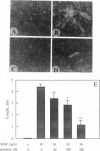
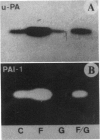
Abstract
Consumption of a plant-based diet can prevent the development and progression of chronic diseases that are associated with extensive neovascularization; however, little is known about the mechanisms. To determine whether prevention might be associated with dietary-derived angiogenesis inhibitors, we have fractionated urine of healthy human subjects consuming a plant-based diet and examined the fractions for their abilities to inhibit the proliferation of vascular endothelial cells. Using gas chromatography-mass spectrometry, we showed that one of the most potent fractions contained several isoflavonoids, which we subsequently synthesized. Of all synthetic compounds, the isoflavonoid genistein was the most potent and inhibited endothelial cell proliferation and in vitro angiogenesis at concentrations giving half-maximal inhibition of 5 and 150 microM, respectively. As we have previously demonstrated, genistein concentrations in urine of subjects consuming a plant-based diet are in the micromolar range, while those of subjects consuming a traditional Western diet are lower by a factor of > 30. The high excretion of genistein in urine of vegetarians and our present results suggest that genistein may contribute to the preventive effect of a plant-based diet on chronic diseases, including solid tumors, by inhibiting neovascularization. Thus, genistein may represent a member of a new class of dietary-derived anti-angiogenic compounds.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Fotsis T., Bannwart C., Wähälä K., Brunow G., Hase T. Isotope dilution gas chromatographic-mass spectrometric method for the determination of lignans and isoflavonoids in human urine, including identification of genistein. Clin Chim Acta. 1991 Jul 15;199(3):263–278. doi: 10.1016/0009-8981(91)90120-2. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Honjo H., Higashi A., Fotsis T., Hämäläinen E., Hasegawa T., Okada H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991 Dec;54(6):1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Musey P. I., Fotsis T., Bannwart C., Wähälä K., Mäkelä T., Brunow G., Hase T. Identification of lignans and phytoestrogens in urine of chimpanzees. Clin Chim Acta. 1986 Jul 30;158(2):147–154. doi: 10.1016/0009-8981(86)90230-5. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl. 1990;201:3–23. [PubMed] [Google Scholar]
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Baggott J. E., Ha T., Vaughn W. H., Juliana M. M., Hardin J. M., Grubbs C. J. Effect of miso (Japanese soybean paste) and NaCl on DMBA-induced rat mammary tumors. Nutr Cancer. 1990;14(2):103–109. doi: 10.1080/01635589009514083. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Blood C. H., Zetter B. R. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990 Jun 1;1032(1):89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- Bouck N. Tumor angiogenesis: the role of oncogenes and tumor suppressor genes. Cancer Cells. 1990 Jun;2(6):179–185. [PubMed] [Google Scholar]
- Chodak G. W., Hospelhorn V., Judge S. M., Mayforth R., Koeppen H., Sasse J. Increased levels of fibroblast growth factor-like activity in urine from patients with bladder or kidney cancer. Cancer Res. 1988 Apr 15;48(8):2083–2088. [PubMed] [Google Scholar]
- Crum R., Szabo S., Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985 Dec 20;230(4732):1375–1378. doi: 10.1126/science.2416056. [DOI] [PubMed] [Google Scholar]
- Denekamp J. Vascular attack as a therapeutic strategy for cancer. Cancer Metastasis Rev. 1990 Nov;9(3):267–282. doi: 10.1007/BF00046365. [DOI] [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Fotsis T., Adlercreutz H. The multicomponent analysis of estrogens in urine by ion exchange chromatography and GC-MS--I. Quantitation of estrogens after initial hydrolysis of conjugates. J Steroid Biochem. 1987 Aug;28(2):203–213. doi: 10.1016/0022-4731(87)90379-7. [DOI] [PubMed] [Google Scholar]
- Fotsis T., Heikkinen R., Adlercreutz H., Axelson M., Setchell K. D. Capillary gas chromatographic method for the analysis of lignans in human urine. Clin Chim Acta. 1982 Jun 3;121(3):361–371. doi: 10.1016/0009-8981(82)90245-5. [DOI] [PubMed] [Google Scholar]
- Fotsis T. The multicomponent analysis of estrogens in urine by ion exchange chromatography and GC-MS--II. Fractionation and quantitation of the main groups of estrogen conjugates. J Steroid Biochem. 1987 Aug;28(2):215–226. doi: 10.1016/0022-4731(87)90380-3. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Mordan L. J., Lau A. F., Kanemitsu M. Y., Boynton A. L. PDGF-induced activation of phospholipase C is not required for induction of DNA synthesis. Science. 1990 Jun 29;248(4963):1660–1663. doi: 10.1126/science.2163545. [DOI] [PubMed] [Google Scholar]
- Linassier C., Pierre M., Le Pecq J. B., Pierre J. Mechanisms of action in NIH-3T3 cells of genistein, an inhibitor of EGF receptor tyrosine kinase activity. Biochem Pharmacol. 1990 Jan 1;39(1):187–193. doi: 10.1016/0006-2952(90)90664-7. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Miller A. B. Diet and cancer. A review. Acta Oncol. 1990;29(1):87–95. doi: 10.3109/02841869009089996. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Montesano R., Pepper M. S., Belin D., Vassalli J. D., Orci L. Induction of angiogenesis in vitro by vanadate, an inhibitor of phosphotyrosine phosphatases. J Cell Physiol. 1988 Mar;134(3):460–466. doi: 10.1002/jcp.1041340318. [DOI] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura A., Arakawa H., Oka H., Yoshinari T., Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Olwin B. B., Krebs E. G. Fibroblast growth factor treatment of Swiss 3T3 cells activates a subunit S6 kinase that phosphorylates a synthetic peptide substrate. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5968–5972. doi: 10.1073/pnas.83.16.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Belin D., Montesano R., Orci L., Vassalli J. D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990 Aug;111(2):743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Montesano R. Proteolytic balance and capillary morphogenesis. Cell Differ Dev. 1990 Dec 2;32(3):319–327. doi: 10.1016/0922-3371(90)90046-y. [DOI] [PubMed] [Google Scholar]
- Proctor A. J., Coombs L. M., Cairns J. P., Knowles M. A. Amplification at chromosome 11q13 in transitional cell tumours of the bladder. Oncogene. 1991 May;6(5):789–795. [PubMed] [Google Scholar]
- Schweigerer L., Breit S., Wenzel A., Tsunamoto K., Ludwig R., Schwab M. Augmented MYCN expression advances the malignant phenotype of human neuroblastoma cells: evidence for induction of autocrine growth factor activity. Cancer Res. 1990 Jul 15;50(14):4411–4416. [PubMed] [Google Scholar]
- Schweigerer L., Christeleit K., Fleischmann G., Adlercreutz H., Wähälä K., Hase T., Schwab M., Ludwig R., Fotsis T. Identification in human urine of a natural growth inhibitor for cells derived from solid paediatric tumours. Eur J Clin Invest. 1992 Apr;22(4):260–264. doi: 10.1111/j.1365-2362.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Fotsis T. Angiogenesis and angiogenesis inhibitors in paediatric diseases. Eur J Pediatr. 1992 Jul;151(7):472–476. doi: 10.1007/BF01957746. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Mergia A., Abraham J. A., Fiddes J. C., Gospodarowicz D. Basic fibroblast growth factor in human rhabdomyosarcoma cells: implications for the proliferation and neovascularization of myoblast-derived tumors. Proc Natl Acad Sci U S A. 1987 Feb;84(3):842–846. doi: 10.1073/pnas.84.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson R. K., Nomura A. M., Grove J. S., Stemmermann G. N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989 Apr 1;49(7):1857–1860. [PubMed] [Google Scholar]
- Troll W., Wiesner R., Shellabarger C. J., Holtzman S., Stone J. P. Soybean diet lowers breast tumor incidence in irradiated rats. Carcinogenesis. 1980 Jun;1(6):469–472. doi: 10.1093/carcin/1.6.469. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Kawada S., Nakano H. Induction of mammalian topoisomerase II dependent DNA cleavage by nonintercalative flavonoids, genistein and orobol. Biochem Pharmacol. 1990 Feb 15;39(4):737–744. doi: 10.1016/0006-2952(90)90153-c. [DOI] [PubMed] [Google Scholar]