Abstract
Nosocomial infection with respiratory syncytial virus (RSV) is an important health risk in pediatric care but is largely preventable by efficient infection control measures. Commonly applied rapid antigen detection tests (RADTs) miss a considerable number of RSV-infected patients. The objective of our analysis was to evaluate whether readily available host parameters are associated with false-negative RADT, and to assess how these parameters could be applied in an optimized RSV isolation strategy.
We retrospectively analyzed a cohort of 242 children under the age of 2 years hospitalized with acute respiratory tract infection to identify host parameters associated with false-negative RADT test result. We subsequently simulated the outcome of different isolation strategies based on RADT result and host parameters in view of the overall isolation efficacy.
Out of 242 hospitalized patients, 134 (55%) patients were found RSV-positive by RT-PCR, whereas 108 (45%) patients were tested negative. The performance of the RADT was compared with the result obtained by reverse transcription polymerase chain reaction on the identical nasopharyngeal wash. Overall, we found that 85 patients (35%) were tested true positive, 108 (45%) were tested true negative, whereas a false-negative test result was obtained in 49 patients (20%). Duration of respiratory symptoms for >3 days and a respiratory admission diagnosis are associated with false-negative RADT result. In comparison with RADT alone, consideration of these clinical parameters and RADT result can decrease the rate of nonisolated RSV-infected patients from approximately 24% to 8% (65% RSV pretest probability).
Consideration of both RADT and clinical parameters associated with false-negative RADT can result in an optimized RSV infection control policy.
INTRODUCTION
Respiratory syncytial virus (RSV) is the single most important cause of severe acute respiratory tract infections (RTIs) in infants and young children.1 By the age of 2 years, most children had been infected with RSV, with about half having experienced ≥2 infections.2 The RSV disease spectrum ranges from mild symptoms such as rhinitis and otitis media to severe illness such as bronchiolitis or pneumonia.3 Especially young infants and immune-compromised individuals are at risk for severe infection, and infection with RSV results in significant morbidity and mortality in these high-risk groups.4 As a consequence, RSV infections are the most frequent cause of hospitalization of infants and young children in industrialized countries.
In Europe and North America, RSV disease occurs as well-defined seasonal epidemic outbreaks during the winter and spring months. RSV has a prolonged survival on skin and clothes,5 and is transmitted by close or direct contact with infectious droplets or fomites.6 Nosocomial RSV infection is an important health risk in pediatric care7 and without adequate infection control measures, a high rate of nosocomial infection occurs.8,9 Madge et al showed in a prospective controlled trial that strict adherence to control measures, including patient cohort isolation and the wearing of gowns and gloves, for all contacts of RSV-infected children can significantly reduce the rate of nosocomial infection, although neither the use of gowns and gloves alone nor cohort nursing alone showed a significant reduction in cross-infection.10
Rapid diagnosis of RSV infection in hospitalized children can therefore not only decrease unnecessary antibiotic use,11 but is also a prerequisite for the introduction of infection control measures like cohort isolation.12,13 As the clinical features of RSV infection are unspecific, confirmation of RSV infection by laboratory analysis is essential. Gold standard of RSV detection is reverse transcription polymerase chain reaction (RT-PCR) analysis.14,15 This method offers highly sensitive test results; however, RT-PCR is not immediately available at the time of admission to inpatient care. In routine pediatric care, many clinicians therefore rely on clinical symptoms and RSV rapid antigen detection tests (RADTs). RADTs detect RSV with high specificity, but unfortunately have only a limited sensitivity of approximately 60% to 89% compared with RT-PCR.16–20 Previous studies reported that RADT sensitivity in children was influenced by clinical manifestation and delay of testing,19 and false-negative RADT test results were significantly associated with older age, prolonged symptoms, and RSV genotype-B infection.21
Policy-making on prehospitalization RSV screening, which includes low-sensitivity RADT testing and isolation practices, is critical for pediatric inpatient health care settings. We therefore directed our efforts toward identifying clinical and laboratory risk factors associated with false-negative RADT results at the time of admission to inpatient care. Second, we simulated potential consequences of 3 different screening and isolation strategies in a decision analytical model, taking into account RADT results obtained by BinaxNow® RSV cards (BN) as well as specific patient's characteristics.
METHODS
Clinical Setting and Case Definition
This is a retrospective cross-sectional analysis of all children under the age of 2 years admitted with acute RTI to the Pediatric Department at the Heidelberg University Hospital between October 19, 2012 and April 28, 2013. During this period, a nasopharyngeal wash (NW) was obtained at presentation from all children under 2 years of age hospitalized with clinical symptoms of upper RTI (URTI) or lower RTI. We identified 242 hospitalized patients that fulfilled our case definition (admission to inpatient care, age below 2 years, respiratory symptoms on admission, BN test on NW obtained before inpatient care, and RT-PCR test obtained on the identical sample) and included these patients in the analysis. Patients were either cohort-isolated, that is isolation in shared rooms, nursing performed with gowns and gloves, or cubicle-isolated, that is, isolation in single bed rooms and nursing performed with gowns and gloves. Medical records were reviewed from all patients to obtain clinical and laboratory data. This study was approved by the Ethical Committee of the University of Heidelberg (S-166/2014).
RSV Detection by RADT and RT-PCR Analysis
NWs were analyzed on admission by RADT with BN according to the manufacturer's instructions before the patient was transferred to inpatient care. Following the analysis by BN, all NWs were stored frozen at −20°C until testing with the RT-PCR gold standard. For RT-PCR analysis, RNA was extracted from respiratory specimens using the QIAamp® viral RNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Amplification and detection of viral RNA was performed with the RealStar® RSV real-time RT-PCR kit (altona Diagnostics, Hamburg, Germany) on a LightCycler® 480 instrument II (Roche, Mannheim, Germany).
Statistical Analysis
To describe the time distribution of admitted RSV cases and pretest probability of RSV infection, we aggregated RSV results as obtained by RT-PCR by calendar month. Demographic and clinical data in our study population were summarized. Groups were compared using χ2 or Fisher exact test for categorical variables and the Student t test or Mann–Whitney U test for continuous variables, as appropriate. Factors potentially associated with a false-negative (versus true-negative) RADT result seen in the univariate comparisons were further analyzed using descriptive statistics and logistic regression models; odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. All comparisons with p < 0.05 were considered statistically significant. Stata/IC13.0 (StataCorp. LP, College Station, TX) was used for all statistical analyses.
Decision Tree Modeling of RSV Screening and Isolation Strategies
A decision tree was constructed to simulate the performance of different screening strategies (TreeAge Pro, Version 2012, TreeAge Software Inc., One Bank Street, Williamstown, MA). The decision tree simulations covered 3 differing scenarios: conservative isolation scenario, cohort isolation (isolation in shared rooms, nursing with gowns and gloves) of all children with positive RADT test results and cubicle isolation (isolation in single rooms and nursing with gowns and gloves) of all negatively tested children; intermediate isolation scenario, cohort isolation of all children with positive RADT test results, cubicle isolation of children with increased risk of false-negative RADT test result; liberal isolation scenario, cohort isolation of all children with positive RADT test result, no isolation of other children.
Parameters associated with increased risk of false-negative RADT outcome were identified as described above, and results from the primary data analysis were used as input data to the decision analytical model. To assess the uncertainty in key model parameters and the robustness of the results to model assumptions, we used a Monte Carlo sampling method. Groups of 100 children were followed for 10,000 times through the decision analytical model.
RESULTS
Descriptive Epidemiology
Of 242 hospitalized patients that fulfilled our case definition, a total of 134 (55%) patients were found RSV-positive by RT-PCR, whereas 108 (45%) patients were tested negative (Table 1). The performance of the RADT was compared with the result obtained by RT-PCR on the identical NW. Overall, we found that 85 patients (35%) were tested true positive, 108 (45%) were tested true negative, whereas a false-negative test result was obtained in 49 patients (20%) (Table 1). The rapid test thereby resulted in a sensitivity of 0.63 (95% CI 0.55–0.72), specificity of 1, and a negative predictive value of 0.69 (95% CI 0.61–0.76).
TABLE 1.
Baseline Characteristics of Patients Under 2 Years of Age Hospitalized With Acute RTI at Initial Presentation
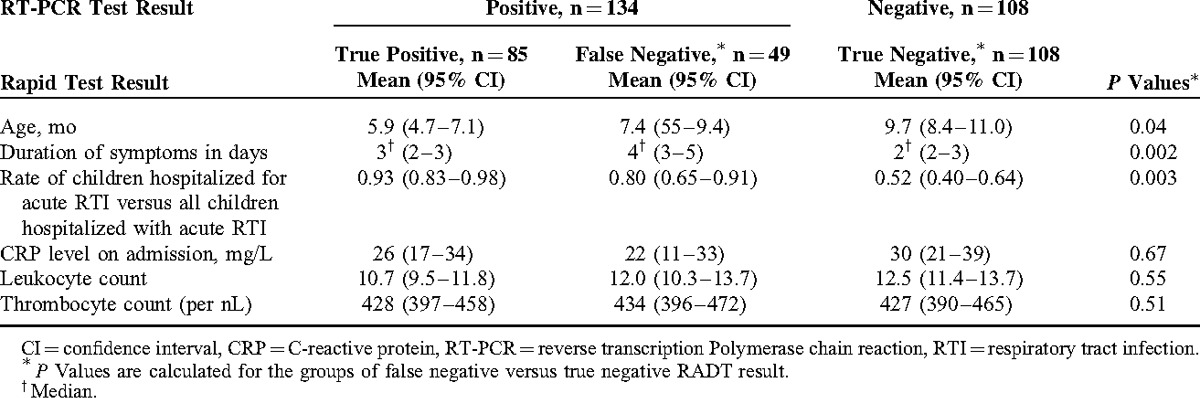
We evaluated the association of admission diagnosis with a false-negative RADT result. The admission diagnosis is the main diagnosis leading to hospitalization and is assigned based on clinical judgment by the admitting physician at the time of admission. At that point, physicians were usually aware of rapid test results, but were unaware of RT-PCR test results. The admission diagnosis was available in 182 of 242 children (75%). We grouped the admission diagnosis into 5 groups, including cases of URTI (ICD J06.9; n = 11), bronchitis (ICD J20.9 and J40; n = 79), bronchiolitis (International Classification o Diseases [ICD] J21.0 and J21.9; n = 25), and pneumonia (ICD J12.1, J15.3, J18.0, J18.9; n = 20), whereas nonrespiratory diagnoses (n = 47) represented children that were admitted for nonrespiratory reasons and concomitant acute RTI. The nonrespiratory diagnoses group includes a variety of different diagnoses, such as febrile convulsions (ICD 56.9), diarrhea, or concussion. False-negative RADT results occurred more frequently in children hospitalized for acute RTI compared with children hospitalized for nonrespiratory reasons (Figure 1A). Within our analysis, we subsequently assessed the rate of false-negative RADT results for children hospitalized for acute RTI versus children hospitalized for nonrespiratory diagnoses and concomitant acute RTI. We found that respiratory admission diagnoses were significantly associated with false-negative RADT outcome (Table 1, P = 0.003). When comparing patients with false-negative RSV versus patients with true-negative test results, false-negative rapid test results were significantly associated with younger age (mean age on admission 7.4 vs 9.7 months, P = 0.04, Table 1).
FIGURE 1.
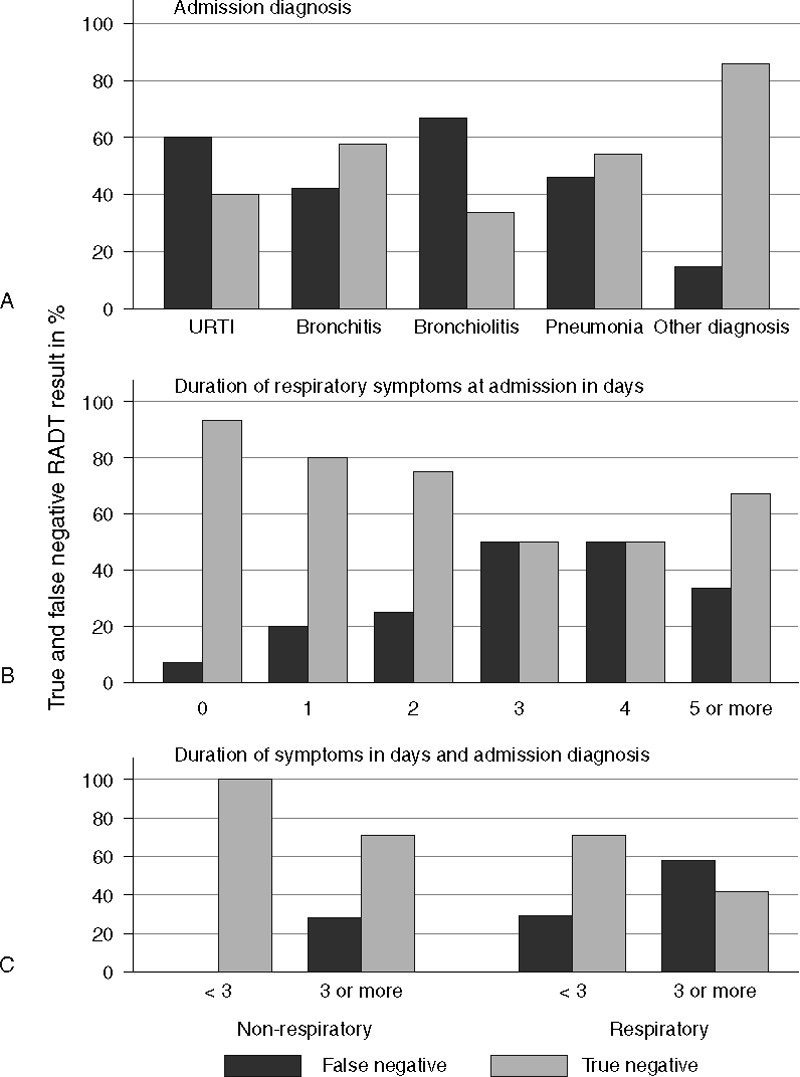
Percentage of true- and false-negative RADT results, (A) depending on admission diagnosis, (B) depending on the duration of respiratory symptoms at admission, and (C) depending on the combination of respiratory symptoms and admission diagnosis.
We further assessed the duration of respiratory symptoms at presentation. From medical records, the precise duration of respiratory symptoms was assessable for 170 of the 242 children (70%). Caretakers reported a significantly longer duration of symptoms (median duration of respiratory symptoms 4 vs 2 days) in children with false-negative as compared with children with true-negative RADT result (P = 0.002, Table 1 and Figure 1B). Regarding laboratory parameters, mean CRP levels as well as leukocyte and thrombocyte count did not differ significantly between true- and false-negative RADT results (Table 1). Of note, children with false-negative test result had significantly lower viral load in RT-PCR analysis as compared with children with true-positive RADT test results (mean ct value 26.9 [95% CI 25.2–28.6] vs 16.9 [95% CI 16.2–17.6] respectively; P < 0.0001).
Multivariate Analysis of Risk Factors Associated With False-negative Test Results
All parameters with P values <0.2 in the univariate analyses, which were not adjusted for multiple comparison (Table 1), were subjected to a multivariate model to identify parameters independently associated with false negative RADT results. We considered age at admission, short (<3 days) vs long (≥3 days) duration of symptoms at presentation, and hospitalization for acute RTI (admission diagnosis URTI, bronchitis, bronchiolitis, and pneumonia) vs all children hospitalized with acute RTI. Logistic regression revealed that among these parameters, respiratory admission diagnosis and prolonged duration of symptoms were significant risk factors associated with false-negative RADT results (Table 2).
TABLE 2.
Independent Risk Factors of False-negative (Versus True-negative) RADT result, logistic regression

Based on these results, we defined a clinical high-risk group for false-negative RADT result, which included children with a respiratory admission diagnosis and duration of respiratory symptoms for ≥3 days (long duration of symptoms). The rate of false-negative RADT result within this high-risk group was 58% (95% CI 41%–74%, Figure 1C).
Simulation of Different Isolation Strategies
For computing the outcome of different screening strategies, we assumed that in an intermediate screening scenario, all children with positive RADT test results are isolated in cohorts, whereas children with negative RADT results that belong to the clinical high-risk group are referred to cubicle isolation. The decision analytical model is illustrated in Figure 2, and all input parameters and their distribution are listed in Table 3.
FIGURE 2.
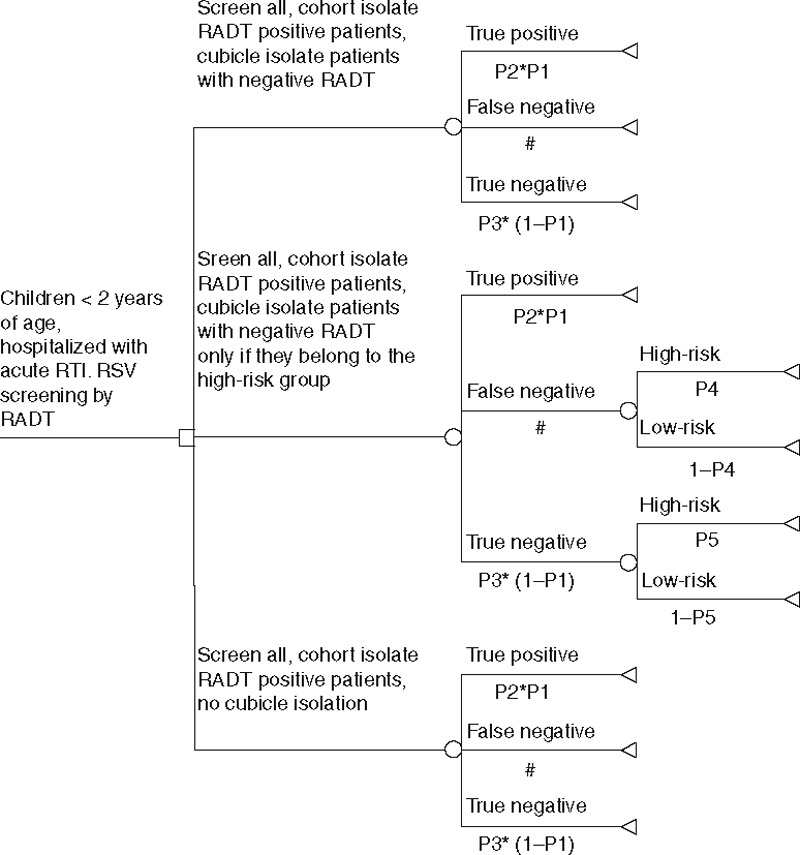
Decision analytical model. The decision tree illustrates 3 different isolation strategies and respective transition probabilities according to the data provided in Table 4.
TABLE 3.
Input Variables
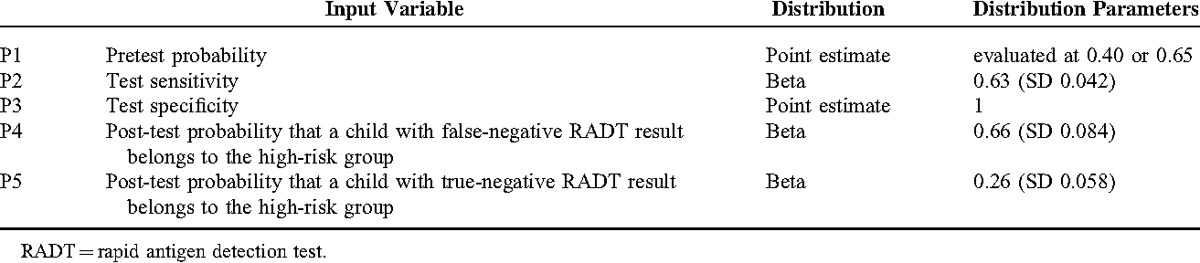
Given a pretest RSV infection probability of 40%, (representing a moderate RSV transmission intensity,22 corresponding to the situation in December 2012 and April 2013 in our patient cohort), we found that the liberal scenario (only cohort isolation of children with positive rapid test results) correctly isolates 25 (95% CI 16–34) of 100 hospitalized children with RTI, whereas 15 (95% CI 8–23) children are missed and therefore not subjected to infection control measures (Table 4). In the intermediate scenario (isolation of all children with positive rapid test result in cohorts, cubicle isolation of children admitted for respiratory diagnosis and of children <3 months of age), 25 (95% CI 16–34) children are isolated in cohorts, whereas 25 (95% CI 15–37) are isolated in cubicles. In this scenario, targeted infection control measures are missed in 5 (95% CI 1–11) RSV-infected children. In the conservative scenario, infection control measures are introduced in all hospitalized patients, including 25 (95% CI 16–34) children with positive test results in cohort isolation, whereas the remaining 75 (95% CI 66–84) children are isolated in cubicles (Table 4)
TABLE 4.
Simulated Performance of Different Isolation Strategies
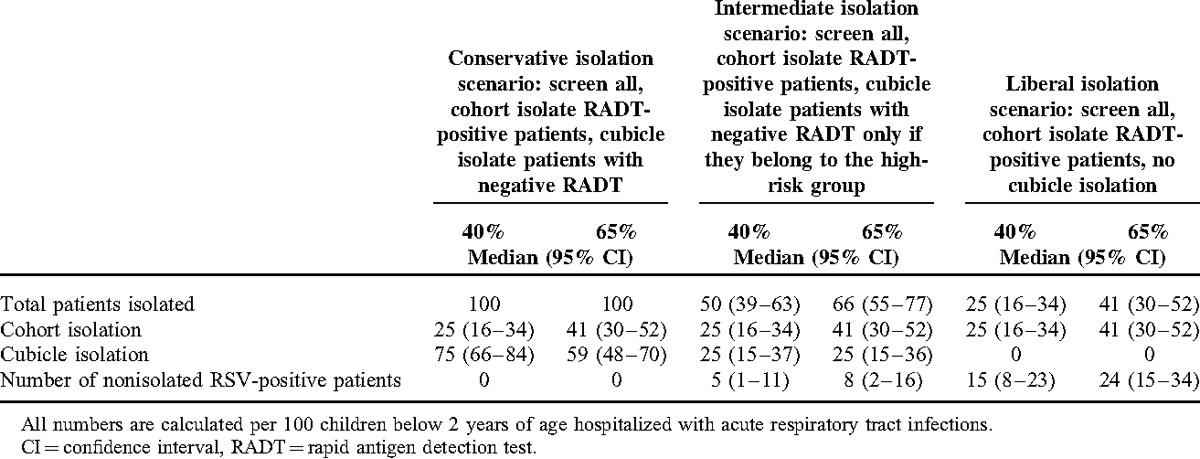
In a situation of a 65% pretest RSV infection probability (high RSV transmission intensity,23 representing the local situation in January and February 2013), the liberal scenario correctly isolates 41 (95% CI 30–52) of 100 hospitalized children with RTI, whereas 24 (95% CI 15–34) children are missed and therefore not subjected to infection control measures. In the intermediate scenario, 41 (95% CI 30–52) children are isolated in cohorts, whereas 25 (95% CI 15–36) are isolated in cubicles. In this scenario, targeted infection control measures are missed in 8 (95% CI 2–16) RSV-infected children. In the conservative scenario, infection control measures are again introduced to all hospitalized patients, including 41 (95% CI 30–52) children with positive test results in cohort isolation, whereas the remaining 59 (95% CI 48–70) children are isolated in cubicles (Table 4).
DISCUSSION
Acute RTI caused by RSV infection is a frequent and important health risk in pediatric care. As RSV infection is associated with significant morbidity, pediatricians need to keep the rate of nosocomial infection under control. The unspecific clinical symptoms of RSV infection and the limited sensitivity of currently available RADT complicate the correct identification of RSV-infected children, and thereby render the implementation of effective hygiene control measures difficult.
There are different ways how pediatricians may approach the diagnostic and logistic challenge of RSV infection control in their local health care situation. Ideally, all children admitted with symptoms of RTI should be isolated in cubicles, and only be transferred to cohort isolation once the infectious agent has been identified by highly sensitive technologies like RT-PCR. This isolation policy, however, requires sufficient resources, both in terms of isolation areas as well as in terms of working time and material required for the appropriate application of hygiene control measures for patients and guardians. In a situation where these resources are limited, hospitals are confronted with major difficulties to sustain adequate hygiene control measures to infectious patients, especially as RSV infections occur in seasonal epidemic outbreaks in winter and spring months,24 concomitant with a magnitude of other highly contagious viral agents.
In view of the high specificity of currently available immunochromatographic RADT, a stringent approach might be to screen young children with RTIs on admission, and subsequently isolate all children with positive RADT results in cohorts. The limited sensitivity of the test however does not exclude RSV infection in negatively screened children. Hence, children with negative test results ideally should be isolated in cubicles until the results from more specific, yet not readily accessible, test methods become available.
A further strategy might be to confine cubicle isolation to children with the highest risk of false-negative RADT results. We therefore questioned whether immediately available parameters, including clinical and laboratory parameters, could be used to identify children with high risk of false-negative rapid test results among all negatively tested children at the time of admission. Our analysis confirms the recent finding that a prolonged duration of clinical symptoms is associated with false-negative RADT result.25 This is most likely explained by diminishing RSV viral load in children with prolonged duration of respiratory symptoms,25 thereby decreasing RADT sensitivity.25 We furthermore found that false-negative RADT results are significantly associated with admission diagnosis of acute RTI, as compared with children admitted for other diagnoses and concomitant RTI. This is again not unexpected, as the RSV pretest probability was higher in children hospitalized for acute RTI as compared with children with concomitant RTI. The finding however highlights the importance of a clear definition of the screened cohort, in particular as some previous reports screened “hospitalized children with a RTI,”17,19,26 whereas other reports limited the screening to children “hospitalized for acute RTI”.21 In our cohort, RSV infection was present in 27% of children with concomitant RTI, which indeed justifies the screening of all children hospitalized with RTI in the respective age group.
Surprisingly, our analysis found that false-negative RADT results were significantly associated with younger age, even though age was not a significant risk factor in the multivariate analysis. In a previous report, negative RADT results were associated with a group of older children aged 24 to 35 months.21 As our analysis only included children below the age of 24 months, we were not able to directly compare our results to these previous findings.
We designed a decision analytical model that incorporated our primary data to simulate 3 different isolation policies taking in consideration RADT results and host parameters associated with false-negative RADT outcome. We thereby intend to inform decision makers who need to select the best possible isolation strategy depending on their local situation. Depending on the RSV pretest probability, cubicle isolation of patients with a high risk of false-negative RADT results will apply to approximately 25 of 100 hospitalized children, and decreases the number of nonisolated, RSV-infected children by about two-thirds as compared with cohort isolation of patients with positive RADT results only. This isolation policy might be an attractive alternative in particular in regular pediatric care, wherein missing relatively few patients with presumably low viral load might be acceptable. In high-risk situations, for example neonatal intensive care units or pediatric oncology wards, a more conservative isolation strategy will be required.
There are several limitations of our analysis. First of all, it has to be mentioned that the RADT sensitivity in our analysis was 63% (95% CI 55–72%), whereas previous studies reported sensitivities of approximately 60% to 89%.16–20 The sensitivity of RADT in our analysis thus represents the lower bounds of previously reported values, which might reflect the test performance when RADTs are applied as a screening assay by physicians in a routine clinical setting within an emergency department. Of note, the problem of limited RADT sensitivity might be overcome in the future if high-sensitive rapid molecular tests become available for point-of-care analysis.26 A further general concern is the size of our study population and the data quality in a retrospective analysis. Our study comprised patients from one season in a single treatment center, and information on the underlying admission diagnosis and the duration of respiratory symptoms on admission was missing in a considerable number of patients. Therefore, we cannot exclude that the findings from our analysis will not or only in parts be applicable to other transmission settings. For the future, it would be desirable to confirm our results in larger, prospective datasets. Finally, an important limitation of this analysis is the focus on RSV infection, whereas data on other viral agents, especially influenza, were unavailable and can therefore not be considered. Our study only demonstrates the impact of differing isolation strategies in view of RSV infection but excludes other viral agents that might expose hospitalized children to the risk of nosocomial infection. Moreover, our findings may not apply outside of similar patient settings (children <2 years hospitalized with acute RTI during peak RSV season) or to other RADT assays.
It is apparent that any isolation strategy that does not introduce cubicle isolation to all patients with clinical symptoms of RTI is imperfect. To offer the best possible protection against nosocomial infections, pediatric hospitals need sufficient resources, including areas for cubicle and cohort isolation, nursing staff, and material. Our analysis demonstrates how pediatricians working under limited resources may use a combination of RADT and host parameters to assure an optimal protection from nosocomial RSV infection in young hospitalized children. Our study shows that at initial presentation, a prolonged duration of respiratory symptoms and admission diagnosis are associated false-negative RADT results. The consideration of these parameters in combination RADT results might be used to derive an optimal isolation strategy at the local health care level.
Acknowledgments
The authors thank the nurses in the Center for Childhood and Adolescent Medicine for collecting NW samples and the technicians in the virology diagnostic laboratory for excellent technical assistance. The authors are grateful to Freimut Nothelfer for his assistance with medical data acquisition. All mentioned persons gave permission to be named.
Footnotes
Abbreviations: BN = BinaxNow® RSV card, CI = confidence interval, NW = nasopharyngeal wash, OR = odds ratio, RADT = rapid antigen detection test, RSV = respiratory syncytial virus, RTI = respiratory tract infection, RT-PCR = reverse transcription PCR.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henrickson KJ, Hoover S, Kehl KS, et al. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J 2004; 23 (1 suppl):S11–S18. [DOI] [PubMed] [Google Scholar]
- 3.Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–546. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady MT, Evans J, Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control 1990; 18:18–23. [DOI] [PubMed] [Google Scholar]
- 6.Hall CB, Douglas RG., Jr Modes of transmission of respiratory syncytial virus. J Pediatr 1981; 99:100–103. [DOI] [PubMed] [Google Scholar]
- 7.Simon A, Muller A, Khurana K, et al. Nosocomial infection: a risk factor for a complicated course in children with respiratory syncytial virus infection-results from a prospective multicenter German surveillance study. Int J Hygiene Environment Health 2008; 211:241–250. [DOI] [PubMed] [Google Scholar]
- 8.Mlinaric-Galinovic G, Varda-Brkic D. Nosocomial respiratory syncytial virus infections in children's wards. Diagn Microbiol Infect Dis 2000; 37:237–246. [DOI] [PubMed] [Google Scholar]
- 9.Geis S, Prifert C, Weissbrich B, et al. Molecular characterization of a respiratory syncytial virus outbreak in a hematology unit in Heidelberg, Germany. J Clin Microbiol 2013; 51:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madge P, Paton JY, McColl JH, et al. Prospective controlled study of four infection-control procedures to prevent nosocomial infection with respiratory syncytial virus. Lancet 1992; 340:1079–1083. [DOI] [PubMed] [Google Scholar]
- 11.Byington CL, Castillo H, Gerber K, et al. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med 2002; 156:1230–1234. [DOI] [PubMed] [Google Scholar]
- 12.Mills JM, Harper J, Broomfield D, et al. Rapid testing for respiratory syncytial virus in a paediatric emergency department: benefits for infection control and bed management. J Hosp Infection 2011; 77:248–251. [DOI] [PubMed] [Google Scholar]
- 13.Lehners N, Schnitzler P, Geis S, et al. Risk factors and containment of respiratory syncytial virus outbreak in a hematology and transplant unit. Bone Marrow Transplant 2013; 48:1548–1553. [DOI] [PubMed] [Google Scholar]
- 14.Mentel R, Wegner U, Bruns R, et al. Real-time PCR to improve the diagnosis of respiratory syncytial virus infection. J Med Microbiol 2003; 52 (Pt 10):893–896. [DOI] [PubMed] [Google Scholar]
- 15.Wishaupt JO, Russcher A, Smeets LC, et al. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics 2011; 128:e1113–e1120. [DOI] [PubMed] [Google Scholar]
- 16.Aslanzadeh J, Zheng X, Li H, et al. Prospective evaluation of rapid antigen tests for diagnosis of respiratory syncytial virus and human metapneumovirus infections. J Clin Microbiol 2008; 46:1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginocchio CC, Swierkosz E, McAdam AJ, et al. Multicenter study of clinical performance of the 3 M Rapid Detection RSV test. J Clin Microbiol 2010; 48:2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munjal I, Gialanella P, Goss C, et al. Evaluation of the 3 M rapid detection test for respiratory syncytial virus (RSV) in children during the early stages of the 2009 RSV season. J Clin Microbiol 2011; 49:1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miernyk K, Bulkow L, DeByle C, et al. Performance of a rapid antigen test (Binax NOW(R) RSV) for diagnosis of respiratory syncytial virus compared with real-time polymerase chain reaction in a pediatric population. J Clin Virol 2011; 50:240–243. [DOI] [PubMed] [Google Scholar]
- 20.Principi N, Esposito S. Antigen-based assays for the identification of influenza virus and respiratory syncytial virus: why and how to use them in pediatric practice. Clin Lab Med 2009; 29:649–660. [DOI] [PubMed] [Google Scholar]
- 21.Papenburg J, Buckeridge DL, De Serres G, et al. Host and viral factors affecting clinical performance of a rapid diagnostic test for respiratory syncytial virus in hospitalized children. J Pediatr 2013; 163:911–913. [DOI] [PubMed] [Google Scholar]
- 22.Bonzel L, Tenenbaum T, Schroten H, et al. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J 2008; 27:589–594. [DOI] [PubMed] [Google Scholar]
- 23.Shafik CF, Mohareb EW, Yassin AS, et al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect Dis 2012; 12:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terletskaia-Ladwig E, Enders G, Schalasta G, et al. Defining the timing of respiratory syncytial virus (RSV) outbreaks: an epidemiological study. BMC Infect Dis 2005; 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson FW, Collier AM, Clyde WA, Jr, et al. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med 1979; 300:530–534. [DOI] [PubMed] [Google Scholar]
- 26.Mahony J, Chong S, Bulir D, et al. Development of a sensitive loop-mediated isothermal amplification assay that provides specimen-to-result diagnosis of respiratory syncytial virus infection in 30 minutes. J Clin Microbiol 2013; 51:2696–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]


