Abstract
The prevalence of metabolic syndrome is increasing rapidly across the globe. Though the prevalence of the disease is similar in population of upper middle income and high income countries, the age of affected population is lower in upper middle income countries. This is attributed to genetic as well as changing life style factors. The contributing factors for type 2 diabetes range from genetic/epigenetic disposal, intra uterine nutrition, dietary pattern to sedentary lifestyle. The role of the gut microbiota in metabolic disorders is increasingly gaining importance. Several studies have reported significant difference in the profile of the gut microbiota in Caucasian population considering obese and type 2 diabetic populations while limited number of studies are available on populations from the developing world. The metabolites from the gut microbes contribute to the gut barrier integrity and a compromised barrier leads to leakage of inflammatory mediators into systemic circulation and hence increases insulin resistance. Attempts have been made at correcting metabolic syndrome through dietary changes by altering the gut microbiota with some success. This report is an attempt to explain the hypothesis of compromised nutrition altering the gut microbiota, gut metabolites, gut barrier function, systemic inflammation and hence insulin response.
Additional Search Terms: dysbiosys, gut metabolites, malnourishment, nutrition, obesity
Introduction
The prevalence of metabolic syndrome (mainly type 2 diabetes, obesity and cardiovascular disease) is increasing rapidly which are of major relevance for public health in developing countries than developed countries. Recent WHO statistics indicates that number of people with type 2 diabetes around the globe was about 382 Million in 2013 and is projected to be about 592 Million by 2035. In addition, 85% of premature deaths from metabolic syndromes occur in developing countries of which about 80% are associated with diabetes. Type 2 diabetes is projected to be the 7th leading cause of death by 2030 (WHO).
International Diabetes Federation (IDF) has projected statistics on prevalence of diabetes by income group and age as shown in Figure 1. The prevalence of type 2 diabetes is similar in population of high and upper middle income countries, but the age group of the affected population is lower in population of lower middle income countries, impacting the economy of the corresponding countries.1 In addition, the mortality due to diabetes is significantly higher in population of lower middle income countries compared to higher income countries (Fig. 2). It is suggested that genetic predisposition is one of the major contributors for higher prevalence of metabolic syndrome. Several genome wide association studies (GWAS) implicated genetic/epigenetic variations as one of the possible reasons for higher incidence of type 2 diabetes in specific ethnic origins.2-5 In addition to the genetic variations, intrauterine growth restriction during the development of the fetus and influence of external environment (mainly those that are obesogenic during the growth phase induce insulin resistance, reduce β cell mass and organ dysfunction) contribute to the development of type 2 diabetes.6,7 Recent WHO report indicates that percentage of underweight children in developing countries is significantly higher compared to that of developed countries. Majority of the children in these countries are malnourished during childhood but as they grow, increased accumulation of increased body fat leads to obesity. The disappropriate fat muscle distribution with high abdominal fat and low skeletal mass suggest8 that nutritional status is also varied in these 2 economies.
Figure 1.
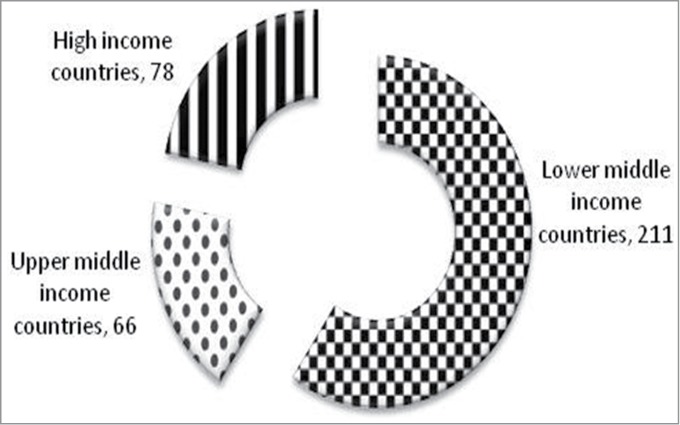
Number of people with diabetes in millions classified based on income groups, IDF 2013. The number of people with diabetes is similar in High income and upper middle income countries but the affected age group is lower in population of lower middle income countries, impacting the economy of the corresponding countries (Source: IDF 2013).
Figure 2.
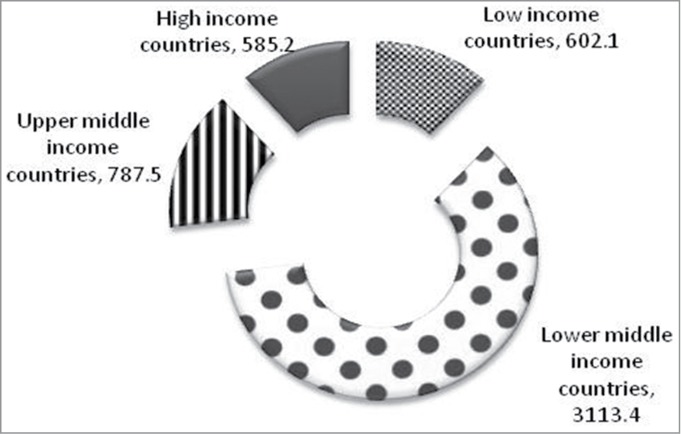
Number of deaths due to diabetes in thousands in 2013 classified based on income groups, IDF. The mortality due to diabetes is significantly higher in population of lower middle income countries compared to higher income countries (Source IDF 2013).
Metabolism of food
Post consumption, food is processed in the gut and subsequently absorbed by the body for either immediate energy requirement or stored for later use. The nutrient processing in the gut is influenced by various factors including the gut microbes. They influence the extraction of energy from the diet and their metabolites also act as signaling molecules.9 Unequivocal evidence demonstrates that gut microbes influence whole body metabolism by affecting the energy balance, gut permeability, metabolic endotoxemia, and inflammation that are associated with several metabolic disorders.10
Microbiota composition of the body
With the first report of Human Microbiome Project (HMP), information on the different roles played by the microbiota on different parts of the body has started emerging.11 In recently performed experiments, which analyzed bacteria in 27 sites of 7-9 healthy adults on 4 occasions, it was shown that biogeography of bacterial communities on the human body, although personalized, varies across body habitats and time and such trends may ultimately reveal how changes in microbiome influence the initiation or progression of diseases.12 The study demonstrated that the extent of diversity within the samples or between subjects varied based on their habitat. Oral and fecal samples showed higher bacterial diversity whereas vaginal samples were least diverse in nature. The uniqueness of each individual's microbial community seemed to be stable over time (relative to the population as a whole), which may be another feature of the human microbiome specifically associated with health.13
Composition of gut microbiota
The human gut harbours around trillions of bacteria and is considered an organ by itself based on the metabolic contributions made by the microbiota. It has also been observed that the relationship between gut microbes and humans is not merely commensal but rather a mutual relationship. The microorganisms of the gut contribute to the host by fermenting unused energy substrates, training the immune system, preventing growth of pathogenic bacteria, regulating the development of the gut, producing vitamins for the host (such as biotin, cobalamin and vitamin K), and stimulating the production of hormones that regulate satiety.
The intestinal microbiota is composed of 6 main phyla – Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, Fusobacteria and Verucomicrobia. At birth, the gut is populated with ∼100 species of bacteria which reaches adult like microbiome i.e. ∼1000 species within the first 3 yrs.14 Bacteroidetes and Firmicutes account for >90% of the total gut microbiota. With increasing age, the proportion of Firmicutes increase and Bacteroides decrease. For optimal health, a symbiotic relationship is maintained between gut microbiota and human host.
Gut microbiota profile changes at different stages of life and is influenced by different factors like age, dietary habits, environment and medicines. In a recent review, an excellent pictorial representation of these changes was depicted as shown in Figure 3.15 Though this is a compilation of data from different studies, significant influence of diet was observed on the profile of the microbiota. The succession of microbiota from babies to centenarians and the influence of diet, drug, nutrition and illness on the diversity of microbiota are depicted though the impact on the functionality needs to be clarified and will need further studies.
Figure 3.
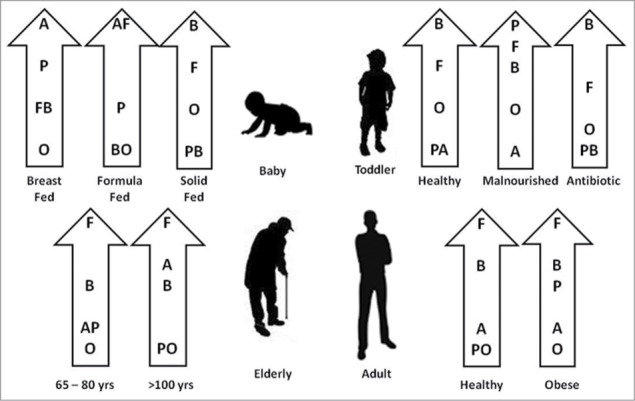
Proportion of different phyla of bacteria in the gut at different stages of life (Adapted from reference 15). Firmicutes (F); Bacteroidetes (B); Actinobacteria (A); Proteobacteria (P); Others (O). Human gut microbiota changes at different stages of life and also influenced by different factors like age, dietary habits, environment and medicines. The succession of microbiota from babies to centenarians and the influence of diet, drug, nutrition and illness on the diversity of microbiota are depicted.
It is proposed that extrinsic influencing factors modulate the diversity and function of the microbiota causing dysbiosis resulting in diseases like metabolic syndrome, inflammatory bowel disease, Non alcoholic fatty Liver disease, gastric ulcer, colon cancer asthma, atrophy, hypertension, mood and behavior through metabolites of the microbiota and hormone signaling.14 In a study group of subjects with low diversity in gut microbiota, demonstrated by low gene copies (LGC) was compared to subjects with high diversity demonstrated by high gene copies (HGC) and the association of gene copies with obese phenotype and serum markers of the same were evaluated. The adiposity phenotype of LGC group was associated with increased serum leptin, decreased serum adiponectin, insulin resistance, hyperinsulinaemia, increased levels of triglycerides and free fatty acids, decreased HDL-cholesterol and a more marked inflammatory phenotype (increased highly sensitive C-reactive protein (hsCRP) and higher white blood cell counts) than seen in HGC group. These associations suggest that the LGC individuals have metabolic disturbances which increase their risk of pre-diabetes and type-2-diabetes.16,17 This also leads to the conclusion that there is a requirement for functional diversity contributing to host metabolism by gut microbes that cater to better health conditions of the host.
Role of Gut microbiota in metabolism
Several animal studies have focused on the role played by gut microbiota on metabolism and metabolic pathways. Recent reports demonstrated that conventionally raised mice have higher serum metabolites from glycolysis and TCA cycle compared to germ free mice indicating that the conventional mice has higher energy harvesting capability. On conventionalization of germ free mice, within 14 days, the germ free mice became obese (accumulated 60% more adiposity) and insulin resistant.18 To investigate further the cause-effect relationship, between gut microbes and obesity, gut microbiota was transferred from lean or obese donor mice to germ free mice. About 25% and 50% increase in body fat was observed in germ free mice when microbiota was transferred from lean or obese mice to germ free mice respectively.19 On transferring Enterobacter species isolated from obese mice to germ free mice compounded with a high fat diet, the recipient mice turned obese. The microbe recipient mice fed with normal diet or the control group that did not receive any bacteria maintained on either high fat or normal diet remained lean.20 It indicates that not only the microbiota but the diet also plays a pivotal role in the outcomes.
In a recent study it was shown that abundance of the bacterial species Akkermansia muciniphila improved the metabolic profile of type 2 diabetic mice. A. muciniphila treatment reversed high-fat diet induced metabolic disorders, including fat mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance. A.muciniphila administration increased the intestinal levels of endocannabinoids that control inflammation, gut barrier function including gut peptide secretion.21 It is becoming more evident that specific species of bacteria that are present in smaller numbers rather than a particular class or phyla of bacteria play a predominant role in disease incidence.
Gut microbiota harvests energy for host by hydrolysis and fermentation of otherwise non digestible polysaccharides and generates monosaccharides and short chain fatty acids (SCFAs). Colonization of germ free mice with the gut microbiota of conventionally raised mice induces the expression of sodium/glucose transporter-1 (SGLT1) in the small intestine. The results reveal that a specific commensal bacterium Bacteroides thetaiotaomicron found in the intestine of both human and mice modulates expression of genes involved in several important intestinal functions, including nutrient absorption, mucosal barrier fortification, xenobiotic metabolism, angiogenesis, postnatal intestinal maturation22 and increases the density of capillaries underlying the small intestinal villus epithelium aiding absorption.23 These findings provide perspectives about the essential nature of the interactions between resident microorganisms and their hosts.
It is known that SCFAs produced by the gut microbiota are predominantly acetate, butyrate and propionate which are absorbed by the intestinal epithelial cells. Butyrate is used by intestinal epithelium and acetate and propionate enter peripheral tissues where it is used for lipogenesis and gluconeogenesis. Acetate enters systemic circulation and reaches peripheral tissues. It is suggested that SCFAs potentially contribute 6–10% of the basal energy requirements of people in countries where there is moderate dietary fiber intake and the contribution could be higher in individuals who consume more dietary fiber.24
SCFA produced by microbial fermentation acts as both energy substrates and signaling molecules. SCFA increase lipogenesis i.e., increases triglycerides, inhibits the inhibitor of lipoprotein lipase in small intestine which results in inhibition of fatty acid release from triglycerides, and hence promotes the cellular uptake of triglycerides resulting in increased storage. SCFAs activate G protein coupled receptors GPR41 (Free Fatty Acid Receptor3 or FFA3) and GPR43 (Free Fatty Acid Receptor 2 or FFA2) on the intestinal epithelial cells. GPR41 stimulation results in the release of peptide PYY which increases the gut transit rate and satiety. It has been demonstrated that fermentation of prebiotics by gut microbiota resulted in reduced hunger and increased satiety, thereby decreasing total energy intake by about 10%.25,26 Activation of GPR 43 reduces inflammation and stimulates GLP1 and GLP2 release from L cells.27 Changes in the distribution and localization of Zonula Occludens-1 (ZO-1) and Occludin (2 tight junction proteins) in intestinal tissue are associated with the increased gut permeability occurring in obese and diabetic rodents. This results in increased transportation of microbial products from the gut to blood triggering higher inflammation. Mice treated with prebiotics showed decreased intestinal permeability and improved tight-junction integrity compared to without treated with prebiotics which subsequently lowered the levels of plasma LPS and cytokines. Prebiotic ingestion increased the endogenous GLP-2 production. It has been observed that increased endogenous GLP-2 production is associated with improved mucosal barrier function via the restoration of tight junction protein expression and distribution.28 Administration of acetate through intravenous route or rectal route in insulin resistant individuals increased the secretion of GLP-1 and PYY. This intervention also reduced TNFα levels in plasma.29 The contribution of these metabolites is summarised in Table 1.14
Table 1.
Metaolites contributed by gut microflora and their potential benefits14
| Metabolites | Related bacteria | Potential biological functions |
|---|---|---|
| Short-chain fatty acids: acetate, propionate, butyrate, isobutyrate, 2-methylpropionate, valerate, isovalerate, hexanoate. | Clostridial clusters IV and XIVa of Firmicutes, species of Eubacterium, Roseburia, Faecalibacterium and Coprococcus | Reduction in colonic pH, lower growth of pathogens; water and sodium absorption; cholesterol synthesis; energy to the colonic epithelial cells, implicated in human obesity, insulin resistance and type 2 diabetes. |
| Bile acids: cholate, hyocholate, deoxycholate, chenodeoxycholate, α, β, ω muricholate, taurocholate, glycocholate, etc | Lactobacillus,Bifidobacteria, Enterobacter, Bacteroides, Clostridium. | Absorb dietary fats and lipid- soluble vitamins, facilitate lipid absorption, maintain intestinal barrier function, signal systemic endocrine functions to regulate triglycerides, cholesterol, glucose and energy homeostasis. |
| Choline metabolites: methylamine, di and tri methylamine, trimethylamine-N-oxide, dimethylglycine, betaine | Faecalibacterium prausnitzii, Bifidobacterium | Modulate lipid metabolism and glucose homeostasis. Involved in NAFLD, diet induced obesity, diabetes. |
| Phenolic, benzoyl, and phenyl derivatives | Clostridium difficile, F. prausnitzii, Bifidobacterium, Subdoligranulum, Lactobacillus | Detoxification of xenobiotics; indicate gut microbial composition and activity; utilize polyphenols. Urinary hippuric acid may be a biomarker of hypertension and obesity in humans. |
| Indole derivatives | Clostridium sporogenes, E. coli | Protect against stress-induced lesions in the GI tract; modulate expression of pro and anti inflammatory genes, strengthen barrier properties. Implicated in GI pathologies, brain-gut axis. |
| Vitamins: vitamin K, vitamin B12, biotin, folate, thiamine, riboflavin, pyridoxine | Bifidobacterium | Provide complementary endogenous sources of vitamins, strengthen immune function; exert epigenetic effects to regulate cell proliferation. |
| Polyamines | Campylobacter jejuni, Clostridium saccharolyticum | Exert genotoxic effects on the host, anti-inflammatory and antitumoral effects. Potential tumor markers. |
| Lipids: conjugated fatty acids, LPS, peptidoglycan, acylglycerols, sphingomyelin, cholesterol, phosphatidylcholines, phosphoethanolamines, triglycerides | Bifidobacterium, Roseburia, Lactobacillus, Klebsiella, Enterobacter, Citrobacter, Clostridium | Impact intestinal permeability, activate intestine brain-liver neural axis to regulate glucose homeostasis; LPS induces chronic systemic inflammation; conjugated fatty acids improve hyperinsulinemia, enhance the immune system and alter lipoprotein profiles. |
| Others: D-lactate, formate, methanol, ethanol, succinate, lysine, glucose, urea, a-ketoisovalerate, creatine, creatinine, endocannabinoids, 2-arachidonoylglycerol (2-AG), N-arachidonoylethanolamide, LPS, etc. | Bacteroides, Pseudobutyrivibrio, Ruminococcus, Faecalibacterium, Subdoligranulum, Bifidobacterium, Atopobium, Firmicutes, Lactobacillus | Direct or indirect synthesis or utilization of compounds or modulation of linked pathways including endocannabinoid system. |
Influence of nutrition on gut microbiota composition and function
The composition of gut microbiota is fairly stable in healthy conditions.30 It was shown that the human gut microbiome is shared among family members, but each person's gut microbial community varies in the specific bacterial lineages present. However, there was a wide array of shared microbial genes among sampled individuals, a 'core microbiome' at the gene level, rather than at the organism lineage level. It was observed that metabolic disorder is associated with phylum-level changes in the microbiota, reduced bacterial diversity, altered representation of bacterial genes and metabolic pathways.30-35
Malnourishment and nutrition in utero
Compromised nutritional status is prevalent in the population of low and middle income countries. It has been stated recently that nutritional problems of population in those countries, constitutes under nutrition, increasing over nutrition and obesity. Higher prevalence of under nutrition in the reproductive age and pregnancy results in restriction of foetal growth and low birth weight.36 In a study with women cohort followed for 3 stages i.e. prior to gestation, pregnancy and post gestation, it has been shown that the pregnant women were deficient in vitamin B12 and not folate which is generally assumed as a nutrient deficiency. Majority of them had methyl malonic acid and homocysteine which is indicative of low vitamin B12. This result in intra uterine growth restriction, smaller newborn babies who gain weight rapidly thereafter and by the age of 6 yrs, developed higher adiposity and insulin resistance.37 It can be noted that during one carbon cycle, dietary folate is used for the synthesis of S-adenosyl methionine (SAM) which is used in DNA methylation resulting in switching on and off of genes. Vitamin B12 is an important cofactor in this cycle. It can also be recalled here that the major source of vitamin B12 is through microbes in the gut.38 It can be hypothesized that the changes in the gut micro flora or the functional genes contributing to the synthesis of Vitamin B12 as a consortium during different stages of pregnancy, different nutritional states could possibly have an influence on the gene expression in a fetus or an individual respectively.
Increasing percentage of underweight, stunted growth and wasted children in developing countries is becoming a big issue. Majority of the children in these countries are malnourished.39 Comparison of gut microbiota from healthy and malnourished child have shown higher amount of human intestinal tissue exfoliation indicative of compromised gut barrier function. In malnourished child, a changed microbiota prone to activate inflammatory response and also malabsorption was observed.40 It is known that malnourishment increases infection and increased infections leads to malnourishment because of low nutrients absorption. So does this indicate that in developing countries, malnourishment leads to increased infection leading to altered gut microbiota function and composition resulting in type 2 diabetes? In gut of cholera infected children a reduction in number of major commensal bacteria of phyla Bacteroidetes, Firmicutes, Actinobacteria, an increase in harmful Proteobacteria to colonize the gut during acute infection state was observed.41 The observed microbiota alteration might explain the prevalent malnutrition in children of Bangladesh where diarrheal diseases are endemic. The contribution of gut microbiota in Kwashiorkor which is an acute form of childhood protein energy malnutrition was also investigated. The study was conducted on Malwian twin pairs followed for first 3 years of life.42 Pairs discordant for Kwashiorkar were compared for their gut microbiota and were treated with a specially designed food. Organisms with the most statistically significant differences, and whose relative proportions were higher in kwashiorkor microbiota, were (i) Bilophila wadsworthia, linked to inflammatory bowel disease (IBD) in human and (ii) Clostridium innocuum, a gut symbiont that can function as an opportunist in immune compromised hosts. When on therapy with specialized food, the metabolic functions of these malnourished kids started correcting itself but started regressing when the food was stopped. On transferring the fecal microbiota from Kwashiorkor affected child to germ free mice, the mice started losing weight and developed Kwashiorkor type of compromised metabolic function which was partially corrected with the therapeutic food. Thirty species-level taxa exhibited statistically significant changes in their representation in kwashiorkor microbiota transplant recipients. In case of gut microbiota transfer from healthy co twin, there was no loss in body weight irrespective of the diet provided. These studies indicate that severely compromised nutritional status alters the gut microbiota leading to poor absorption of nutrients and gut barrier function
Influence of diet on gut microflora
It is known that gut microbiota influences both nutrient absorption and regulates host genes. The shift in nutritional load rapidly influences the change in gut microbiota and about 20% increase in Firmicutes with corresponding decrease in Bacteroides increases the energy harvest by 150 kcal.43 Mice fed high fat diet showed reduced numbers of Bifidobacteria in the gut and increased endotoxemia44 and when these mice were supplemented with a prebiotic the growth of beneficial bacteria restore the levels of Bifidobacteria and also the endotoxemia in these mice.45 In the gut of individuals consuming a diet rich in animal products, Bacteroides were predominant and in individuals consuming plant material rich diet, Prevotella was the predominant species among the gut microbiota. In addition, the bacterial metabolites, the SCFAs were produced in higher amounts in individuals on plant material rich diet and they were beneficial in preventing the growth and establishment of potentially pathogenic microorganisms.46 Similarly in individuals on predominantly plant based diet, the microbiota was represented by enzymes for anabolism of metabolites like riboflavin synthesis, glutamate synthesis and amylase whereas in individuals predominantly on diet based on animal source, the microbiota was represented by enzymes for catabolism of metabolites like enzymes for degradation of amino acids, glycan degradation.47
Impact of gut microbiota composition and function–developed versus developing countries
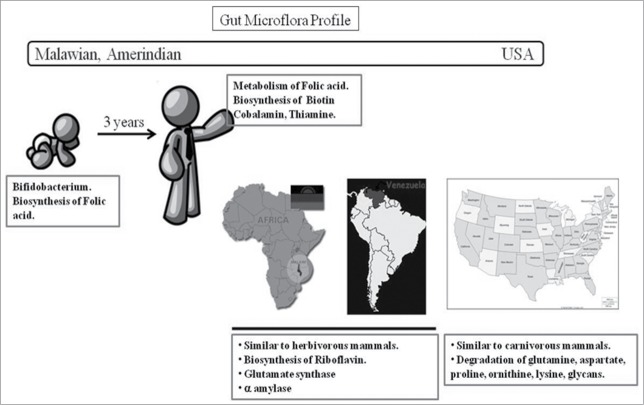
In a recent study the gut microbiota in individuals in developing and developed countries was compared. The comparison was done between Malawian individuals (least developed), Amerindian individuals from Venezuela (less developed) and USA (developed).47 The outcome of the study is shown in Figure 4. It is shown that the Malawian and Amerindian flora was significantly different from that of individuals from USA. The gut microbiota of children was less diverse and significantly different from adult where the bacterial diversity was very high.
Figure 4.
Major differences in the gut microbiota profile between the least developed, developing and dev;loped representative countries.The transformation of the gut microbiota in children to that of adult gut microbiota happens in the span of first 3 years of life. The functionality of the microbiota in children focused on enzymes of folic acid biosynthesis while in adults, the focus was on biosynthesis of biotin, cobalmin and thiamine and breakdown of folic acid. Malawian and Amerindian flora were very similar to that of herbivorous mammals. Enzymes for anabolism of metabolites like riboflavin synthesis, glutamate synthesis and amylase were highly represented. Gut microbiota of USA population is similar to carnivorous mammals, (enzymes for catabolism of metabolites like enzymes for degradation of amino acids, glycan degradation were highly represented).
It has been mentioned earlier, the transformation of the gut microbiota in children to that of adult gut microbiota happens in the span of first 3 years of life. The functionality of the microbiota in children focused on enzymes of folic acid biosynthesis while in adults, the focus was on biosynthesis of biotin, cobalmin and thiamine and breakdown of folic acid. Malawian and Amerindian flora were very similar to that of herbivorous mammals. Enzymes for anabolism of metabolites like riboflavin synthesis, glutamate synthesis and amylase were highly represented.
It was observed that Gut microbiota of USA population is similar to carnivorous mammals, (enzymes for catabolism of metabolites like enzymes for degradation of amino acids, glycan degradation were highly represented).47
Impact of transfer of gut microbiota on metabolic health
The impact of the gut microbiota on health and disease is best understood and appreciated in studies involving the transfer of the same. Several studies using animals and human models were performed to decipher the role of gut microbiota on energy homeostasis, obesity and insulin resistance. Several studies were done using both animal and human model on obesity and insulin resistance. Results of some studies are mentioned previously.18,20,21
Animal studies
Adult germ-free mice were colonized with microbiota harvested from the cecum of obese (ob/ob) or lean donors. It was also noted that the ob/ob donor microbiota had a greater relative abundance of Firmicutes compared with the lean donor microbiota. Strikingly, mice colonized with an ob/ob microbiota exhibited a significantly greater percentage increase in body fat over 2 weeks than mice colonized with a lean microbiota.19 In a study by Turnbaugh et al, a mouse model of obesity was produced by consumption of a prototypic western diet. Diet-induced obesity (DIO) produced a bloom in a single uncultured group of organisms with single ancestor from the Mollicutes class. This increase in the microorganism was diminished by subsequent dietary manipulations that limited weight gain. Microbiota transplantation from mice with DIO to lean germ-free recipients promoted greater fat deposition than transplants from lean donors.48
Human studies
When gut microbiota was transferred from lean individual to an individual with metabolic syndrome, insulin sensitivity of this recipient improved significantly both in peripheral tissues and hepatic tissue. When autologous transfer of gut microbiome was done, there was no change in the insulin sensitivity in these individuals with metabolic syndrome.49
These studies indicate that the role of gut microbiota in metabolic syndrome is an important one and interventions to modulate the microbiota for beneficial outcomes would be a desired target compared to pharmaceutical interventions to manage a disease.
Gut microbiota transfer through duodenum have proven advantageous in recurring infections like clostridia infection. Faecal transfer through colonoscopy supports efficient colonisation by the fecal bacteria because exposure to different digestive juices and conditions are avoided. In addition, bacteria present in smaller numbers and the ones that cannot be cultured outside will still be available to the individual under therapy. In metabolic syndrome where a single microbe cannot be identified as a culprit or a solution, a group of gut bacteria catering to different functions or sequential reactions to produce desired metabolite will be delivered as a group. The outcomes of the transfer can be observed faster compared to dietary interventions where one would need to wait for a longer duration of time to observe change if any.
Modulation of gut microbiota by dietary intervention
It is known that modulation of gut microbiota is possible and directing this to derive beneficial outcomes is desired to reverse progression toward metabolic syndrome. To achieve this, dietary modification would be an easier and acceptable approach. Dietary interventions with fibers, micronutrients and probiotics have demonstrated efficacy in reversing metabolic syndrome. An intervention with Ma-Pi-2 diet (mixture of complex carbohydrates, natural fibers and probiotics) on type 2 diabetic subjects has demonstrated beneficial effects in terms of HbA1c and HOMA-IR.53 In the case of probiotics interventions, not all but specific species and strains of bacteria demonstrate benefit.51 Interventions with probiotics like Lactobacillus or Bifidobacterium species for 4–12 weeks have demonstrated significant improvements in body fat and insulin sensitivity. In addition, interventions with prebiotics like resistance starch, inulin, arabinoxylan demonstrated improvements in biomarkers associated with metabolic syndrome.52 In a recent review, the role of different polysaccharide in diet in bringing about a shift in microbiota has been reported. Interventions with resistant starch and inulin increased the Bifidobacteria and Ruminococcus counts in the gut.53 With a 4-23 weeks intervention with pre and probiotics, they found that the prebiotics promote the growth of beneficial microbiota which reduce endotoxemia, stimulate the endocannabinoid system, reduce gut permeability, stimulate GPR43, thereby limiting inflammation, increase insulin sensitivity and reduce lipolysis. The short-chain fatty acids produced by the fermentation of carbohydrates bind to GPR41 in the intestine and promote the expression of PYY, which slows down the intestinal transit. Some SCFAs also activate GPR43, the expression of which is increased by a high-fat diet in the adipose tissue. This activation decreases lipolysis, increases PPARγ-related differentiation and thereby increases adiposity. In a recent study obese subjects with low gene copies of gut microbiota depicting its richness were subjected to dietary modulation with energy restricted high-protein diet for 6 weeks. The 35% decrease in energy intake after the first 6 weeks was associated with a reduction in body-fat mass, serum triglycerides, adipocytes diameter and improvements in insulin sensitivity measured by HOMA IR, and markers of metabolism and inflammation depicted by hsCRP.54
The long term advantage of modulation in gut microbiota and functions associated with this change in terms of metabolic syndrome will need longer duration of studies.
Conclusion
The role played by microbiota in health and disease is gathering evidence since the completion of the human microbiome project. The interactions between nutrition and gut microbiota is a 2 way process. Though this relationship is established, the cause or effect or both roles of microbiota and type 2 diabetes will not be clear until there is a long-term follow up study, but recent evidences (e.g. transfer studies etc) indicate more involvement towards cause than effect. The subject warrants further research to delineate the cause and effect relationship in the context of course of disease progression in diverse population. For example, the gut microbiota and its function in healthy individuals in developing nations or specific ethnicities are not adequately studied. The gut microbiota modulation from healthy to prediabetic and diabetic individuals has also not been well investigated. It is also becoming increasingly evident that the function of the microbes in the population becomes predominant rather than to what class or phylum it belongs to.
The profile of the gut microbiota in population from developing countries needs to be further evaluated and established. In addition to microbiomics, to understand the gene expression patterns and hence metabolic contribution, meta-transcriptomics and metabolomics also need attention. The modulation of this profile over a period of time in the general population and in prediabetic and diabetic population compared to healthy will indicate the cause or effect relationship of gut microbiota with the metabolic syndrome. Comparison of this profile with the profile of the population from developed countries might support the hypothesis of altered gut microbiota and function being one of the reasons for increased incidence of diabetes in the developing world.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf [Google Scholar]
- 2. Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, Been LF, Chia KS, Dimas AS, Hassanali N, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet 2011; 43(10):984-9; PMID:21874001; http://dx.doi.org/ 10.1038/ng.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rees SD, Hydrie MZ, Shera AS, Kumar S, O'Hare JP, Barnett AH, Kelly MA. Replication of 13 genome-wide association (GWA)-validated risk variants for type 2 diabetes in Pakistani populations. Diabetologia 2011; 54(6):1368-74; PMID:21350842; http://dx.doi.org/ 10.1007/s00125-011-2063-2 [DOI] [PubMed] [Google Scholar]
- 4. Lu S, Xie Y, Lin K, Li S, Zhou Y, Ma P. Lv Z, Zhou X. Genome-wide association studies-derived susceptibility loci in type 2 diabetes: confirmation in a Chinese population. Clin Invest Med 2012; 35(5):E327; PMID:23043714 [DOI] [PubMed] [Google Scholar]
- 5. Saxena R, Saleheen D, Been LF, Garavito ML, Braun T, Bjonnes A, Young R, Ho WK, Rasheed A, Frossard P, et al. Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in sikhs of Punjabi origin from India. Diabetes 2013; 62(5):1746-55; PMID:23300278; http://dx.doi.org/ 10.2337/db12-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 2004; 134(1):205-10; PMID:14704320 [DOI] [PubMed] [Google Scholar]
- 7. Kanaka-Gantenbein C. Fetal origins of adult diabetes. Ann N Y Acad Sci 2010; 1205: 99-105; PMID:20840260; http://dx.doi.org/ 10.1111/j.1749-6632.2010.05683.x [DOI] [PubMed] [Google Scholar]
- 8. Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutrit 2009; 102(4): 632- 641; PMID:19203416; http://dx.doi.org/ 10.1017/S0007114508207221 [DOI] [PubMed] [Google Scholar]
- 9. Vangaveti V, Shashidhar V, Jarrod G, Baune BT, Kennedy RL. Free fatty acid receptors: emerging targets for treatment of diabetes and its complications. Ther Adv Endocrinol Metab 2010; 1(4): 165-75; PMID:23148161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012. 489: 242-9; PMID:22972297; http://dx.doi.org/ 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 11. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012; 13:260-70; PMID:22411464; http://dx.doi.org/ 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009; 326: 1694-7; PMID:19892944; http://dx.doi.org/ 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 2012; 486: 207-214; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-Gut microbiota metabolic interactions. Science 2012; 336: 1262-7; PMID:22674330 [DOI] [PubMed] [Google Scholar]
- 15. Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol 2012; 2 (104): 1-11; PMID:22919693; http://dx.doi.org/ 10.3389/fcimb.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 500: 541-6; PMID:23985870; http://dx.doi.org/ 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 17. Fang S, Evans RM. Wealth management in the gut. Nature 2013; 500: 538-9; PMID:23985869 [DOI] [PubMed] [Google Scholar]
- 18. Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004; 101(44):15718-23; PMID:15505215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027-31; PMID:17183312 [DOI] [PubMed] [Google Scholar]
- 20. Fei N, Zhao L. An opportunistic pathogen isolated from the gut of obese human causes obesity in germfree mice. ISME J 2013; 7 (4): 880-4; PMID:23235292; http://dx.doi.org/ 10.1038/ismej.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013; 110(22):9066-71; PMID:23671105; http://dx.doi.org/ 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001; 291 (5505): 881-4; PMID:11157169 [DOI] [PubMed] [Google Scholar]
- 23. Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002; 99(24):15451-5; PMID:12432102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutrit 1984; 39: 338-42; PMID:6320630 [DOI] [PubMed] [Google Scholar]
- 25. Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutriti 2006; 60, 567-72; PMID:16340949 [DOI] [PubMed] [Google Scholar]
- 26. Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 2009; 90, 1236-43; PMID:19776140 [DOI] [PubMed] [Google Scholar]
- 27. Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Therapeut 2011. 130(2): 202-12; PMID:21295072; http://dx.doi.org/ 10.1016/j.pharmthera.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 28. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009. 58(8):1091-03; PMID:19240062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br J Nutrit 2010; 103(3):460-6; PMID:19818198; http://dx.doi.org/ 10.1017/S0007114509991863 [DOI] [PubMed] [Google Scholar]
- 30. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 2009; 457(7228):480-4; PMID:19043404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hullar MA, Lampe JW. The gut microbiome and obesity. Nestle Nutr Inst Workshop Ser 2012; 73:67-79; PMID:23128767 [DOI] [PubMed] [Google Scholar]
- 32. Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA Surg 2013; 148 (6): 563-9; PMID:23571517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect 2013;19(4): 305-13; PMID:23452229 [DOI] [PubMed] [Google Scholar]
- 34. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med 2013; 34 (1):39-58; PMID:23159341; http://dx.doi.org/ 10.1016/j.mam.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 35. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013; 341: 1241214-1-1241214-10; PMID:24009397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet 2013; 382: 427-51; PMID:23746772 [DOI] [PubMed] [Google Scholar]
- 37. Yajnik CS, Deshmukh US. Fetal programming: maternal nutrition and role of one-carbon metabolism. Rev Endocr Metab Disord. 2012. 13(2):121-7; PMID:22415298; http://dx.doi.org/ 10.1007/s11154-012-9214-8 [DOI] [PubMed] [Google Scholar]
- 38. Raux E, Schubert HL, Warren MJ. Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum. Cell Mol Life Sci 2000; 57(13-14):1880-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. http://data.worldbank.org/child-malnutrition [Google Scholar]
- 40. Sengupta S, Mohammed MH, Ghosh TS, Kanungo S, Nair GB, Mande SS. Metagenome of the gut of a malnourished child. Gut Pathogens 2011; 3:7; PMID:21599906; http://dx.doi.org/ 10.1186/1757-4749-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Monira S, Nakamura S, Gotoh K, Izutsu K, Watanabe H, Alam NH, Nakaya T, Horii T, Ali SI, Iida T, Alam M. Metagenomic profile of gut microbiota in children during cholera and recovery. Gut Pathog 2013; 5(1):1; PMID:23369162; http://dx.doi.org/ 10.1186/1757-4749-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. Gut Microbiomes of Malawian Twin Pairs Discordant for Kwashiorkor. Science 2013; 339: 548-54; PMID:23363771; http://dx.doi.org/ 10.1126/science.1229000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jumpertz R, Le D, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 2011; 94: 58-65; PMID:2154353; http://dx.doi.org/ 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007. 56(7):1761-72; PMID:17456850; http://dx.doi.org/ 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 45. Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microbiota improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007; 50 (11): 2374-2383; PMID:17823788 [DOI] [PubMed] [Google Scholar]
- 46. Filippo CD, Cavalieri D, Paola MD, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS 2010. 107(33): 14691-14696; PMID:20679230; http://dx.doi.org/ 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Gloria M, Bello D, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486 (7402): 222-7; PMID:22699611; http://dx.doi.org/ 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mMouse distal gut microbiome. Cell Host Microbe 2008; 3:213-23; PMID:18407065; http://dx.doi.org/ 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vrieze A, Nood EV, Holleman F, Salojarvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913-6; PMID:22728514 [DOI] [PubMed] [Google Scholar]
- 50. Fallucca F, Porrata C, Fallucca S, Pianesi M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab Res Rev 2014; 30(Suppl. 1): 48-54; PMID:24532292; http://dx.doi.org/ 10.1002/dmrr.2518 [DOI] [PubMed] [Google Scholar]
- 51. Yin YN, Yu QF, Fu N, Liu XW. and Lu FG. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol 2010; 16(27): 3394-401; PMID:20632441; http://dx.doi.org/ 10.3748/wjg.v16.i27.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delzenne NM, Neyrinck AM, Backhed F. and Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 2011; 7:639-46; PMID:21826100; http://dx.doi.org/ 10.1038/nrendo.2011.126 [DOI] [PubMed] [Google Scholar]
- 53. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012; 9:577-89; PMID:22945443; http://dx.doi.org/ 10.1038/nrgastro.2012.156 [DOI] [PubMed] [Google Scholar]
- 54. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Chatelier EL, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500:585-8; PMID:23985875 [DOI] [PubMed] [Google Scholar]