343:43-53; PMID:16988332; http://dx.doi.org/ 10.1385/1-59745-130-4:43 [DOI] [PubMed] [Google Scholar]
- 41. Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium -mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735-43; PMID:10069079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


