Abstract
Previous studies have shown bismuth subsalicylate (BSS) has antimicrobial properties, but few studies have addressed the mechanism of action. Furthermore, following BSS ingestion other bismuth salts form throughout the gastrointestinal tract including bismuth oxychloride (BiOCl) that also act upon enteric pathogens. To further understand the antimicrobial activity of bismuth in infectious diarrhea, the antimicrobial effect of BSS and BiOCl on Clostridium difficile, Salmonella, Shigella, Shiga toxin-producing Escherichia coli strains and norovirus (NoV) were measured. Bacterial enteric pathogens in pure culture or in human fecal material were exposed to 35mg/ml BSS or BiOCl with or without a vehicle suspension. BSS and BiOCl treated samples were quantified and visualized by transmission electron microscopy. To measure the effect on NoV, reduction of infectious murine NoV (MNV), a surrogate for human NoV, and Norwalk virus RNA levels were measured by viral plaque assay and RT-qPCR, respectively. BSS and BiOCl reduced bacterial growth by 3–9 logs in all strains with majority resulting in populations of <10 cfu/ml within 24 h. Similar results were found when fecal material was included. Microscopy images detected bismuth on bacterial membranes and within the bacterial organisms at 30 min post-treatment. At 8.8mg/ml BSS and BiOCl reduced infectivity of MNV significantly by 2.7 and 2.0 log after 24 h of exposure. In addition, both BSS and BiOCl slightly reduced the level of Norwalk replicon-bearing cells suggesting that bismuth may inhibit NoV in vivo. Collectively, our results confirm and build on existing data that BSS has antimicrobial properties against a wide-range of diarrhea-causing pathogens.
Keywords: bismuth subsalicylate, bismuth oxychloride, Clostridium difficile, diarrhea, enteric pathogens, Escherichia coli, Salmonella, Shigella, norovirus
Abbreviations
- BSS
bismuth subsalicylate
- BiOCl
bismuth oxychloride
- NoV
norovirus
- MNV
murine norovirus
- SS
sodium salicylate
- TD
traveler's diarrhea
- ETEC
enterotoxigenic Eschericia coli
- GI
gastrointestinal
- MIC
minimum inhibitory concentration
- TEM
transmission electron microscopy
Introduction
Bismuth subsalicylate (BSS), an insoluble salt that contains a trivalent heavy metal and salicylic acid, has been used for over 100 y to relieve several digestive ailments including diarrhea. Several studies have shown that BSS is safe and effective in preventing and treating traveler's diarrhea (TD). Dupont et al. found that US students that acquired diarrhea in Mexico and treated with BSS had significant reduction in number of unformed stools and severity of associated symptoms.1 In similar cohorts, BSS prophylaxis prevented TD with a protection rate >60%.2,3 Significant reduction in diarrhea rates also was detected in Swiss travelers to West Africa and volunteers challenged with enterotoxigenic Escherichia coli (ETEC), a primary cause of TD, when taking BSS prophylactically vs. placebo.4,5 To help explain the effectiveness of BSS in relieving infectious diarrhea, in vitro experiments published in the 80s and 90s have demonstrated this active drug possesses antimicrobial properties against bacteria and viruses.6-9 Along with BSS, other bismuth salts that form in the gastrointestinal (GI) tract elicit similar activity. As BSS passes through the stomach, it undergoes hydrolysis by stomach acid resulting in the release of salicylate that gets absorbed into the bloodstream while the bismuth remains in the GI tract forming other insoluble salts including bismuth oxychloride (BiOCl).10 Despite these favorable results for BSS, the evidence describing the underlying mechanisms of how BSS controls infectious diarrhea requires further investigation.
There are several bacterial pathogens that instigate infectious diarrhea. Some of these bacteria have been studied in relation to antimicrobial activity of bismuth, such as ETEC and Salmonella, while other species and strains of enteric bacteria that are causing public health concerns have not been evaluated to the same extent. One example is Clostridium difficile, which has been a major issue in hospitals, other healthcare facilities and in the community. C. difficile infections are difficult to eradicate with antibiotics, and morbidity and mortality rates of C. difficile associated-diarrhea have risen dramatically in the last decade.11-15 Contributing to this increased incidence is a new antibiotic-resistant hypervirulent strain that is emerging in hospitals and, alarmingly, infecting healthy individuals out in the community.16,17 Over the last 30 y another pathogen that has been problematic for healthcare providers is Shiga toxin-producing E. coli (STEC), most notably E. coli O157:H7. It is estimated that this strain has caused 17 outbreaks and 75,000 illnesses each year in the US.18 Although the incidence rate has decreased considerably over the last few years due to improved prevention and surveillance strategies, E. coli O157:H7 is still among the top 5 pathogens contributing to foodborne hospitalizations.19,20 Other than rehydration therapy, there is no specific treatment for this type of infection and antibiotics are not recommended as they may increase the risk of hemolytic uremic syndrome (HUS). Another STEC serotype, O104:H4, has recently gained public attention when it caused the 2011 outbreak in Germany with 3,950 illnesses, 908 HUS cases and 53 deaths.21,22 As with O157:H7, the O104:H4 strain causes HUS even in the presence of antibiotic therapy. In addition to the lack of data regarding bismuth activity on these pathogens, the in vitro studies mentioned above have only investigated the antimicrobial effect of bismuth on pure cultures. While this is the first logical step of assessing any antibacterial compound, single-specie cultures are extremely different from the GI environment where trillions of microorganisms reside.
Another major pathogen of infectious diarrhea is human norovirus (NoV). NoV is the leading cause of epidemic acute gastroenteritis and causes 50% of all diarrheal outbreaks worldwide.23,24 The economic burden of NoV infections is estimated to be greater than $2 billion including healthcare expenses and loss of productivity. As a NoV vaccine is not available yet, implementation of personal and environmental hygiene is recommended to prevent NoV infections.25 In a previous clinical study of inoculated healthy volunteers, BSS has been shown to be able to reduce the severity and duration of NoV symptoms, although the mechanism has yet to be elucidated.26
To further study how BSS and BiOCl may control infectious diarrhea, we evaluated their effect on several key enteric pathogens using conventional and molecular methods.
Results
MICs of BSS +/− vehicle and BiOCl +/− vehicle on common bacterial enteric pathogens
The MICs for BSS +/− vehicle ranged from 2–8 mg/ml while the BiOCl +/− vehicle MICs were 4–64 mg/ml (Table 1). The MICs of BiOCl +/− vehicle were 2- to 8-fold higher compared to MICs of BSS +/− vehicle. SS alone had similar MICs to BSS +/− vehicle at 2–4 mg/ml. C. difficile was the most susceptible organism tested to the bismuth agents having the lowest MIC of 4 mg/ml for BiOCl and low end MICs (2–8 mg/ml) for the other 3 bismuth treatments compared to the Gram-negative pathogens.
Table 1.
Susceptibilities of various enteric pathogens to bismuth subsalicylate (BSS) +/− vehicle, bismuth oxychloride (BiOCl) +/− vehicle and sodium salicylate (SS)
| MIC (mg/ml) |
|||||
|---|---|---|---|---|---|
| Organism | BSS | BiOCl | BSS+Vehicle | BiOCl+Vehicle | SS |
| ETEC | 8 | 16–32 | 4–8 | 32–64 | 2–4 |
| S. Typhimurium | 4–8 | 16–32 | 4–8 | 8–32 | 4 |
| S. sonnei | 2–8 | 16–32 | 4 | 16–32 | 4 |
| C. difficile | 2 | 4 | 4 | 8 | 4 |
Establishment of Soleris to replace conventional plate counts for in vitro antimicrobial assay
Soleris is a rapid optical instrument, formerly known as BioSys, that has the capability of indirectly measuring CO2 production from bacteria in a closed vial system (see Materials and Methods). The amount of CO2 produced is proportional to the amount of bacteria in a sample. Precision and accuracy of Soleris to measure various bacterial concentrations in samples were evaluated by generating 3 standard curves with fresh cultures of ETEC in a BSS+vehicle background and comparing them to standard plate counts (Fig. 1). BSS+vehicle was the background of choice because it was the most complex matrix out of the 4 bismuth preparations. The detection times produced by Soleris for each 10-fold dilution were within 0.8 h as indicated by the narrow error bars. The detection times from Soleris also were strongly correlated with the results from plate counts with an R2-value >0.99. The average exponential equation from the 3 standard curves was y = (5.7 × 109)−1.007x, where x is the detection time and y is the bacterial concentration in cfu/ml. This equation was utilized to convert detection times to cfu/ml for the in vitro antimicrobial assay with ETEC.
Figure 1.
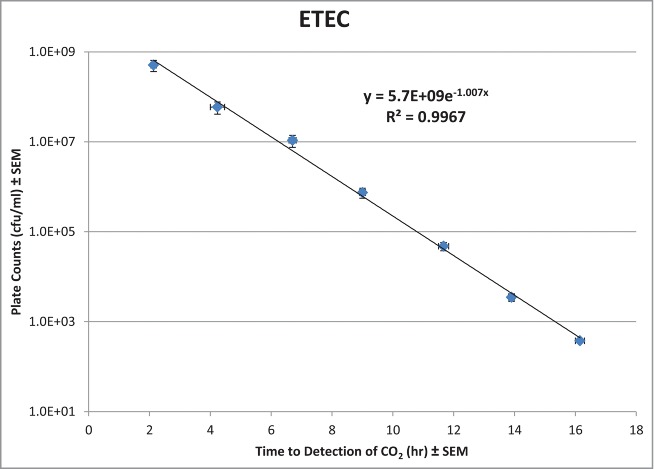
Correlation of plate count results and Soleris detection times for serial dilutions of ETEC. Each () represents the mean ± SEM for each dilution run in triplicate.
Bacteriostatic and bactericidal activity of BSS +/− vehicle and BiOCl +/− vehicle on enteric pathogens
An in vitro antibacterial assay was performed to track the activity of BSS +/− vehicle and BiOCl +/− vehicle (35 mg/ml) over time against common bacterial pathogens that cause diarrhea. Furthermore, a rapid and less labor intensive technology, Soleris, was utilized in place of serial dilutions and plate counts to quantify the concentration of ETEC in each sample. Results from Soleris show that BSS +/− vehicle and BiOCl significantly reduced viable numbers of ETEC over a 48 h period (Fig. 2). By 24–48 h no bacteria were detected with those 3 treatments indicating <10 cfu/ml were present in the flask as the lower limit of detection for this assay is 10 cfu/ml. Although BiOCl+vehicle never did achieve undetectable levels of bacteria and the viable numbers remained constant over the period of 2 to 24 h, the results were still statistically significant and there was a 3-log reduction of ETEC compared to the untreated control by 48 h.
Figure 2.
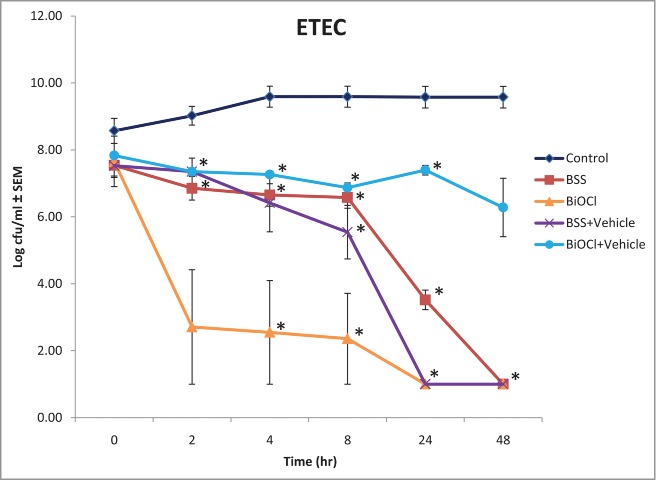
Soleris results from the in vitro antibacterial assay with ETEC are depicted as the mean log cfu/ml ± SEM for untreated and treated bacteria over a 48 h period. All bismuth treatments were tested at 35 mg/ml. (*p < 0.05)
For the other bacterial species tested and quantified by plate counts, the bismuth treatments at 35 mg/ml reduced bacterial growth by 3–9 logs when compared to the control at 24 h of exposure (Table 2 and Fig. S1). More than half (65%) of the experimental trials resulted in no bacterial growth on the agar plates, again indicating <10 cfu/ml were present. This signifies that the bismuth preparations were able to eliminate ≥99.99% of the initial inoculum within 24 h. BSS +/− vehicle was more potent than the hydrolyzed forms against Shigella sonnei, and BiOCl had minimal activity against E. coli O157:H7 compared to the other treatments. All bismuth forms had similar toxicity at 24 h against C. difficile, E. coli O104:H21, and Salmonella Typhimurium. C. difficile was the most susceptible pathogen; within 2–8 h of exposure to each treatment no bacteria were detected. S. Typhimurium was second most susceptible with no detectable bacteria within 8–24 h of exposure to each treatment. Although the results from the bismuth treatments at 17.5 mg/ml were not as dramatic as the 35 mg/ml concentration, they had similar inhibitory effects against the pathogens (data not shown).
Table 2.
Log reduction of bacterial growth vs. control at 24 h of treatment (35 mg/ml)
| Organism | BSS | BiOCl | BSS+Vehicle | BiOCl+Vehicle |
|---|---|---|---|---|
| S. Typhimurium | 9.0 | 9.0 | 9.0 | 9.0 |
| S. sonnei | 9.3 | 6.5 | 9.3 | 4.5 |
| C. difficile | 7.4 | 7.4 | 7.4 | 7.4 |
| E. coli O157:H7 | 8.0 | 3.2 | 8.0 | 8.0 |
| E. coli O104:H21 | 5.8 | 3.3 | 3.1 | 4.0 |
When a complex fecal environment was introduced to the assay, the bismuth complexes at 35 mg/ml maintained their antibacterial activity toward the pathogens. Eighty-three percent of the experimental runs resulted in the bismuth agents eradicating the bacterial pathogens below the lower level of detection (Table 3 and Fig. S2). Only S. sonnei was still detected after 24 h when it was exposed to BiOCl and BSS+vehicle, but with a >3 log reduction vs. the control.
Table 3.
Log reduction of bacterial growth vs. control at 24 h of treatment (35mg/ml) in a fecal environment
| Organism | BSS | BiOCl | BSS+Vehicle | BiOCl+Vehicle |
|---|---|---|---|---|
| S. Typhimurium | 7.4 | 7.4 | 7.4 | 7.4 |
| S. sonnei | 6.4 | 3.3 | 3.5 | 6.4 |
| C. difficile | 7.2 | 7.2 | 7.2 | 7.2 |
Binding of BSS to ETEC and accumulation within bacterial cells
To complement the results from the in vitro antibacterial assay, TEM analysis was conducted to visualize how BSS interacts with bacteria at the cellular level. In 0.5 h of BSS treatment, multiple dark particles were bound to the surface of ETEC and had penetrated into the cells (Fig. 3). Similar results also were detected at 4 and 8 h of exposure. These dense particles continued to accumulate within the bacterial cells indicated by the growing number and size of the particles at 24 h. Energy dispersive X-ray spectrometry confirmed that the dark particles attached to the bacterial cell membrane and located within the bacteria were bismuth (Fig. S3).
Figure 3.
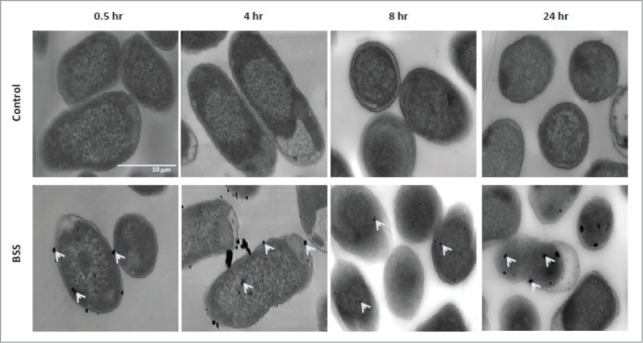
TEM images of ETEC cells that were incubated in media with or without 0.35 mg/ml BSS at 0.5, 4, 8, and 24 h. Bismuth particles (indicated by arrows) bound to bacterial membrane and accumulated within the cells.
Interference effect of bismuth on entry of murine norovirus in RAW 267.4 cells
At 0.1 mg/ml bismuth compounds did not affect the plaque formation of murine norovirus (MNV; Table 4), whereas at >2.1 mg/ml they resulted in cytotoxicity of the RAW 267.4 cells. Between 0.5─2.1 mg/ml BSS and BSS+vehicle was able to reduce the number of MNV plaques by ≥98.0% and ≥85.9% (p <0.05), respectively (Table 4). At 2.1 mg/ml BiOCl reduced infectious MNV by 48.3% (p <0.05) while BiOCl+vehicle had little effect.
Table 4.
Norovirus reduction vs. control at 1.5 h post-infection
| Reduction ± SD (%) of MNV 1 |
||||
|---|---|---|---|---|
| Concentration of test ingredient (mg/ml) | BSS | BiOCl | BSS+Vehicle | BiOCl+Vehicle |
| 0.1 | 4.9 ± 10.9 | 2.9 ± 8.6 | 11.7 ± 16.8 | 3.4 ± 8.6 |
| 0.3 | 30.0 ± 28.2* | 7.6 ± 20.0 | 26.8 ± 30.0 | 17.3 ± 29.4 |
| 0.5 | 98.0 ± 13.8* | 31.4 ± 32.5 | 85.9 ± 15.8* | 36.7 ± 33.4 |
| 1.1 | 100 ± 6.0* | 29.1 ± 27.1 | 98.0 ± 2.6* | 6.8 ± 13.6 |
| 2.1 | 100 ± 0.0* | 48.3 ± 34.1* | 100 ± 0.0* | 26.7 ± 26.4 |
percentage reduction of infectious MNV in treated cells compared to mock-infected cells
p <0.05 vs. untreated cells
Effect of bismuth on MNV
Infectivity of untreated MNV (PBS control) was reduced by 1.2 and 1.8 log10 PFU/ml after 24 and 48 h at 37°C, respectively. At 2.1 mg/ml none of the bismuth compounds statistically affected MNV infectivity compared to untreated PBS control (Table 5). However, when treated with BSS or BiOCl at 8.8 mg/ml, MNV infectivity was reduced by 2.7 and 2.0 log10 after 24 h and by 3.0 and 2.9 log10 after 48 h of exposure, respectively (Table 5). In contrast, after exposure to BSS+vehicle or BiOCl+vehicle MNV infectivity was not reduced compared to untreated controls.
Table 5.
Antimicrobial activity of bismuth on murine norovirus
| Reduction of infectivity ± SD (log10 PFU) |
|||||
|---|---|---|---|---|---|
| Concentration of test ingredient (mg/ml) | Contact time (hr) | BSS | BiOCl | BSS+Vehicle | BiOCl+Vehicle |
| 2.1 | 24 | 1.2 ± 0.7 | 0.8 ± 0.4 | 0.4 ± 0.2 | 0.5 ± 0.2 |
| 48 | 2.2 ± 0.8 | 1.6 ± 0.3 | 1.1 ± 0.5 | 1.2 ± 0.5 | |
| 8.8 | 24 | 2.7 ± 0.2* | 2.0 ± 0.2* | 0.7 ± 0.2 | 0.6 ± 0.2 |
| 48 | 3.0 ± 0.3* | 2.9 ± 1.0* | 1.2 ± 0.1 | 1.2 ± 0.6 | |
p <0.05 vs. PBS treated cells
The effect of bismuth on the replication of Norwalk virus replicon in HG 23 cells
The effect of BSS +/− vehicle and BiOCl +/− vehicle on Norwalk replicon-bearing HG 23 cells was examined (data not shown). Incubation with bismuth compounds at concentrations higher than 0.26 mg/ml resulted in cytotoxicity of the HG cells. Exposure to BSS and BiOCl at concentrations ranging from 0.004 to 0.13 mg/ml reduced the level of Norwalk RNA copies in HG cells by ≥47.3% compared to mock treatment (p < 0.05). BSS+vehicle resulted in reduction of Norwalk virus RNA when tested between 0.03 and 0.13 mg/ml (p < 0.05).
Discussion
BSS and BiOCl possess antimicrobial properties against common diarrhea-causing pathogens by inhibiting bacterial growth or killing the bacteria, or with respect to viruses it can prevent viral invasion of host cells and deter viral replication. A few studies from the 1980s–90s have demonstrated the antimicrobial effects of BSS on enteric pathogens, but the in-vitro antimicrobial data is not available for several important pathogens.6–9 In addition, little attention was focused on the relative contribution of BiOCl, which is an additional form of bismuth in the small and large intestine where the pathogens would be residing. In comparison to the current MICs presented here, Cornick et al. reported analogous MICs for BSS and SS on similar genera and species of bacteria, but MICs for BiOCl were not done.6 The most likely cause for the BSS +/− vehicle MICs to be lower than the BiOCl +/− vehicle MICs is removal of the salicylate from bismuth during hydrolysis. It is known salicylates have antimicrobial effects on bacteria and per MIC results salicylate alone has equivalent potency as BSS +/− vehicle.6,27,28 This does not suggest bismuth lacks antimicrobial activity because clearly it does in the form of BiOCl as shown in the MIC, antibacterial and NoV assays. Furthermore, the antimicrobial effects of salicylate on enteric pathogens in the lower GI tract are irrelevant in vivo as after salicylate disassociates from bismuth in the stomach nearly all of it is absorbed into the bloodstream whereas >99% of bismuth remains within the GI tract after each dose.10 C. difficile, a Gram-positive microbe, was the most susceptible to the bismuth insults compared to the other 3 pathogens that are Gram-negative. This implies that the antibacterial activity of BSS and BiOCl is influenced by the physiological structure of the bacterial cell wall; however more Gram-positive and Gram-negative strains need to be tested to confirm this notion.
Soleris has been used successfully in food and consumer products industries to rapidly determine the presence or absence of microbes in finished products. For our in vitro antibacterial assay, Soleris was used to replace conventional plating because it was faster, cheaper and less labor intensive. The values generated by Soleris for the standard curves were strongly correlated to ETEC concentrations determined by plate counts and were even consistent on separate days with different cultures. With the standard curves an average exponential equation was able to be calculated and utilized for quantifying bacteria in the unknown experimental samples. To our knowledge, this is the first published study to use Soleris with this mathematical approach for enumerating bacteria. This technology allowed for multiple trials to be done more quickly and easily for statistical analysis.
The in vitro antibacterial assay found that BSS and BiOCl inhibited bacterial growth or reduced viable numbers below initial values in as little as 2–24 h. This time frame parallels what has been observed in clinical studies for diarrhea relief.2,29 Manhart found similar inhibition from BSS on ETEC, Salmonella, Shigella and some rare clinical isolates in a dose-dependent manner ranging from 0.35–17.5 mg/ml.7 The current study generated comparable results for all bismuth treatments at 17.5 mg/ml. When the concentration was doubled to 35 mg/ml, which is equivalent to or higher than the majority of MICs that were measured, it drastically decreased the number of organisms in a 24 h period. Furthermore, these results were reproducible when fecal material with GI microflora was introduced. The plethora of microorganisms from the fecal slurry could have acted as competitive inhibitors on the bismuth and reduce its activity toward the pathogens, but this did not occur in our assay.
Besides the common enteric species (ETEC, Salmonella, Shigella) that were tested in the past with bismuth, other current and emerging strains related to public health also were included in the antibacterial assay. BSS and BiOCl had relative effects on C. difficile, E. coli O157:H7, and E. coli O104:H21. As expected from the MICs, C. difficile was the most susceptible pathogen to the bismuth challenges in the antibacterial assay with both pure culture and fecal slurries. These pathogens can cause severe to fatal illness and can be difficult to treat with antibiotics. These new findings suggest that BSS could be used as an alternative to antibiotics in treating these problematic diseases, or in conjunction with antibiotic therapy as is the recommended strategy for resolving Helicobacter pylori infections.30 More in vivo testing would need to be done in animal models and clinical trials to confirm efficacy of BSS against these specific infections, but nonetheless an area that is worth exploring.
The TEM images provide visual evidence of how bismuth from BSS interacts with a common enteric pathogen over time. BSS has the ability to bind to the bacterial membrane of ETEC and accumulate within the cells in as little as 0.5 h of exposure, and this activity continues to occur over 24 h. These images correlate well with the antibacterial data where bacterial growth is inhibited by 8 h and bacteria are eradicated by 24 h, and they are consistent with previously published ultrastructural images generated to show bismuth interacting with bacteria. Electron microscopy images of H. pylori and Yersinia enterocolitica exposed to BSS, and C. difficile incubated with a synthetic bismuth compound showed bismuth deposits on the membrane and inside the bacteria within one hr resulting in bactericidal activity.31-33 There is evidence that bismuth may exert its antibacterial activity by a number of mechanisms including cell wall degradation, inhibiting plasma membrane function and hindering protein and ATP synthesis.8,34-36 However, more specific insights on the cellular targeting by bismuth in its molecular level interactions remain to be investigated.
In addition to antibacterial affects, BSS and BiOCl also showed antiviral activity. Similar to a previous study with rotavirus,9 BSS and BSS+vehicle at ≥0.5 mg/ml reduced MNV infection possibly by inhibition of the attachment to its host cells. Though BiOCl is much weaker in inhibiting MNV plaque formation compared to BSS, both BSS and BiOCl concentrations as low as 0.004 mg/ml were able to reduce Norwalk RNA levels. However, it remains unclear how relevant these results are for a potential in vivo treatment of NoV infections. Additional clinical trials using modern methods will better clarify this in vitro antiviral activity of BSS.
In conclusion, BSS and BiOCl that is generated in the digestive tract as BSS passes through the stomach have similar antimicrobial effects on various enteric bacterial pathogens within 24 h of exposure, which confirms data from previous clinical studies. BSS and BiOCl also have antiviral activity against NoV as demonstrated by the data from our MNV and Norwalk virus replicon studies. Collectively, our results confirm and build on existing data that BSS has antimicrobial properties against a wide-range of diarrhea-causing pathogens.
Materials and Methods
Testing agents
Dry powder USP grade BSS was manufactured by Procter & Gamble Co. (Cincinnati, OH). BSS+vehicle solution consisted of the active ingredient suspended in magnesium aluminum silicate and methylcellulose with preservatives, flavoring and dyes (Pepto-Bismol, Procter & Gamble Co.). Sodium salicylate (SS) and all components for fasted state simulated gastric fluid (FaSSGF) and taurocholate-cefoxitin-cycloserine-fructose agar (TCCFA) were purchased from Sigma-Aldrich Corp. For hydrolyzing BSS, 10 g of BSS was stirred for 1 h in 500 ml of FaSSGF (pH 1.6) composed of 34.2 mM sodium chloride, 0.08 mM sodium taurochlate, 0.02 mM lecithin and 0.1 mg/ml pepsin in sterile water. Afterwards, the precipitate was allowed to settle, the top clear solution was decanted, and the bottom solution with precipitate was centrifuged and washed twice with sterile water. The remaining precipitate was weighed and resuspended with sterile water to a specific concentration. Four-hundred ml of BSS+vehicle containing 7g of BSS was hydrolyzed and prepared in a similar fashion with 600 ml FaSSGF. More than 99% of the hydrolyzed BSS +/−vehicle was confirmed to be bismuth oxychloride (BiOCl) by X-ray diffraction analysis using a Bruker D8 Advance diffractometer.
Bacterial and viral strains
All bacterial agents were purchased from American Type Culture Collection and included Clostridium difficile (BAA-1382; clinical isolate from Switzerland that contains toxin A and toxin B genes), enterotoxigenic Escherichia coli (ETEC, ATCC 31705), E. coli O157:H7 (ATCC 35150), E. coli O104:H21 (BAA-178; STEC strain isolated from a 1994 Montana outbreak), Salmonella enterica serovar Typhimurium (ATCC 13311), and Shigella sonnei (ATCC 11836). Before each experiment, one ml of frozen bacterial stock was thawed and transferred to 90 ml of trypticase-soy broth (TSB; BD Difco) and incubated in a 5% CO2 incubator at 37oC for 16 h except C. difficile, which was cultivated in brain heart infusion broth (BHI; BD Difco) with 10% L-cysteine (Sigma-Aldrich) and incubated in an anaerobic chamber at 37oC for 48 h. Liquid cultures then were centrifuged at 16,000 x g for 5 min, resuspended in saline or appropriate growth media and the optical density adjusted to 1.0 at 610nm (∼3–5 × 108 cfu/ml). Initial bacterial concentrations were confirmed by standard serial dilution and plate counts on trypticase-soy agar (TSA; BD Difco) or BHI agar (BD Difco) with 10% L-cysteine.
Strain CW3 of murine NoV (MNV) was used as a surrogate for human NoV and propagated in RAW 264.7 cells (ATCC# TIB-71) using Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% low endotoxin fetal bovine serum (Hyclone Laboratories), 100 U/liter penicillin and 100 μg/liter streptomycin, 1 mM HEPES, and 1 mM of sodium pyruvate, as previously described.37 Aliquots of MNV with a viral titer of 106.6 PFU/ml were stored at −80°C and thawed only once.
Minimum inhibitory concentration agar dilution assay
Minimum inhibitory concentrations (MIC) for BSS +/− vehicle and BiOCl +/− vehicle were measured by the Clinical and Laboratory Standards Institute agar dilution assay due to the high insolubility of the bismuth salts in liquid media.38 Briefly, BSS +/− vehicle and BiOCl +/− vehicle were diluted by 2-fold increments in liquid TSA or BHI+10% L-cysteine agar in a total volume of 15 ml at concentrations ranging from 0.5 to 256 mg/ml. Plates were poured and allowed to cool and dry. Prepared cultures of C. difficile, ETEC, S. Typhimurium and S. sonnei were diluted 1:100 in saline followed by spot inoculating each plate with 10 μl of 104 cfu. After the spot inoculations dried, plates were incubated appropriately and the presence or absence of bacterial growth was examined at 24 h for the aerobes and at 48 h for C. difficile to determine the MIC.
In vitro antibacterial assay
Flasks containing 30 ml of TSB or BHI+10% L-cysteine with or without BSS +/− vehicle or BiOCl +/− vehicle at concentrations of 17.5 mg/ml or 35 mg/ml were inoculated separately with ∼106–107 cfu of each bacterial strain. The bismuth concentrations were chosen based on the concentrations found in BSS-containing products that are on the market. Flasks then were incubated under optimal growth conditions for each strain on an orbital shaker at 250 rpm for 24 h. One ml aliquot samples were collected from each flask at 0, 2, 4, 8, 24, and 48 h post-inoculation. Samples were neutralized and diluted 1:10 in modified letheen broth with 1.5% tween 80 (MLBT; BD Difco), serially diluted in saline and cultured onto appropriate agar plates to acquire a cfu/ml concentration.
To simulate the complex environment of the lower GI tract, the assay was repeated with the addition of gut microflora via fecal slurries and incubating the flasks under anaerobic conditions. Approximately 106–107 cfu of C. difficile, S. Typhimurium or S. sonnei were added to flasks containing 15 ml of media with or without one of the 4 bismuth treatments at 35 mg/ml and 15 ml of 200 mg/ml of pooled fecal material homogenized in saline buffer from 4 healthy volunteers. Flasks were incubated at 37oC in an anaerobic chamber while shaking. After samples were neutralized and serially diluted they were plated on selective-differential agar. C. difficile was quantified on self-made TCCFA. This was prepared by adding 40g proteose peptone 3, 5g Na2HPO4, 1g KH2PO4, 2g NaCl, 0.1g MgSO4, 6g fructose, and 20g agar to 1L of water and then autoclaved. Afterwards 1g taurcholate, 0.25g D-cycloserine, and 16mg cefoxitin were combined with the liquid agar. SS agar (BD Difco) was used to quantify S. Typhimurium and S. sonnei.
Soleris (Neogen, Lansing, MI), a rapid optical instrument formerly known as BioSys, also was utilized for this assay with a pure culture of ETEC to replace standard serial dilution and plating. Soleris has the capability of indirectly measuring CO2 production, which is proportional to the amount of bacteria in the sample, by using a closed vial system containing MLBT with 1% lecithin (MLBTL) and a liquid indicator that changes from blue to yellow as CO2 levels are elevated. Three standard curves were generated on separate days with fresh cultures of ETEC and compared to conventional plate counts. Each standard curve consisted of NF-MLBTL vials (Neogen) with one ml of BSS+vehicle to serve as background matrix and inoculated with 10-fold dilutions of ETEC. The vials were inserted into the Soleris machine and incubated for 48 h at 33oC. Using Soleris Microbiological System v6.1.2f software, the threshold was set at 12 with a skip factor of one. Standard curves were plotted as cfu/ml from plate counts vs. detection time in hr from Soleris and best fit exponential equations were calculated. The in vitro assay was repeated 3 times with ETEC as described above. Samples were neutralized 1:10 in NF-MLBTL vials and analyzed by Soleris under the same conditions and settings as the standard curves. The average of the 3 exponential equations from the standard curves then was used to convert detection times to cfu/ml.
Electron microscopy and energy dispersion spectrometry analysis
For transmission electron microscopy (TEM), 108 cfu of ETEC were exposed to 0.35 mg/ml BSS in TSB at 37oC while shaking. The 0.35mg/ml concentration was chosen because higher concentrations gave too much background noise and proved difficult to visualize the bacterial cells in the TEM images. At 0.5, 4, 8, and 24 h of exposure bacterial aliquots were fixed in 2.5% glutaraldehyde in 0.1M cacodylate buffer for one hr at room temperature, and washed and centrifuged (16,000 × g for 5 min) 3 times with 0.1M cacodylate buffer. Samples then were stained with 1% osmium tetroxide in 0.1M cacodylate buffer, dehydrated in increasing concentrations of acetone followed by infiltration with increasing concentrations of Embed-It (Polysciences Inc.) and cured overnight at 65oC. Next, 70 nm sections were cut by a Reichert-Jung Ultra E ultramicrotome (Buffalo, NY) with a diamond knife and placed on a 100 mesh nickel grid. Finally, all sections were examined by a Hitachi S-5200 STEM at 30KeV with PCI software (Tokyo, Japan) for imaging analysis and a Burker Esprit 1.9 for energy dispersive X-ray spectrometry for elemental analysis to confirm the presence of bismuth.
NoV interference assay
Confluent monolayers of raw cells were infected with a mixture of 100 PFU of MNV and bismuth compounds in concentrations ranging from 0.1 to 17.5 mg/ml and incubated for 1.5 h at 37°C. Viral plaques were enumerated as described previously.37 The difference in viral titer between bismuth compound-treated cells and untreated cells was compared. Uninfected cells were used to determine possible cytotoxicity of the bismuth compounds.
MNV plaque assay
A suspension test was used to measure the virucidal effect of bismuth solutions on MNV as described previously.37 Briefly, 0.9 ml of each bismuth compound (BSS +/− vehicle and BiOCl +/− vehicle) at 2 concentrations (8.8 and 2.1 mg/ml) was mixed with 0.1 ml of MNV, and incubated at 37°C. After 24 and 48 h, 100 μl of the bismuth-virus mixture was transferred into 900 μl of PBS containing 10% fetal bovine serum (PBS-F), followed by the centrifugation at 12,000 x g for 1 min. Each supernatant was 10-fold serially diluted in PBS-F and assayed by plaque assay. At least 6 replicates from 3 independent experiments were analyzed for each variable.
Norwalk virus replication inhibition assay
Huh-7 based Norwalk virus replicon-bearing cells (HG23 cells), kindly provided by Dr. Chang (Kansas State University), were propagated in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (HyClone Laboratories), 1% nonessential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1 mg/ml geneticin (DMEM; Life Technologies). The human NoV replicon system (HG23 cells), consisting of a human liver cell line transfected with human NoV RNA, was used to assess the dose response of each compound on the Norwalk replication level.39 Specifically, HG cells were seeded at the density of 2 × 105 replicon cells in 96-well plates and incubated at 37oC. After 24 h, culture medium was aspirated, and replenished with 100 μl of DMEM having one of the bismuth compounds (BSS +/− vehicle or BiOCl +/− vehicle) ranging from 0.0003 to 2.13 mg/ml. After incubation for 48 h, cells were washed 3 times with PBS, lysed and viral RNA was extracted using a total RNA extraction kit (Life Technologies). NoV RNA was measured by RT-qPCR as described previously.40 Morphological changes or cytotoxic effect of bismuth compound treated HG23 cells were monitored daily by light microscopy up to 7 d. To compensate for intra- and inter-kinetic RT-qPCR variations, ß-actin RNA was used to normalize results as described previously.39
Statistical Analysis
Student t-tests were conducted on the results from the antibacterial assay with Soleris by comparing control samples to each of the 4 bismuth-treated samples for each time point. The effect of the bismuth compounds on MNV plaque formation and Norwalk replicon RNA levels were analyzed with one-way analysis of variance (ANOVA) using PASW Statistic 18 (IBM SPSS Inc., New York, NY).41 Each pairwise treatment comparison was statistically tested at a significance level of 5%.
Disclosure of Potential Conflicts of Interest
Adam M Pitz and Ying L Boissy are full-time employees at The Procter & Gamble Company.
Acknowledgments
We would like to thank Jeff Anderson and our P&G Professional business for funding the NoV work. Also we would like to acknowledge Russ Poehner for conducting the MIC and antibacterial assays, Averrin Mwalupindi for hydrolyzing the BSS, and Michael Dicks for performing the X-ray diffraction analysis.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
References
- 1. DuPont HL, Sullivan P, Pickering LK, Haynes G, Ackerman PB. Symptomatic treatment of diarrhea with bismuth subsalicylate among students attending a Mexican university. Gastroenterology 1977; 73:715-718; PMID:330307 [PubMed] [Google Scholar]
- 2. DuPont HL, Ericsson CD, Johnson PC, Bitsura JA, DuPont MW, de la Cabada FJ. Prevention of travelers' diarrhea by the tablet formulation of bismuth subsalicylate. JAMA 1987; 257:1347-1350; PMID:3820443; http://dx.doi.org/ 10.1001/jama.1987.03390100085031 [DOI] [PubMed] [Google Scholar]
- 3. DuPont HL, Sullivan P, Evans DG, Pickering LK, Evans DJ, Jr, Vollet JJ, Ericsson CD, Ackerman PB, Tjoa WS. Prevention of traveler's diarrhea (emporiatric enteritis). Prophylactic administration of bismuth subsalicylate. JAMA 1980; 243:237-241; PMID:6985681; http://dx.doi.org/ 10.1001/jama.1980.03300290019013 [DOI] [PubMed] [Google Scholar]
- 4. Steffen R, DuPont HL, Heusser R, Helminger A, Witassek F, Manhart MD, Schär M. Prevention of traveler's diarrhea by the tablet form of bismuth subsalicylate. Antimicrob Agents Chemother 1986; 29:625-627; PMID:3518624; http://dx.doi.org/ 10.1128/AAC.29.4.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham DY, Estes MK, Gentry LO. Double-blind comparison of bismuth subsalicylate and placebo in the prevention and treatment of enterotoxigenic Escherichia coli-induced diarrhea in volunteers. Gastroenterology 1983; 85:1017-1022; PMID:6352386 [PubMed] [Google Scholar]
- 6. Cornick NA, Silva M, Gorbach SL. In vitro antibacterial activity of bismuth subsalicylate. Rev Infect Dis 1990; 12(Suppl 1): S9-10; PMID:2305178; http://dx.doi.org/ 10.1093/clinids/12.Supplement_1.S9 [DOI] [PubMed] [Google Scholar]
- 7. Manhart MD. In vitro antimicrobial activity of bismuth subsalicylate and other bismuth salts. Rev Infect Dis 1990; 12(Suppl 1): S11-15; PMID:2406851; http://dx.doi.org/ 10.1093/clinids/12.Supplement_1.S11 [DOI] [PubMed] [Google Scholar]
- 8. Sox TE, Olson CA. Binding and killing of bacteria by bismuth subsalicylate. Antimicrob Agents Chemother 1989; 33:2075-2082; PMID:2694949; http://dx.doi.org/ 10.1128/AAC.33.12.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ward RL, Sander DS, Knowlton DR. In vitro activities of bismuth salts against rotaviruses and other enteric viruses. Antimicrob Agents Chemother 1985; 27:306-308; PMID:2986544; http://dx.doi.org/ 10.1128/AAC.27.3.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bierer DW. Bismuth subsalicylate: history, chemistry, and safety. Rev Infect Dis 1990; 12(Suppl 1): S3-S8; PMID:2406853; http://dx.doi.org/ 10.1093/clinids/12.Supplement_1.S3 [DOI] [PubMed] [Google Scholar]
- 11. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 2012; 55(Suppl 2): S65-S70; PMID:22752867; http://dx.doi.org/ 10.1093/cid/cis319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011; 32:387-390; PMID:21460491; http://dx.doi.org/ 10.1086/659156 [DOI] [PubMed] [Google Scholar]
- 13. Gravel D, Miller M, Simor A, Taylor G, Gardam M, McGeer A, Hutchinson J, Moore D, Kelly S, Boyd D, Mulvey; Canadian Nosocomial Infection Surveillance Program. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian nosocomial infection surveillance program study. Clin Infect Dis 2009; 48:568-576; PMID:19191641; http://dx.doi.org/ 10.1086/596703 [DOI] [PubMed] [Google Scholar]
- 14. Burckhardt F, Friedrich A, Beier D, Eckmanns T. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis 2008; 14:691-692; PMID:18394306; http://dx.doi.org/ 10.3201/eid1404.071023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999-2004. Emerg Infect Dis 2007; 13:1417-1419; PMID:18252127; http://dx.doi.org/ 10.3201/eid1309.061116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumyati G, Stevens V, Hannett GE, Thompson AD, Long C. Maccanell D, Limbago B. Community-associated Clostridium difficile infections, Monroe County, New York, USA. Emerg Infect Dis 2012; 18:392-400; PMID:22377231; http://dx.doi.org/ 10.3201/eid1803.102023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005; 353:2433-2441; PMID:16322603; http://dx.doi.org/ 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 18. Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis 1999; 5: 607-625; PMID:10511517; http://dx.doi.org/ 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilliss D, Cronquist A, Cartter M, Tobin-D'Angelo M, Blythe D, Smith K, Lathrop S, Birkhead G, Cieslak P, Dunn J, et al. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food — foodborne diseases active surveillance network, 10 US sites, 1996—2010. MMWR 2011; 60:749-75521659984 [Google Scholar]
- 20. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011; 17:7-15; PMID:21192848; http://dx.doi.org/ 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. E. coli: rapid response in a crisis [Internet] European Food Safety Authority; 2012 July 11. [cited 2013 Oct 17]. Available from: http://www.efsa.europa.eu/en/press/news/120711.htm [Google Scholar]
- 22. Outbreaks of E. coli O104:H4 infection: update 30 [Internet] World Health Organization; 2011 July 22. [cited 2013 Oct 17]. Available from: http://www.euro.who.int/en/where-we-work/member-states/germany/sections/news/2011/07/outbreaks-of-e.-coli-o104h4-infection-update-30 [Google Scholar]
- 23. Hoffmann S, Batz MB, Morris JG, Jr. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 2012; 75:1292-1302; PMID:22980013; http://dx.doi.org/ 10.4315/0362-028X.JFP-11-417 [DOI] [PubMed] [Google Scholar]
- 24. Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med 2009; 361:1776-1785; PMID:19864676; http://dx.doi.org/ 10.1056/NEJMra0804575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopman B, Gastañaduy P, Park GW, Hall AJ, Parashar UD, Vinjé J. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol 2012; 2:96-102; PMID:22440972; http://dx.doi.org/ 10.1016/j.coviro.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 26. Steinhoff MC, Douglas RG, Jr, Greenberg HB, Callahan DR. Bismuth subsalicylate therapy of viral gastroenteritis. Gastroenterology 1980; 78:1495-1499; PMID:7372069 [PubMed] [Google Scholar]
- 27. Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumonia by sodium salicylate. Infect Immun 1989; 57:3778-3782; PMID:2680983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Price CT, Lee IR, Gustafson JE. The effects of salicylate on bacteria. Int J Biochem Cell Biol 2000; 32:1029-1043; PMID:11091136; http://dx.doi.org/ 10.1016/S1357-2725(00)00042-X [DOI] [PubMed] [Google Scholar]
- 29. Steffen R, Mathewson JJ, Ericsson CD, DuPont HL, Helminger A, Balm TK, Wolff K, Witassek F. Travelers' diarrhea in West Africa and Mexico: fecal transport systems and liquid bismuth subsalicylate for self-therapy. J Infect Dis 1988; 157:1008-1013; PMID:2896219; http://dx.doi.org/ 10.1093/infdis/157.5.1008 [DOI] [PubMed] [Google Scholar]
- 30. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection—the Masstricht IV/ Florence Consensus Report. Gut 2012; 61:646-664; PMID:22491499; http://dx.doi.org/ 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 31. Mahony DE, Lim-Morrison S, Bryden L, Faulkner G, Hoffman PS, Agocs L, Briand GG, Burford N, Maguire H. Antimicrobial activities of synthetic bismuth compounds against Clostridium difficile. Antimicrob. Agents Chemother 1999; 43:582-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nadeau OW, Gump DW, Hendricks GM, Meyer DH. Evidence that bismuth salts reduce invasion of epithelial cells by enteroinvasive bacteria. Med Microbiol Immunol 1992; 181:131-143; PMID:1522823; http://dx.doi.org/ 10.1007/BF00202054 [DOI] [PubMed] [Google Scholar]
- 33. Marshall BJ, Armstrong JA, Francis GJ, Nokes NT, Wee SH. Antibacterial action of bismuth in relation to Campylobacter pyloridis colonization and gastritis. Digestion 1987; 37(Suppl 2): 16-30; PMID:3622946; http://dx.doi.org/ 10.1159/000199555 [DOI] [PubMed] [Google Scholar]
- 34. Nilius M, Ströhle A, Bode G, Malfertheiner P. Coccoid like forms (CLF) of Helicobacter pylori. Enzyme activity and antigenicity. Zentralbl Bakteriol 1993; 280:259-272; PMID:8280950; http://dx.doi.org/ 10.1016/S0934-8840(11)80964-3 [DOI] [PubMed] [Google Scholar]
- 35. Slomiany BL, Kasinathan C, Slomiany A. Lipolytic activity of Campylobacter pylori: effect of colloidal bismuth subcitrate (De-Nol). Am J Gastroenterol 1989; 84:1273-1277; PMID:2801678 [PubMed] [Google Scholar]
- 36. Armstrong JA, Wee SH, Goodwin CS, Wilson DH. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro–an ultrastructural study. J Med Microbiol 1987; 24:343-350; PMID:3694664; http://dx.doi.org/ 10.1099/00222615-24-4-343 [DOI] [PubMed] [Google Scholar]
- 37. Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinjé J. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot 2010; 73:2232-2238; PMID:21219741 [DOI] [PubMed] [Google Scholar]
- 38. Clinical and Laboratory Standards Institute Agar dilution procedure. In: Larrisey MP, editor. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 9th ed. Wayne (PA): Clinical and Laboratory Standards Institute; c2012. Chapter 9. [Google Scholar]
- 39. Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Stable expression of a norwalk virus RNA replicon in a human hepatoma cell line. Virology 2006; 353:463-473; PMID:16843517; http://dx.doi.org/ 10.1016/j.virol.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 40. Costantini VP, Whitaker T, Barclay L, Lee D, McBrayer TR, Schinazi RF, Vinjé J. Antiviral activity of nucleoside analogues against norovirus. Antivir Ther 2012; 17:981-91; PMID:22910194; http://dx.doi.org/ 10.3851/IMP2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D'Agostino SR, Sullivan L, Beiser A. Introductory Applied Biostatistics. Thomson, Brooks/Cole; 2006; 1: 457-62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


