Abstract
BACKGROUND
Research targeting glycosylated hemoglobin (HbA1c) to < 6.5% to prevent coronary heart disease (CHD) events has conflicting results. We previously observed the haptoglobin (Hp) Hp2-2 genotype is associated with a ~10-fold increased CHD risk among individuals with HbA1c ≥ 6.5%, and thus might be useful in identifying those at high risk of CHD who would benefit from maintaining HbA1c < 6.5%.
OBJECTIVES
We modeled whether HbA1c ≥ 6.5% in the Hp2-2 genotype is associated with CHD in a prospective case-control study nested within the Health Professionals Follow-Up Study (HPFS).
METHODS
HbA1c concentration and Hp genotype were determined for 695 incident cases of CHD from 1994–2010 and matched controls. Logistic regression models calculated relative risk (RR) and 95% confidence intervals (CI), for the first and second halves of follow-up, adjusting for confounding variables. A dataset from the Nurses’ Health Study (NHS) served as a replication cohort.
RESULTS
Prevalence of Hp2-2 genotype in HPFS was 39%. Compared to HbA1c < 6.5%, RR of CHD for HbA1c ≥ 6.5% for the Hp2-2 genotype over full follow-up was 3.07 (1.37–6.86)-- 3.88 (1.31–11.52) during the first half of follow-up and 2.16 (0.61–7.61) in the second half. The corresponding RRs (95% CI) for the Hp1-1 + Hp2-1 genotypes were 2.19 (1.14–4.24) (full follow-up), 1.60 (0.73–3.53) (first half), and 4.72 (1.26–17.65) (second half).
CONCLUSIONS
In 2 independent cohorts, risk of CHD associated with HbA1c ≥ 6.5% is pronounced in the Hp2-2 genotype, particularly in early cases. The Hp2-2 genotype may identify individuals at greatest CHD risk from hyperglycemia.
Keywords: acute myocardial infarction, coronary disease, epidemiology, genetic association, glycoproteins
Introduction
Large randomized controlled trials that have targeted glycosylated hemoglobin (HbA1c) to a concentration < 6.5% among participants with diabetes mellitus have found significant reduction in cardiovascular outcomes in some (1,2) but not all (3,4) studies. This inconsistency may be due in part to different unknown characteristics among patient subgroups which have not yet been explored (5). The common haptoglobin (Hp) copy number variant (CNV) (rs72294371) has 3 genotypes (Hp1-1, Hp2-1, and Hp2-2) with frequencies that differ among populations (6) and may potentially explain differences in the efficacy of strict glycemic control between study populations. The primary function of Hp is to protect against oxidative damage from extracorpuscular hemoglobin (7). The 3 Hp genotypes produce structurally different Hp proteins, of which the Hp2-2 is the least functional--an impairment that is further accentuated when hemoglobin is glycosylated (8). Currently available genome-wide association study (GWAS) technologies have not been found to capture the Hp CNV polymorphism, explaining why the association of this polymorphism with cardiovascular disease in the setting of elevated blood glucose has not been investigated in cohorts screened only by GWAS (9–11).
In 2 independent populations, we have observed that participants with both the Hp2-2 genotype (homozygous for presence of CNV) and HbA1c ≥ 6.5% had a 10-fold higher risk of coronary heart disease (CHD) compared to those with at least 1 Hp1 allele and HbA1c < 6.5% (12). However, a recent analysis of cardiovascular disease among adults in the Bruneck study did not observe a similar association (13). The discrepancy may be due to several study design factors, especially the combined endpoint of CHD and stroke (the majority of cases were stroke), since stroke has been associated with the Hp1-1 genotype rather than the Hp2-2 genotype (14,15). However, the Bruneck study is of interest because it contained repeated measures of HbA1c and provides suggestive evidence for a potential time-dependent bias against the Hp2-2 genotype as participants aged that supports previous findings of increased longevity among individuals with the Hp1-1 genotype (16,17). Thus, if Hp2-2 individuals are at increased risk of early disease and death, it strengthens the growing evidence behind the hypothesis that targeted screening and treatment for the Hp2-2 genotype in cases of HbA1c ≥ 6.5% could prevent coronary events.
In the present study, we used a large prospective case-control study nested within a cohort of healthy men at baseline to further examine the interaction between the Hp polymorphism and HbA1c concentration on risk of CHD, testing the hypothesis that targeted screening for the Hp2-2 genotype in the setting of HbA1c ≥ 6.5% may be prudent. Because previous long-term studies have reported that the association between HbA1c and risk of CHD is strongest in the earliest years of follow-up (18) we examined the first and second halves of follow-up separately as well as together. We then sought replication of our full analysis in a complementary, but independent, cohort of women.
METHODS
COHORT: THE HEALTH PROFESSIONALS FOLLOW-UP STUDY
The Health Professionals Follow-up Study (HPFS) is a prospective cohort of 51,529 male health professionals who were 40 to 75 years of age at baseline in 1986. Information on anthropometric and lifestyle factors is obtained through self-administered questionnaires every 2 years and diet every 4 years. Blood samples were collected from participants free of cancer and without prior cardiovascular events in 1993–1995. Men who had an incident myocardial infarction (MI) or fatal CHD between the date of blood draw and January 2010 were identified and matched 1:1 to controls with the same age, smoking status, and month of blood draw. Participants were excluded from the final sample in the present analysis if they were missing data on the exposures or the outcome. Using 2-tailed tests, the type I error probability of 0.05, a prevalence in controls of 0.39 for the Hp2-2 genotype, and our final HPFS sample size of 695 cases matched 1:1 to controls, we have > 90% power to detect an odds ratio of 1.50. The majority (> 95%) of participants in this case-control sample are Caucasian. Anthropometric and lifestyle variables were derived from the questionnaire administered in 1994, with missing information substituted from the two previous questionnaires. The validity of the questionnaires and the reproducibility of the measurements have been previously reported (19). The institutional review board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard T. H. Chan School of Public Health approved the study protocol.
REPLICATION COHORT: THE NURSES’ HEALTH STUDY
The Nurses’ Health Study (NHS) is a prospective cohort of 121,700 female US registered nurses who were 30 to 55 years of age at baseline in 1976. Information on anthropometric and lifestyle factors is obtained through self-administered questionnaires every 2 years and diet every 4 years. From 1989 to 1990, a blood sample was provided by 32,826 women. Women who had an incident MI or fatal CHD between the date of blood draw and June 2004 were identified and matched 1:1 to controls for age, smoking status, fasting status, and month of blood draw, as described elsewhere (20). Details of the genotype frequencies and baseline characteristics of the specific sample used in this present study have been previously reported (12) but the hypothesis tested is new in the present study. Using 2-tailed tests, the type I error probability of 0.05, a prevalence in controls of 0.39 for the Hp2-2 genotype, and our final NHS sample size of 400 cases matched 1:1 to controls, we have >80% power to detect an odds ratio of 1.50.
HAPTOGLOBIN TYPING
Although recently given the identifier rs72294371 (21), the Hp polymorphism is not a single nucleotide polymorphism; it is a CNV defined by the absence (Hp1 allele) or presence (Hp2 allele) of a 1.7 kb partial in-frame intragenic duplication of exons 3 and 4. Hp type was determined in both cohorts by protein gel electrophoresis of hemoglobin enriched serum (22). This procedure produces a fingerprint banding pattern for each Hp type and has been shown to correspond completely with the Hp genotype (23). Currently available GWAS platforms do not include the Hp CNV polymorphism (9–11). Genotype frequencies in the HPFS were in Hardy-Weinberg equilibrium in the whole sample (p = 0.08) and also within cases (p = 0.28) and controls (p = 0.17) separately. Genotype frequencies in the NHS were also in Hardy-Weinberg equilibrium, as previously reported (12).
CORONARY HEART DISEASE CASE ASSESSMENT
As previously detailed in HPFS (24) and NHS (25), CHD was similarly defined in the 2 datasets as non-fatal MI or fatal CHD, but does not include the development of coronary atherosclerosis which has not resulted in an acute coronary syndrome. MI was diagnosed on the basis of the criteria of the World Health Organization (symptoms plus either diagnostic electrocardiographic changes or altered levels of cardiac enzymes) (26). After a participant reported a non-fatal event on a questionnaire, the case was confirmed through the review of medical records by physicians blinded to the self- report. Deaths were identified from state vital records and the National Death Index or reported by the participant’s next of kin or the postal system. Fatal CHD was confirmed by an examination of hospital or autopsy records.
STATISTICAL ANALYSIS
Participant characteristics were compared between cases and controls using t-tests for continuous variables and χ2 tests for categorical variables. For skewed variables (physical activity, alcohol, CRP, HDL, HbA1c, and triglycerides), p-values from log-transformed analyses and median and interquartile ranges were determined. The primary hypothesis we tested was that HbA1c ≥ 6.5% in the risk genotype (Hp2-2) is associated with CHD in a prospective nested case-control study. Because of the nested case-control study design, relative risks (RRs) of CHD were estimated by incidence rate ratios from logistic regression with adjustment for the matching factors. In addition to matching factors, analyses were adjusted for BMI, alcohol, physical activity, parental MI before the age of 60, diet quality score (the 2010 Alternate Healthy Eating Index (AHEI) (27)), history of high cholesterol, history of high blood pressure, and medications for high blood pressure. We used an a prioi cut-point of 6.5% for HbA1c, as this is the level for complications from hyperglycemia as established by the International Expert Committee composed of members of the American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation (28). Because of the low frequency of the Hp1-1 genotype (~15%) and the structure (29) and function (30) of the different Hp proteins, we used a common approach of combining the Hp1-1 and Hp 2-1 genotype for most analyses (13). Because a single measure of HbA1c at baseline has been shown to be differently associated with short-term CHD risk than long-term CHD risk (18), we conducted analyses within halves of follow-up (first 8 years and second 8 years), in addition to examining the full follow-up period. To pool the risk estimates from multiple study cohorts, we used the weighted average of regression estimates in a random-effects meta-analysis, and tested for between-study heterogeneity (31). All analyses were conducted using SAS software version 9.3 (SAS Institute), at a 2-tailed alpha level of 0.05.
RESULTS
The distribution of Hp genotype frequencies in the HPFS cohort was 16% (Hp1-1), 45% (Hp2-1), and 39% (Hp2-2). The Hp genotype frequency did not differ between cases and controls. Baseline characteristics by case-control status are described in Table 1. As expected, cases drank less alcohol and had a lower diet quality than controls, and had higher BMI, family history of CHD prevalence of hypertension, diabetes, hypercholesterolemia, and greater medication use at baseline.
Table 1.
Baseline characteristics by case status among men 46–80 years of age at blood draw from the nested case-control study of coronary heart disease events in the Health Professionals Follow-up Study (1994–2010).
| Cases | Controls | p | |
|---|---|---|---|
| n | 695 | 696 | -- |
| Age* (years) | 63.1 ± 8.7 | 63.0 ± 8.7 | -- |
| Age at event/matched event (years) | 70.0 ± 8.2 | 70.0 ± 8.2 | -- |
| Time to event (years) | 6.9 ± 4.4 | 6.9 ± 4.4 | -- |
| Smoking Status* | |||
| Never | 317 (46) | 318(46) | -- |
| Past | 327 (47) | 336 (48) | |
| Current | 51 (7) | 42 (6) | |
| Married | 614 (88) | 620 (89) | 0.66 |
| BMI (kg/m2) | 26.3 ± 3.3 | 25.7 ± 3.5 | 0.001 |
| Activity (MET hrs/week) | 24.2 (9.5–47.5) | 25.6 (11.2–49.8) | 0.19 |
| Alcohol (g/day) | 4.4 (0.0–14.0) | 7.0 (1.0–17.6) | < 0.0001 |
| AHEI 2010 Diet Quality Score | 53.2 ± 11.5 | 54.5 ± 11.4 | 0.03 |
| Parental MI < 60 years of age | 281 (40) | 234 (34) | 0.009 |
| History of hypercholesterolemia | 346 (50) | 259 (37) | < 0.0001 |
| History of hypertension | 261 (38) | 184 (26) | < 0.0001 |
| History of diabetes | 57 (8) | 19 (3) | < 0.0001 |
| Diabetes† | 117 (17) | 40 (6) | < 0.0001 |
| Aspirin ≥5 days/week | 179 (26) | 150 (22) | 0.07 |
| Cholesterol-lowering drug use | 55 (8) | 45 (6) | 0.29 |
| Antihypertensive drug use | 188 (27) | 135 (19) | 0.0007 |
| Diabetes medication† | 75 (11) | 32 (5) | < 0.0001 |
| CRP (mg/l) | 1.40 (0.65–2.72) | 0.93 (0.47–1.87) | < 0.0001 |
| Triglycerides (mg/dl) | 138 (93–195) | 114 (83–164) | < 0.0001 |
| HbA1c (%) | 5.70 (5.44–5.96) | 5.62 (5.36–5.84) | < 0.0001 |
| HbA1c ≥ 6.5% | 65 (9) | 23 (3) | < 0.0001 |
| Total cholesterol (mg/dl) | 206 ± 37 | 200 ± 33 | 0.0006 |
| HDL cholesterol (mg/dl) | 41 (35–48) | 45 (38–54) | < 0.0001 |
| LDL cholesterol (mg/dl) | 135 ± 34 | 129 ± 31 | 0.0005 |
| Apolipoprotein B (mg/dl)*** | 98 ± 24 | 90 ± 20 | < 0.0001 |
| Haptoglobin genotype | |||
| 1-1 | 112 (16) | 109 (695) | 0.78 |
| 2-1 | 317 (46) | 312 (45) | |
| 2-2 | 266 (38) | 275 (39) | |
AHEI = alternate healthy eating index; CRP = high sensitivity C-reactive protein; HbA1c = glycosylated hemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MET = metabolic equivalent.
Data presented are n (%), mean ± SD, or median (interquartile range). Participant characteristics were compared between genotypes using a t-test for continuous variables and χ2 tests for categorical variables. For skewed variables (physical activity, alcohol, CRP, HDL, HbA1c and triglycerides), p-values from log-transformed analyses and median and interquartile ranges are reported.
Age and smoking status were matching factors, and thus no differences were observed.
cases were updated as having diabetes if they developed diabetes before their CHD event (controls if diagnosed with diabetes before their matched case had a CHD event). Diabetes medication use includes medications initiated during follow-up.
n = 424 cases and 421 controls for Apolipoprotein B.
For the Hp2-2 genotype and the Hp1 allele carriers separately, Table 2 presents the multivariate-adjusted relative risk (RR) and 95% confidence intervals (CI) of CHD when HbA1c was ≥ 6.5% compared to when HbA1c < 6.5%. HPFS participants with the Hp2-2 genotype had a multivariate relative risk of 3.07 (1.37–6.86) if their HbA1c was ≥ 6.5% compared to if it was < 6.5%, and the corresponding relative risk for the Hp1-1 + Hp2-1 genotypes (Hp 1 allele carriers) was 2.19 (1.14–4.24). Although with limited power, when earlier and later CHD events were examined separately, the risk of CHD associated with HbA1c ≥ 6.5% for the Hp2-2 genotype was greater in the earlier HPFS cases (RR = 3.88, 95% CI: 1.31 to 11.52 for events that occurred in the first 8 years) and less so in the later cases (RR = 2.16, 95% CI: 0.61 to 7.61 for events that occurred in the last 8 years). The reverse pattern was observed for the HPFS participants with the Hp1-1+Hp2-1 genotypes.
Table 2.
Adjusted relative risk (RR) of coronary heart disease (CHD) with 95% confidence intervals (CI) for HbA1c ≥ 6.5% stratified by haptoglobin (Hp) genotypes in men 46–80 years of age at blood draw from the nested case-control study of CHD events in the Health Professionals Follow-up Study (HPFS) (1994–2010) and in women 44–69 years of age at blood draw from the nested case-control study of CHD events in the Nurses’ Health Study (NHS) (1990–2004).
| Haptoglobin Genotype | P, Interaction | ||||
|---|---|---|---|---|---|
|
| |||||
| Hp1-1 + Hp2-1 | Hp2-2 | ||||
|
| |||||
| HbA1c < 6.5% | HbA1c ≥ 6.5% | HbA1c < 6.5% | HbA1c ≥ 6.5% | ||
| HPFS
| |||||
| All Participants Together | |||||
| N, Cases/Controls | 393/407 | 36/14 | 237/266 | 29/9 | |
| RR (95% CI) | 1.00 (Ref) | 2.19 (1.14–4.24) | 1.00 (Ref) | 3.07 (1.37–6.86) | 0.76 |
|
| |||||
| First half (8 years) of follow-up | |||||
| N, Cases/Controls | 231/235 | 21/11 | 135/150 | 19/5 | |
| RR (95% CI) | 1.00 (Ref) | 1.60 (0.73–3.53) | 1.00 (Ref) | 3.88 (1.31–11.52) | 0.41 |
|
| |||||
| Second half of follow-up | |||||
| N, Cases/Controls | 162/172 | 15/3 | 102/116 | 10/4 | |
| RR (95% CI) | 1.00 (Ref) | 4.72 (1.26–17.65) | 1.00 (Ref) | 2.16 (0.61–7.61) | 0.42 |
|
| |||||
| NHS
| |||||
| All Participants Together | |||||
| N, Cases/Controls | 212/239 | 33/11 | 135/148 | 24/2 | |
| RR (95% CI) | 1.00 (Ref) | 2.12 (0.98–4.60) | 1.00 (Ref) | 10.59 (2.34–47.91) | 0.05 |
|
| |||||
| First 8 years of follow-up | |||||
| N, Cases/Controls | 104/108 | 25/5 | 47/77 | 17/1 | |
| RR (95% CI) | 1.00 (Ref) | 3.46 (1.14–10.55) | 1.00 (Ref) | 28.62 (3.27–250.72) | 0.09 |
|
| |||||
| After first 8 years of follow-up | |||||
| N, Cases/Controls | 108/131 | 8/6 | 88/71 | 7/1 | |
| RR (95% CI) | 1.00 (Ref) | 0.92 (0.27–3.07) | 1.00 (Ref) | 4.98 (0.54–46.16) | 0.17 |
|
| |||||
| NHS and HPFS Pooled
| |||||
| All Participants | |||||
| N, Cases/Controls | 605/646 | 69/25 | 372/418 | 53/11 | |
| RR (95% CI) | 1.00 (Ref) | 2.16 (1.31–3.57) | 1.00 (Ref) | 4.80 (1.49–15.42) | 0.30 |
|
| |||||
| First 8 years of follow-up | |||||
| N, Cases/Controls | 335/343 | 46/16 | 182/231 | 36/6 | |
| RR (95% CI) | 1.00 (Ref) | 2.12 (1.02–4.39) | 1.00 (Ref) | 8.38 (1.25–56.32) | 0.15 |
|
| |||||
| After 8 years of follow-up | |||||
| N, Cases/Controls | 270/303 | 23/9 | 190/187 | 17/5 | |
| RR (95% CI) | 1.00 (Ref) | 2.03 (0.41–10.13) | 1.00 (Ref) | 2.64 (0.88–7.92) | 0.78 |
The multivariate model is adjusted for matching factors (age and smoking) and also: BMI, alcohol, physical activity, parental CHD before the age of 60, diet quality score, history of high cholesterol, history of high blood pressure, and medications for high blood pressure.
We have previously reported the preliminary results from the NHS which found an elevated risk of CHD among participants with both HbA1c ≥ 6.5% and the Hp2-2 genotype (12). When we re-analyzed the NHS with identical methods to our present HPFS analysis and pooled results, our findings were strong and consistent. The risk of CHD associated with HbA1c ≥ 6.5% for the Hp2-2 genotype was substantial (RR = 10.59; 95% CI: 2.34 to 47.91), but even potentially greater when we restricted the analysis to the first 8 years after blood draw (RR = 28.62; 95% CI: 3.27 to 250.72), and was not significant after the first 8 years of follow-up (RR = 4.98; 95% CI: 0.54 to 46.16) (Table 2). Among NHS participants, the RR of CHD for HbA1c ≥ 6.5% was not significant for the Hp1-1+Hp2-1 genotypes (RR = 2.12; 95% CI: 0.98 to 4.60). Pooling the results from the HPFS and NHS together substantially increased the power and highlighted the differences in the RR of CHD for HbA1c ≥ 6.5% among the Hp 2-2 genotypes (RR = 8.38; 95% CI: 1.25 to 56.32) and Hp1 carriers (RR = 2.12; 95% CI: 1.02 to 4.39) during the first 8 years of follow-up. The multivariate-adjusted p-values for the tests of interaction were not significant. Results were not appreciably altered by further adjustment for cholesterol-lowering medications such as statins, including those taken during the follow-up period.
In keeping with the format of previous analyses (12), pooled risk of CHD was calculated for HPFS and NHS participants together, with participants grouped by the combination of their Hp genotype and HbA1c status (Online Figure 1). Compared to participants who carried an Hp1 allele and had HbA1c < 6.5%, those with the Hp2-2 genotype and HbA1c ≥ 6.5% had a 4.25-fold increased risk of CHD (1.05–17.22), while those with the Hp2-2 genotype and HbA1c < 6.5% did not have a significant risk of CHD (RR = 0.92; 95% CI: 0.69 to 1.23).
DISCUSSION
In a prospective nested case-control study design with 16 years of follow-up, HbA1c ≥ 6.5% was a strong and significant predictor of CHD among men with the Hp2-2 genotype, especially in the cases most proximal to blood draw. Our results are strengthened by the replication of these findings in women, using data from a second cohort.
In 2 independent cohorts with a broad range of HbA1c concentrations (the Israel Cardiovascular Events Reduction With Vitamin E Study, and the same Nurses’ Health Study dataset used as a replication cohort in the present study), we have previously reported that individuals with the Hp2-2 genotype and HbA1c ≥ 6.5% had a > 10-fold increased risk of CHD compared to those with an Hp1 allele and HbA1c < 6.5% (12). Previously reported results from the Strong Heart Study data support additional replication (12,32). However, a recent longitudinal analysis of cardiovascular disease among adults in the Bruneck study did not report that HbA1c ≥ 6.5% in the Hp2-2 genotype was predictive of cardiovascular disease (13). The authors did not directly present the RR of CHD for HbA1c ≥ 6.5% among the Hp2-2 subgroup, but did present a non-significant risk (multivariate-adjusted RR = 0.35; 95%C1: 0.08 to 1.52) for the Hp2-2 versus Hp2-1+Hp1-1 genotypes in the strata of participants with HbA1c > 6.5%. The discrepancy in findings may be due to several distinguishing factors. For example, the Bruneck study included participants with prevalent disease at baseline and had a small number of cases during the 15 years of follow-up (123 cases, among which only 48 cases were CHD). The endpoint of CHD was combined with stroke, an endpoint which has been associated with the Hp1-1 genotype rather than Hp2-2 (14,15). It has been suggested that the protection conferred by the Hp1-1 genotype against CHD development is connected to function of Hp as the scavenger of free hemoglobin, while the role of Hp in angiogenesis may confer the protection against stroke associated with the Hp2-2 phenotype (33,34).
A single biomarker at baseline may be a better proxy for exposure over the first several years, and possibly become less correlated with exposure over a longer period of time. In a recent 14-year study of HbA1c and risk of CHD, when we stratified according to the midpoint of follow-up, the association was strongest among cases and controls in the first half (first 7 years) of follow-up, even when limited to only participants with HbA1c < 6.5% (18). In the present analysis, when we analyzed the HPFS data in halves of follow-up (two 8-year long studies instead of one 16-yearlong study), we observed an increased risk of CHD in the Hp2-2 participants with HbA1c ≥ 6.5% in the first half but not the second half of follow-up, and an increased risk of CHD in the Hp1-1+ Hp2-1 participants with HbA1c ≥ 6.5% in the second half of follow-up but not the first half. We observed the same pattern in the NHS data as well. It is possible that our single measurement of HbA1c may not be a strong predictor of risk beyond 8 years. In our pilot studies, HbA1c did have a strong intra-class correlation of 0.73 over a 3-year period (r between draws = 0.88, n = 83), but when HbA1c was measured in blood samples collected 10 years apart, the intra-class correlation was 0.45 (r between draws = 0.68, n = 244), which suggests good within-person reproducibility of HbA1c over periods of time as long as a decade, but that this reproducibility still decays over time. The fact that the correlations between draws are higher than the intra-class correlations indicates that the HbA1c is changing in the same direction for participants. Indeed, HbA1c levels are positively associated with age, even among populations without impaired fasting glucose (35,36), suggesting that HbA1c ≥ 6.5% may be a weaker marker of risk among older populations. However, the increase in HbA1c with age is considered to be modest (37) and age-specific diagnostic and treatment criteria do not exist.
If individuals with the Hp2-2 genotype are more susceptible to CHD, they may have cardiovascular events at an earlier age and have overall reduced longevity than the other genotypes, creating a survivor bias or depletion of susceptibles that may explain in part why we observe a stronger association in the earlier follow-up years and even a reverse pattern in the later years. Analyses in Bruneck participants stratified by age displayed an interesting pattern: in participants aged 75 years or older only, the risk of cardiovascular disease for the Hp2-2 type compared to the Hp1-1 type was 0.11 (0.01–0.95) among those with HbA1c ≥ 6.5%, suggesting a potential survivor bias against the Hp2-2 genotype with increased age. This is consistent with previous studies that suggest increased longevity among individuals with the Hp1-1 genotype (16,17).
BIOLOGICAL MECHANISMS
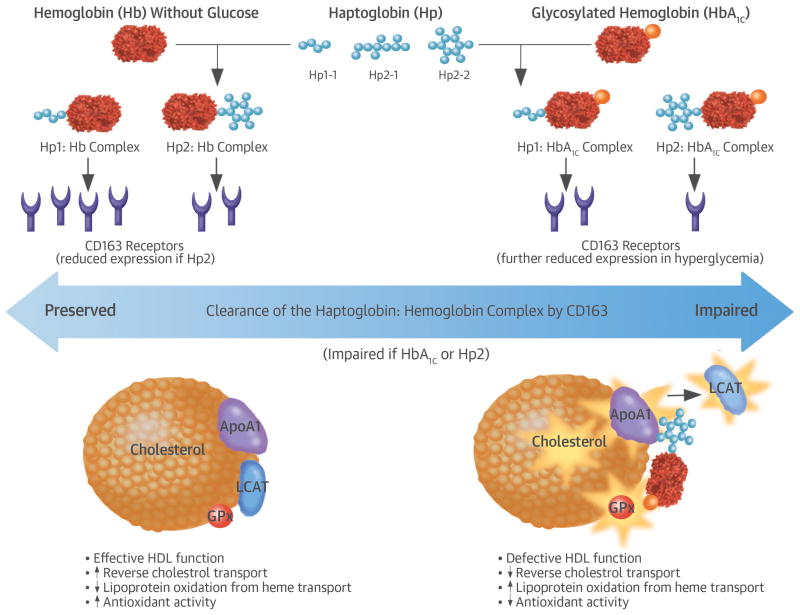
The large difference in size of the Hp CNV’s 2 alleles, Hp1 and Hp2, results in structurally and functionally different proteins being formed by each of the 3 genotypes (38). Hb released intravascularly from erythrocytes is rapidly bound by Hp protein to form an Hp-Hb complex that is cleared by the monocyte/macrophage scavenger receptor CD163 (Figure 1). However, this clearance by CD163 is impaired in Hp2-2 individuals as well as under hyperglycemic conditions, resulting in increased amounts of circulating Hp2-2:Hb complex in Hp2-2 individuals with hyperglycemia (8,39). Further, the glycosylation of hemoglobin impairs the ability of the Hp2-2 protein to act as an antioxidant, thus resulting in increased oxidative activity of the glycosylated Hp2:Hb complex (8). This pro-oxidant Hp2-2:Hb complex can bind to HDL and produce reactive oxygen species that oxidize HDL and its related components such as apolipoprotein A, glutathione peroxidase, and lecithin-cholesterol acyltransferase, thereby decreasing the function of HDL as both an antioxidant and in its role in reverse cholesterol transport, and paradoxically turning the HDL into a proatherogenic prothrombotic molecule (8,39–43). Our results were stronger in our analysis of women than of men, potentially due to higher HDL among women and thus more influence by the dysfunctional HDL associated with the Hp2-2 genotype in the setting of hyperglycemia. However we were unable to assess this potential sex difference directly in the present study.
IMPLICATIONS
Replication is required to confirm findings from the present study, and should be attempted in a randomized trial targeting HbA1c to concentrations < 6.5%, such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study (4), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial (1), and the Veterans Affairs Diabetes Trial (VADT) (3). These studies have reported conflicting results regarding the efficacy of achievement of HbA1c concentrations < 6.5% to prevent cardiovascular disease, an inconsistency that may be due in part to different unknown characteristics among patient subgroups which have not yet been explored, as recently suggested in a meta-analysis (5). Unfortunately, currently available versions of single nucleotide polymorphism (SNP) chips and tagging SNPs cannot be used to query the common Hp polymorphism (9), so replication or confirmation of our findings by GWAS is not possible. However, several genotyping and phenotyping methods exist that easily allow for direct assessment of the Hp CNV polymorphism and could be used to determine Hp genotype in ACCORD, ADVANCE, or VADT. Such an investigation, with a follow-up length of 3–5 years, could further reduce the risk of survival bias for the Hp2-2 genotype subgroup, and could also reflect a more contemporary sample than our own, due to medication use and biomarker concentrations that better reflect current clinical settings. If our findings are replicated, Hp genotyping could potentially assist in identifying the genetically susceptible individuals who would most benefit from targeted clinical management of HbA1c.
STUDY STRENGTHS AND LIMITATIONS
Strengths of the present analysis include comprehensive data gathered prospectively with a long duration of follow-up, replication in a second cohort, and a validated Hp genotyping method. The main limitation of the present study is that we only had a single measurement of HbA1c, taken at baseline, which became less proximal during each year of follow-up. Thus, random error caused by normal fluctuations and increases in HbA1c with age (35,36) could cause underestimation of true relative risks, or the biomarker may lose effectiveness as a measure of glycemia over time. We had a large number of cases and our results were significant, but with stratification by HbA1c and Hp genotype. The CIs that we observed are wide, and thus the exact risk estimate is difficult to estimate with precision and our main hypothesis needs confirmation within an intervention study design. Tests of interaction were underpowered, especially when adjusted for covariates. Bias from residual confounding is a potential limitation; however, our cases and controls were matched on age and smoking (the 2 strongest CHD risk factors), and our multivariate model showed little attenuation by known CHD risk factors. Another important limitation is that the participants we studied are predominantly Caucasian, so it is unknown whether the associations we observed would be similar in non-Caucasian populations.
CONCLUSIONS
In 2 independent cohorts, the risk of CHD associated with HbA1c ≥ 6.5% was intensified in individuals with the Hp2-2 genotype, an association that was pronounced in the earlier half of cases in this prospective nested case-control study. If replicated, the results of the present study suggest that targeted screening for the Hp polymorphism among individuals with HbA1c ≥ 6.5% could help to identify the patients who would most benefit from interventions to lower glycemia to HbA1c concentrations < 6.5%. The present study also suggests that aging and time-dependent bias limit observational research of the Hp polymorphism, and thus results would best be confirmed within a randomized clinical trial study design.
Supplementary Material
Central illustration. HbA1c, Hp2-2 genotype, and coronary heart disease: Proposed biological mechanism to explain why individuals with the Hp2-2 genotype and hyperglycemia have a pronounced risk of CHD.
Free hemoglobin (Hb) and glycosylated Hb (HbA1c) released from red blood cell lysis are rapidly bound by haptoglobin (Hp) protein to form a complex that is cleared by scavenger receptor CD163. Expression of CD163 is reduced in hyperglycemic conditions. The clearance by CD163 is further impaired in Hp2, resulting in increased amounts of circulating Hp2:Hb complex in Hp2-2 individuals with hyperglycemia. The glycosylated H2:Hb complex has weakened ability to act as an antioxidant, resulting in increased oxidative activity. This pro-oxidant Hp2:HbA1c complex can bind to HDL and produce reactive oxygen species that oxidize cholesterol and its related components such as apolipoprotein A (ApoA1), glutathione peroxidase (GPx) and lecithin-cholesterol acyltransferase (LCAT), thereby decreasing the function of HDL as both an antioxidant and in reverse cholesterol transport.
PERSPECTIVES.
Competency in Medical Knowledge
Previous randomized trials of interventions targeting strict glycemic control (hemoglobin A1c levels <6.5%) to reduce cardiovascular events have yielded inconsistent results.
Translational Outlook
Further studies are needed to determine whether aggressive glycemic control is more effective in preventing cardiovascular events when applied to individuals with specific haptoglobin genotypes.
Acknowledgments
Funding Sources: This work was supported by the NIH (Bethedsa, Maryland) DK085226, UM1 CA167552, R01 HL35464, R01 HL034594, R01 CA49449, UM1 CA186107, and the Israel Science Foundation, and additionally by a Canadian Institutes of Health Research (Ottawa, Ontario) Postdoctoral Fellowship to Dr. Leah Cahill, and the American Diabetes Association grant #1-15-JF-30 to Dr. Jensen. These funding agencies did not have any role in the study design, analysis, or preparation of the manuscript.
The authors would like to sincerely thank Dr. Sherry Sawyer and Dr. Kim Bertrand for their work running the quality control of the biomarkers, Mira Kaufman and Robert Sheahan for their work managing the case-control selection and samples, and also the staff and participants of the Nurses’ Health Study and the Health Professionals Follow-up Study.
Abbreviations List
- AHEI
Alternate Healthy Eating Index
- CHD
coronary heart disease
- CNV
copy number variant
- CRP
high sensitivity c-reactive protein
- GWAS
genome-wide association study
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- Hp
haptoglobin
- MI
myocardial Infarction
- RR
relative risk
Footnotes
Disclosures: Dr Andrew Levy is the author of a patent owned by his university regarding use of Hp genotype to predict susceptibility to cardiovascular disease in individuals with diabetes.
References
- 1.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 2.Saremi A, Schwenke DC, Bahn G, et al. The effect of intensive glucose lowering therapy among major racial/ethnic groups in the Veterans Affairs Diabetes Trial. Metabolism. 2015;64:218–25. doi: 10.1016/j.metabol.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Genuth S, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. New Engl J Med. 2011;364:818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 6.Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol. 2007;29:92–110. doi: 10.1111/j.1751-553X.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 7.Gutteridge JM. The antioxidant activity of haptoglobin towards haemoglobin-stimulated lipid peroxidation. Biochim Biophys Acta. 1987;917:219–23. doi: 10.1016/0005-2760(87)90125-1. [DOI] [PubMed] [Google Scholar]
- 8.Asleh R, Marsh S, Shilkrut M, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 9.Cahill LE, Jensen MK, Chasman DI, Hazra A, Levy AP, Rimm EB. Currently available versions of genome-wide association studies cannot be used to query the common haptoglobin copy number variant. J Am Coll Cardiol. 2013;62:860–1. doi: 10.1016/j.jacc.2013.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez S, Williams DM, Guthrie PA, et al. Molecular and population analysis of natural selection on the human haptoglobin duplication. Ann Hum Genet. 2012;76:352–62. doi: 10.1111/j.1469-1809.2012.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams JN, Cox AJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Genetic analysis of haptoglobin polymorphisms with cardiovascular disease and type 2 diabetes in the Diabetes Heart Study. Cardiovasc Diabetol. 2013;12:31. doi: 10.1186/1475-2840-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill LE, Levy AP, Chiuve SE, et al. Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J Am Coll Cardiol. 2013;61:728–37. doi: 10.1016/j.jacc.2012.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pechlaner R, Kiechl S, Willeit P, et al. Haptoglobin 2–2 genotype is not associated with cardiovascular risk in subjects with elevated glycohemoglobin-results from the Bruneck Study. J Am Heart Associ. 2014;3:e000732. doi: 10.1161/JAHA.113.000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staals J, Pieters BM, Knottnerus IL, Rouhl RP, van Oostenbrugge RJ, Delanghe JR, et al. Haptoglobin polymorphism and lacunar stroke. Curr Neurovasc Res. 2008;5:153–8. doi: 10.2174/156720208785425675. [DOI] [PubMed] [Google Scholar]
- 15.Costacou T, Secrest AM, Ferrell RE, Orchard TJ. Haptoglobin genotype and cerebrovascular disease incidence in type 1 diabetes. Diab Vasc Dis Res. 2014;11:335–42. doi: 10.1177/1479164114539713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Napolioni V, Gianni P, Carpi FM, Concetti F, Lucarini N. Haptoglobin (HP) polymorphisms and human longevity: A cross-sectional association study in a Central Italy population. Clin Chim Acta. 412:574–7. doi: 10.1016/j.cca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Hamad M, Awadallah S. Age group-associated variations in the pattern of Hp type distribution in Jordanians. Clin Chim Acta. 2000;300:75–81. doi: 10.1016/s0009-8981(00)00301-6. [DOI] [PubMed] [Google Scholar]
- 18.Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB. Hemoglobin a1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2:e000077. doi: 10.1161/JAHA.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 20.Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ, Rimm EB. Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol. 2009;29:134–9. doi: 10.1161/ATVBAHA.108.171066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corti R, Fuster V, Fayad ZA, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years’ follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002;106:2884–7. doi: 10.1161/01.cir.0000041255.88750.f0. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg I, Roguin A, Nikolsky E, Chanderashekhar PV, Cohen S, Levy AP. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis. 2002;161:441–6. doi: 10.1016/s0021-9150(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 23.Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem. 2002;48:1377–82. [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 25.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. New Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 26.Rose GA, Blackburn H. Monograph series no 58. 2. World Health Organization; 1982. Cardiovascular survey methods. [PubMed] [Google Scholar]
- 27.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diab Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wejman JC, Hovsepian D, Wall JS, Hainfeld JF, Greer J. Structure and assembly of haptoglobin polymers by electron microscopy. J Mol Biol. 1984;174:343–68. doi: 10.1016/0022-2836(84)90342-5. [DOI] [PubMed] [Google Scholar]
- 30.Costacou T, Levy AP, Miller RG, et al. Effect of vitamin E supplementation on HDL function by haptoglobin genotype in type 1 diabetes: results from the HapE randomized crossover pilot trial. Acta Diabetol. 2015 May 24; doi: 10.1007/s00592-015-0770-8. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Levy AP, Hochberg I, Jablonski K, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol. 2002;40:1984–90. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 33.Costacou T, Rosano C, Aizenstein H, et al. The haptoglobin 1 allele correlates with white matter hyperintensities in middle-aged adults with type 1 diabetes. Diabetes. 2015;64:654–9. doi: 10.2337/db14-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X, Song S, Sun G, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci. 2009;29:15819–27. doi: 10.1523/JNEUROSCI.3776-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31:1991–6. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins RA, Jones JG, Cumming SP, Coelho e Silva MJ, Teixeira AM, Verissimo MT. Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol. 2012;11:13. doi: 10.1186/1475-2840-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuttall FQ. Effect of age on the percentage of hemoglobin A1c and the percentage of total glycohemoglobin in non-diabetic persons. J Lab Clin Med. 1999;134:451–3. doi: 10.1016/s0022-2143(99)90165-8. [DOI] [PubMed] [Google Scholar]
- 38.Levy AP, Asleh R, Blum S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12:293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 39.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435–41. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 40.Levy AP, Levy JE, Kalet-Litman S, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thrombo Vasc Biol. 2007;27:134–40. doi: 10.1161/01.ATV.0000251020.24399.a2. [DOI] [PubMed] [Google Scholar]
- 41.Melamed-Frank M, Lache O, Enav BI, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 42.Guetta J, Strauss M, Levy NS, Fahoum L, Levy AP. Haptoglobin genotype modulates the balance of Th1/Th2 cytokines produced by macrophages exposed to free hemoglobin. Atherosclerosis. 2007;191:48–53. doi: 10.1016/j.atherosclerosis.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 43.Asleh R, Blum S, Kalet-Litman S, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2–2 genotype. Diabetes. 2008;57:2794–800. doi: 10.2337/db08-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.