Abstract
Background
Vitamin D plays a role in several immune-mediated diseases, but its association with inflammatory bowel disease (IBD) is unclear. We conducted a systematic review and meta-analysis to assess the association between IBD and vitamin D deficiency.
Methods
We searched electronic databases from inception to December 2014 for observational studies reporting the presence of vitamin D deficiency (defined as serum 25-hydroxycholecalciferol [25(OH)D] level of ≤20 ng/ml) in IBD patients and having a control group without IBD. Odds ratios (OR) were combined using a random effects model. Meta-regression was performed using latitude as a moderator. Study quality was assessed using the Newcastle-Ottawa scale.
Results
Out of 816 citations, 14 eligible studies were identified, comprising 1891 participants (938 IBD cases and 953 controls). Meta-analysis showed that patients with IBD had 64% higher odds of vitamin D deficiency when compared to controls (OR = 1.64; 95% CI: 1.30, 2.08; I2 = 7%; p < 0.0001). UC patients had more than double the odds of vitamin D deficiency when compared to normal controls (OR = 2.28; 1.18, 4.41; I2 = 41%; p=0.01). Latitude did not influence the association between IBD and vitamin D deficiency (p = 0.34). Generalizability of our results might be limited as we summarized unadjusted ORs, due to non-availability of adjusted ORs in individual studies.
Conclusions
IBD is significantly associated with having higher odds of vitamin D deficiency. Well-designed RCTs and longitudinal studies are needed to further clarify the role of vitamin D in IBD pathogenesis and its therapy.
Keywords: Meta-analysis, Inflammatory Bowel Disease, Crohn’s Disease, Ulcerative Colitis, Vitamin D
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic, relapsing-remitting systemic disease that includes two major forms, Crohn’s disease (CD) and Ulcerative colitis (UC). CD primarily involves the ileum and colon, but it may affect any region of the gastrointestinal tract, while UC is mostly limited to the colon and/or rectum. The prevalence of IBD is increasing worldwide, with approximately 3 million people affected in Europe and 1.5 million in the USA and rapidly increasing trends observed in the Asia-Pacific regions1–4. IBD has a significant impact on health related quality-of-life5. It poses a significant economic burden, with estimated annual direct medical costs of nearly 3 billion US dollars6, 7.
The exact etiology of IBD has not been fully elucidated; however, it is thought to result from an inappropriate and ongoing activation of the immune system against environmental triggers in genetically predisposed individuals8, 9. Risk factors associated with IBD include altered intestinal flora9, 10, a diet rich in carbohydrates and fats11, oral contraceptives12 and living in urban areas13. A stressful lifestyle is considered to exacerbate the disease14. In this setting, an aberrant innate immune response to gut luminal agents, possibly facilitated by an impaired mucosal barrier function, results in the stimulation of dendritic cells and subsequent activation of the inflammatory cascade, leading to intestinal inflammation15, 16.
Vitamin D is a pleiotropic hormone with a diverse range of effects ranging from immune modulation to cell differentiation and intercellular adhesion. Several in vivo and in vitro studies have examined the role of vitamin D in immune-mediated diseases like IBD17–19. The consequences of vitamin D deficiency on the gastrointestinal tract include, but are not limited to, decreased colonic bacterial clearance20, reduced expression of tight junctions in the intestinal epithelium21, and elevated Th1-driven inflammation at the gut level22.
Hypovitaminosis D is reported to be as high as 60% in IBD patients23, although it is not clear whether it results from IBD-related malabsorption due to intestinal mucosal damage24, or whether it is a possible contributor to disease onset and progression25, 26. Evidence from observational studies remains questionable, as some studies report lower circulating vitamin D levels in IBD27–43, while others44–53 do not. Given the lack of clarity regarding the association of vitamin D deficiency with IBD, we decided to conduct a systematic review and meta-analysis of observational studies looking at the association of IBD and its subtypes with vitamin D deficiency.
MATERIALS AND METHODS
Study protocol
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines54. A comprehensive search of major electronic databases was conducted for articles from inception through December 2014. The following databases were included: 1) PubMed, 2), the COCHRANE library 3) EMBASE, and 4) CINAHL. The search utilized the terms ‘Vitamin D’, ‘ergocalciferol’, ‘Inflammatory bowel disease’, ‘Crohn’s disease’ and ‘Ulcerative colitis’ in several combinations. The detailed search strategy is presented in the online appendix. In addition, review articles on the topic were searched for eligible articles. The search strategy was not limited by language. We did not attempt to contact the authors of the articles for retrieving additional information or clarifications.
Inclusion and exclusion criteria
Two authors (RDP and DP) independently reviewed abstracts and articles for eligibility. Conflicts were resolved in consultation with a senior author (FC)55. Inclusion of studies was limited to case-control, cohort and cross-sectional studies with a control group that reported dichotomous outcomes of vitamin D deficiency in adult or pediatric subjects. Vitamin D deficiency was defined as circulating 25(OH)D ≤20 ng/ml (≤50 nmol/L), according to the Endocrine Society Guidelines56. We did not exclude studies on the basis of disease parameters (IBD activity, severity, duration of disease or region/extent of involvement), previous or current IBD therapy (including use of corticosteroids, salicylates and biologics), history of IBD-related surgery and vitamin D supplementation.
The following data were extracted (Table 1):
Study characteristics: primary author, year of publication, time period of study, country, latitude of the area where the study was conducted, seasonality data, number of patients with IBD and in the control group
Patient characteristics: age, sex, race/ethnicity, BMI, smoking status, serum vitamin D levels, current IBD-related therapy, prior IBD-related surgery, vitamin D supplementation and tanning habits
Disease characteristics: distribution of IBD subtypes (CD vs. UC), disease activity, site/s and extent of involvement and disease duration
Assay characteristics: type of assay used for circulating vitamin D assessment (such as RIA or ELISA) and inter/intra-assay coefficients of variability
Outcome measures: Prevalence of vitamin D deficiency in participants with and without IBD, as defined by the number or percentage of participants with circulating vitamin D levels ≤20 ng/ml or adjusted ORs and a measure of variability such as 95% confidence interval (CI) or standard error (SE).
Table 1.
Characteristics of the included studies.
| Author, year |
Country, latitude (°)1 |
Time period of study |
25(OH)D assay |
IBD/control | N. | Sex (M/F) |
Race/Ethnicity (%) | Age (years) (SD) |
Mean 25(OH)D (SD or CI)§,* |
|---|---|---|---|---|---|---|---|---|---|
| Grunbaum 2013 | Canada, Montréal 45.46 |
March 2009–April 2011 | RIA | IBD (CD/UC) | 55 (34/21) | 21/34 | Caucasian-95%. Jewish-51% | CD: 39.9 (12.3) UC: 44.2 (13.7) | 71.2 (32.8)§ |
| Non-IBD | 48 | 10/38 | Caucasian-79%. Jewish-42% | 39.6 (13.8) | 68.3 (26.2)§ | ||||
| Souza 2008 | Brasil, Curitiba 25.42 |
N/A | RIA | IBD (CD/UC) | 76 (39/37) | 33/43 | N/A | CD: 32.1 (8.7) UC: 35.0 (8.5) |
CD: 25.9 (8.2)* UC: 21.8 (8.0)* |
| Non-IBD | 40 | 16/24 | N/A | 34 (7) | 34.4 (12.8)* | ||||
| Silvennoinen 1996 | Finland, Oulu 65.01 |
April–May 1993 | RIA | IBD (CD/UC) | 150 (76/67) | 79/71 | N/A | 40 (9.3) | 28.4 (12.0)§ |
| Non-IBD | 73 | 35/38 | N/A | 40.8 (9.3) | 36.1 (16.7)§ | ||||
| Suibhne 2012 | Irland, Dublin 53.34 |
All seasons | RIA | IBD (CD/UC) | 81 (81/−) | 33/48 | Caucasian-100% | 36.4 (11) | 47.76 (27.27)§ |
| Non-IBD | 70 | 28/42 | Caucasian-100% | 36.3 (9.5) | 51.86 (24.53)§ | ||||
| Garg 2013 | Australia, Melbourne 37.86 |
All seasons | ECLA | IBD (CD/UC) | 71 (40/31) | 39/32 | Australian/NZ-72%, European-18%, Other-8% | CD: 41 (23–76) UC: 44 (22–82) |
CD: 70 (61–78)§ UC: 70 (58–81)§ |
| Non-IBD | 23 | 10/13 | Australian/NZ-70%, European-9%, Other-26% | 39 (22–68) | 66 (55–76)§ | ||||
| Gilman 2006 | Irland, Cork 51.89 |
All seasons | ELISA | IBD (CD/UC) | 73 (47/26) | ns | N/A | CD: 36.0 (11.6) UC: 40.5 (11.0) |
CD: 71.6 (33)§ UC: 63.9 (20.5)§ |
| Non-IBD | 73 | ns | N/A | CD ctr: 35.9(11.5) UC ctr: 40.3(11.2) |
CD ctr: 133 (69.2)§ UC ctr: 109 (50.8)§ |
||||
| Duggan 2004 | Irland, Cork 51.89 |
September–October 2002 | ELISA | IBD (CD/UC) | 44 (44/−) | 15/29 | N/A | 36.9 (11) | 75 (28.7)§ |
| Non-IBD | 44 | 15/29 | N/A | 36.7 (11) | 105.3 (55.5)§ | ||||
| Prosnitz 2013 | Pennsylvania, Philadelphia 40.00 |
All seasons | RIA | IBD (CD/UC) | 78 (78/−) | 44/34 | Black-10%, Non-Black-90% | 12.7 (2.8) | Black: 10.5 (4.6)* Non-Black: 23.5 (9.2)* |
| Non-IBD | 221 | 112/109 | Black-28%, Non-Black-72% | 13.5 (4.4) | Black: 15.8 (7.9)* Non-Black: 25.3 (8.7)* |
||||
| Laakso¥ 2012 | Finland, Helsinki 60.17 |
June 2004–December 2005 | HPLC | IBD (CD/UC) | 80 (49/28) | 37/43 | N/A | 14.9 (5.1–20.1) | N/A |
| Non-IBD | 80 | 37/43 | N/A | 14.4 (7.4–18.8) | N/A | ||||
| Tajika 2004 | Japan, Nagoya 35.16 |
December 2001–January 2002 | CPBA | IBD (CD/UC) | 44 (33/11) | 31/13 | Asian-100% | CD: 37.6 (7.5) UC: 47.6 (12.4) |
CD: 15.2 (6.5)* UC: 17.6 (4.7)* |
| Non-IBD | 15 | 8/7 | Asian-100% | 37.7 (10) | 16.9 (5.2)* | ||||
| De Bruyn 2014 | Netherlands, Amsterdam 52.37 |
September–December 2012 | CLIA | IBD (CD/UC) | 101 (101/−) | 31/70 | Caucasian-83% | 41 (30–50) | 51.6 (26.6)§ |
| Non-IBD | 41 | 8/33 | Caucasian-88% | 28 (24–39) | 60.8 (27.6)§ | ||||
| Veit 2014 | Massachussets, Worcester 42.27 |
January 2007–June 2013 | CLIA | IBD (CD/UC) | 58 (40/18) | 31/27 | White-88%, Black-3%, Multiethnicity-3%, Unknown-5% | CD: 16.6 (2.2) UC: 16.1 (1.9) |
CD: 61.69 (24.43)§ UC: 53.26 (25.51)§ |
| Non-IBD | 116 | 49/67 | White-80%, Black-8%, Multiethnicity-5%, Unknown-4% | 14.5 (4.3) | 65.32 (27.97)§ | ||||
| Dumitrescu 2014 | Romania, Iasi 47.13 |
March 2011–June 2012 | HPLC | IBD (CD/UC) | 47 (14/33) | 25/22 | N/A | CD: 36 (9) UC: 42 (14) |
24 (10)¶ |
| Non-IBD | 94 | 50/44 | N/A | 42 (12) | 31 (13)¶ | ||||
| Salacinski 2013 | Pennsylvania, Pittsburgh 41.94 |
October–November | HPLC | IBD (CD/UC) | 19 (19/−) | 9/10 | N/A | 44.16 (10.28) | 32.0 (9.1)* |
| Non-IBD | 19 | 9/10 | N/A | 41.68 (11.19) | 35.3 (11.1)* |
degrees of latitude as reported by the included studies or, if data not available, derived from the region where research was conducted (see text)
N/A = not available
circulating 25(OH)D expressed as nmol/l
circulating 25(OH)D expressed as ng/ml
circulating 25(OH)D expressed as mcg/l
winter values were used (IBD: n=41; non-IBD: n=76).
RIA: radio-immuno assay
ECLA: electro-chemiluminescence assay
ELISA: enzyme linked immuno-sorbent assay
CPBA: competitive-protein binding assay
HPLC: high-performance liquid chromatography
NZ: New Zealander
Assessment of study quality
Quality of included articles was assessed using the Newcastle-Ottawa Scale for case-control studies57. The following items were assessed:
Adequacy of definition of cases: IBD cases had to be confirmed by clinical, histological and radiographic confirmation
Representativeness of the defined cases
Criteria used for selection of controls
Comparability of cases and controls. Age/sex were considered the most important matching factors, and an additional star was awarded if the study controlled for at least one additional confounder such as race, BMI, sun exposure, vitamin D supplementation, smoking, socioeconomic status or absence of bone pathology
Method of ascertainment of exposure, i.e. assessment of vitamin D levels in cases and controls.
Statistical Analysis
Using a random effects model, studies were pooled to calculate the odds of vitamin D deficiency in the IBD group in comparison to the control group. We used adjusted ORs whenever available, otherwise dichotomous data were used to calculate unadjusted ORs. Heterogeneity between studies was assessed by the I2 statistic as defined by the Cochrane handbook for systematic reviews58. Accordingly, an I2 value of 50% or more was considered to represent a substantial heterogeneity. Review manager 5 was used to generate forest plots, and generated funnel plots were used to test for publication bias58. Since studies reported the prevalence of vitamin D deficiency by specific groups, namely adults or children, we conducted stratified meta-analyses based on these groups. We also conducted stratified analysis based on the two IBD subtypes, CD and UC. Since latitude affects sunlight exposure and thereby serum vitamin D levels as well, we decided to perform meta-regression using latitude as a moderator. The ‘metafor’ package59 in R software was used to perform random-effects meta-regression and plot the graph60. We also performed sensitivity analysis based on two different cut-offs for vitamin D deficiency (i.e. ≤20 ng/ml and <15 ng/ml).
RESULTS
Out of 816 citations, 14 articles with a total of 1891 patients met our predefined inclusion and exclusion criteria. The study flow is presented in Figure 1. The descriptive characteristics of the included studies are presented in Table 1. Women comprised 50.7% of the total IBD population. Thirteen studies reported on previous surgery and 22% of 788 IBD patients had had a history of bowel resection. Thirteen studies reported on vitamin D supplementation and 24.3% of 862 IBD cases and 14.3% of 913 controls were on vitamin D supplements (Supplementary Table 1). Data on disease location and extent and seasonality are reported in Supplementary Table 2.
Figure 1.
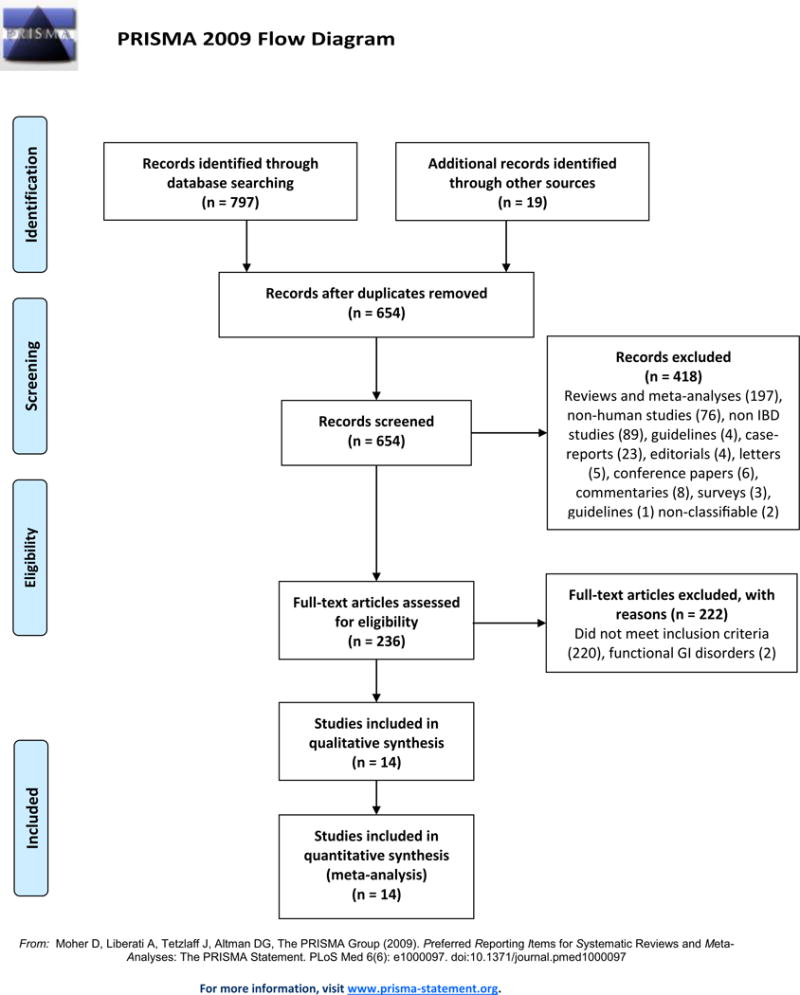
PRISMA flow diagram.
The methodological quality of these studies based on the Newcastle-Ottawa scale is described in Supplementary Table 3. Three studies matched for age/sex and at least one other a priori defined confounding variable, while 8 studies only matched for age/sex. Studies had a quality score between 6 and 9 stars.
Description of excluded studies
The study by Alkhouri et al. was excluded as a higher cut-off for vitamin D deficiency was used (30 ng/ml)47. The study by McCarthy et al. was excluded as the prevalence of vitamin D deficiency was examined in two different seasons resulting in unit-of-analysis errors when combined29. The study by Sylvester et al. was excluded, as events were not observed in the examined groups32. Two studies42, 43 where the control group participants comprised of persons with functional gastrointestinal disorders were excluded. Three other studies30, 33, 51 that reported extractable data for only one of the two groups, i.e. either for cases or controls only, were also excluded.
Results of the meta-analysis
Vitamin D deficiency in IBD cases vs. non-IBD controls
Meta-analysis of 14 studies including 1891 patients (938 IBD cases and 953 controls) showed that patients with IBD had 64% higher odds of vitamin D deficiency when compared to controls (OR = 1.64; 95% CI: 1.30, 2.08; p<0.0001) (Figure 2). Heterogeneity between studies was low (I2 = 7%). We did not assess for publication bias using funnel plots due to the lack of sufficient studies.
Figure 2.
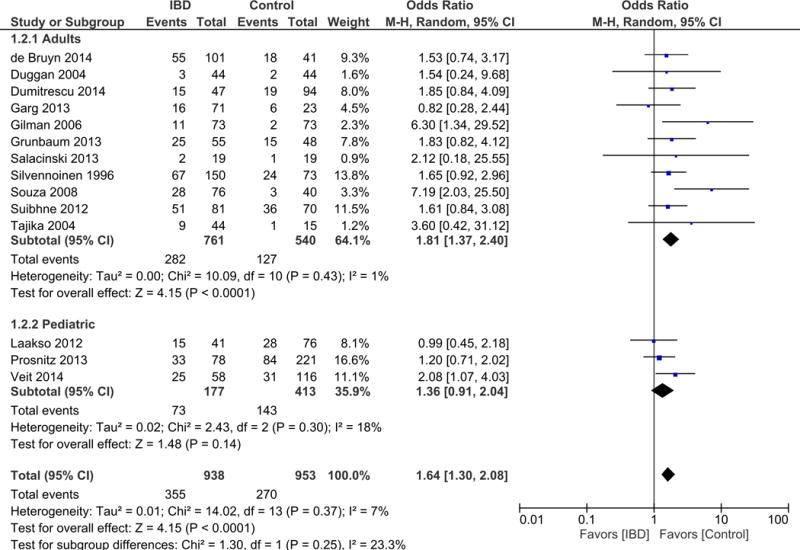
Meta-analysis of Vitamin D deficiency in IBD cases compared to non-IBD controls. Stratified analysis based on adult versus pediatric participants.
Stratified analysis based on adult vs. pediatric participants
Of the 14 included studies, 11 reported on adult participants, whereas 3 were on pediatric participants. Therefore, we conducted a stratified meta-analysis based on age (adult vs. pediatric). Meta-analysis of the 11 studies reporting on adults showed that 761 adult participants with IBD had nearly double the odds of vitamin D deficiency when compared to 540 controls (OR = 1.81; 95% CI: 1.37, 2.40; I2 = 1%; p < 0.0001). Meta-analysis of the 3 studies reporting on children showed that 177 pediatric cases with IBD had a higher, though not significant odds of vitamin D deficiency compared to 413 non-IBD controls (OR = 1.36; 95% CI: 0.91, 2.04; I2 = 18%; p = 0.14) (Figure 2).
Vitamin D deficiency in CD and UC cases vs. controls
We also conducted meta-analysis of studies that reported vitamin D deficiency by type of IBD. Meta-analysis of 12 studies reporting on vitamin D deficiency in CD showed that 570 participants with CD had a significantly higher odds of vitamin D deficiency compared to 778 controls (OR = 1.63; 95% CI: 1.24, 2.13; p = 0.0004) (Figure 3). Heterogeneity between studies was not detected (I2 = 0%). Meta-analysis of 7 studies reporting on prevalence of vitamin D deficiency in UC showed that 177 participants with UC had more than double the odds of vitamin D deficiency compared to 362 controls (OR = 2.28; 95% CI: 1.18, 4.41; p = 0.01) (Figure 4). Heterogeneity between studies was moderate (I2 = 41%).
Figure 3.
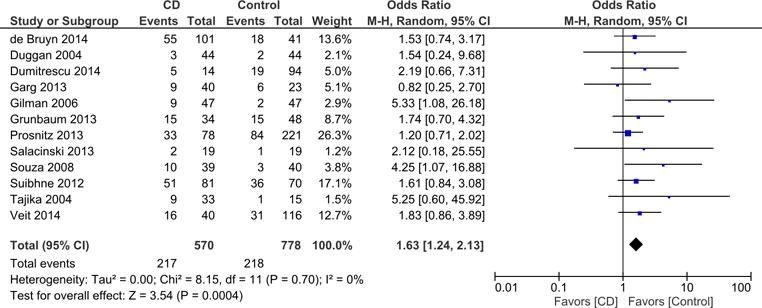
Stratified meta-analysis of Vitamin D deficiency in CD cases compared to controls.
Figure 4.
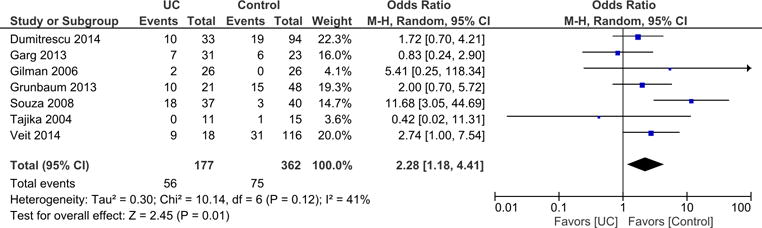
Stratified meta-analysis of Vitamin D deficiency in UC cases compared to controls.
Meta-regression using latitude as a moderator
Whenever latitude data was available in studies, we extracted this information. When studies did not provide latitude data, we used the region of the hospital where the study was conducted (or using the region of the source of cases and controls) to obtain the latitude as we felt that this would be a reasonable approximation of the true latitude. We performed meta-regression analysis on the main meta-analysis (IBD vs. controls), which showed that latitude had no effect on the association between IBD and serum vitamin D status (p = 0.34) (Supplementary Figure 1).
Sensitivity analysis based on Vitamin D deficiency cut-offs
Out of the 14 studies, 5 studie27, 35, 40, 46, 50 reported on vitamin D deficiency using a more stringent cut-off of 15 ng/ml61. Therefore, we conducted sensitivity analyses to check if the exclusion of these studies would change the effect estimate. Exclusion of these 5 studies from the meta-analysis did not substantially influence the summary estimate (OR = 1.65; 95% CI: 1.25, 2.19; I2 = 11%; p = 0.0004).
DISCUSSION
Our meta-analysis shows that vitamin D deficiency is significantly higher in IBD patients, as well as its subtypes, when compared to non-IBD subjects. UC, in particular, was found to be associated with more than double the odds of vitamin D deficiency compared to the absence of the disease. Stratified analysis based on age showed that adult IBD patients had nearly twice the odds of vitamin D deficiency when compared to healthy adult controls, whereas a similar comparison in the pediatric population showed a higher odds of vitamin D deficiency in the presence of IBD, but didn’t reach statistical significance, likely due to a small sample size. Latitude did not seem to moderate the association between IBD and serum vitamin D. Sensitivity analysis after excluding studies that used a lower cut-off of 15 ng/ml did not substantially influence the pooled effect estimate. All studies were of moderate-high quality as assessed by the Newcastle-Ottawa scale, with a rating between 6 and 9 stars.
Prior studies62–64 have reviewed the scientific evidence regarding the role of vitamin D in IBD and concluded that crucial aspects of this relationship are still to be elucidated. In particular, whether effective preventive or therapeutic strategies with vitamin D supplementation can be adopted in IBD, and how they should be conducted to obtain meaningful clinical results, still remains an open question. Therefore, we believe that our meta-analysis evaluating vitamin D status in a relatively large cohort of 1891 patients might provide useful information for future investigations.
Hypovitaminosis D in IBD may have several explanations. It is of significance that both conditions are associated with common environmental factors such as air pollution, industrialization, high latitude, and seasonality13. Hypovitaminosis D in the context of IBD may be the consequence of malabsorption, due to bowel inflammation or surgical resection24; reduced outdoor activities with less UV exposure, as a consequence of IBD symptomatology63; or increased uptake of vitamin D by inflammatory cells in the affected sites44. Consistent with the latter point, enhanced 25(OH)D uptake has been demonstrated in peripheral monocyte/macrophages from HIV-infected patients with hypovitaminosis D after in vitro stimulation with the viral envelope protein gp120 or lipopolysaccharide (LPS)65. Low vitamin D levels may also negatively affect the gut barrier and immune system functions, thus potentially impacting IBD onset and progression. In particular, vitamin D has been demonstrated to inhibit several pro-inflammatory pathways66, 67, modulate autophagy67, decrease oxidative stress68, reduce white cells differentiation and activation67, 69, 70, and enhance expression of tight junctions in the intestinal epithelium, thereby influencing mucosal permeability and tissue integrity21. In vivo studies show that vitamin D receptor (VDR) knockout mice are more susceptible to bowel inflammation71, and genetic studies have also linked VDR and vitamin D binding protein (VDBP) polymorphisms to IBD72, 73.
The Nurses’ Health Study, a large longitudinal study of 72,719 adult women in the United States followed from 1986 to 2008 showed that higher pre-diagnosis vitamin D levels were associated with a significant reduction in risk of incident CD and a nonsignificant reduction in risk of incident UC in the examined cohort26. A retrospective study of 504 IBD patients not only demonstrated the high prevalence of vitamin D deficiency (defined as serum vitamin D<20 ng/ml) in the study population (~50%), but also showed that vitamin D deficiency is independently associated with greater disease activity, as well as lower quality-of-life in CD patients74. Low plasma 25(OH)D levels have been shown to be associated with an increased risk of IBD-related surgery, as well as increased hospitalizations75. Vitamin D might also enhance the durability of anti-TNF therapy in IBD and its insufficiency has been found to be associated with earlier cessation of anti-TNFα therapy, particularly in CD76. A recent randomized, double-blind placebo-controlled study on 94 CD patients with inactive disease, assigned to either 1200 IU vitamin D3 daily or placebo for 12 months, showed that the IBD relapse rate had a trend towards being lower in the treatment group (p = 0.06)77. Other authors have observed short-term beneficial effect on disease activity in CD patients treated with vitamin D, and this was particularly true for those patients receiving the active form of the vitamin78. Similarly, a recent prospective randomized controlled trial on 18 patients with UC and hypovitaminosis D showed that vitamin D3 supplementation improved quality-of-life and reduced UC disease activity, especially at higher doses (4000 IU daily vs. 2000 IU daily)79.
Our meta-analysis demonstrated an association between IBD and low serum vitamin D levels only in adults, but not in the pediatric population. There could be several reasons for this, including, but not limited to shorter disease duration, more frequent outdoor activities leading to increased sunlight exposure, and greater use of vitamin D fortified foods when compared to adults. Furthermore, a physiological decline in cutaneous levels of the vitamin D precursor, 7-dehydrocholesterol, associated with aging, may profoundly affect the skin’s vitamin D production capability, particularly when sun exposure is limited, which might explain the significantly higher vitamin D deficiency in adults80.
Our meta-analysis produced an interesting finding in that UC patients had a higher odds of vitamin D deficiency than CD patients. This is likely a sample size issue as there were fewer total patients and fewer events in the UC meta-analysis in comparison to the CD meta-analysis. However, there might be other pathophysiological mechanisms; particularly, alterations in the vitamin D metabolic pathway that could potentially explain our findings. These include vitamin D activation and deactivation processes mediated by cytochromes (CYP2R1 and CYP27B1 for activation and CYP24A1 for deactivation), its transportation in blood and across cell membranes mediated by proteins (DBP, megalin/cubulin) and its genetic effects mediated by cellular complexes such as VDR/RXR and transcriptional activators/repressors81. In addition, genetic polymorphisms25, 73, 82 and disease related impairments (altered protein turnover83, protein-losing enteropathies84, 85 and dysbiosis86, 87) might also modify the association between IBD and serum vitamin D levels.
Our meta-regression analysis seemed to indicate that latitude does not moderate the association between IBD and vitamin D levels. However, it would be simplistic to dismiss this association as the relationship between latitude, vitamin D levels and IBD is likely more complex. Secondly, the effect of latitude could also not be measured precisely because most studies did not provide the latitude of the region where they conducted the study. Nevertheless, we felt that using the region of the hospital where the studies were conducted (or using the region of the source of cases and controls) was a reasonable approximation of the true latitude. Most importantly, because of the variable nature of seasonality data (as different studies were conducted in different seasons), the effect of latitude, if any, might have been suppressed. Finally, it should be noted that meta-regression itself typically has low power to detect statistically significant relationships88 and hence, the lack of such a relationship should be interpreted with caution.
Despite considerable diagnostic and therapeutic achievements in recent years, IBD still represents a challenge in terms of treatment89. The available therapies are not curative and their side effects may considerably impact patients’ general health. Current drug research is therefore highly oriented towards the study of novel therapies that target specific pathogenetic pathways90. In parallel with disease-modifying agents, there are promising results from other approaches aimed at modulating the gut environment. Intestinal microbiota and the innate immune system have therefore become interesting targets for complementary therapies, such as probiotic formulations. Vitamin D as a therapeutic agent, in particular, has also shown promise in lowering relapse rates and bettering quality-of-life in IBD, but larger, well-designed randomized controlled trials investigating the long-term effectiveness of vitamin D in IBD are needed to substantiate these findings from early trials.
This meta-analysis had several strengths. First, the number of included studies (n=14) provided a sufficiently large sample size. Second, study quality was systematically assessed using the Newcastle-Ottawa scale57 and the included studies were of reasonably high quality. Third, subgroup and sensitivity analyses were conducted, the results of which were congruent with our findings in the main meta-analysis. Finally, heterogeneity was moderate or not present in all the meta-analyses we conducted. Our study was not without limitations. The entire body of evidence was observational, which is often biased due to unmeasured confounders. Included studies, with the exception of one39, did not provide baseline adjusted data; therefore, in the absence of adjusted measures of risk, unadjusted measures (unadjusted ORs) were used, which limits generalizability of our results. In that study, even after adjusting for several confounders like age, sex, race, season, and vitamin D supplementation, the odds of vitamin D deficiency was still twice as high in IBD cases in comparison to healthy controls. Few studies reported stratified results on vitamin D deficiency based on important parameters such as surgery, disease location or disease activity and hence, stratified meta-analyses based on these criteria were not possible. Different 25(OH)D assays were also used in the studies (table 1); consequently, inter-assay variability is possible, due to different sensitivity of each assay method to vitamin D2 or D3.
In summary, this meta-analysis shows that IBD is associated with a higher odds of vitamin D deficiency compared to the absence of the disease. Further studies, particularly longitudinal studies in different settings, are needed for corroborating our findings. Well-designed, large randomized controlled trials using variable doses of vitamin D supplementation in different IBD statuses can help us better understand the therapeutic significance of vitamin D in IBD.
Supplementary Material
Supplemental methods: Search strategies. PubMed search strategy; COCHRANE search strategy; EMBASE search strategy; CINAHL search strategy.
Supplementary Table 1: Use of vitamin D supplementation, smoking status, mean BMI, mean disease duration, previous IBD-related surgery, disease activity, and use of medications as reported in the included studies.
Supplementary table 2: Disease location and extent, seasonality, and tanning habits in the included studies.
Supplementary Table 3: Methodological quality of the included studies based on the Newcastle-Ottawa Quality Assessment Scale for case-control studies.
Supplementary Figure 1: Meta-regression analysis on the association between IBD and serum vitamin D levels using latitude as a moderator. Red solid line represents the linear model, whereas blue dashed line represents the polynomial fractional best fitting model. No interaction was found between serum vitamin D levels and latitude.
Acknowledgments
We wish to thank Dr. Shari Bolen and Dr. Kristen Arseneau for critical revision of the manuscript. We acknowledge continued support from the National Institutes of Health: DK 042191, DK 055812 and DK 091222 to FC.
Grant support: We acknowledge continued support from the National Institutes of Health: DK 042191, DK 055812 and DK 091222 to FC.
Footnotes
Competing Interest
Authors declare that they have no competing interest.
Authors’ Contributions
RDP and DP devised and designed the study. RDP and DP performed database searches and data extraction. AKC conducted the meta-analyses and sensitivity analyses. DP conducted the meta-regression analysis. RDP and AKC performed the quality assessment and wrote the first draft. FC and CF supervised the study selection and quality assessment, interpreted the results and implemented the manuscript draft. All Authors reviewed the study findings, and read and approved the final version before submission.
References
- 1.Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013;29:357–62. doi: 10.1097/MOG.0b013e32836229fb. [DOI] [PubMed] [Google Scholar]
- 2.Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol. 2013;5:237–47. doi: 10.2147/CLEP.S33961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 4.Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am J Gastroenterol. 2009;104:2100–9. doi: 10.1038/ajg.2009.190. [DOI] [PubMed] [Google Scholar]
- 5.Bernklev T, Jahnsen J, Aadland E, et al. Health-related quality of life in patients with inflammatory bowel disease five years after the initial diagnosis. Scand J Gastroenterol. 2004;39:365–73. doi: 10.1080/00365520310008386. [DOI] [PubMed] [Google Scholar]
- 6.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–13. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnarsson C, Chen J, Rizzo JA, et al. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57:3080–91. doi: 10.1007/s10620-012-2289-y. [DOI] [PubMed] [Google Scholar]
- 8.Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365–9. doi: 10.1097/MOG.0b013e3281c55eb2. [DOI] [PubMed] [Google Scholar]
- 9.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60:290–8. doi: 10.1007/s10620-014-3350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hold GL, Smith M, Grange C, et al. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192–210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentschew L, Ferguson LR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res. 2012;56:524–35. doi: 10.1002/mnfr.201100630. [DOI] [PubMed] [Google Scholar]
- 12.Cornish JA, Tan E, Simillis C, et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394–400. doi: 10.1111/j.1572-0241.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- 13.Molodecky NA, Panaccione R, Ghosh S, et al. Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:1792–9. doi: 10.1002/ibd.21511. [DOI] [PubMed] [Google Scholar]
- 14.Rampton DS. The influence of stress on the development and severity of immune-mediated diseases. J Rheumatol Suppl. 2011;88:43–7. doi: 10.3899/jrheum.110904. [DOI] [PubMed] [Google Scholar]
- 15.Cominelli F. Cytokine-based therapies for Crohn’s disease–new paradigms. N Engl J Med. 2004;351:2045–8. doi: 10.1056/NEJMp048253. [DOI] [PubMed] [Google Scholar]
- 16.Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390–400. doi: 10.3748/wjg.14.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardizzone S, Cassinotti A, Trabattoni D, et al. Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol. 2009;22:63–71. doi: 10.1177/039463200902200108. [DOI] [PubMed] [Google Scholar]
- 18.Di Rosa M, Malaguarnera G, De Gregorio C, et al. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36–43. doi: 10.1016/j.cellimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Cantorna MT, Munsick C, Bemiss C, et al. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 20.Lagishetty V, Misharin AV, Liu NQ, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–32. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 23.Abraham BP, Prasad P, Malaty HM. Vitamin D deficiency and corticosteroid use are risk factors for low bone mineral density in inflammatory bowel disease patients. Dig Dis Sci. 2014;59:1878–84. doi: 10.1007/s10620-014-3102-x. [DOI] [PubMed] [Google Scholar]
- 24.Farraye FA, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17:2116–21. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 25.Xue LN, Xu KQ, Zhang W, et al. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: a meta-analysis. Inflamm Bowel Dis. 2013;19:54–60. doi: 10.1002/ibd.22966. [DOI] [PubMed] [Google Scholar]
- 26.Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of crohn’s disease. Gastroenterology. 2012;142:482–489. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilman J, Shanahan F, Cashman KD. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1007–16. doi: 10.1111/j.1365-2036.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 28.Hadj Taieb S, Kallel L, Feki M, et al. Unbalanced bone remodeling in Tunisian patients with inflammatory bowel diseases. Tunis Med. 2013;91:273–7. [PubMed] [Google Scholar]
- 29.McCarthy D, Duggan P, O’Brien M, et al. Seasonality of vitamin D status and bone turnover in patients with Crohn’s disease. Aliment Pharmacol Ther. 2005;21:1073–83. doi: 10.1111/j.1365-2036.2005.02446.x. [DOI] [PubMed] [Google Scholar]
- 30.El-Hodhod MA, Hamdy AM, Abbas AA, et al. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol. 2012;12:44. doi: 10.1186/1471-230X-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza HN, Lora FL, Kulak CA, et al. Low levels of 25-hydroxyvitamin D (25OHD) in patients with inflammatory bowel disease and its correlation with bone mineral density. Arq Bras Endocrinol Metabol. 2008;52:684–91. doi: 10.1590/s0004-27302008000400015. [DOI] [PubMed] [Google Scholar]
- 32.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 33.Driscoll RH, Jr, Meredith SC, Sitrin M, et al. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology. 1982;83:1252–8. [PubMed] [Google Scholar]
- 34.de Bruyn JR, van Heeckeren R, Ponsioen CY, et al. Vitamin D deficiency in Crohn’s disease and healthy controls: A prospective case-control study in the Netherlands. J Crohns Colitis. 2014;8:1267–73. doi: 10.1016/j.crohns.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Silvennoinen J. Relationships between vitamin D, parathyroid hormone and bone mineral density in inflammatory bowel disease. J Intern Med. 1996;239:131–7. doi: 10.1046/j.1365-2796.1996.420765000.x. [DOI] [PubMed] [Google Scholar]
- 36.Tan B, Li P, Lv H, et al. Vitamin D levels and bone metabolism in Chinese adult patients with inflammatory bowel disease. J Dig Dis. 2014;15:116–23. doi: 10.1111/1751-2980.12118. [DOI] [PubMed] [Google Scholar]
- 37.Garg M, Rosella O, Lubel JS, et al. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2634–43. doi: 10.1097/01.MIB.0000436957.77533.b2. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen SP, Hvas CL, Agnholt J, et al. Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7:e407–13. doi: 10.1016/j.crohns.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Prosnitz AR, Leonard MB, Shults J, et al. Changes in vitamin D and parathyroid hormone metabolism in incident pediatric Crohn’s disease. Inflamm Bowel Dis. 2013;19:45–53. doi: 10.1002/ibd.22969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duggan P, O’Brien M, Kiely M, et al. Vitamin K status in patients with Crohn’s disease and relationship to bone turnover. Am J Gastroenterol. 2004;99:2178–85. doi: 10.1111/j.1572-0241.2004.40071.x. [DOI] [PubMed] [Google Scholar]
- 41.Dumitrescu G, Mihai C, Dranga M, et al. Serum 25-hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J Gastroenterol. 2014;20:2392–6. doi: 10.3748/wjg.v20.i9.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. 2011;56:825–9. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 43.Joseph AJ, George B, Pulimood AB, et al. 25 (OH) vitamin D level in Crohn’s disease: association with sun exposure & disease activity. Indian J Med Res. 2009;130:133–7. [PubMed] [Google Scholar]
- 44.Abreu MT, Kantorovich V, Vasiliauskas EA, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–36. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suibhne TN, Cox G, Healy M, et al. Vitamin D deficiency in Crohn’s disease: prevalence, risk factors and supplement use in an outpatient setting. J Crohns Colitis. 2012;6:182–8. doi: 10.1016/j.crohns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Tajika M, Matsuura A, Nakamura T, et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol. 2004;39:527–33. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 47.Alkhouri RH, Hashmi H, Baker RD, et al. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:89–92. doi: 10.1097/MPG.0b013e31826a105d. [DOI] [PubMed] [Google Scholar]
- 48.Grunbaum A, Holcroft C, Heilpern D, et al. Dynamics of vitamin D in patients with mild or inactive inflammatory bowel disease and their families. Nutr J. 2013;12:145. doi: 10.1186/1475-2891-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ardizzone S, Bollani S, Bettica P, et al. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn’s disease and ulcerative colitis. J Intern Med. 2000;247:63–70. doi: 10.1046/j.1365-2796.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 50.Laakso S, Valta H, Verkasalo M, et al. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcif Tissue Int. 2012;91:121–30. doi: 10.1007/s00223-012-9617-2. [DOI] [PubMed] [Google Scholar]
- 51.Vazquez MA, Lopez E, Montoya MJ, et al. Vertebral fractures in patients with inflammatory bowel disease compared with a healthy population: a prospective case-control study. BMC Gastroenterol. 2012;12:47. doi: 10.1186/1471-230X-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salacinski AJ, Regueiro MD, Broeder CE, et al. Decreased neuromuscular function in Crohn’s disease patients is not associated with low serum vitamin D levels. Dig Dis Sci. 2013;58:526–33. doi: 10.1007/s10620-012-2372-4. [DOI] [PubMed] [Google Scholar]
- 53.Veit LE, Maranda L, Fong J, et al. The vitamin D status in inflammatory bowel disease. PLoS One. 2014;9:e101583. doi: 10.1371/journal.pone.0101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 55.Landis JR, Koch GG. The measurement of observer agreement for categorical data. biometrics. 1977:159–174. [PubMed] [Google Scholar]
- 56.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 57.Wells GA, S B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Reserach Institute Web site; Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 25, 2015. [Google Scholar]
- 58.Higgins JPT, G Se. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Vol. 2014. The Cochrane Collaboration; 2011. [updated March 2011] [Google Scholar]
- 59.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 60.Ihaka R, Gentleman R. R: a language for data analysis and graphics. Journal of computational and graphical statistics. 1996;5:299–314. [Google Scholar]
- 61.Hanley DA, Davison KS. Vitamin D insufficiency in North America. J Nutr. 2005;135:332–7. doi: 10.1093/jn/135.2.332. [DOI] [PubMed] [Google Scholar]
- 62.Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:125–36. doi: 10.1111/apt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palmer MT, Weaver CT. Linking vitamin d deficiency to inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2245–56. doi: 10.1097/MIB.0b013e31828a3b6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys. 2012;523:103–6. doi: 10.1016/j.abb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinzone MR, Di Rosa M, Celesia BM, et al. LPS and HIV gp120 modulate monocyte/macrophage CYP27B1 and CYP24A1 expression leading to vitamin D consumption and hypovitaminosis D in HIV-infected individuals. Eur Rev Med Pharmacol Sci. 2013;17:1938–50. [PubMed] [Google Scholar]
- 66.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–97. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verway M, Behr MA, White JH. Vitamin D, NOD2, autophagy and Crohn’s disease. Expert Rev Clin Immunol. 2010;6:505–8. doi: 10.1586/eci.10.31. [DOI] [PubMed] [Google Scholar]
- 68.Polidoro L, Properzi G, Marampon F, et al. Vitamin D protects human endothelial cells from H(2)O(2) oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6:221–31. doi: 10.1007/s12265-012-9436-x. [DOI] [PubMed] [Google Scholar]
- 69.Cantorna MT. Why do T cells express the vitamin D receptor? Ann N Y Acad Sci. 2011;1217:77–82. doi: 10.1111/j.1749-6632.2010.05823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konijeti GG, Boylan MR, Song Y, et al. Sa1779 Vitamin D Modulates T Cell-Mediated Immunity: Results From a Randomized Controlled Trial of Low-Dose and High-Dose Vitamin D3. Gastroenterology. 2014;146:S-294. [Google Scholar]
- 71.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simmons JD, Mullighan C, Welsh KI, et al. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47:211–4. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eloranta JJ, Wenger C, Mwinyi J, et al. Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics. 2011;21:559–64. doi: 10.1097/FPC.0b013e328348f70c. [DOI] [PubMed] [Google Scholar]
- 74.Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308–16. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 75.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1921–7. doi: 10.1097/MIB.0b013e3182902ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zator ZA, Cantu SM, Konijeti GG, et al. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-alpha therapy in inflammatory bowel diseases. JPEN J Parenter Enteral Nutr. 2014;38:385–91. doi: 10.1177/0148607113504002. [DOI] [PubMed] [Google Scholar]
- 77.Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn’s disease – a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 78.Miheller P, Muzes G, Hritz I, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis. 2009;15:1656–62. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 79.Mathur J, Mills P, Naing S, et al. Su1385 Supplementation of Vitamin D3 (Cholecalciferol) in Patients With Ulcerative Colitis and Hypovitaminosis D: A Prospective Randomized Trial. Gastroenterology. 2014;146:S-454. [Google Scholar]
- 80.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–8. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3) Semin Dial. 2007;20:316–24. doi: 10.1111/j.1525-139X.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 82.Xia S, Xia X, Wang W, et al. [Associations of ulcerative colitis with vitamin D receptor gene polymorphisms and serum levels of 25-hydroxyl vitamin D] Zhonghua Yi Xue Za Zhi. 2014;94:1060–6. [PubMed] [Google Scholar]
- 83.Klein S, Meyers S, O’Sullivan P, et al. The metabolic impact of active ulcerative colitis. Energy expenditure and nitrogen balance J Clin Gastroenterol. 1988;10:34–40. doi: 10.1097/00004836-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 84.Hebuterne X, Filippi J, Al-Jaouni R, et al. Nutritional consequences and nutrition therapy in Crohn’s disease. Gastroenterol Clin Biol. 2009;33(Suppl 3):S235–44. doi: 10.1016/S0399-8320(09)73159-8. [DOI] [PubMed] [Google Scholar]
- 85.Campos FG, Waitzberg DL, Teixeira MG, et al. Inflammatory bowel diseases: principles of nutritional therapy. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:187–98. doi: 10.1590/s0041-87812002000400009. [DOI] [PubMed] [Google Scholar]
- 86.Yoon SS, Sun J. Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol Res Pract. 2011;2011:971938. doi: 10.1155/2011/971938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010;26:591–5. doi: 10.1097/MOG.0b013e32833d4b9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 89.D’Haens GR, Sartor RB, Silverberg MS, et al. Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014;8:726–34. doi: 10.1016/j.crohns.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 90.Bamias G, Cominelli F. Novel strategies to attenuate immune activation in Crohn’s disease. Curr Opin Pharmacol. 2006;6:401–7. doi: 10.1016/j.coph.2006.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods: Search strategies. PubMed search strategy; COCHRANE search strategy; EMBASE search strategy; CINAHL search strategy.
Supplementary Table 1: Use of vitamin D supplementation, smoking status, mean BMI, mean disease duration, previous IBD-related surgery, disease activity, and use of medications as reported in the included studies.
Supplementary table 2: Disease location and extent, seasonality, and tanning habits in the included studies.
Supplementary Table 3: Methodological quality of the included studies based on the Newcastle-Ottawa Quality Assessment Scale for case-control studies.
Supplementary Figure 1: Meta-regression analysis on the association between IBD and serum vitamin D levels using latitude as a moderator. Red solid line represents the linear model, whereas blue dashed line represents the polynomial fractional best fitting model. No interaction was found between serum vitamin D levels and latitude.


