Abstract
Background and Purpose
Oligodendrogenesis is essential for white matter repair after stroke. Although agonists of peroxisome proliferator-activated receptors γ (PPAR-γ) confer neuroprotection in models of cerebral ischemia, it is not known whether this effect extends to white matter protection. This study tested the hypothesis that the PPAR-γ agonist rosiglitazone enhances oligodendrogenesis and improves long-term white matter integrity after ischemia/reperfusion.
Methods
Male adult C57/BL6 mice (25-30g) were subjected to 60 minutes middle cerebral artery occlusion (MCAO) and reperfusion. Rosiglitazone (3mg/kg) was injected intraperitoneally once daily for 14d beginning 2 hours after reperfusion. Sensorimotor and cognitive functions were evaluated up to 21d after MCAO. Immunostaining was used to assess infarct volume, myelin loss, and microglial activation. Bromodeoxyuridine (BrdU) was injected for measurements of proliferating NG2+ oligodendrocyte precursor cells (OPCs) and newly generated APC+ oligodendrocytes. Mixed glial cultures were used to confirm the effect of rosiglitazone on oligodendrocyte differentiation and microglial polarization.
Results
Rosiglitazone significantly reduced brain tissue loss, ameliorated white matter injury, and improved sensorimotor and cognitive functions for at least 21d after MCAO. Rosiglitazone enhanced OPC proliferation and increased the numbers of newly generated mature oligodendrocytes after MCAO. Rosiglitazone treatment also reduced the numbers of Iba1+/CD16+ M1 microglia and increased the numbers of Iba1+/CD206+ M2 microglia after stroke. Glial culture experiments confirmed that rosiglitazone promoted oligodendrocyte differentiation, perhaps by promoting microglial M2 polarization.
Conclusions
Rosiglitazone treatment improves long-term white matter integrity after cerebral ischemia, at least in part by promoting oligodendrogenesis and facilitating microglial polarization toward the beneficial M2 phenotype.
Keywords: white matter, inflammation, oligodendrogenesis, microglial polarization
Introduction
White matter is composed mainly of axonal fibers, oligodendrocytes, and other glial cells, and is highly vulnerable to ischemic injury.1-2 White matter injury contributes to nearly half of the infarct volume in human ischemic stroke.3 Oligodendrocytes are the myelin-producing cells of the central nervous system (CNS). The myelin that they produce is wrapped around the internodes of axons, thereby facilitating saltatory conduction of nerve impulses and protecting axons from damage. Death of oligodendrocytes can result in myelin loss, axonal injury, and ultimately may be manifested as neurological deficits in stroke victims.4 Stroke and experimental ischemia can induce the proliferation of oligodendrocyte precursor cells (OPCs).4-5 However, most of these OPCs fail to develop into mature oligodendrocytes, resulting in insufficient remyelination and unsuccessful white matter repair.6,7 Therefore, promoting oligodendrogenesis and OPC differentiation may represent a potential therapeutic strategy to enhance white matter integrity and improve neurological recovery after stroke.
Administration of peroxisome proliferator-activated receptor γ (PPAR-γ) agonists has been shown to reduce infarct volume and improve neurological outcomes after stroke through multiple mechanisms, such as by mitigating excitotoxicity,8 apoptosis,9 inflammation10-11 and microvascular damage.12 However, the effects of PPAR-γ agonists on white matter integrity after stroke have not been investigated. Recent in vitro studies have documented that several PPAR-γ agonists promote OPC differentiation into mature oligodendrocytes.13-15 These findings shed light on the therapeutic potential of PPAR-γ agonists for oligodendrogenesis and white matter repair in CNS diseases with demyelination.
In this study, we show for the first time that post-ischemic treatment with rosiglitazone, a PPAR-γ agonist, can promote oligodendrocyte replacement and white matter repair in a mouse model of stroke with middle cerebral artery occlusion (MCAO). We further demonstrate that rosiglitazone promotes post-stroke microglial polarization toward the beneficial M2-like phenotype, which has recently been shown to be important for OPC differentiation and the process of remyelination.16
Materials and Methods
Murine Transient Focal Ischemia Model and Drug Administration
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male 8- to 10-week-old C57/BL6 mice (Jackson Laboratory, Bar Harbor, Maine USA) were subjected to MCAO as previously described.10 In brief, focal cerebral ischemia was induced by intraluminal occlusion of the left middle cerebral artery (MCA) for 1 hour under anesthesia with 1.5% isoflurane in a 30% O2/68.5% N2O mixture. Laser Doppler flowmetry was used to measure regional cerebral blood flow of all stroke animals, and only animals with cerebral blood flow reduction > 70% of preischemia baseline levels during MCAO were included for further investigations. Sham-operated animals underwent anesthesia and surgical exposure of arteries but without MCAO. Rectal temperature was maintained at 37 ± 0.5 °C with a heating pad during surgical procedures.
Animals were randomly assigned to Sham, MCAO + Vehicle, and MCAO + Rosiglitazone groups by using a lottery-drawing box. Rosiglitazone (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO) and further diluted with saline before use (0.5% v/v). Animals were administered 3 mg/kg rosiglitazone intraperitoneally daily for consecutive 14 days, initiated 2 hours following MCAO. This dose of rosiglitazone was chosen on the basis of our previous studies showing that 3 mg/kg is the minimum dosage to provide neuroprotection against brain infarcts.10 The control group received an equivalent volume and concentration of saline-diluted DMSO use (0.5% v/v). Animals were sacrificed 21 days after MCAO. Brains were removed and sectioned into 25-μm free-floating coronal cryosections using a microtome.
BrdU Injections
In order to label proliferating cells, all animals were intraperitoneally injected with the thymidine analogue 5′-bromo-2′-deoxy-uridine (BrdU, 50 mg/kg) twice a day–with an interval of at least 8 hours–for 5 days, beginning at 3 days after MCAO.
Neurological function evaluation
The Rotarod test (motor coordination), corner (sensorimotor asymmetry),17 and Morris water maze tests (spatial learning and memory)18 were performed as previously described to assess neurological functions before and after surgery by investigators who were blinded to experimental group assignments.
Immunofluorescence Staining and Quantification
Immunostaining was performed on free-floating cryosections. Sections were incubated in the following primary antibodies overnight at 4 °C: rabbit anti-myelin basic protein (MBP; Abcam), mouse anti-nonphosphorylated neurofilament H (SMI32; Calbiochem), rabbit anti-Iba1 (Wako), goat anti-CD206 (R&D Systems), rat anti-CD16 (BD biosciences), mouse anti-BrdU (BD bioscience), rabbit anti NG2 (Millipore), and mouse anti-adenomatous polyposis coli protein (APC; Calbiochem). For BrdU staining, brain sections were pretreated with 1N HCl for 1 hour followed by 0.1 mol/L boric acid (pH 8.5) for 10 min at 37 °C. Images were captured using a confocal laser scanning microscope (Olympus Fluoview FV1000; Olympus). The immunostaining intensity with MBP and SMI32 antibodies, as well as the numbers of target immunopositive cells were quantified by a blinded investigator using Image J software. Three randomly selected microscopic fields within the external capsule, cortex, striatum, subventricular zone (SVZ), and subgranular zone (SGZ) on each of 3 consecutive sections were analyzed for each brain by a blinded investigator. White matter injury was expressed as the mean ratio of SMI32 to MBP immunostaining. Immunopositive cell counts were presented as the mean number of cells per square millimeter.
Brain Tissue Loss
Brain tissue loss was determined by immunostaining using rabbit microtubule-associated protein 2 (MAP2) antibodies (Santa Cruz Biotechnology). Images were captured by a blinded investigator with a 1.25× objective. A series of 6 sections in the MCA territory were selected in each mouse brain. The area of brain tissue loss for each section was measured with the following equation: MAP2-positive staining in contralateral hemisphere – MAP2-positive staining in ipsilateral hemisphere. Brain volume loss was then determined by multiplying the mean area of tissue loss by the thickness of the evaluated tissue.
Primary Mixed Glial Cells and Microglia-depleted Cultures
Mixed glial cells were prepared from the whole brains of 1-day-old Sprague-Dawley rat pups, as described previously.19 Cells were seeded in poly-D-lysine coated 175-cm2 flasks at a density of 1.5 × 107cells/mL and maintained in glial cell culture media (DMEM/F12 containing L-glutamine, MEM non-essential amino acids, sodium pyruvate, penicillin/streptomycin and fetal bovine serum). Culture media was changed on the following day and subsequently every three days until a confluent monolayer of cells was achieved. For microglia depletion, 1.5 mM L-leucine methyl ester (LME) was added to the in vitro cultures beginning at day 2 and was exchanged every 3 days. This protocol resulted in less than 0.1% microglia. Fourteen-day-old cultures were then used for treatments.
Quantitative Real Time PCR
Quantitative RT-PCR was performed as previously described19. In brief, total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. The first strand of cDNA was synthesized with 1 μg RNA using the Superscript First-Strand Synthesis System (Invitrogen). Quantitative RT-PCR was performed on the Opticon 2 Real-Time PCR Detection System (Bio-Rad) using SYBR green PCR Master Mix (Invitrogen). Primers used are as follows: MBP forward primer: 5'- CTCCCAGCTTAAAGATTTTGGAAA -3', reverse primer: 5'-AAATCGGCTCACAAGGGATTC -3'; PLP forward primer: 5'-GCAAGGATCTTTCACCCTTAGAAA -3', reverse primer: 5'-TGGCTGAGTTAGGGCTTAAATAGTC -3'; CD206 forward primer: 5'-CAAGGAAGGTTGGCATTTGT-3', reverse primer: 5'-CCTTTCAGTCCTTTGCAAGC-3'; GAPDH forward primer: 5'-GTGAAGGTCGGTGTGAACGG-3'; reverse primer: 5'-GTTTCCCGTTGATGACCAG-3'. Expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA served as an internal control. Gene expression at the mRNA levels was normalized to GAPDH mRNA and expressed as fold changes versus control.
Flow cytometry
Cells were stained with anti-rat CD206, CD11b, NG2, O4 and the appropriate isotype controls following manufacturer’s instructions (eBioscience). Flow cytometric analysis was performed using a FACS flow cytometer (BD Biosciences).
Statistical analyses
All results are presented as mean values ± standard error of mean. Unless otherwise indicated, multiple comparisons were made using a one-way ANOVA followed by the Bonferroni post hoc test. The Student’s t test was used for two-group comparisons. Results were deemed statistically significant at P ≤ 0.05.
Results
Rosiglitazone Reduces Brain Tissue Loss after MCAO
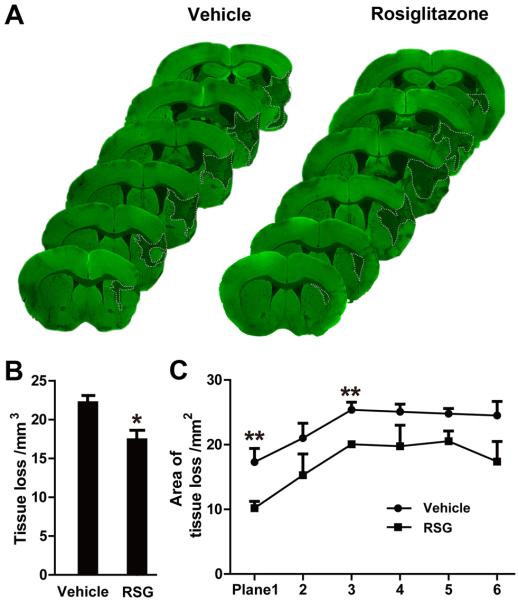
Loss of brain tissue volume was significantly reduced in rosiglitazone-treated mice compared with vehicle-treated animals at 21 days after MCAO, as shown by MAP2 immunohistochemistry (P = 0.015; Figure 1A and 1B). Specifically, rosiglitazone treatment significantly reduced the area of brain tissue loss in coronal sections at 1.0 mm and 0.14 mm rostral to bregma (P = 0.007 and 0.002, respectively; Figure 1C).
Figure 1. Rosiglitazone Reduces Brain Tissue Loss 21 days after MCAO.
A, Representative MAP2-stained coronal sections in vehicle or rosiglitazone-treated mice sacrificed 21 days following MCAO. Dotted lines indicate infarct areas. B, Total volume of brain tissue loss (mm3) in vehicle-treated and rosiglitazone (RSG)-treated mice. C, Infarct areas in 6 consecutive coronal sections throughout the MCA territory, spaced 1 mm apart. N=5-6 per group. Data are expressed as mean ± SEM. * P ≤ 0.05, ** P ≤ 0.01 vs vehicle.
Rosiglitazone Promotes White Matter Integrity after MCAO
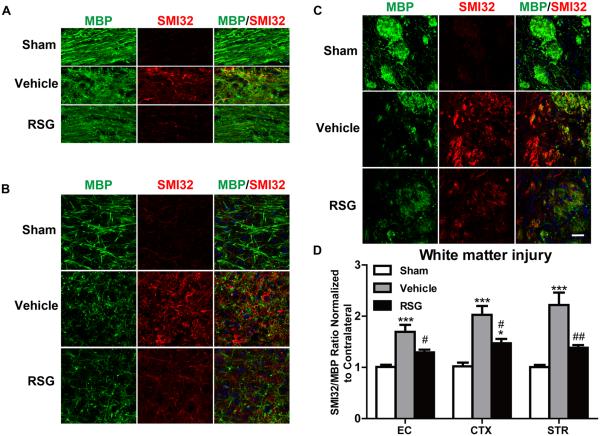
White matter damage is a critical component of ischemic brain injury. Therefore, we examined the effect of rosiglitazone on post-stroke white matter integrity using immunofluorescent double labeling for MBP, a marker of myelin integrity, and SMI32, a marker for axonal damage.20 Marked myelin loss accompanied by severe axonal damage was detected in the ischemic penumbra of vehicle-treated brains at 21 days after MCAO. MBPy+ myelin structures were absent around SMI32-immunoreactive injured axons in the ischemic penumbra (Figure 2A-2C). Treatment with rosiglitazone significantly preserved white matter integrity after MCAO, as measured by the decreased ratio of SMI32 to MBP staining intensity relative to vehicle-treated controls (P = 0.027 in EC, P = 0.010 in CTX, P = 0.006 in STR; Figure 2D).
Figure 2. Rosiglitazone Promotes White Matter Integrity 21 days after MCAO.
A-C, Representative images of MBP (green) and SMI32 (red) immunostaining in the external capsule (EC, A), cortex (CTX, B) and striatum (STR, C). Scale bar = 50 μm. D, The relative ratio of SMI32 vs MBP immunostaining intensity in the ipsilateral hemisphere was expressed as a function of contralateral values, and expressed as mean ± SEM. N=5-7 per group. *P ≤ 0.05, *** P ≤ 0.001 vs sham, # P ≤ 0.05, ## P ≤ 0.01 vs vehicle.
Rosiglitazone Improves Long-term Recovery of Neurological Function after MCAO
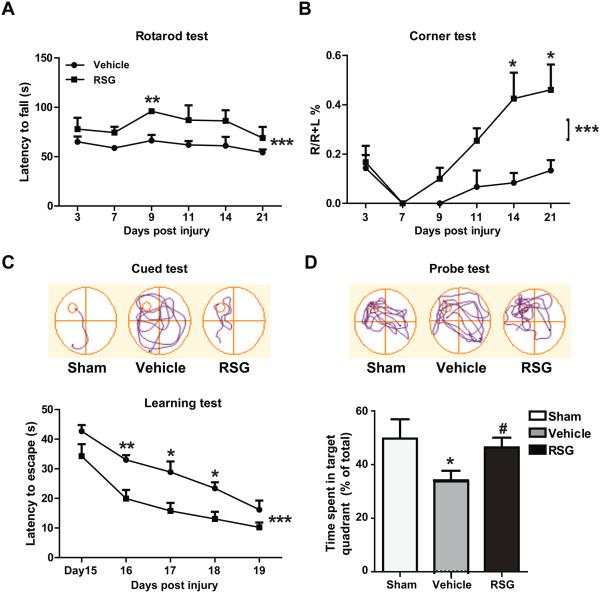
Next we tested whether the beneficial effects of rosiglitazone are associated with enhanced neurological functional recovery. Rosiglitazone treatment significantly reduced sensorimotor deficits after ischemic stroke, as demonstrated by an increased latency to fall off the accelerating Rotarod (P < 0.001; P = 0.008 at day 9 post injury; Figure 3A), and a reduced tendency to turn towards the unlesioned side in the corner test (P < 0.001; P = 0.020 and P =0.012 at day 14 and 21 post injury, respectively; Figure 3B).
Figure 3. Rosiglitazone Improves Long-term Recovery of Neurological Function after MCAO.
A-B, Sensorimotor dysfunction was significantly attenuated in rosiglitazone (RSG)-treated mice up to 21 days after ischemia, as assessed by the Rotarod test (A) and corner test (B). Corner test performance was expressed by the percentage of right turns out of ten turn trials. The performance in the Rotarod test was expressed as the time spent on the rotating rod before falling off. C-D, The Morris water maze test was performed to measure cognitive deficits after cerebral ischemia. C, Latency to find the hidden platform in the cued test (spatial learning). D, Time spent in target quadrant in the probe test (memory consolidation). Data are expressed as mean ± SEM. A-C, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 vs vehicle. D, * P ≤ 0.05 vs sham, # P ≤ 0.05 vs vehicle. N=6-8 per group.
The Morris water maze test revealed that rosiglitazone treatment facilitated spatial learning recovery after MCAO, as evidenced by less time spent finding the hidden platform during the cued trials in the rosiglitazone-treated group (P < 0.001; P =0.002 at day 16, P =0.013 at day 17, P =0.018 at day 18 post injury; Figure 3C). In the probe test, where the platform was removed, rosiglitazone-treated MCAO mice spent significantly more time in the target quadrant (P = 0.030; Figure 3D). There was no significant difference in swimming speed between the two groups (not shown). These findings demonstrate that rosiglitazone facilitates spatial learning and memory consolidation without any confounding effect on swimming speed.
Rosiglitazone Enhances Oligodendrogenesis after MCAO
Regeneration of mature myelinating oligodendrocytes is essential for remyelination and functional recovery after cerebral ischemia. Thus, we sought to determine whether rosiglitazone treatment acted on oligodendrocyte lineage development after MCAO, thereby facilitating white matter restoration. First, we found that MCAO dramatically increased the number of NG2+ OPCs in the peri-infarct areas (external capsule, cortex, and striatum) within 21 days, and this endogenous response was further enhanced by rosiglitazone (P = 0.044 in EC, P = 0.023 in CTX, P < 0.001 in STR; Figure 4A and 4Ca). The BrdU incorporation assay demonstrated that ischemia stimulated OPC proliferation, as reflected by increased numbers of BrdU+NG2+ cells in vehicle-treated groups compared to the sham control group. Rosiglitazone further amplified this endogenous response in OPC proliferation, as revealed by increased numbers of NG2+OPCs with BrdU incorporation in peri-infarct areas (P = 0.011 in EC, P = 0.021 in CTX, P = 0.029 in STR; Figure 4A and 4Cb). The SVZ lining the lateral ventricles and the SGZ of the hippocampus are two critical structures for neurogenesis and oligodendrogenesis in the adult brain.21,22 We found that rosiglitazone greatly increased the number of NG2+ OPCs in these two areas at 21d after MCAO (P = 0.042 in SVZ, P = 0.003 in SGZ; Figure 4A and 4Cc). The number of BrdU+ proliferating OPCs was also elevated by rosiglitazone (P = 0.017 in SVZ, P = 0.006 in SGZ; Figure 4A and 4Cd). These data suggest that rosiglitazone enhances oligodendrogenesis in both peri-infarct areas and in the neural stem cell pools (SVZ and SGZ) after cerebral ischemia.
Figure 4. Rosiglitazone Enhances Oligodendrogenesis and Oligodendrocyte Replacement after MCAO.
A, Representative images of BrdU (green) and NG2 (red) immunostaining 21 days after cerebral ischemia. Scale bar = 50 μm. B, Representative image showing the colocalization of BrdU, NG2, and DAPI staining at high magnification. Scale bar = 20 μm. C, Numbers of NG2-positive OPCs (a) and BrdU/NG2-dual labeled proliferating OPCs (b) in ipsilateral external capsule (EC), cortex (CTX), and striatum (STR). Numbers of NG2-positive OPCs (c) and BrdU/NG2-dual labeled proliferating OPCs (d) in ipsilateral SVZ and SGZ. D, Rosiglitazone enhances oligodendrocyte replacement after MCAO. Left, Representative images of BrdU (green) and APC (red) immunostaining 21 days after cerebral ischemia in ipsilateral EC, CTX, and STR. Scale bar = 50 μm. Right, numbers of BrdU/APC-dual labeled oligodendrocytes in peri-infarct areas were expressed as cells / mm2. Data are expressed as mean ± SEM. N=6-8 per group. ** P ≤ 0.01, *** P ≤ 0.001 vs sham, # P ≤ 0.05, ### P ≤ 0.001 vs vehicle.
Rosiglitazone Treatment Increases Generation of New Oligodendrocytes after MCAO
To determine whether increased OPC proliferation leads to the generation of new oligodendrocytes, brain sections were double-stained with BrdU and anti-APC (also known as CC1), a marker for mature oligodendrocyte cell bodies. As expected, increases in the colocalization of APC and BrdU were detected in peri-infarct areas in vehicle-treated MCAO mice compared with sham controls, suggesting that spontaneous generation of new oligodendrocytes occurs at 21 days after MCAO (P = 0.019 in EC, P = 0.016 in CTX, P = 0.037 in STR; Figure 4D). Treatment with rosiglitazone further augmented oligodendrocyte replacement, as evidenced by greater numbers of APC+BrdU+ new mature oligodendrocytes in peri-infarct areas. These data indicate that post-ischemia treatment with rosiglitazone enhances the generation of new oligodendrocytes at least 21 days after focal cerebral ischemia.
Rosiglitazone Drives M2 Microglial Polarization after MCAO
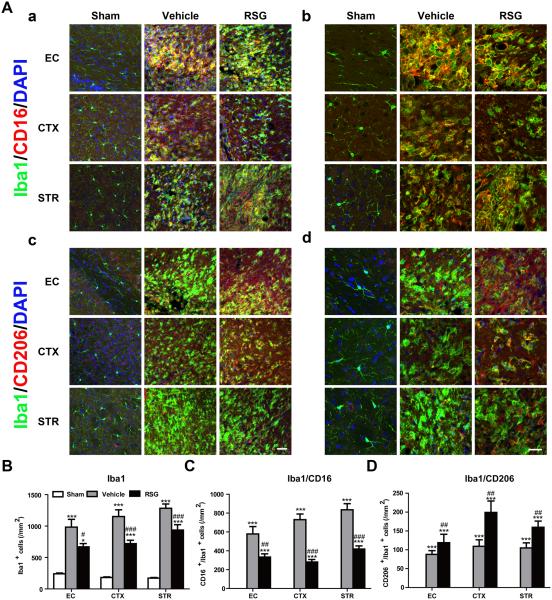
Microglia with different phenotypes have distinct impacts on the survival and differentiation of oligodendrocyte lineage cells.16 For example, M1 microglia are characterized by pro-inflammatory effects and lead to exacerbation of tissue damage, whereas M2 microglia resolve local inflammation and facilitate tissue repair.23 We previously reported that rosiglitazone inhibited microglia-mediated neuroinflammation after acute cerebral ischemia.10 Here, we further tested whether administration of rosiglitazone could modulate microglial phenotype during the recovery phase of stroke. Consistent with our previous studies, Iba1 immunostaining revealed that rosiglitazone treatment attenuated microglial activation around the ischemic zone at 21 days after MCAO (P = 0.042 in EC, P < 0.001 in CTX, P < 0.001 in STR; Figure 5A and 5B). Vehicle-treated MCAO mice exhibited increases in both M1 and M2 microglia in peri-infarct areas. Intriguingly, rosiglitazone treatment decreased the number of M1 microglia (P = 0.008 in EC, P < 0.001 in CTX, P < 0.001 in STR; Figure 5A and 5C) while increasing the number of M2 microglia (P = 0.003 in EC, P = 0.001 in CTX, P =0.005 in STR; Figure 5A and 5D). Collectively, these findings suggest that rosiglitazone promotes microglial polarization to the beneficial M2 phenotype after ischemia.
Figure 5. Rosiglitazone Drives M2 Microglial Polarization after MCAO.
A, Representative images of Iba1 (green), CD16 (red, a,b), and CD206 (red, c,d) immunostaining 21 days after cerebral ischemia in ipsilateral external capsule (EC), cortex (CTX), and striatum (STR). Scale bar in a, c = 100 μm. Scale bar in b, d = 30 μm. B-D, Numbers of Iba1-positive microglia (B), Iba1/CD16-dual labeled M1 microglia (C) and Iba1/CD206-dual labeled M2 microglia (D) were quantified and expressed as cells / mm2. Data are expressed as mean ± SEM. N=8-10 per group. * P ≤ 0.05, *** P ≤ 0.001 vs sham, # P ≤ 0.05, ## P ≤ 0.01, ### P ≤ 0.001 vs vehicle.
Rosiglitazone Promotes Oligodendrocyte Differentiation and M2 Microglial Polarization in mixed glial cultures
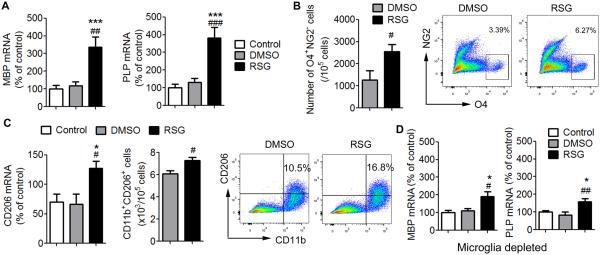
To verify our in vivo results and confirm that rosiglitazone promotes oligodendrocyte differentiation, we treated mixed glial cultures with rosiglitazone. As shown in Figure 6A, rosiglitazone treatment enhanced the mRNA expression of MBP and PLP, two markers for mature oligodendrocytes in mixed glial cultures (P = 0.007 in MBP, P < 0.001 for PLP). Flow cytometry confirmed that rosiglitazone treatment increased the expression of oligodendrocyte differentiation marker O4, a marker of differentiating oligodendrocytes, and reduced the expression of NG2, a marker of OPCs, in mixed glial cultures (P = 0.048; Figure 6B). These findings demonstrate enhanced oligodendrocyte differentiation. We also found that the expression of the M2 marker CD206 was increased in rosiglitazone-treated mixed glial cultures (P = 0.015; Figure 6C). Flow cytometry further revealed enhanced CD206 staining in CD11b+ microglia. Importantly, when microglia were depleted from the mixed glial culture system, rosiglitazone was less efficient in inducing oligodendrocyte maturation (P = 0.031; Figure 6D). These data suggest that rosiglitazone may promote OPC differentiation into oligodendrocytes and that this effect may be partially dependent on the presence of microglia.
Figure 6. Rosiglitazone Promotes Oligodendrocyte Differentiation and M2 Microglial Polarization in Mixed Glial Cultures.
A-C, Mixed glial cultures were treated with 0.5 μM rosiglitazone or DMSO vehicle for 7 days. A, mRNA expression levels of MBP and PLP were measured by real time PCR. B, Flow cytometry analyses of NG2−O4+ differentiating oligodendrocytes in mixed glial cultures. C, mRNA expression of CD206 was measured by real time PCR (Left). Flow cytometry analyses of CD206+CD11b+ microglia in mixed glial cultures. D. Mixed glial cultures were treated with 1.5 mM LME to deplete the microglia and then treated with 0.5 μM rosiglitazone or DMSO vehicle for 7 days. mRNA expression levels of MBP and PLP were measured by real time PCR. Data are expressed as mean ± SEM. N=4/group. *P ≤ 0.05, *** P ≤ 0.001 vs control, # P ≤ 0.05, ## P ≤ 0.01, ### P ≤ 0.001 vs DMSO.
Discussion
PPAR-γ agonists have been shown to possess neuroprotective effects in stroke patients and in animal models of stroke. 10, 24-25 However, the long-term effects of PPAR-γ agonists on white matter injury after stroke remain unknown. In the present study, we found that the activation of PPAR-γ by rosiglitazone enhanced white matter integrity during the recovery phase of stroke in the MCAO model. Consistent with previous studies,4 we found that transient focal cerebral ischemia induced severe myelin loss and axonal damage in peri-infarct areas such as the corpus callosum, external capsule, striatum, and cortex. These pathological changes in the white matter were significantly ameliorated by rosiglitazone treatment initiated 2 h after stroke. Preserving or restoring white matter integrity could promote neurological behavioral functions, including sensorimotor and cognitive functions, after stroke by enhancing the precise and efficient transmission of neuronal signals between different brain areas. Thus, rosiglitazone can elicit protection of both white matter and gray matter,10 thereby facilitating long-term functional recovery after stroke.
The mechanisms underlying rosiglitazone-afforded white matter protection could be manifold. First, we demonstrated that rosiglitazone treatment enhanced oligodendrogenesis and the generation of new oligodendrocytes after stroke. Oligodendrocytes are known to be highly susceptible to ischemic injury, 1-2, 4 and damage to oligodendrocytes leads to myelin loss and axonal injury. Successful regeneration of oligodendrocytes is essential for remyelination after brain injuries, because injured mature oligodendrocytes no longer produce functional myelin and mature oligodendrocytes are not proliferative.5 OPCs are distributed throughout the CNS26 and OPCs derived from neural stem cells in the SVZ27 actively proliferate after ischemic stroke in a concerted effort at regeneration, and migrate to the peri-infarct areas. We demonstrated here that rosiglitazone enhanced the proliferation of OPCs in peri-ischemic areas and in the SVZ. In addition, we showed that the number of proliferating OPCs in the SGZ of the hippocampus was also increased in rosiglitazone-treated ischemic brains, indicating that neural stem cells in the SGZ might also give rise to a new population of OPCs after ischemia. Despite the active proliferation of OPCs after stroke, very few give rise to new mature myelin-producing oligodendrocytes,28 which may contribute to poor white matter recovery.29 In this study, we showed that rosiglitazone not only enhanced OPC proliferation, but, more importantly, increased the maturation of new oligodendrocytes. This result is in line with previous in vitro studies showing that PPAR-γ agonists promoted OPC differentiation into mature myelinating oligodendrocytes.13, 30 Therefore, the promotion of OPC proliferation and differentiation into mature oligodendrocytes may be important mechanisms underlying the increase in white matter integrity by rosiglitazone.
In addition to stimulating oligodendrogenesis, rosiglitazone may enhance white matter integrity through protection of OPCs and oligodendrocytes. Oxidative stress and excitotoxicity are the two main mechanisms leading to cell death of oligodendrocytes and OPCs under ischemic conditions.2 Many PPAR-γ-regulated genes, such as superoxide dismutase,31-32 catalase,33 and glutamate transporter GLT1/EAAT234 possess strong anti-oxidant and anti-excitotoxic properties. Thus, it is possible that rosiglitazone protects OPCs and oligodendrocytes by blunting oxidative and excitotoxic injuries.
Recent studies have emphasized the importance of microglia/macrophage responses in white matter injury and repair.23, 35 Intriguingly, M1 and M2 microglia/macrophages, the two extreme phenotypes along the continuum, have been shown to exert distinct functions in oligodendrocyte survival31 and OPC differentiation.16 It is becoming increasingly accepted that microglia activation status should be shifted toward the beneficial M2 phenotype in place of blanketed suppression of all microglia.23 We demonstrated in this study that rosiglitazone favored microglial activation toward the M2 phenotype during the recovery phase of cerebral ischemia, which might enhance oligodendrocyte survival and promote OPC differentiation after stroke. In vitro data confirmed that the increase in OPC differentiation by rosiglitazone in the mixed glial culture was partially dependent on the presence of microglia. In agreement with our results, another PPAR-γ agonist, pioglitazone, has been reported to promote the M1 to M2 switch in animal models of Alzheimer’s disease.36 Therefore, it is likely that PPAR-γ agonists also regulate oligodendrogenesis indirectly through the modulation of microglial polarization after stroke. Further studies are warranted to confirm the direct effect of rosiglitazone on microglial polarization and the underlying mechanisms.
In conclusion, our data demonstrate that post-ischemia treatment with rosiglitazone improves white matter integrity after stroke. Rosiglitazone exerted both direct and indirect effects on white matter by promoting endogenous oligodendrogenesis and favoring microglial polarization toward the M2 phenotype. Additional in vitro and in vivo studies have shown that rosiglitazone treatment can protect neurons,10 reduce oxidative stress, and mitigate excitotoxicity,8 all of which are likely to contribute to long-term recovery after stroke, especially when combined with increased oligodendrogenesis and M2 polarization of microglia. There are few other treatments that have such multimodal properties in stroke models. Thus, rosiglitazone can be considered a multipurpose molecule warranting further investigation as a therapeutic agent for stroke.
Acknowledgments
Sources of Funding
This work was supported by the NIH/National Institute of neurological disorders and stroke (NINDS) grants NS095671, NS045048, NS036736, and NS089534 (to J.C) and NS092618 (to X.H), U.S. Department of Veterans Affairs Research Career Scientist Award and RR&D Merit Review I01RX000420 (to J.C.), and grant 13SDG14570025 from the American Heart Association (to X.H). L.H. was supported by pre-doctoral fellowship from the Chinese Scholarship Council. Y.X. was supported by China Natural Science Foundation grants (No. 81230026 and No. 81171085). P.L was supported by the Science and Technology Commission of Shanghai Municipality (13ZR1452200) and National Natural Science Foundation grant (No. 81400956)
Footnotes
Disclosures
None.
References
- 1.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- 2.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 3.Ho PW, Reutens DC, Phan TG, Wright PM, Markus R, Indra I, et al. Is white matter involved in patients entered into typical trials of neuroprotection? Stroke. 2005;36:2742–2744. doi: 10.1161/01.STR.0000189748.52500.a7. [DOI] [PubMed] [Google Scholar]
- 4.McIver SR, Muccigrosso M, Gonzales ER, Lee JM, Roberts MS, Sands MS, et al. Oligodendrocyte degeneration and recovery after focal cerebral ischemia. Neuroscience. 2010;169:1364–1375. doi: 10.1016/j.neuroscience.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman SA, Osorio J. So many progenitors, so little myelin. Nat Neurosci. 2014;17:483–485. doi: 10.1038/nn.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, et al. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuhayra M, Zhao Y, von Forstner C, Henze E, Gohlke P, Culman J, et al. Activation of cerebral peroxisome proliferator-activated receptors gamma (ppargamma) reduces neuronal damage in the substantia nigra after transient focal cerebral ischaemia in the rat. Neuropathol Appl Neurobiol. 2011;37:738–752. doi: 10.1111/j.1365-2990.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- 9.Kinouchi T, Kitazato KT, Shimada K, Yagi K, Tada Y, Matsushita N, et al. Activation of signal transducer and activator of transcription-3 by a peroxisome proliferator-activated receptor gamma agonist contributes to neuroprotection in the peri-infarct region after ischemia in oophorectomized rats. Stroke. 2012;43:478–483. doi: 10.1161/STROKEAHA.111.618926. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Journal of neurochemistry. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- 11.Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, et al. N2 neutrophils, novel players in brain inflammation after stroke: Modulation by the ppargamma agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- 12.Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A. Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1683–1694. doi: 10.1038/jcbfm.2009.87. [DOI] [PubMed] [Google Scholar]
- 13.Bernardo A, De Simone R, De Nuccio C, Visentin S, Minghetti L. The nuclear receptor peroxisome proliferator-activated receptor-gamma promotes oligodendrocyte differentiation through mechanisms involving mitochondria and oscillatory ca2+ waves. Biol Chem. 2013;394:1607–1614. doi: 10.1515/hsz-2013-0152. [DOI] [PubMed] [Google Scholar]
- 14.Paintlia AS, Paintlia MK, Singh AK, Orak JK, Singh I. Activation of ppar-gamma and pten cascade participates in lovastatin-mediated accelerated differentiation of oligodendrocyte progenitor cells. Glia. 2010;58:1669–1685. doi: 10.1002/glia.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim FJ, Lang JK, Ali TA, Roy NS, Vates GE, Pilcher WH, et al. Statin treatment of adult human glial progenitors induces ppar gamma-mediated oligodendrocytic differentiation. Glia. 2008;56:954–962. doi: 10.1002/glia.20669. [DOI] [PubMed] [Google Scholar]
- 16.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during cns remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. Journal of neuroscience methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 18.Pu H, Guo Y, Zhang W, Huang L, Wang G, Liou AK, et al. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1474–1484. doi: 10.1038/jcbfm.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 20.Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: Axonal damage, progenitor responses and mri correlates. Journal of neuroscience methods. 2009;180:261–272. doi: 10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maki T, Liang AC, Miyamoto N, Lo EH, Arai K. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci. 2013;7:275. doi: 10.3389/fncel.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falcao AM, Marques F, Novais A, Sousa N, Palha JA, Sousa JC. The path from the choroid plexus to the subventricular zone: Go with the flow! Front Cell Neurosci. 2012;6:34. doi: 10.3389/fncel.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nature reviews. Neurology. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Reding M. Effects of thiazolidinediones on stroke recovery: A case-matched controlled study. Neurochem Res. 2007;32:635–638. doi: 10.1007/s11064-006-9138-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 26.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult cns. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 27.Ortega F, Gascon S, Masserdotti G, Deshpande A, Simon C, Fischer J, et al. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to wnt signalling. Nat Cell Biol. 2013;15:602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Perez O, Alvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev. 2011;67:147–156. doi: 10.1016/j.brainresrev.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu M, Hu X, Lu S, Gan Y, Li P, Guo Y, et al. Focal cerebral ischemia activates neurovascular restorative dynamics in mouse brain. Frontiers in bioscience. 2012;4:1926–1936. doi: 10.2741/513. [DOI] [PubMed] [Google Scholar]
- 30.Roth AD, Leisewitz AV, Jung JE, Cassina P, Barbeito L, Inestrosa NC, et al. Ppar gamma activators induce growth arrest and process extension in b12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res. 2003;72:425–435. doi: 10.1002/jnr.10596. [DOI] [PubMed] [Google Scholar]
- 31.Doonan F, Wallace DM, O'Driscoll C, Cotter TG. Rosiglitazone acts as a neuroprotectant in retinal cells via up-regulation of sestrin-1 and sod-2. Journal of neurochemistry. 2009;109:631–643. doi: 10.1111/j.1471-4159.2009.05995.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, et al. Peroxisome proliferator-activated receptor gamma (ppar-gamma) and neurodegenerative disorders. Molecular neurobiology. 2012;46:114–124. doi: 10.1007/s12035-012-8259-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-prostaglandin j2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]
- 34.Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, et al. Ischemic preconditioning reveals that glt1/eaat2 glutamate transporter is a novel ppargamma target gene involved in neuroprotection. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of alzheimer's disease. J Neurosci. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]