Abstract
The nucleoskeleton contains mainly nuclear intermediate filaments made of lamin proteins. Lamins provide nuclear structure and also play a role in various nuclear processes including signal transduction, transcription regulation and chromatin organization. The disparate functions of lamins may be related to the intrinsic disorder of the tail domains, which allows for altered and promiscuous binding. Here, we show modulation of lamin tail domain structures in the presence of divalent cations. We utilize changes in fluorescence of tryptophan residues within the Ig-fold flanked by disordered regions to experimentally measure protein thermodynamics. Using spectroscopy experiments and molecular dynamics simulations, we show that the tail domain of lamin B1 shows enhanced association with both Ca2+ and Mg2+ compared to the tail domain of lamin A. Binding curves show a similar KD between protein and ion (250–300 μM) for both proteins with both ions. However, we observe a maximum binding of ions to lamin B1 tail domain which is 2–3 times greater than that for lamin A tail domain by both experiment and simulation. Using simulations, we show that divalent ion association alters the Ig-fold by pinning flanking regions. With cells in culture, we observe altered lamin B1 organization in the presence of excess Mg2+ more so than for lamin A. We suggest that the differential sensitivity to divalent cations contributes to the vastly different functionalities and binding of the 2 proteins.
Keywords: intrinsically disordered proteins, lamin, molecular dynamics, nucleoskeleton
Abbreviations
- GST
glutathione S-transferase
- HFF
human foreskin fibroblasts
- LA
lamin A
- LA-TD
the tail domain of lamin A
- LB1
lamin B1
- LB1-TD
the tail domain of lamin B1
- MD
molecular dynamics
- PME
particle mesh Ewald
- preLA
prelamin A
- preLA-TD
the tail domain of prelamin A
- REMD
replica exchange molecular dynamics
- TD
tail domain
- trLA-TD
lamin A tail domain truncated to be the same length as lamin B tail domain
- ΔI/Io
change in intensity normalized to initial intensity
Introduction
The nucleoskeleton also called the nuclear lamina is the structural protein meshwork at the inner nuclear membrane, and is composed primarily of intermediate filaments of A-type and B-type lamin proteins.1,2 A-type lamins, primarily lamin A and lamin C, are splice variants of the LMNA gene; B-type lamins, lamin B1 and lamin B2, are encoded from different genes, LMNB1 and LMNB2.3 A-type lamins, by virtue of the fact that they are associated with large numbers of disease-causing mutations, are more extensively studied than B-type lamins.2 However, B-type lamins are ubiquitously expressed in every cell type, including embryonic stem cells whereas A-type lamins are differentially expressed later in development.4 Like most intermediate filaments, lamins have a globular head domain, a coiled rod domain and a globular tail domain (TD); the lamin TD is significantly larger than other intermediate filaments and mediates numerous interactions.3,5 The TD has an s-type Ig-fold flanked by mostly intrinsically disordered regions.3,6-8
A-type and B-type lamins form separate filament networks,9 have different binding partners, and perform different functions within the cell.10 Lamin binding to inner nuclear membrane lamin associated proteins, DNA and the nuclear membrane itself occurs via the TD. Lamin A binds to emerin, LAP-2α, MAN1, actin, DNA and many other binding partners through the TD.11 The TD of lamin B is also essential for binding with partner proteins and DNA.2 Through binding of the TD to numerous lamin-binding proteins, lamins are involved with chromatin organization, nuclear assembly and regulation of transcription and replication.12
After translation, B-type lamins (lamin B1 and lamin B2) are modified with the addition of a farnesyl group and carboxymethyl group to the C-terminal CaaX domain in the TD.13 An A-type lamin precursor, prelamin A also goes through similar processing of the C-terminus, but C-terminus is then cleaved to remove the farnesyl group to form a mature lamin A that incorporated into the lamina nucleoskeleton.14 Aberrant farnesylation of lamin A, either by a mutation that removes the cleavage region or a loss of the cleavage enzyme, causes severe diseases including Hutchinson Gilford progeria syndrome and restrictive dermopathy, respectively.15 Lamin C does not contain the CaaX domain and therefore does not undergo modification. Lamin C is found in both the nucleoplasm and nucleoskeleton with increased mobility over lamin A and lamin B proteins.3 Interestingly, mice expressing lamin C only (no lamin A expressed from LMNA) do not show any disease phenotype.9
Thus, retention of the farnesyl group in lamin B proteins is part of normal function whereas farnesylation of lamin A leads to devastating disease. However, there is little understanding of how the farnesyl group differentially impacts lamin B1 and lamin B2 versus lamin A or how farnesylation is modulated in the protein. Recent studies of farnesyltransferases in cells show a potentially deleterious impact on B-type lamins.13 Previously we have shown the role of divalent cations in mediating the exposure of the farnesyl group of prelamin A and a mutant lamin A TD by altering the conformation of the protein.7 Here, we examine changes in protein structure of lamin B1 (LB1) and lamin A (LA) TDs when exposed to Ca2+ and Mg2+. We hypothesize that responsiveness to divalent cations may help explain the differential behavior of LA and LB1 including: different binding partners, formation of independent networks and altered farnesyl state in the mature protein. Due to the intrinsic disordered nature of the lamin TDs, we measure changes in protein conformation by monitoring the intrinsic fluorescence of tryptophan residues.7 We also perform molecular dynamics (MD) simulations to visualize the change in protein conformation confirming differential association of divalent cations and associated changes in protein structure in the Ig fold.
Materials and Methods
Protein production and characterization
Lamin A and lamin B1 TD cDNAs (from amino acid R386 to the C-terminus at amino acid Y646 for LA and M586 for LB1) were produced from full length cDNA by PCR and subcloned into the (Glutathione S Transferase) GST-parallel vector, similar to previous work.7 The plasmids were expressed in E. coli BL21 Codon-Plus cells (Agilent) at 37°C. Purification was performed with glutathione magnetic beads (Pierce) and the protein was cleaved enzymatically with proTEV cleavage enzyme (Promega) at 30°C for 5–7 hrs. The cleaved protein was further purified by exposure to agarose glutathione beads (Pierce) to remove excess GST.
Purified LA-TD or LB1-TD were dialyzed (Slide-A-Lyzer Dialysis Cassettes) into diH2O or aqueous solutions of NaCl. Concentration was measured by Bradford assay, and protein concentration was adjusted to 3 μM. In our previous study, we fully characterized protein purity using mass spectroscopy and gel electrophoresis as well as structure using fluorescence spectroscopy, calorimetry and circular dichroism.7 Fluorescence intensity is impacted by both protein concentration and concentrations of salt in the buffer, and the intensity impacts the signal to noise ratio but not ΔI/Io. On testing a wide range of buffer concentrations ranging from 50 mM to 500 mM we found that 250 mM NaCl solution is the ideal concentration: TDs did not aggregate, but the NaCl did not impact the ion-dependent structural changes.7
Tryptophan fluorescence
Protein conformational changes were measured using fluorometry of tryptophan residues. Both LA-TD and LB1-TD have 4 tryptophan residues which are located in the Ig fold. Tryptophan ring structures have inherent fluorescence with an excitation at 295 nm and emission at 340–345 nm; fluorescence intensity is quenched with exposure to solvent and emission can shift.16 The peak emission was used for analysis and was normalized by the peak without Ca2+ and Mg2+ for both samples. We measured fluorescence using a Fluorolog fluorometer (Horiba) with excitation at 295 nm and emission spectra from 315 to 400 nm.
Fluorescence curve fitting
Unlike previous studies, changes in fluorescence intensity could not be well-fit to a Hill model.7 Rather, we considered Ca2+ or Mg2+ bound to charged residues determined by simulation. We modeled this binding similar to enzyme-substrate analysis where:
Can be modeled as:
Thus, plotting date of inverse ΔI/Io vs. inverse [Ca2+] yields a Lineweaver-Burk plot with an intercept of inverse bindingmax and a slope of KD/bindingmax. Fits were considered by both linear fits of inverse data and by nonlinear fit to unbiased data. In both cases, r2 was greater than 0.9 (with dropped point).
Molecular modeling of lamin tail domains
The structure identification of lamin TD was carried out by using the Replica exchange molecular dynamics (REMD)17 implemented in the CHARMM c35b1 simulation software.18 The replica management and conformation analysis are done by using the MMTSB script package19 in implicit solvent condition with CHARMM19 force field. We have previously used this technique to obtain representative conformations of LA-TD at global-minimum free energy states.8 Further refinement in explicit solvent condition is performed by using the CHARMM27 force field which accounts for the accurate description of ion effect in simulations.7 For LB1-TD, we use structure homology method to build its initial geometry (amino acids 387 to 586) according to the sequence and structure of LA-TD (amino acids 386 to 646). Sequence alignment and modeling of the molecular structure are performed by using the homology modeling algorithm20,21 using comparative studies of the large database of known protein structures.
Molecular dynamics simulation of lamin TDs
Classical full atomistic molecular dynamics (MD) simulations were used to equilibrate the LB1-TD and LA-TD in different solvent conditions (one of 200 mM NaCl without CaCl2 and the other one with CaCl2). The net charge of the system is zero and each ion was initially distributed randomly in water with at least 5 Å from the protein structure. The simulations were performed in the NPT ensemble (constant pressure of 1 atm and constant temperature of 310 K) with the shrinkable volume for 20 ns in the purpose of getting an equilibrated structure in different solvent conditions. The interacting force and energy among particles was defined by the CHARMM27 all-atom energy function for protein and solvent models. The simulation time step was 2 fs with rigid bonds model for all the covalent bonds between hydrogen atoms and other heavy atoms. We used particle mesh Ewald (PME) function with a grid width <1 Å to calculate the electronic interaction as an efficient method to accurately include all the long-distance electrostatics interactions.
Post-processing analysis of simulation results
We analyzed the trajectory of all the deformation and movement of protein and ions during each simulation to provide their interaction and the response of the protein structure in different solvent conditions. We computed the number of ions within 5 Å of each amino acid as a function of time during MD simulation. We computed the dihedral angle of the Ig-fold by using the coordinates of the Cα atoms of orthogonal amino acid S429, S437, L478 and I497 for LA-TD and the coordinates in amino acids S430, T438, V479 and I497 for LB1-TD, corresponding to the alignment result. For statistics, we compared the dihedral angle of the Ig-fold during the last 5 ns of the simulation time. The dihedral angle was measured for every 20 ps and 250 pair of dihedral angles were compared with t-test (for p>0.05). We have also measured the average dihedral angle for every 500 ps and repeat the t-test for 10 pair of dihedral angles. The result was consistent with the first comparison.
Cell culture, treatment, fixation and fluorescence imaging
Human foreskin fibroblasts (HFF-1, ATCC SCRC-1031) of passage 8 were cultured in Dubelcco's Modified Eagle's Medium (Life Technologies) with 15% FBS and 1% penicillin/streptomycin. Cells were either untreated (normal medium) or treated with twice the amount of Mg2+. DMEM High Glucose has 0.81 mM MgSO4; MgCl2 (Cl- is already in much higher concentration in media than SO42-) was added for a final Mg2+ concentration of 1.62 mM for 24 hours. Following treatment, cells were fixed using 3.7% formaldehyde in phosphate buffered saline (PBS) solution. Cells were then permeabilized using 0.2% Triton X-100 in PBS. Cell blocking was done using 0.2% BSA. For lamin A/C, immunostaining was done using a primary lamin A/C antibody (sc7292 from Santa Cruz Biotechnology) and secondary antibody with Alex Fluor 594. Lamin B was immunostained using a primary lamin B antibody (sc6217 Santa Cruz Biotechnology) and secondary antibody with Alex Fluor 488. DNA was stained using DAPI (Life Technology). Imaging was done using a 63x (1.4 NA) oil immersion objective of an inverted microscope (DMI6000, Leica).
Post-processing analysis of imaging results
Ten different fields of view were imported into ImageJ and 1–2 nuclei were chosen at random from each image; care was taken to find fields of view with different localized cell density so as not to bias samples with cell crowding. The intensity profile was plotted along the ferret diameter of the nucleus (the longest chord transecting a spheroid) and imported into MS Excel. The position was normalized to have the region of intensity associated with the border of the nucleoskeleton start at 0.1 to provide an extranuclear reference as ∼10% of the image. The intensity along the length was then calculated to the other end of the nucleus typically 0.7 (up to as much as 1). The length was not normalized to show the intensity profiles of similar sized nuclei. See Figure S1 for a schematic of image analysis.
Results
Comparison of LA-TD and LB1-TD
For this study we consider the tail domains of lamin A and lamin B1; lamin B1 is the better studied of the B-type lamins. On comparing the sequences of LA-TD and LB1-TD we observe the lowest sequence homology in the TD. For comparison, the rod domains have a maximum sequence similarity of ∼75%, the head domain shows ∼60% sequence homology. However the TDs of LA and LB1 show low similarity: more than 50% of the sequence differs between the 2 proteins. This includes a large stretch (>70 amino acids at the C-terminus) in LA not found in LB1 (Fig. 1).
Figure 1.

Sequence comparison of LA-TD and LB1-TD. The tail domain of mature LA from 386 to 646 with LB1 from 387 to 586 is shown with homology and amino acid similarity (+). The Ig-folds are underlined and the tryptophans (W), used for fluorescence spectroscopy, are boxed.
The exact percentage of mismatch is not important for this study; rather the regions of charge and regions of charge bridging are what we will consider later. Most of the sequence mismatch is located in the region of 405–424 amino acid and 560–583. Generally, in comparing the TDs, there are nearly similar numbers of negatively charged residues (28 D,E in LB1 and 27 D,E in LA). However, in the last 50 residues of the LA-TD there is only one negatively charged residue, whereas the charged regions of the LB1-TD is closer to the C-terminus. Previous sequence comparisons between the TDs of LA and LB between species (Xenopus vs. human) have suggested that lamin B is an ancestral lamin and lamin A is a variant thereof.22
Recombinant TD show structural changes when exposed to divalent cations
Aside from the Ig-fold, lamin tail domains are intrinsically disordered,6,8 which limits structural determination through traditional techniques. We indirectly measure protein conformational changes by quantifying the tryptophan fluorescence in the Ig-fold (see Methods). The Ig-folds (underlined in Fig. 1) of both LA-TD and LB1-TD have 4 tryptophans (W's are boxed). With an excitation at 295 nm, the emission spectra showed a maximum at 342 nm for both LA-TD and LB1-TD (Fig. 2). With no divalent cations in the solution, LA-TD showed maximum peak intensity at 1.4 × 106 whereas LB1-TD showed a ∼9.5 × 105 maximum (Fig. 2). The fluorescence intensities are arbitrary but comparable with one another. With the addition of either Mg2+ or Ca2+, the peak emission intensity at 342 nm decreased for both LA-TD and LB1-TD (Fig. 2). In each case, the maximum intensity was always 342 nm.
Figure 2.
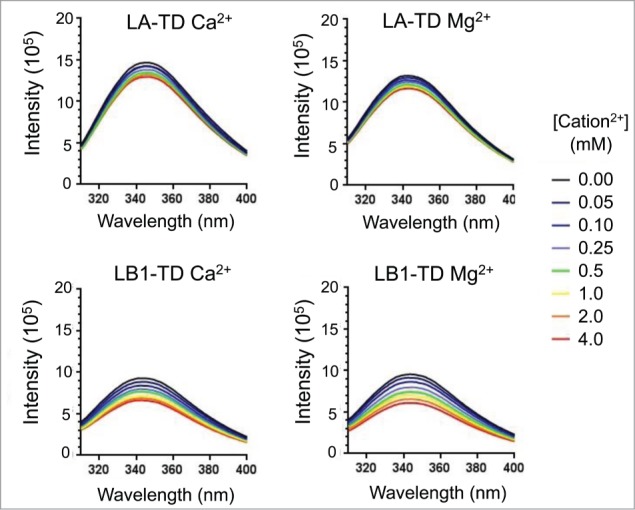
Fluorescence of tryptophan residues in TDs. With an excitation of tryptophan residues at 295 nm, we measure emission (maximum at 340–345) spectrum and intensity (black line). Increasing divalent cation concentration reduces the intensity of fluorescence emission (blue – red), but does not show significant shift in the wavelength of the maximum intensity.
On examining mismatched sequences in the Ig fold (Fig. 1, underlined residues), we observe different amino acids near some tryptophan (W) bases; there are different polarities near W470 and W517. The difference in polarities near the W bases may be responsible for the difference in initial fluorescence intensity: LB1-TD has a 33% lower baseline fluorescence intensity compared with LA-TD (Fig. 2).
Differential responsiveness of lamin TDs to calcium and magnesium
Plotting the fractional intensity increase (ΔI/Io see Methods) vs. divalent ion concentration ([Ca2+] or [Mg2+]), we observe an increased responsiveness of LB1-TD compared with LA-TD (increased ΔI/Io in Fig. 3A). Although not significantly different from one another using this methodology (p>0.05 t-test comparing replicates at 2 and 4 mM), TDs of both LA and LB1 appear to be more responsive to Mg2+ than Ca2+ (Fig. 3A).
Figure 3.
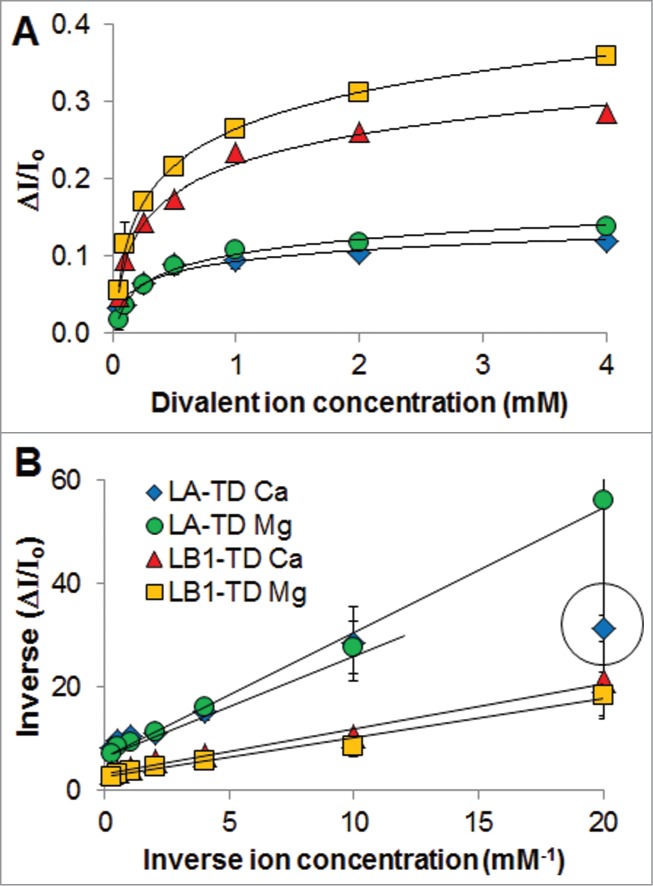
Binding curves of tail domains with divalent cations. (A) For multiple experiments, the plots of change in fluorescence intensity (ΔI/Io) versus divalent ion concentration show higher sensitivity of LB1-TD for divalent cations. Logarithm fits show r2 > 0.9. (B) Plotting of data in A to a Lineweaver-Burk plot (inverse-inverse of plot in A) allows us to extrapolate KD of binding and max binding. The datapoint in the circle was not considered for the fit: this very low ion concentration showed the highest uncertainty, and we did not want to bias the data to this point. For these experiments, n is 2–3 independent experiments starting from transformed E. coli.
Plots of inverse ΔI/Io versus inverse divalent ion concentration (Fig. 3B) allow us to determine KD of binding and maximum binding of divalent cations to TDs (see Methods). The KD, determined from the slope and intercept of the plots in Fig. 3B, was 310 μM for LA-TD and 260 μM for LB1-TD. Within the limit of detection, we observe ∼300 μM binding for both proteins to both divalent cations. This is in agreement with our previous reports of calcium binding to lamin TDs,7 and represents a non-specific intermolecular interaction. The maximum binding of divalent cations to the lamin TDs was determined from the intercept (see Methods) of the plots in Figure 3B. We calculate a binding of 0.14 and 0.33 for LA-TD and LB1-TD, respectively. While the absolute numbers may not be quantitatively useful, we observe a 2.3x increase in max binding of divalent cations to LB1-TD vs. LA-TD. We suggest that LB1-TD has an increased number of binding sites than LA-TD, and each site has roughly the same affinity. Note that this is not a conventional sense of specific protein-ligand interactions with a singular KD; these interactions are numerous non-specific intermolecular interactions each with a strength of KD with the number of interactions quantified by bindingmax.
Simulations show increased affinity and duration of Ca2+ binding to lamin TDs
Since there is no single equilibrium structure for the lamin TDs, we obtained a collection of pseudo-equilibrium states using MD simulation (Fig. S2). To observe the maximum number of potential interaction sites, we simulated LB1 and the longer, precursor to LA, prelamin A (preLA); we show a sample equilibrated structure of both preLA-TD and LB1-TD (Fig. 4A). To the simulation we add Ca2+ and measure the number of divalent cations which associate within 5Å of the protein. Here we show Ca2+, but the only difference between Ca2+ and Mg2+ in the simulation is the size and mass of the ion, so we expect similar results for the 2 ions. It is also shown by experiments that both Ca2+ and Mg2+ have same qualitative effect on the structure change of other intermediate filament tails.23 We find that LB1-TD has a significantly higher binding with Ca2+, as shown by stably associated Ca2+ (Ca2+ as red spheres in Fig. 4A). When tracking Ca2+ association with every amino acid over the course of a 20 ns simulation, we find that Ca2+ binds to more amino acids in LB1-TD, and Ca2+ tends to associate at the same location through the entire simulation (Fig. 4B, time along x-axis) compared with preLA-TD (Fig. 4B).
Figure 4.

The simulated lamin TD structures and association with Ca2+. From REMD simulations we find the equilibrated LA-TD and LB1-TD structures (A). Ca2+ within 5 Å of the protein structures are highlighted by red spheres. (B) Associated Ca2+ are shown with each amino acid of the LA-TD or LB1-TD during equilibrium for 20 ns.
We computed the average time that a Ca2+ binds to an amino acid on TD surface during the simulation, which provides a relative dissociation rate constant, koff (Table 1). At equilibrium, we calculate the number of Ca2+ ions that bind to the protein (Table 1, # Ca2+ bound), and with this concentration we determine kon (Table 1). Comparing these temporal and concentration values, we observe a ∼2.7x increase in maximum binding of divalent cations to LB1-TD vs. preLA-TD, which agrees with what experiments found. There are quantitative differences from experiments, mainly because of the uncertain value of koff, as a larger extrapolation is performed by inferring statistical long-timed massive events from short-time simulations. We also examined a truncated form of LA-TD, trLA-TD which has a similar number of amino acids as LB1-TD. Interestingly, we observed slightly increased Ca2+ binding to the truncated version compared with preLA-TD but not as much binding as to LB1-TD (Fig. S3).
Table 1.
Binding coefficients determined by simulation.
| preLA-TD | LB1-TD | |
|---|---|---|
| koff (s-1) | 5.0 ×109 | 9.2 × 108 |
| kon (M-1s-1) | 2.0 × 1012 | 6.3 × 1012 |
| # Ca2+ bound | 8.9 | 24.3 |
The regions of increased Ca2+ interaction are associated with sequences of higher glutamic acid content in LB1-TD (Figure S4) Generally LB1-TD has more glutamic acids (11% for LB1-TD and 5% for LA-TD) including a stretch of 8 residues in LB1-TD starting at E552 (Fig. S4C).
Differential molecular conformations with exposure to Ca2+
From the full protein conformation, we measured the opening of the Ig-fold as determined by the dihedral angle of the orthogonal β-sheets (see Methods and7). The Ig-fold in LB1-TD in the presence of Ca2+ has a larger dihedral angle (64.6 ± 2.6º) compared with that of LA-TD (61.1 ± 2.9º), which represents a more solvent accessible conformation (Fig. 5A, B). Simulations suggest that the amino acids of LB1-TD interact with Ca2+ in a more cooperative way than LA-TD: the side chains of several amino acids are pinned together by Ca2+ (Fig. 5C). Such a mechanism effectively increases the tension on each half of the Ig-fold, causing bending and distortion of the Ig-fold. This molecular insight explains the larger dihedral angle and altered structure of LB1-TD in Ca2+ environment. Thus, despite sequence similarity of the Ig-fold region, there is an altered structure due to interactions with divalent cations of flanking regions.
Figure 5.
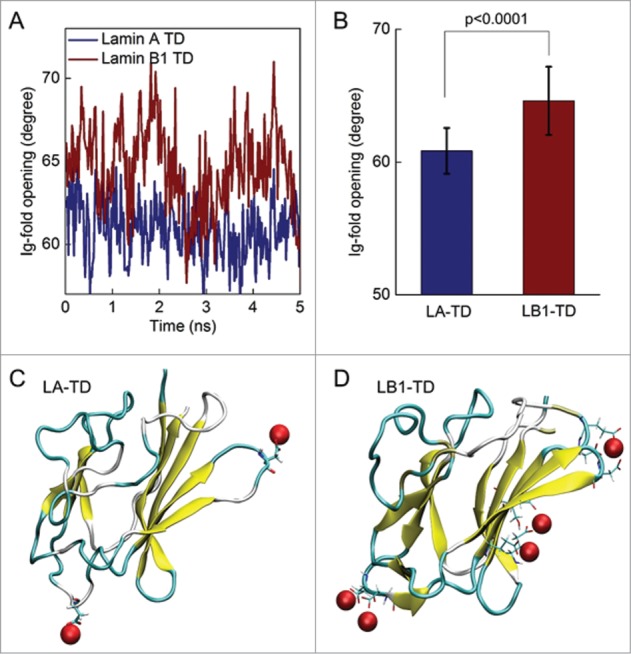
Altered Ig-fold structures of LA and LB1 in equilibrium with solvent with calcium. (A) We examined the dihedral angles between the β-sheets within the Ig-fold as functions of time during last 5 ns of the equilibration simulation to determine how open the Ig-fold is for each protein. (B) LB1-TD almost exclusively shows a larger opening of the Ig-fold over time and with a statistical comparison. (C) Snapshots of the LA Ig-fold at the end of the simulation shows associated Ca2+ in red. A similar snapshot of the LB1 Ig-fold highlights amino acids that interact (within 5 Å) with Ca2+ as a stick model. The Ca2+ interacting with the LB1 Ig-fold show a pinning or bridging of amino acids, causing the larger deformation of the Ig-fold as shown in B.
Altered nucleoskeletal structure in the presence of divalent cations
To test the effects of increased divalent cations in situ, we incubated human foreskin fibroblasts (HFF) with twice the amount of Mg2+ found in cell culture media (see Methods). Imaging of nucleoskeletal lamins (see Methods) showed that Mg2+ did not qualitatively alter lamin A distribution whereas lamin B1 has an increased “rim stain” (Fig. 6A) associated with increased lamins in the nucleoskeleton. Analysis of the intensity profile of nuclei from 10 fields of view (see Methods) shows that lamin B1 has increased intensity at nucleoskeleton periphery, as seen by spikes in the y-axis along the projection (Fig. 6B and Fig. S1).
Figure 6.
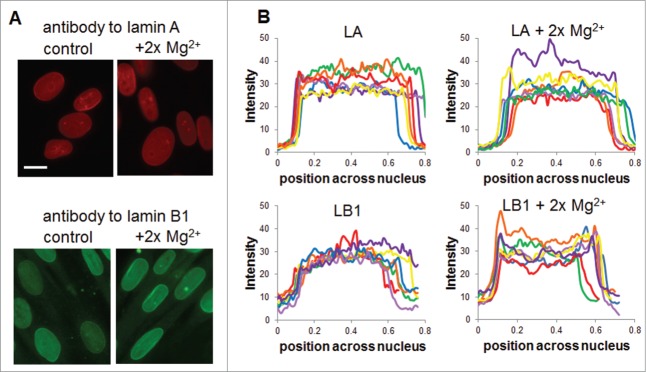
Nucleoskeleton redistribution with divalent cations. Human foreskin fibroblasts grown in standard cell culture media or in media spiked with 2x Mg2+ were fixed and labeled with antibodies for lamin A and lamin B1. (A) Representative image of each group shows mild changes in lamin A intensity or localization in the presence of Mg2+ whereas lamin B1 shows altered localization to the nuclear periphery. (B) Quantification of localization in nuclei from more than 10 fields of view each using the scheme shown in Figure S1 shows altered lamin B localization. Scale bar is 20 μm.
Discussion
Here, we examine the interaction of the divalent cations Ca2+ and Mg2+ with TDs of LA and LB1 using experimentation and simulation. Previous studies with LA-TDs have shown aggregation in the presence of other divalent cations found in the nucleus including Mn2+ and Zn2+.24 Our experimental results show that LB1 is 2–3 times more responsive to divalent cations than LA, and simulations show a similar increased affinity as well as binding duration. Although simulations suggest that divalent cations bind to amino acid regions at some distance from the Ig-fold (where the experimentally measured tryptophan residues reside), simulations confirm altered Ig-fold structure from nonadjacent calcium binding. This is likely due to the flexibility of the intrinsically disordered regions outside of the Ig-fold, which can impact the stability of the fold. The Ig-fold is altered more in LB1-TD than LA-TD in the presence of divalent cations.
This altered structure, particularly in response to divalent cation concentration, may influence the differential association of lamin B1 and lamin A with binding partners. LA-TD binds lamin-associated proteins including emerin, MAN1 and Lap2α while LB1-TD shows greater affinity to other proteins like LBR and F-actin.11 Generally, LA and LB1 form distinct nucleoskeletal networks and have different binding partners.25 Their altered binding of the TDs to divalent cations may add yet another complexity to the altered higher-ordered structures they form inside the nucleus.
Divalent cations in the cell and nucleus
Ca2+ levels are altered significantly during intracellular signal transduction, and generally nuclear Ca2+ concentration is greater than cytosolic. Intracellular Ca2+ concentration varies, typically in the 100s of nM, but levels can increase dramatically during various signaling processes to 10–100s of μM.26,27 Cytosolic Mg2+ levels are higher and do not fluctuate as much as Ca2+; typically Mg2+ concentrations are 200 μM-2 mM depending on the cell type.28 Ca2+ and Mg2+ contribute almost 18% the neutralization of the negatively charged DNA in cell nuclei.29 Here, we have shown a KD of 200–300 μM for the lamin TDs to divalent cations with a consistent but not statistically different experimental result that both LA-TD and LB1-TD are more responsive to Mg2+ than Ca2+. Similarly, the results of the simulations conducted with Ca2+ will be similar to Mg2+ since the ion is nonspecific divalent charge and changes only slightly with size. Combined, the free divalent cation concentration is sufficient to elicit structural transitions. Generally, the highly charged nucleoplasm, including the poly-anionic DNA and highly-positive histones, provide an environment of high ionic strength and localized regions of multivalent charge.
In situ divalent cations and mechanical cellular structures
Within the cell, we observe impacts of increased Mg2+ on the lamin B1 nucleoskeletal structure. We chose to alter Mg2+ concentration since increases in Ca2+ concentration can alter numerous cellular signaling processes and cell structures. Within the nucleus, Ca2+ helps maintain nuclear envelope structures including pore-linked filaments.30 Although it is nearly impossible to perform a perfectly controlled cellular experiment with alteration of Mg2+ due to numerous cell changes – including chromatin structure,31 cytoskeletal and focal adhesion structure,32 and cellular signaling - this study suggests the possibility that Mg2+ alters lamin B1 localization more strongly than lamin A. The concept of localization of lamin B1 to the nuclear envelope may seem redundant since lamin B1 is permanently farnesylated. However, we have found that farnesylation does not dictate membrane association, and the KD of farnesylated lamin tail domain binding to membranes is relatively weak.7 Also, there are numerous factors that influence protein-membrane association including protein-protein interaction within the lamina nucleoskeleton and the presence of divalent cations.33
Influence on farnesylation-membrane association
As noted in the introduction, lamin B1 is permanently farnesylated whereas lamin A is transiently farnesylated, and aberrant farnesylation of lamin A leads to devastating diseases. Some of the differences could be a function of filament and network assembly of lamin A vs. lamin B: the lamin A nucleoskeleton is stiffer than the lamin B1.9 However, we have shown previously that divalent cations play a vital role in the binding of farnesylated lamins to membranes.7 Thus, we suggest the differential interaction of divalent cations with LB1-TD and LA-TD may act to modulate membrane interactions and may play a role in determining why farnesylation can be retained in lamin B (but not in lamin A) without causing any disease states. Also, the increased opening of the Ig-fold of LB1-TD compared with LA-TD in the presence of divalent cations may suggest an inherent increased flexibility of the molecule. In previous studies, we have found that the Ig-fold opening of lamin A determined by simulation matches with heat denaturation.8
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors acknowledge as well as funding from the Progeria Research Foundation (KND), NIH (NIA-F30-AG030905 to AK), NSF (CBET-0954421 to KND and CMMI-0642545 to MB) ONR-PECASE (N000141010562 to MB) and ARCS Foundation (STS), Bertucci Fellowship (STS) and James C. Meade Fellowship (STS).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
Author Contributions
SG: protein production, purification and fluorescence experiments and analysis; ZQ: simulations and analysis; SS: cell culture and imaging experiments; MB: PCR production of LB1-TD and assisted in protein production; KC: assisted in protein purification and fluorescence; AK: PCR production of LA-TD; MB: supervision of simulations; KND: supervision of experiments
References
- 1. Dittmer TA, Misteli T. The lamin protein family. Genome Biol 2011; 12:222; PMID:21639948; http://dx.doi.org/ 10.1186/gb-2011-12-5-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma 2013; 122:13-31; PMID:23475188; http://dx.doi.org/ 10.1007/s00412-013-0399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 2007; 8:562-73; PMID:17551517; http://dx.doi.org/ 10.1038/nrm2197 [DOI] [PubMed] [Google Scholar]
- 4. Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 2006; 24:177-85; PMID:16179429; http://dx.doi.org/ 10.1634/stemcells.2004-0159 [DOI] [PubMed] [Google Scholar]
- 5. Kapinos LE, Schumacher J, Mucke N, Machaidze G, Burkhard P, Aebi U, Strelkov SV, Herrmann H. Characterization of the head-to-tail overlap complexes formed by human lamin A, B1 and B2 "half-minilamin" dimers. J Mol Biol 2010; 396:719-31; PMID:20004208; http://dx.doi.org/ 10.1016/j.jmb.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 6. Dhe-Paganon S, Werner ED, Chi YI, Shoelson SE. Structure of the globular tail of nuclear lamin. J Biol Chem 2002; 277:17381-4; PMID:11901143; http://dx.doi.org/ 10.1074/jbc.C200038200 [DOI] [PubMed] [Google Scholar]
- 7. Kalinowski A, Qin Z, Coffey K, Kodali R, Buehler MJ, Losche M, Dahl KN. Calcium causes a conformational change in lamin A tail domain that promotes farnesyl-mediated membrane association. Biophysical J 2013; 104:2246-53; PMID:23708364; http://dx.doi.org/ 10.1016/j.bpj.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin Z, Kalinowski A, Dahl KN, Buehler MJ. Structure and stability of the lamin A tail domain and HGPS mutant. J Structural Biol 2011; 175:425-33; PMID:21635954; http://dx.doi.org/ 10.1016/j.jsb.2011.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, Cote N, Gavino B, Qiao X, Chang SY, Young SR, et al. . Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest 2006; 116:743-52; PMID:16511604; http://dx.doi.org/ 10.1172/JCI27125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic 'network of networks'. Nat Rev Mol Cell Biol 2011; 12:695-708; PMID:21971041; http://dx.doi.org/ 10.1038/nrm3207 [DOI] [PubMed] [Google Scholar]
- 11. Zastrow MS, Vlcek S, Wilson KL. Proteins that bind A-type lamins: integrating isolated clues. J Cell Sci 2004; 117:979-87; PMID:14996929; http://dx.doi.org/ 10.1242/jcs.01102 [DOI] [PubMed] [Google Scholar]
- 12. Dorner D, Gotzmann J, Foisner R. Nucleoplasmic lamins and their interaction partners, LAP2alpha, Rb, and BAF, in transcriptional regulation. FEBS J 2007; 274:1362-73; PMID:17489094; http://dx.doi.org/ 10.1111/j.1742-4658.2007.05695.x [DOI] [PubMed] [Google Scholar]
- 13. Adam SA, Butin-Israeli V, Cleland MM, Shimi T, Goldman RD. Disruption of lamin B1 and lamin B2 processing and localization by farnesyltransferase inhibitors. Nucleus 2013; 4:142-50; PMID:23475125; http://dx.doi.org/ 10.4161/nucl.24089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young SG, Fong LG, Michaelis S. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria–new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res 2005; 46:2531-58; PMID:16207929; http://dx.doi.org/ 10.1194/jlr.R500011-JLR200 [DOI] [PubMed] [Google Scholar]
- 15. Young SG, Meta M, Yang SH, Fong LG. Prelamin A farnesylation and progeroid syndromes. J Biol Chem 2006; 281:39741-5; PMID:17090536; http://dx.doi.org/ 10.1074/jbc.R600033200 [DOI] [PubMed] [Google Scholar]
- 16. Vivian JT, Callis PR. Mechanisms of tryptophan fluorescence shifts in proteins. Biophysical J 2001; 80:2093-109; PMID:11325713; http://dx.doi.org/ 10.1016/S0006-3495(01)76183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugita Y, Okamoto Y. Replica-exchange moleculardynamics method for protein folding. Chem Physics Lett 1999; 314; 1–2:141–51; doi: 10.1016/S0009-2614(99)01123-9 [DOI] [Google Scholar]
- 18. Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. Charmm: a program for macromolecular energy, minimization, and dynamic calculations. J Computational Chem 1983; 4:187-217; http://dx.doi.org/ 10.1002/jcc.540040211 [DOI] [Google Scholar]
- 19. Feig M, Karanicolas J, Brooks CL. MMTSB Tol Set: enhanced sampling and multiscale modeling methods for applications in structural biology. J Mol Graph Model 2004; 22:377-95; PMID:15099834; http://dx.doi.org/ 10.1016/j.jmgm.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 20. Miteva MA, Alexov E, Villoutreix BO. Protein Structure Analysis Online. Wiley, 2007; Nov; Chapter 2:Unit 2.13.. doi: 10.1002/0471140864.ps0213s50 [DOI] [PubMed] [Google Scholar]
- 21. Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A. Comparative protein structure modeling using MODELLER. Current Protocol Protein Sci 2007; 2:2 9; PMID:18429317; http://dx.doi.org/ 10.1002/0471140864.ps0209s50 [DOI] [PubMed] [Google Scholar]
- 22. Stick R. The gene structure of Xenopus nuclear lamin A: a model for the evolution of A-type from B-type lamins by exon shuffling. Chromosoma 1992; 101:566-74; PMID:1521501; http://dx.doi.org/ 10.1007/BF00660316 [DOI] [PubMed] [Google Scholar]
- 23. Lin YC, Broedersz CP, Rowat AC, Wedig T, Herrmann H, Mackintosh FC, Weitz DA. Divalent cations crosslink vimentin intermediate filament tail domains to regulate network mechanics. J Mol Biol 2010; 399:637-44; PMID:20447406; http://dx.doi.org/ 10.1016/j.jmb.2010.04.054 [DOI] [PubMed] [Google Scholar]
- 24. Kalinowski A, Yaron PN, Shenoy S, Losche M, Dahl KN. Interfacial binding and aggregation of lamin A tail domains associated with Hutchinson-Gilford progeria syndrome. Biophys Chem 2014; 195:43-8; PMID:25194277; http://dx.doi.org/ 10.1016/j.bpc.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. . The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 2008; 22:3409-21; PMID:19141474; http://dx.doi.org/ 10.1101/gad.1735208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chandra S, Gross D, Ling YC, Morrison GH. Quantitative imaging of free and total intracellular calcium in cultured cells. Proc Nat Acad Sci U S A 1989; 86:1870-4; PMID:2928310; http://dx.doi.org/ 10.1073/pnas.86.6.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J 1997; 16:7166-73; PMID:9384593; http://dx.doi.org/ 10.1093/emboj/16.23.7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ebel H, Gunther T. Magnesium metabolism: a review. J Clin Chem Clin Biochem 1980; 18:257-70; PMID:7000968 [DOI] [PubMed] [Google Scholar]
- 29. Morgan JE, Blankenship JW, Matthews HR. Polyamines and acetylpolyamines increase the stability and alter the conformation of nucleosome core particles. Biochem 1987; 26:3643-9; PMID:3651402; http://dx.doi.org/ 10.1021/bi00386a058 [DOI] [PubMed] [Google Scholar]
- 30. Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, Wilson KL. Actin- and protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci 2004; 117:2481-90; PMID:15128868; http://dx.doi.org/ 10.1242/jcs.01098 [DOI] [PubMed] [Google Scholar]
- 31. Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J 2005; 89:2855-64; PMID:16055543; http://dx.doi.org/ 10.1529/biophysj.105.062554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dahl KN, Kalinowski A. Nucleoskeleton mechanics at a glance. J Cell Sci 2011; 124:675-8; PMID:21321324; http://dx.doi.org/ 10.1242/jcs.069096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bera M, Kotamarthi HC, Dutta S, Ray A, Ghosh S, Bhattacharyya D, Ainavarapu SR, Sengupta K. Characterization of unfolding mechanism of human lamin A Ig fold by single-molecule force spectroscopy-implications in EDMD. Biochem 2014; 53:7247-58; PMID:25343322; http://dx.doi.org/ 10.1021/bi500726f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


