Abstract
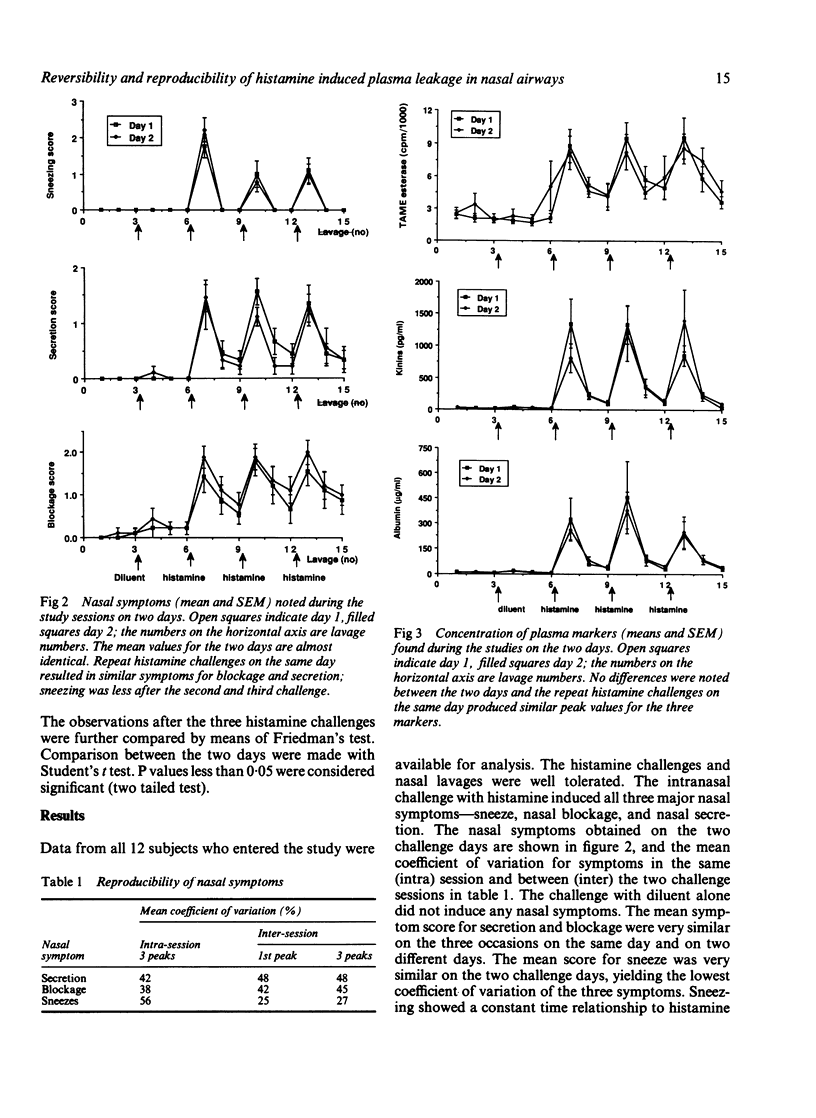
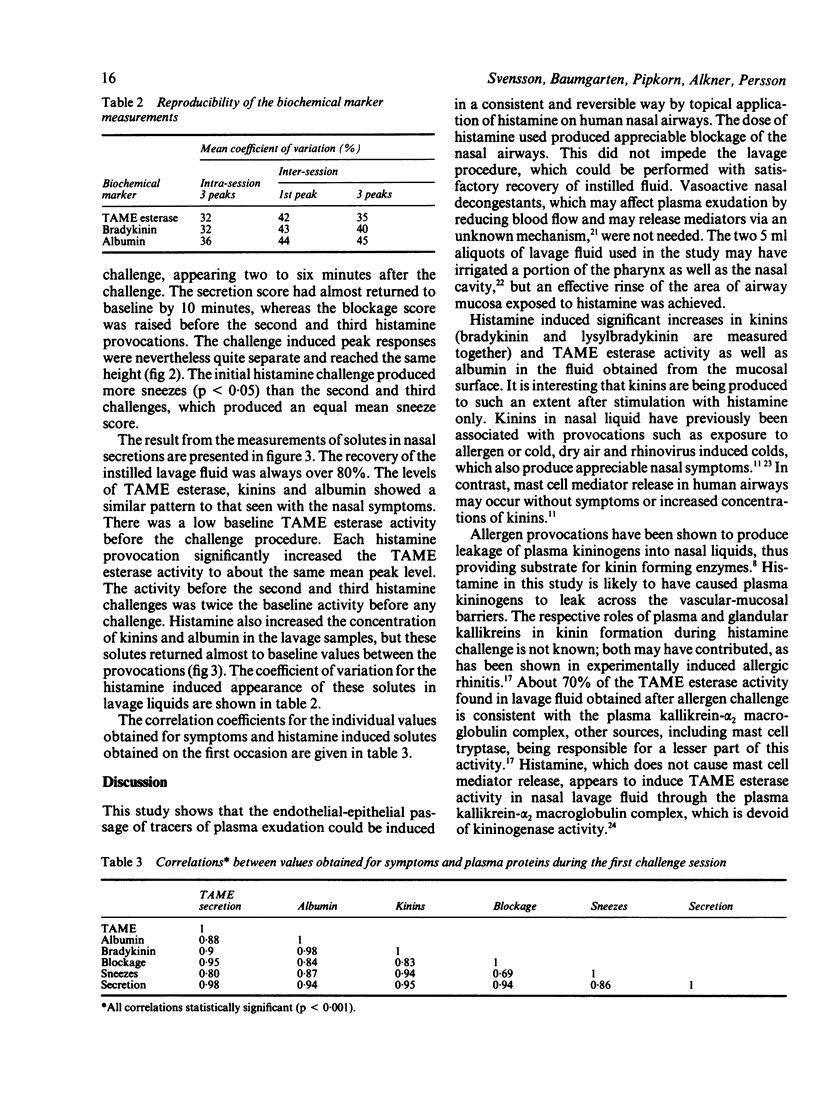
Plasma exudation is one cardinal factor in airways defence and inflammation. In inflammatory airway diseases such as rhinitis and asthma, however, plasma leakage may also have a pathogenetic role. Experimental data from animals indicate that highly sensitive, active, and reversible processes regulate the vascular and mucosal permeability to macromolecules. With the use of a nasal lavage model for the recovery of liquids on the mucosal surface the effect of histamine on the macromolecular permeability of the airway endothelial-epithelial barriers was studied in normal subjects. The concentrations of albumin, kinins, and N-alpha-beta-tosyl-L-arginine-methyl esterase (TAME) in nasal lavage fluid were measured and nasal symptoms assessed by a scoring technique. The reproducibility of three repeated challenges with 30 minute intervals on the same day was studied in 12 subjects and compared with the same procedure (three challenges) on a different day. Sneezing decreased significantly (p less than 0.05) after the first histamine challenge but was maintained thereafter. Otherwise, the mean values for symptoms and for markers of vascular leakage were very similar both for the three challenges in the same session and for the two challenge sessions on a different day. Sneezing, blockage, and secretions were associated with increased concentrations of TAME esterase (maximum 9000 cpm/ml), kinins (1.4 ng/ml), and albumin (0.3 g/l) in lavage fluid. Both the symptoms and the measures of plasma exudation were reversible and reproducible in the three repeat histamine challenges and at two challenge sessions on different days. These findings support the view that non-injurious, active processes regulate the inflammatory flow of macromolecules across airways endothelial-epithelial barriers. The present experimental approach would be suitable for studies of the modulatory effects of inflammatory stimulus induced plasma leakage and symptoms in human airways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten C. R., Nichols R. C., Naclerio R. M., Lichtenstein L. M., Norman P. S., Proud D. Plasma kallikrein during experimentally induced allergic rhinitis: role in kinin formation and contribution to TAME-esterase activity in nasal secretions. J Immunol. 1986 Aug 1;137(3):977–982. [PubMed] [Google Scholar]
- Baumgarten C. R., Togias A. G., Naclerio R. M., Lichtenstein L. M., Norman P. S., Proud D. Influx of kininogens into nasal secretions after antigen challenge of allergic individuals. J Clin Invest. 1985 Jul;76(1):191–197. doi: 10.1172/JCI111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H., Krogsgaard O. W., Mygind N. Measurement of secretion in nasal lavage. Clin Sci (Lond) 1987 Aug;73(2):217–222. doi: 10.1042/cs0730217. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Ranga V., Paré P. D., Inoue S., Moroz L. A., Hogg J. C. Effect of histamine and methacholine on guinea pig tracheal permeability to HRP. J Appl Physiol Respir Environ Exerc Physiol. 1978 Dec;45(6):939–948. doi: 10.1152/jappl.1978.45.6.939. [DOI] [PubMed] [Google Scholar]
- Brofeldt S., Mygind N., Sørensen C. H., Readman A. S., Marriott C. Biochemical analysis of nasal secretions induced by methacholine, histamine, and allergen provocations. Am Rev Respir Dis. 1986 Jun;133(6):1138–1142. doi: 10.1164/arrd.1986.133.6.1138. [DOI] [PubMed] [Google Scholar]
- Dolovich J., Back N., Arbesman C. E. Kinin-like activity in nasal secretions of allergic patients. Int Arch Allergy Appl Immunol. 1970;38(4):337–344. doi: 10.1159/000230287. [DOI] [PubMed] [Google Scholar]
- Harpel P. C. Human plasma alpha 2-macroglobulin. An inhibitor of plasma kallikrein. J Exp Med. 1970 Aug 1;132(2):329–352. doi: 10.1084/jem.132.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson G., Pipkorn U., Andreasson L. Substance P and human nasal mucociliary activity. Eur J Clin Pharmacol. 1986;30(3):355–357. doi: 10.1007/BF00541544. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naclerio R. M., Meier H. L., Kagey-Sobotka A., Adkinson N. F., Jr, Meyers D. A., Norman P. S., Lichtenstein L. M. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983 Oct;128(4):597–602. doi: 10.1164/arrd.1983.128.4.597. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Togias A. G., Adkinson N. F., Jr, Meyers D. A., Kagey-Sobotka A., Plaut M., Norman P. S., Lichtenstein L. M. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med. 1985 Jul 11;313(2):65–70. doi: 10.1056/NEJM198507113130201. [DOI] [PubMed] [Google Scholar]
- Persson C. G. Plasma exudation and asthma. Lung. 1988;166(1):1–23. doi: 10.1007/BF02714025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C. G. Role of plasma exudation in asthmatic airways. Lancet. 1986 Nov 15;2(8516):1126–1129. doi: 10.1016/s0140-6736(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Pipkorn U., Proud D., Lichtenstein L. M., Schleimer R. P., Peters S. P., Adkinson N. F., Jr, Kagey-Sobotka A., Norman P. S., Naclerio R. M. Effect of short-term systemic glucocorticoid treatment on human nasal mediator release after antigen challenge. J Clin Invest. 1987 Oct;80(4):957–961. doi: 10.1172/JCI113188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Togias A., Naclerio R. M., Crush S. A., Norman P. S., Lichtenstein L. M. Kinins are generated in vivo following nasal airway challenge of allergic individuals with allergen. J Clin Invest. 1983 Nov;72(5):1678–1685. doi: 10.1172/JCI111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. Bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jan;135(1):250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Joyner W. L. The effects of intermittent and continuous stimulation of microvessels in the cheek pouch of hamsters with histamine and bradykinin on the development of venular leaky sites. Microcirc Endothelium Lymphatics. 1984 Aug;1(4):381–396. [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Togias A. G., Naclerio R. M., Warner J., Proud D., Kagey-Sobotka A., Nimmagadda I., Norman P. S., Lichtenstein L. M. Demonstration of inhibition of mediator release from human mast cells by azatadine base. In vivo and in vitro evaluation. JAMA. 1986 Jan 10;255(2):225–229. [PubMed] [Google Scholar]


