Abstract
Xanthoceras sorbifolium, a tree species endemic to northern China, has high oil content in its seeds and is recognized as an important biodiesel crop. The plant is characterized by late-acting self-incompatibility (LSI). LSI was found to occur in many angiosperm species and plays an important role in reducing inbreeding and its harmful effects, as do gametophytic self-incompatibility (GSI) and sporophytic self-incompatibility (SSI). Molecular mechanisms of conventional GSI and SSI have been well characterized in several families, but no effort has been made to identify the genes involved in the LSI process. The present studies indicated that there were no significant differences in structural and histological features between the self- and cross-pollinated ovules during the early stages of ovule development until 5 days after pollination (DAP). This suggests that 5 DAP is likely to be a turning point for the development of the selfed ovules. Comparative de novo transcriptome analysis of the selfed and crossed ovules at 5 DAP identified 274 genes expressed specifically or preferentially in the selfed ovules. These genes contained a significant proportion of genes predicted to function in the biosynthesis of secondary metabolites, consistent with our histological observations in the fertilized ovules. The genes encoding signal transduction-related components, such as protein kinases and protein phosphatases, are overrepresented in the selfed ovules. X. sorbifolium selfed ovules also specifically or preferentially express many unique transcription factor (TF) genes that could potentially be involved in the novel mechanisms of LSI. We also identified 42 genes significantly up-regulated in the crossed ovules compared to the selfed ovules. The expression of all 16 genes selected from the RNA-seq data was validated using PCR in the selfed and crossed ovules. This study represents the first genome-wide identification of genes expressed in the fertilized ovules of an LSI species. The availability of a pool of specifically or preferentially expressed genes from selfed ovules for X. sorbifolium will be a valuable resource for future genetic analyses of candidate genes involved in the LSI response.
Introduction
Self-incompatibility (SI) is considered to be the most important and widespread mechanism promoting outcrossing in flowering plants. Various SI systems have been described in which self-fertilization can be prevented at any stage from the first contact of the pollen and the stigma to the fertilization of the ovule, indicating considerable diversity of SI mechanisms within plants [1].
The genetically characterized SI systems fall into two broad categories: gametophytic and sporophytic self-incompatibility (GSI and SSI) [2]. All characterized SSI systems show inhibition of incompatible pollen on the stigma surface, whereas in GSI systems, inhibition of incompatible pollen tubes frequently occurs within the style. In addition to these two SI systems, there are ovarian or late-acting SI systems (OSI or LSI), in which self-pollinated flowers consistently fail to form fruits or seeds, despite the fact that pollen tubes grew to the ovaries and penetrated the ovules; however, the ovules are rejected either just before fertilization or at some stage after fertilization [3–4].
A major goal of recent research into SI has been to identify and characterize genes that control SI. The molecular mechanisms of GSI and SSI reactions have been established in detail in only five families, but these families are widely diverse [5]. Most of the genetically well-characterized GSI and SSI systems are controlled by a single locus with multiple alleles, the S locus. The S-locus comprises at least two tightly linked, polymorphic genes, one of which encodes the male determinant and the other encodes the female determinant. In the GSI families Solanaceae, Rosaceae and Plantaginaceae, stylar inhibition of an incompatible pollen tube is mediated through an interaction between a stylar S-RNase (female determinant) and a pollen tube-borne F-box protein (male determinant), SLF or SFB, in which incompatibility degrades pollen tube RNA [6–8]. In addition to these primary male and female determinants, many other genes are also known or predicted to reside at the S locus [9]. Genetic data also show that other pistil factors not linked to the S locus, such as a small asparagine-rich protein (HT-B), 120K, and 4936 factor, are important for fully functional SI [10].
In GSI in the Papaveraceae family, the SI female determinant is a stigma-expressed S-glycoprotein ligand, a small extracellular signaling molecule named PrsS. The male determinant is a pollen-expressed Ca2+-channel protein named PrpS. With incompatible pollinations, PrsS reacts with its cognate trans-membrane receptor PrpS to trigger a Ca2+ -dependent signaling cascade, resulting in the inhibition of pollen tube growth [11–12].
In SSI in Brassica, the principal female determinant is a stigma-specific S-locus receptor kinase (SRK) that consists of an extracellular domain in the stigmatic pellicle, a trans-membrane domain and an intracellular serine/threonine kinase domain [13–14]. The male determinant is a small cysteine-rich protein (<10 kDa, termed SP11 or SCR) located in the pollen coat [15–16]. SSI is regulated by an S-haplotype-specific protein interaction in which SRK is activated by its cognate ligand SCR/Sp11 leading to an intracellular signal transduction cascade [17]. This interaction involves the formation of a receptor complex involving SRK, SCR and a cytoplasmic kinase, MLPK (M-locus protein kinase), and autophosphorylation of SRK [18–19].
Compared to GSI and SSI, the genetic mechanisms of LSI are poorly understood because genetic analysis of late-acting SI is more difficult than that of prezygotic SI [4]. In addition, the presence of LSI has often been correlated with a woody perennial habit [20]. The perennial nature of trees with LSI makes genetic analysis very time-consuming or impracticable [4,21].
Despite the difficulties inherent in the genetic characterization of LSI, a limited number of studies showed that LSI is under genetic control [22–23]. The genetic basis of LSI systems is frequently hypothesized to be gametophytic [24]. Crossing experiments in several species suggested that the LSI response is controlled by at least one locus and is most likely controlled by multiple loci with multiple alleles [2,23,25–26]. However, no effort has been made to identify the genes of the SI loci, so the genes for the loci on both the pollen and pistil sides remain unknown.
Xanthoceras sorbifolium, a tree species of Sapindaceae endemic to northern China, is an oilseed crop that has high oil content of up to 40%. Its seed oil is of good quality for dietary applications because of its high unsaturated fatty acid content. In addition, it fulfills many of requirements for biodiesel production and is recognized as an important biodiesel crop in China. X. sorbifolium is characterized by late-acting self-incompatibility [27]. Pollen tubes penetrate ovules and effect double fertilization after self-pollination, but selfed ovules are uniformly rejected at the endosperm syncytial stage [27].
Little information is available regarding the molecular mechanisms regulating the LSI response. The aims of the present study were to identify candidate genes involved in pollen-ovule interactions and LSI mechanisms. These genes have been identified by comparing the transcriptomes of the self- and cross-pollinated ovules of X. sorbifolium at the whole genome level using high-throughput next-generation sequencing technology to perform an RNA-seq analysis. To achieve this goal and to gain a better understanding of the LSI process in X. sorbifolium, further detailed investigations of the histology of developing ovules and observations of LSI phenomena were also performed.
Materials and Methods
Plant materials
Three cultivated ten-year-old X. sorbifolium trees were used in this study. All the experimental materials, including young ovules at various stages of development and fertilized ovules after self- and cross-pollination, were harvested from the three unrelated trees A, B, and C.
Morphological and histological analysis
The young, fertilized ovules were dissected from pistils and fixed in formalin—acetic acid—alcohol (FAA) for light and scanning electron microscopy (LM and SEM), respectively. Fixed specimens for LM were dehydrated through a tertiary butyl alcohol series, embedded in paraffin, and sectioned at 6–8 μm. Sections were stained with 1.0% aqueous safranin and 0.05% fast green. For SEM, fixed specimens were dehydrated in an alcohol series, critical point dried, coated with gold, and viewed with a Hitachi S-4800 scanning electron microscope.
RNA extraction, library construction and Illumina sequencing
For the RNA-seq sampling, ovules were harvested from the pistils that were self- and cross-pollinated at 5 DAP. Each pollination treatment was represented by three biological replicates from the trees A, B and C, resulting in a total of four samples from two pollination treatments. Each replicate consisted of 6 ovules taken from random positions on each of ten bunches on that tree. The samples were flash frozen in liquid nitrogen and stored at −80°C until further use.
The total RNA from the ovules with the two pollination treatments was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and purified using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturers’ protocols. The quality of the total RNA was determined using a NanoDrop 2000 Spectrophotometer (Thermo Fisher, USA). The mRNA was purified from the total RNA samples using a Dynabead mRNA Purification Kit according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and the quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Waldbronn, Germany). Double-stranded cDNA was synthesized using the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Specific adapters were ligated to the fragmented cDNA and denatured to generate single-stranded cDNA followed by emulsion PCR amplification. The sequencing was performed using an Illumina HiSeq 2000 sequence analyzer at Hanyu Genomics Institute (Shanghai, China). RNA-seq read data were deposited in the NCBI Sequence Read Archive (SRA) under accession number SRP062402.
De novo transcriptome assembly
The raw reads were filtered to obtain high quality de novo transcriptome sequence data. We discarded the reads with adapter contamination, those with more than 5% unknown nucleotides, and those of low quality (≥20% of the bases with a quality score (Q)≤10) using a Perl script. Clean reads were assembled using the Trinity de novo assembler (http://trinityrnaseq.sourceforge.net/) [28]. Trinity is a 3-module assembler composed of inchworm, chrysalis and butterfly. Inchworm assembles clean reads into a set of full length linear contigs for the major isoform as well as unique portions of minor spliced variants. Chrysalis then connects these isoforms into components that were likely to represent alternative splice forms and closely related paralogs by finding shared subsequences in the contigs, and build de Bruijn transcript graphs for each component. Finally, butterfly processes each generated graph and enumerates full length alternatively spliced isoforms and transcripts from paralogous genes. The following parameters were used in Trinity: min_glue = 3, V = 10, edge-thr = 0.05, min_kmer_cov = 3, path_reinforcement_distance = 85, group_pairs_distance = 250, and the other parameters were set as the default.
The assembled contigs that were shorter than 200 bp were removed using the Perl script seqclean. Redundant contigs were trimmed by using a self-cross BlastN, searching with a cutoff E value ≤1×10−10, identity ≥95%, covered length ≥90%. Compared with ribosomal RNA (rRNA) databases (http://www.girinst.org), the contigs meeting a cutoff (E value ≤1×10−10, identity ≥80% and covered length of query ≥80%) were removed. We identified and discarded any potential contaminated sequence from microbes using BlastN against databases of microbial genomes downloaded from NCBI (ftp://ftp.ncbi.nih.gov/refseq/release/microbial/) with the cutoff E value ≤1×10−10, identity ≥80%, and alignment length ≥90%.
Functional annotation and classification
All assembled unigenes were annotated with getorf from the EMBOSS package. The ORFs were then aligned with Swiss-Prot and NCBI NR peptide databases with thresholds of E-value = 1e-5 and the ORF with the highest score was used to annotate the contig. For the contigs that did not have hits in these databases, the longest ORF were annotated as ‘‘hypothetical protein”. Domain-based alignments were performed against the KOG database at NCBI with a cutoff E-value = 1e-5. GO annotations were carried out using the Blast2GO software. WEGO software (http://wego.genomics.org.cn/cgi-bin/wego/index.pl) was used to produce GO functional classification for all unigenes [29]. Another GO analysis tool, SEA, was used to identify overrepresented GO terms [30]. KEGG pathways annotations were performed using the KEGG Automatic Annotation Server (KAAS) (http://www.genome.jp/kaas-bin/kaas_main?modeest_b) with the bi-directional best hit information method [31]. KAAS annotates every submitted sequence with KEGG orthology (KO) identifiers that represent an orthologous group of genes directly linked to an object in the KEGG pathways and BRITE functional hierarchy [31–32]. Transcription factors (TFs) were analyzed with all the unigenes by BLASTX searches against the Plant Transcription Factor Database (PlnTFDB) (version 3.0) (E-value = 1e-10).
Analysis of differentially expressed genes (DEGs)
To identify differentially expressed genes in the selfed and crossed ovules, a rigorous algorithm was developed based on the method described by Audic and Claverie [33]. The number of reads for each of the contigs from the samples of two pollination treatments was converted to reads per kilobase per million (RPKM). The false discovery rate was used to determine the threshold of the P-value in multiple tests and analyses. We used an FDR of < 1e-05, the absolute value of log2 ratio > 2, and fold change >1 as thresholds to define significant differences in gene expression [34].
Quantitative real time reverse transcription-PCR analysis
Quantitative RT-PCR was carried out on cDNA generated from three biological replicates harvested as described above, one of which corresponded to the sample subjected to Illumina sequencing for RNA-seq analysis. The total RNA (10 μg) was reverse-transcribed with an oligo (dT) primer for cDNA synthesis using a SuperScript III First-Strand Synthesis Kit (Invitrogen). Amplification of the X. sorbifolium actin gene was used as an internal control to normalize all data. Gene-specific primers were designed using PRIMEREXPRESS software (Applied Biosystems). The primer sequences are listed in S3 Table. Quantitative PCR assays were performed in three technical replications using SYBR Green Real-time PCR Master Mix (Toyobo, Osaka, Japan) with a Bio-Rad CFX96 Real-Time Detection System. The quantitative variation in the different replicates was calculated using the delta delta threshold cycle relative quantification method.
Results
Structural and histological analyses of ovule development
The mature ovules of X. sorbifolium consisted of a short funicle, an inner and outer integument, a nucellus and an embryo sac (Fig 1A, 1B, 1C and 1D). There were 9 to 12 layers of cells in the outer integument and 4 to 7 layers in the inner integument at 5 DAP (Fig 1C and 1D). The innermost 3 to 5 layers of the outer integument on the funicular side gave rise to many lignified cells that contained tannin-like substances after fertilization. Later, several layers of cells on the abaxial side also produced lignified cell walls and accumulated tannin-like substances. The integumentary parenchyma became compressed and was absorbed following expansion of the fertilized embryo sac.
Fig 1. Developing ovules of Xanthoceras sorbifolium. A, B. Scanning electron microscope images.
A: Young ovules from a floral bud. B: A selfed ovule at 5 d after pollination (DAP). C and D: Longitudinal section of crossed and selfed ovules at 5 DAP, respectively. Arrows show free nuclear endosperm. Abbreviations: ES, embryo sac; HY, hypostase; NU, nucellus; OI, outer integument; II, inner integument.
The nucellus was well developed and exhibited conspicuous curvature in the fertilized ovules. A beak-shaped outgrowth pointing toward the micropyle (nucellus beak) was formed at the nucellar apex. A massive, cup-like hypostase situated above the chalazal vasculature was differentiated at the nucellar and chalazal tissue (Fig 1C). The hypostase cells became filled with tannin-like substances after fertilization, with the cell walls thickening with suberin and lignin deposition.
A large embryo sac was embedded within the massive nucellar tissue. After fertilization, the embryo sac expanded dramatically in both length and width and became progressively curved. The primary endosperm nucleus migrated to the basal cytoplasm of the central cell and divided without formation of interzone phragmoplasts or a cell wall between sister nuclei within 30 h after self- and cross-pollination. Mitosis continued more or less synchronously and approximately two hundred free nuclei were generated by 5 DAP. The nuclei in the endosperm coenocyte became evenly dispersed in a thin layer of cytoplasm around the periphery of a large central vacuole. Resting zygotes were observed at 5 DAP, and they never divided in the selfed ovules.
Morphological observations of late acting self-incompatibility
All the ovules in an ovary were penetrated by pollen tubes, followed by double fertilization after either self- or cross- pollination. Ovule development within an ovary was homogeneous during the first 5 days after pollination. However, some of the ovules ceased developing and showed visible signs of degeneration at 6 d after pollination. The mean area of the median longitudinal section of the ovules and embryo sacs was significantly smaller after self-pollination than it was after cross-pollination at 7 DAP (Fig 2A and 2B). Lignification of the cell wall and accumulation of tannin-like substances in the outer integument also occurred more quickly and more heavily after self- than cross-pollination. A greater proportion of ovules degenerated after self- than cross-pollination by 8 DAP. All of the ovules in an ovary degenerated 8–15 d after self-pollination depending on the tree. The pistils in which more than 60% of the ovules degenerated would abort after either self- or cross-pollination.
Fig 2. Mean area of the median longitudinal section of ovule and embryo sac after cross- and self- pollination of Xanthoceras sorbifolium.

A: Ovule area; B: Embryo sac area.
Identification of genes expressed specifically or preferentially in the selfed ovules
To identify the genes involved in selfed ovule rejection and late acting self-incompatibility, we performed a comparative RNA-seq analysis on the selfed and crossed ovules of X. sorbifolium. These two samples enabled us to distinguish selfed ovule specific or preferential transcripts from transcripts that contribute to common biological processes and cellular activities. As stated above, there are no discernable differences in structural and histological features between the selfed and crossed ovules during the early stage of development until 5 DAP. After that time, the selfed ovules begin to show differences in histology compared with the crossed ones. These observations suggest that 5 DAP is likely to be a turning point for development of selfed ovules and that the reprogrammed gene expression at this stage possibly represents the molecular mechanisms modulated by the LSI genes that may play crucial roles in ovule development. Thus, selfed and crossed ovules at 5 DAP were sampled for the present study of comparative transcriptomics (Fig 3A and 3B).
Fig 3. The selfed and crossed ovules were harvested from the pistil at 5 d after pollination for the study of RNA-seq and morphology.
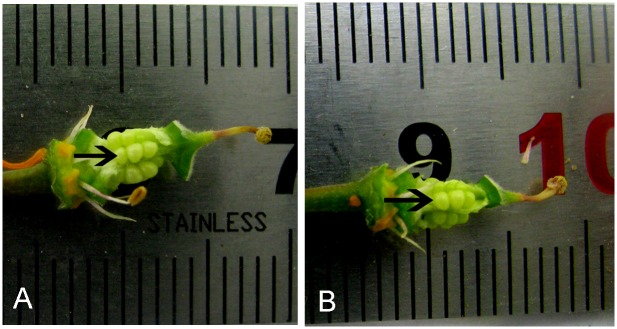
A and B showing the crossed and selfed ovules (arrows), respectively.
The mRNA from the samples of two pollination treatments was used to construct cDNA libraries, which were then sequenced on an Illumina HiSeq 2000 system. A total of 35.8 and 24.1 million raw reads were generated from the selfed and crossed ovules, respectively. After removing low quality reads, including reads with adapter sequences, those with unknown nucleotides comprising more than 5%, and those of low quality (≥20% of the bases with a quality score (Q)≤10), 34,743,319 and 23,135,380 high-quality reads were obtained from the selfed and crossed ovules, respectively. All of the high-quality reads were pooled together for de novo transcriptome assembly into contigs using Trinity software (version v2013-02-25). The assembled sequences were then filtered to remove the contigs that were shorter than 200 bp, those that were either viral or bacterial in origin, those that contained redundant sequences, and those that contained ribosomal RNA sequences. Ultimately, we obtained 36,117 unigenes that ranged in length from 201 to 32,445 bp. The average and N50 lengths of the unigenes were 1,137 and 1,771 bp, respectively. The length distribution of unigenes is shown in Fig 4. Approximately forty-six percent of the unigenes (16,602) were longer than 800 bp.
Fig 4. Distribution of contig length.

The X-axis indicates contig length (bp). The Y-axis indicates number of unigenes.
Using both a fold change of >1 and a false discovery rate (FDR) of <1e-05 as a cutoff to identify differentially expressed genes, 274 unigenes were found that were significantly up-regulated in the selfed ovules compared to the crossed ovules (S1 Table). These up-regulated unigenes were considered to be specifically (fold change >5) or preferentially expressed in the selfed ovules (hereafter designated as the selfed ovule dataset). Using the same standard, we also identified 42 unigenes specifically or preferentially expressed in the crossed ovules (S2 Table).
Confirmation of genes expressed specifically or preferentially in the selfed ovule by quantitative real-time RT-PCR analysis
To validate the RNA-seq data and to check whether the candidate genes are specifically or preferentially expressed in the selfed ovules, 16 genes were randomly selected from the selfed ovule dataset for quantitative real-time RT-PCR analysis (the primer sequences are available in S3 Table). The expression of all tested genes was validated using PCR in the selfed and crossed ovules. Scatterplots were generated by comparing the log2-fold change determined by the transcriptome analysis and quantitative real-time RT-PCR. The correlation between these two analyses was then evaluated. The results showed that the expression patterns of these genes examined using quantitative real-time RT-PCR were well correlated with those by RNA-seq (R2 = 0.754), thus verifying the reliability of the RNA-seq technique (Fig 5).
Fig 5. Validation of RNA-seq results by quantitative real time RT-PCR.

Correlation plots indicating the relationship between qPCR results (fold change; Y-axis) of 16 selected genes expressed in the selfed and crossed ovules and the corresponding data from RNA-seq analysis (X-axis).
Functional annotation of the transcriptome gene models
The ORFs of all the gene models were predicted using getorf (EMBOSS 6.2.0). A total of 36,101 (99.96%) unigenes were predicted to have ORFs longer than 30 amino acids (aa). The ORF of each predicted protein was aligned against the Swiss-Prot and NCBI non-redundant (NR) databases using BLASTP with an E-value cutoff of 1e-5. The homology search results showed that 16,714 (46.27%) and 9,106 (25.21%) of the 36,117 X. sorbifolium unigenes had significant matches with sequences in the NCBI NR and Swiss-Prot protein databases, respectively. Altogether, 16,722 (46.30%) unigenes were successfully annotated using these two public databases. The species distribution of the best match for each NR annotated unigene showed 5,604 (33.53%) matches with Vitis vinifera sequences, 4,351 (26.03%) with Ricinus communis, 3,928 (23.50%) with Populus trichocarpa, 818 (4.89%) with Glycine max, 284 (1.70%) with Arabidopsis, and 202 (1.21%) with Medicago.
The assembled unigenes were further annotated with Gene Ontology (GO) terms. Of the 36,117 unigenes, 6,809 sequences were assigned one or more GO terms (S4 Table). These 6,809 unigenes were categorized into 51 GO functional groups under three main categories: molecular function, biological process and cellular component (Fig 6). Within the biological process category, the terms cellular process, metabolic process, and biological regulation were dominant. In the cellular component category, most unigenes were assigned to cell, cell part, and organelle. In the molecular function category, the most highly represented GO terms were binding and catalytic activity.
Fig 6. Gene Ontology categories of assembled unigenes.
Unigenes were assigned to three main categories (biological processes, cellular components, and molecular functions) and 51 subcategories. The X-axis indicates GO term. The Y-axis indicates number of unigenes.
All unigenes were further annotated and classified based on EuKaryotic Orthologous Groups (KOG) category. A total of 6,343 unigenes were assigned KOG functional annotation and grouped into 24 functional categories (Fig 7). Among these categories, signal transduction mechanisms (864, 13.62%); general function prediction only (813, 12.81%); and posttranslational modification, protein turnover, chaperones (720, 11.35%) were dominant, followed by carbohydrate transport and metabolism (404, 6.37%), and translation, ribosomal structure and biogenesis (399, 6.29%). For the category signal transduction mechanisms, the most abundant type of unigene was serine/threonine protein kinases (310, 35.92%).
Fig 7. KOG function classification.
The unigenes were aligned to the KOG database to predict and categorize possible functions. A total of 6343 unigenes were assigned to 24 categories.
We also annotated 5,696 unigenes with a K number to BRITE functional hierarchies, and 3,724 of them were assigned with an EC number (S5 Table). The BRITE functional mapping revealed the most common classifications and categorized the unigenes into 252 KEGG pathways (S6 Table). Among these pathways, ribosome (164), biosynthesis of amino acids (158), carbon metabolism (134), protein processing in endoplasmic reticulum (124), spliceosome (117), RNA transport (108), phagosome (100), purine metabolism (97), RNA polymerase (97), and plant hormone signal transduction (91) were most highly represented.
Functional annotation of genes expressed differentially in the selfed- and crossed ovules
Of the 274 genes that were specifically or preferentially expressed in the selfed ovules, 76 were annotated with KOG functions (S7 Table). The 76 genes with KOG annotation were grouped into 17 functional categories. The five largest categories were general function prediction only (13.16%); secondary metabolites biosynthesis, transport and catabolism (11.84%); posttranslational modification, protein turnover, chaperones (10.53%); transcription (10.53%); and signal transduction mechanisms (9.21%) (Fig 8). The most abundant genes in the second largest category encode cytochrome P450 monooxygenases, of which 3 belong to the cytochrome P450 CYP2 family.
Fig 8. KOG function classification of the genes expressed specifically or preferentially in the selfed ovules of Xanthoceras sorbifolium.

The X-axis indicates KOG function classification. The Y-axis indicates number of unigenes.
To further evaluate the potential functions of genes in the selfed ovule dataset, Gene Ontology categories were assigned to the 274 specifically or preferentially expressed genes in the selfed ovules. Ninety-three (33.94%) genes were assigned one or more GO terms (S8 Table), and 92 of these were assigned to biological process and were further classified into 17 subcategories (Fig 9). Among the 17 subcategories, metabolic and cellular processes; biological regulation; and response to stimulus were predominant. Under cellular component, cell parts; cell; organelle and organelle part were the largest subcategories. Binding (nucleotide binding, protein binding, chromatin binding) and catalysis were the most abundant subcategories within molecular function (Fig 9).
Fig 9. GO function classification of the identified genes expressed specifically or preferentially in the selfed ovules of Xanthoceras sorbifolium.

Fourteen unigenes expressed specifically or preferentially in the crossed ovules were assigned with GO terms. In cell component category, cell and cell part subcategories are dominant, followed by organelle, macromolecular complex, and membrane-enclosed lumen. Biological process category includes response to stimulus, metabolic process, cellular process, and multi-organism process. Binding is the largest subcategory within molecular function category (Fig 10).
Fig 10. GO function classification of the identified genes expressed specifically or preferentially in the crossed ovules of Xanthoceras sorbifolium.

For large groups of genes, statistically enriched GO terms can give insights into the biological pathways that are likely to be highly active by comparing them to the frequency at which those GO terms appear in the whole transcriptome. A singular enrichment analysis (SEA) [30] was performed to identify the significantly enriched GO terms in genes specifically or preferentially expressed in the selfed ovules of X. sorbifolium. The results showed that 18 GO terms were overrepresented in the selfed ovules based on the P-value <0.001 and the FDR ≤0.05 cutoffs, which included 15 cellular component categories and 3 molecular function categories (Table 1). The genes involved in the plastid, chloroplast part, plastid part and thylakoid were overrepresented based on the GO cellular component analysis. In the molecular function category, the selfed ovule was enriched in GO terms related to iron ion binding, tetrapyrrole binding and oxidoreductase activity.
Table 1. The overrepresented functional GO terms of the genes specifically or preferentially in the selfed ovules of Xanthoceras sorbifolium.
| GO term | Ontogeny | Description | Query item number | Query total | Number in BG/Ref | Bg total | p-value | FDR |
|---|---|---|---|---|---|---|---|---|
| GO:0046906 | F | tetrapyrrole binding | 11 | 93 | 143 | 6809 | 8.20E-06 | 0.0012 |
| GO:0005506 | F | iron ion binding | 12 | 93 | 186 | 6809 | 2.00E-05 | 0.0015 |
| GO:0016705 | F | oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen | 7 | 93 | 75 | 6809 | 9.80E-05 | 0.0049 |
| GO:0009579 | C | thylakoid | 12 | 93 | 185 | 6809 | 1.90E-05 | 0.0019 |
| GO:0009522 | C | photosystem I | 5 | 93 | 23 | 6809 | 1.50E-05 | 0.0019 |
| GO:0044435 | C | plastid part | 19 | 93 | 451 | 6809 | 5.10E-05 | 0.0026 |
| GO:0044434 | C | chloroplast part | 19 | 93 | 443 | 6809 | 4.10E-05 | 0.0026 |
| GO:0031976 | C | plastid thylakoid | 10 | 93 | 167 | 6809 | 0.00016 | 0.0055 |
| GO:0009534 | C | chloroplast thylakoid | 10 | 93 | 167 | 6809 | 0.00016 | 0.0055 |
| GO:0031984 | C | organelle subcompartment | 10 | 93 | 186 | 6809 | 0.00038 | 0.0097 |
| GO:0009521 | C | photosystem | 5 | 93 | 44 | 6809 | 0.00038 | 0.0097 |
| GO:0009507 | C | chloroplast | 27 | 93 | 935 | 6809 | 0.00089 | 0.017 |
| GO:0055035 | C | plastid thylakoid membrane | 8 | 93 | 140 | 6809 | 0.00093 | 0.017 |
| GO:0042651 | C | thylakoid membrane | 8 | 93 | 142 | 6809 | 0.001 | 0.017 |
| GO:0009535 | C | chloroplast thylakoid membrane | 8 | 93 | 140 | 6809 | 0.00093 | 0.017 |
| GO:0009536 | C | plastid | 27 | 93 | 949 | 6809 | 0.0011 | 0.017 |
| GO:0034357 | C | photosynthetic membrane | 8 | 93 | 147 | 6809 | 0.0013 | 0.018 |
| GO:0044436 | C | thylakoid part | 8 | 93 | 157 | 6809 | 0.0019 | 0.02 |
Of 274 unigenes in the selfed ovule dataset, 37 were annotated with a K number to BRITE functional hierarchies, and 26 of 37 unigenes were assigned an EC number (Table 2). The BRITE functional mapping categorized the gene models into 22 KEGG pathways. Photosynthesis; photosynthesis-antenna proteins; cysteine and methionine metabolism; phenylalanine metabolism; and ribosome were the most abundant pathways.
Table 2. The Xanthoceras sorbifolium selfed ovule specifically or preferentially expressed genes were annotated to BRITE functional hierarchies.
| Gene-ID | Ko-ID | Enzymes/Proteins | EC Number | Pathways |
|---|---|---|---|---|
| comp11446_c0_seq1 | K08914 | LHCB3; light-harvesting complex II chlorophyll a/b binding protein 3 | 00196 Photosynthesis—antenna proteins | |
| comp12163_c0_seq1 | K02638 | petE; plastocyanin | 00195 Photosynthesis | |
| comp12271_c0_seq1 | K02975 | RP-S25e, RPS25; small subunit ribosomal protein S25e | 03010 Ribosome | |
| comp15623_c0_seq1 | K14498 | SNRK2; serine/threonine-protein kinase SRK2 | [EC:2.7.11.1] | 04075 Plant hormone signal transduction |
| comp15703_c0_seq1 | K02639 | petF; ferredoxin | 00195 Photosynthesis | |
| comp15750_c0_seq1 | K00430 | E1.11.1.7; peroxidase | [EC:1.11.1.7] | 00360 Phenylalanine metabolism |
| comp16174_c0_seq3 | K15398 | CYP86A4S; cytochrome P450, family 86, subfamily A, polypeptide 2/4/7/8 (fatty acid omega-hydroxylase) | [EC:1.14.-.-] | 00073 Cutin, suberine and wax biosynthesis |
| comp16267_c0_seq2 | K00878 | thiM; hydroxyethylthiazole kinase | [EC:2.7.1.50] | 00730 Thiamine metabolism |
| comp16909_c0_seq1 | K04125 | E1.14.11.13; gibberellin 2-oxidase | [EC:1.14.11.13] | 00904 Diterpenoid biosynthesis |
| comp18656_c0_seq1 | K15813 | LUP4; beta-amyrin synthase | [EC:5.4.99.39] | 00909 Sesquiterpenoid and triterpenoid biosynthesis |
| comp18849_c0_seq4 | K13356 | FAR; fatty acyl-CoA reductase | [EC:1.2.1.-] | 00073 Cutin, suberine and wax biosynthesis |
| comp19771_c0_seq1 | K02152 | ATPeVG, ATP6G1; V-type H+-transporting ATPase subunit G | [EC:3.6.3.14] | 00190 Oxidative phosphorylation |
| comp21740_c0_seq1 | K10111 | malK, mtlK, thuK; multiple sugar transport system ATP-binding protein | [EC:3.6.3.-] | 02010 ABC transporters |
| comp22480_c0_seq1 | K13789 | GGPS; geranylgeranyl diphosphate synthase, type II | [EC:2.5.1.1 2.5.1.10 2.5.1.29] | 00900 Terpenoid backbone biosynthesis |
| comp23147_c0_seq1 | K15746 | crtZ; beta-carotene 3-hydroxylase | [EC:1.14.13.129] | 00906 Carotenoid biosynthesis |
| comp23211_c0_seq1 | K05907 | APR; adenylyl-sulfate reductase (glutathione) | [EC:1.8.4.9] | 00920 Sulfur metabolism |
| comp23344_c0_seq1 | K14498 | SNRK2; serine/threonine-protein kinase SRK2 | [EC:2.7.11.1] | 04075 Plant hormone signal transduction |
| comp24050_c0_seq1 | K00454 | LOX2S; lipoxygenase | [EC:1.13.11.12] | 00591 Linoleic acid metabolism |
| comp24115_c0_seq1 | K12153 | CYP79A2; cytochrome P450, family 79, subfamily A, polypeptide 2 (phenylalanine N-monooxygenase) | [EC:1.14.13.124] | 00460 Cyanoamino acid metabolism |
| comp24691_c0_seq1 | K00522 | FTH1; ferritin heavy chain | [EC:1.16.3.1] | 00860 Porphyrin and chlorophyll metabolism |
| comp24825_c0_seq1 | K08905 | psaG; photosystem I subunit V | 00195 Photosynthesis | |
| comp24912_c0_seq3 | K05279 | E2.1.1.76; flavonol 3-O-methyltransferase | [EC:2.1.1.76] | 00944 Flavone and flavonol biosynthesis |
| comp26604_c0_seq1 | K02900 | RP-L27Ae, RPL27A; large subunit ribosomal protein L27Ae | 03010 Ribosome | |
| comp31612_c0_seq1 | K13034 | ATCYSC1; L-3-cyanoalanine synthase/ cysteine synthase | [EC:2.5.1.47 4.4.1.9] | 00270 Cysteine and methionine metabolism |
| comp32523_c0_seq1 | K00588 | E2.1.1.104; caffeoyl-CoA O-methyltransferase | [EC:2.1.1.104] | 00360 Phenylalanine metabolism |
| comp32688_c0_seq1 | K00547 | mmuM; homocysteine S-methyltransferase | [EC:2.1.1.10] | 00270 Cysteine and methionine metabolism |
| comp33345_c0_seq1 | K00430 | E1.11.1.7; peroxidase | [EC:1.11.1.7] | 00360 Phenylalanine metabolism |
| comp5793_c0_seq1 | K15086 | TPS14; (3S)-linalool synthase | [EC:4.2.3.25] | 00902 Monoterpenoid biosynthesis |
| comp6957_c0_seq1 | K03236 | EIF1A; translation initiation factor 1A | 03013 RNA transport | |
| comp7253_c0_seq1 | K02692 | psaD; photosystem I subunit II | 00195 Photosynthesis | |
| comp7608_c0_seq1 | K02905 | RP-L29e, RPL29; large subunit ribosomal protein L29e | 03010 Ribosome | |
| comp7631_c0_seq1 | K01761 | E4.4.1.11; methionine-gamma-lyase | [EC:4.4.1.11] | 00270 Cysteine and methionine metabolism |
| comp7882_c0_seq1 | K08909 | LHCA3; light-harvesting complex I chlorophyll a/b binding protein 3 | 00196 Photosynthesis—antenna proteins | |
| comp8062_c0_seq1 | K00090 | E1.1.1.215; gluconate 2-dehydrogenase | [EC:1.1.1.215] | 00030 Pentose phosphate pathway |
| comp8280_c0_seq1 | K02126 | ATPeF0A, MTATP6; F-type H+-transporting ATPase subunit a | [EC:3.6.3.14] | 00190 Oxidative phosphorylation |
| comp8351_c0_seq1 | K01188 | E3.2.1.21; beta-glucosidase | [EC:3.2.1.21] | 00460 Cyanoamino acid metabolism |
| comp9794_c0_seq2 | K08912 | LHCB1; light-harvesting complex II chlorophyll a/b binding protein 1 | 00196 Photosynthesis—antenna proteins |
Transcription factors in the fertilized ovules of X. sorbifolium
Transcription factors (TFs) play a pivotal role in regulating the spatial and temporal expression of genes in all living organisms. This regulation ensures accurate development and functioning of an organism. To understand transcription factor expression patterns in the fertilized ovules of X. sorbifolium, all the assembled transcripts were aligned with known TF protein sequences of other sequenced plants listed in PlnTFDB (E-value ≤ 1e-10) using BLASTX. In total, 3,812 putative TF-encoding transcripts, distributed over at least 60 families, were identified, representing 10.55% of the total ovule transcripts detected in the present study. The top 25 TF gene families are depicted in Fig 11. The largest TF family was FAR1, which contained 509 unigenes. The next largest families were PHD, MADS, C3H, bHLH, MYB, NAC, and WRKY family TFs.
Fig 11. Top 25 transcription factor families of the Xanthoceras sorbifolium selfed ovule dataset.
The X-axis indicates the top 25 TF families. The Y-axis indicates the number of unigenes assigned to a specific TF family.
TF identification is useful for studying the transcriptional regulatory switches involved in plant morphology and functional competence and also in generating responses to changing condition. Hence, it was of great interest to perform a comparative analysis of TF gene expression between the selfed and crossed ovules in an LSI species. Of 274 unigenes expressed specifically or preferentially in the selfed ovules of X. sorbifolium, 28 encode for putative transcription factors belonging to 12 different families (Table 3). The most frequently represented genes in the largest category encode for the FAR1 TF family, which contains eight genes. The most highly expressed TF-encoding transcripts with RPKM values of above 10 in the selfed ovules encode the FAR1, HB, NAC, and MYB families. Among 42 genes expressed specifically in the crossedovules, 4 encode for NAC, FAR1, MYB and LIM TF families.
Table 3. Twenty-eight specifically or preferentially expressed unigenes in the selfed ovules of Xanthoceras sorbifolium encode for the putative transcription factors belonging to 12 different families.
RPKM: Reads per Kilobase per Million reads.
| Gene_ID | Transcription factor families | RPKM |
|---|---|---|
| comp7866_c0_seq1 | FAR1 | 70.68928969 |
| comp13650_c0_seq1 | HB | 37.96544751 |
| comp13995_c0_seq1 | FAR1 | 16.56510458 |
| comp10460_c0_seq2 | FAR1 | 16.21917041 |
| comp7496_c0_seq1 | FAR1 | 14.15901078 |
| comp10091_c0_seq2 | NAC | 13.74973919 |
| comp15623_c0_seq1 | HB | 13.23787232 |
| comp15750_c0_seq1 | MYB | 11.74753425 |
| comp16204_c0_seq1 | bZIP | 8.663881644 |
| comp12230_c0_seq1 | C3H | 8.615953788 |
| comp12503_c0_seq1 | OFP | 8.326351607 |
| comp6837_c0_seq1 | FAR1 | 7.182740113 |
| comp16650_c0_seq1 | C2H2 | 6.861621657 |
| comp17938_c0_seq1 | C2C2-CO-like | 6.756090309 |
| comp8243_c0_seq1 | FAR1 | 5.846955337 |
| comp17767_c0_seq2 | FHA | 5.838119077 |
| comp13002_c0_seq2 | HB | 5.48346443 |
| comp17703_c0_seq2 | SBP | 5.327418492 |
| comp12970_c0_seq1 | HB | 5.121261816 |
| comp12417_c0_seq1 | MYB-related | 4.838350968 |
| comp8062_c0_seq1 | HB | 4.269904026 |
| comp8351_c0_seq1 | HB | 4.187594785 |
| comp7986_c0_seq2 | MYB-related | 4.152074582 |
| comp18100_c0_seq2 | PLATZ | 3.765540213 |
| comp17167_c0_seq1 | C2H2 | 3.757697173 |
| comp17403_c0_seq2 | MYB-related | 3.163581425 |
| comp5928_c0_seq1 | FAR1 | 2.7641009 |
| comp6110_c0_seq2 | FAR1 | 2.134615466 |
Discussion
Historically, most studies on SI have focused on the dynamics of interactions between pollen and the stigma or the style, with little attention given to events occurring in the ovary. Late-acting SI has been neglected and was often treated as an anomaly of limited importance by researchers studying the conventional SI systems, in which SI barriers occur at stigmatic and stylar levels of the pistil. This situation has recently changed because OSI was revealed in many angiosperm species [20,24,27,35–44]and hence is known to play an important role, as do other forms of SI, in reducing inbreeding and its harmful effects [45]. However, in most instances the critical structural and histological investigations of ovule development were required to distinguish whether self-sterility results from a true OSI based on self-recognition with major gene control or due to the effects of early acting inbreeding depression (EID), caused by the expression of deleterious recessive alleles. Discrimination between LSI and EID may be a difficult task [21]. This situation is particularly contentious for the cases of post-zygotic rejection of selfed pistils in presumed LSI species, as in X. sorbifolium.
To distinguish between late-acting SI and early acting ID, one of the principal criteria is the timing of ovule abortion [21]. Early acting ID is expected to cause embryo failure at a variety of developmental stages, whereas a uniform failure of ovules at a single developmental stage would be interpreted as late-acting SI. The morphological investigations in the present study sufficiently demonstrate the occurrence of double fertilization in all the selfed ovules of X. sorbifolium and the uniform failure of zygotes arising from self-pollination at an initial stage prior to cell division, with the early stages of free-nuclear endosperm formation apparently proceeding normally. Similar post-penetration events in selfed ovules were also observed in other LSI species studied, such as in Bignoniaceae species [37–38,46], Pseudowintera axillaris [20], Gasteria verrucosa [47], Narcissus triandrus [48], and Ipomopsis aggregate [41]. No embryo divisions occurred in any of these species and these plants show the phenomenon of ‘resting zygotes’. The failure of zygotic division is followed by the rejection of an entire ovule.
The morphological observations in X. sorbifolium also indicated that self-pollen or pollen tubes elicit a reduction in embryo sac size, quick deposition of thick, lignified cell wall, and pronounced accumulation of tannin-like substances in the outer integument in comparison to cross-pollen or pollen tubes. These results suggested that the process of self-recognition and rejection in X. sorbifolium may entail long-distance signaling between pollen or pollen tubes and ovarian tissues, which results in the modification of post-pollination stimulatory functions of pollen or pollen tubes on ovule development. Late-acting SI may start early, and self-pollen tubes growing in the style may project ‘hostile’ or ‘adverse’ signals that may set in motion a subsequent chain of events that lead to ovule rejection.
Long-distance signaling from the pollen tube to the ovules has been implicated in some LSI taxa in which the presence of incompatible pollen or pollen tubes influence ovule integument development, embryo sac viability, starch metabolism, and transmitting tissue secretion in ovarian tissue before and after penetration by pollen tubes [41,47]. It has been suggested for several species exhibiting LSI that self-pollen tubes do not provide the appropriate signals for stimulation of ovule and seed development. In Gasteria verrucosa [47], Theobroma cacao [22], and Asclepias exaltata [26], integumentary growth fails to proceed normally after entry of a self-pollen tube into the ovule. Sears suggested that interaction of compatible pollen tubes with integuments might be important for stimulation of normal seed development [47]. In Prunus dulcis, embryo sac development was strongly affected by pollen tube activity in the pistil. Cross-pollen tubes had a greater stimulatory effect than self-pollen tubes and irregularities in embryo sac development were more frequent after self-pollination [49]. Sage et al. showed that embryo sac degeneration after self-pollination might result from the absence of a required stimulus for normal ovule development in Narcissus triandru [48]. Sage et al. reported that ovules in self-pollinated flowers of Ipomopsis aggregate indicated an absence of embryo sac expansion, little starch storage, disorderly development of the integumentary tapetum and adjacent cells before pollen tube entry into the ovary [41]. Indoleacetic acid, gibberellic acid, ethylene, and ethylene precursors have been posited to play a role in post-pollination stimulation events by pollen tubes [50].
Where free-nuclear endosperm and the resting zygote develop, the ovule must possess or produce some substance that actively inhibits the growth of selfed endosperm and division of the zygotes in the LSI species. Early priming for ovule degeneration triggered by possible adverse signals from self-pollen tubes might be revealed by searching for early molecular indicators of biological processes. The present study identified 274 genes predicted to be specifically or preferentially expressed in the selfed ovules of X. sorbifolium using high-throughput next-generation sequencing technology to perform an RNA-seq analysis. It is likely that at least some of these genes function in pollen-ovule interactions and LSI mechanisms. This study represents the first genome-wide identification of genes expressed in the ovules of a late-acting SI species.
The genes expressed specifically or preferentially in the selfed ovules at 5 DAP were predicted to encode proteins that may perform crucial functions in the switch from normal to aberrant development of ovules. Our analysis found that overrepresented functional categories in the transcripts expressed specifically or preferentially in selfed ovules include signal transduction mechanisms, secondary metabolites biosynthesis, transcription, inorganic ion transport and metabolism. We hypothesize that at least some of these genes are potentially involved in the pollination compatibility responses and the LSI process.
Cell-cell communication and signal transduction possibly implicated in LSI responses
Genes predicted to function in cell-cell communication and signal transduction are of particular interest in the context of pollen-ovule interactions and LSI. The selfed ovule dataset contained a significant proportion of genes in these categories. The most noteworthy are genes encoding Ca2+/calmodulin-dependent protein kinase, serine/threonine protein kinase, serine/threonine protein phosphatase.
Protein phosphorylation/dephosphorylation by specific protein kinases/phosphatases is one of the most important mechanisms whereby cells respond to extracellular signals [51]. It is well known that protein phosphorylation events play a crucial role in the signaling cascade of Papaver GSI and Brassica SSI. Interaction between pollen S-ligand (SCR/SP11) and its cognate receptor (SRK) results in transphosphorylation of the kinase domain of SRK, leading to activation of elements of a signaling cascade in Brassica SI [52]. Phosphorylation of the p26 pyrophosphatases and p56 (a mitogen-activated protein kinase, MAPK) is crucial for the SI response in Papaver [53–55]. MAPKs are Ser/Thr protein kinases that are activated by phosphorylation. Activated MAPKs trigger diverse signaling cascades in response to a variety of signals and stimuli [56]. In Nicotiana alata, a species with S-RNase SI, a pollen Ca2+-dependent protein kinase has been shown to specifically phosphorylate the S-RNase [57]. It is particularly notable that three unigenes encoding protein kinase and three encoding protein phosphatase were observed in the X. sorbifolium selfed ovule dataset. Among these six potential candidate genes encoding signaling-related components, 3 (comp13650_c0_seq1, comp15623_c0_seq1 and comp17403_c0_seq2) were confirmed using PCR in the selfed and crossed ovules. Although we are far from understanding the precise role of these protein kinases and phosphatases in the X. sorbifolium LSI mechanisms, it is likely that these proteins are potentially involved in the LSI process and that their roles may be analogous to those in the incompatibility responses of the other species studied.
Ca2+ ions are a most versatile second messenger used in signal transduction in all eukaryotic organisms. In plants, temporally and spatially distinct changes in cytosolic Ca2+ concentrations that are evoked in response to different stimuli, designated as “Ca2+ signatures”, represent a central mechanistic principle to present defined stimulus-specific information [58]. The Ca2+ signatures are detected, decoded and transmitted downstream by Ca2+ sensors [59–60]. Several classes of calcium-sensing proteins have been identified in higher plants, including calmodulin, calmodulin-like, calcineurin B-like proteins, and calcium-dependent protein kinases [61–62]. The X. sorbifolium selfed ovule dataset contains a unigene predicted to encode Ca2+/calmodulin-dependent protein kinase. This gene implicates the involvement of Ca2+-mediated signaling and Ca2+-dependent protein kinase in the X. sorbifolium LSI response.
Transcription factors likely involved in coordinated activation of genes related to LSI
The processes underlying the pollen-ovule interaction and LSI require the concerted action of genes that are regulated by transcription factors. Transcription factors are sequence-specific DNA-binding proteins that may simultaneously function as an activator of one set of functionally related genes and a repressor of others. TFs are responsible for selective gene regulation and are often expressed in a tissue-specific, developmental stage-specific or stimulus-dependent manner [63].
We identified 28 TF genes in the selfed ovule dataset that are likely to play critical regulatory roles in controlling developmental events unique to the selfed ovule development of X. sorbifolium. Therefore 28 is the lower limit of the number of regulators in the selfed ovule dataset because of the stringent filtering process we used in analyzing RNA-seq datasets generated in this study. Most of these TFs (58%) belonged to three families: FAR1 (FAR-RED IMPAIRED RESPONSE1), HB and MYB. Among these families, the FAR1 genes (8) form the largest category. FAR1 functions as a positive and key transcription factor in directly regulating chlorophyll biosynthesis in Arabidopsis. FAR1 and FHY3 (FAR-RED ELONGATED HYPOCOTYL 3) work together to modulate phytochrome A (phyA) nuclear accumulation and phyA responses through directly activating gene expression of a pair of downstream targets, FHY1 and FHY1-LIKE. FHY1 and FHL are two small plant-specific proteins required for nuclear accumulation of light-activated phyA. We observed 3 unigenes predicted to encode chlorophyll a-b binding protein in the selfed ovules dataset. This suggests a general trend to enhance photosynthesis in the selfed ovules of X. sorbifolium during incompatible pollen-ovule interactions.
Most MYB proteins function as transcription factors with varying numbers of MYB domain repeats conferring their ability to bind DNA [64–65]. MYB proteins can be divided into four classes. Most plant MYB genes encode proteins of the R2R3-MYB class [64]. Numerous R2R3-MYB proteins are involved in the control of plant-specific processes including: primary and secondary metabolism, cell fate and identity, developmental processes, and responses to biotic and abiotic stresses [65]. For instance, the R2R3-MYB proteins encoded by AtMYB5 and AtMYB23 regulate tannin biosynthesis in Arabidopsis [66]. AtMYB5 also regulates outer seed coat differentiation. AtMYB52, AtMYB54 and AtMYB69 are proposed to regulate lignin, xylan and cellulose biosynthesis [67]. AtMYB58, AtMYB63 and AtMYB85 activate lignin biosynthesis in fibers and/or vessels [68], whereas AtMYB68 negatively regulates lignin deposition in roots [69]. Our histological analyses indicated that cell wall lignification and accumulation of tannin-like substances in the outer integument occurred more quickly and more heavily after self-pollination than cross- pollination in X. sorbifolium. We hypothesize that some of the MYB genes detected by the present study are potentially involved in tannin and lignin biosynthesis in the integuments of X. sorbifolium.
Phosphorylation is important in determining MYB protein activity. The transcriptional activity of the R2R3-MYB PtMYB4 protein in Pinus taeda is positively regulated by PtMAPK6, which phosphorylates a Ser in the C-terminal activation domain, and similar phosphorylation might regulate other R2R3-MYB proteins, such as AtMYB46 in Arabidopsis [70]. It is likely that some specifically or preferentially expressed protein kinases in the selfed ovules in X. sorbifolium are also involved with regulation of MYB protein activity.
The selfed ovule dataset also contains many TF genes not previously identified as ovule-specific in other species. These include OFP, SBP, SNF2 and PLATZ. The identification of novel TF genes in the X. sorbifolium selfed ovule dataset is particularly interesting as this species possesses an LSI system, which most likely operates through a different mechanism from other conventional SI and self-compatibility (SC) systems. Therefore, the X. sorbifolium selfed ovule dataset is expected to contain TF genes potentially involved in mediating the female side of LSI.
Biosynthesis of secondary metabolites possibly related to rejection of selfed ovules
Incompatible pollen-pistil interactions are sometimes associated with the accumulation of intermediates of secondary metabolite pathways [71], such as the phenylpropanoid pathway, which also occurs in the plant’s response to pathogens and stress [72]. The X. sorbifoilum selfed ovule dataset contains a relatively large number of cytochrome P450 unigenes. P450 enzymes are an ancient superfamily of heme-containing monooxygenase proteins found in all domains of life [73], most of which catalyze NADPH and O2-dependent hydroxylation reactions. Plant P450s are involved in a wide range of biochemical pathways, including those devoted to the synthesis of the following: lignin intermediates; phenylpropanoids; alkaloids; terpenoids; lipids; cyanogenic glycosides; glucosinolates; and plant growth regulators such as gibberellins, jasmonic acid, and brassinosteroids [72,74 – 75]. The CYP superfamily has a total of 977 families, of which 69 are present in animals [76]. The CYP2 gene family is the largest and most complex of the 18 CYP gene families in vertebrates. The number of genes per CYP2 subfamily is variable and can be quite large in some species. CYP2s play a significant role in the metabolism of a variety of exogenous and endogenous compounds [77–78]. The present studies identified 4 unigenes homologous to members of CYP2 family. It will be of interest to investigate whether these CYP2 genes are involved in pollen-ovule interactions and LSI.
Conclusion
The present study demonstrated that there were no significant differences in structural and histological features between the selfed and crossed ovules during the earliest stages of development. After 5 DAP, some of the selfed ovules ceased developing and showed visible signs of degeneration. These observations suggest that 5 DAP is likely a turning point for development of selfed ovules and that the reprogrammed gene expression at this stage possibly represents the molecular mechanisms essential for the rejection of the selfed ovules. The comparative de novo transcriptome analysis of the selfed and crossed ovules at 5 DAP using high-throughput next-generation sequencing technology resulted in the identification and functional classification of 274 genes that were specifically or preferentially expressed in the selfed ovules. Although the biological roles of these genes have yet to be determined, it is likely that at least some of the genes expressed specifically or preferentially in the selfed ovule have functions related to the development of the selfed ovules and LSI mechanisms. To our knowledge, there were no data on genes related to the LSI process prior to our study. This study represents the first genome-wide identification of genes expressed in the fertilized ovules of a late-acting SI species. The valuable genomic resources obtained here will trigger new interesting research on molecular mechanisms of LSI and promote a better understanding of LSI more comparable to those of conventional GSI and SSI systems.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Jie Wen and Fengqin Dong for help with the experiment works. This study was funded by the National Natural Science Foundation of China (31370611 and 31570680).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the National Natural Science Foundation of China.
References
- 1. Takayama S, Isogai A. Self-incompatibility in plants. Ann Rev Plant Biol. 2005; 56: 467–489. 10.1146/annurev.arplant.56.032604.144249 [DOI] [PubMed] [Google Scholar]
- 2. Allen AM, Hiscock SJ (2008) Evolution and phylogeny of self-incompatibility systems in angiosperms In: Franklin-Tong VE, ed. Self-incompatibility in flowering plants—evolution, diversity and mechanisms. Berlin, Germany: Springer. [Google Scholar]
- 3. de Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants. Second ed Springer, Heidelberg. [Google Scholar]
- 4. Gibbs PE. Late-acting self-incompatibility—the pariah breeding system in flowering plants. New Phytol. 2014; 203 (3): 717–734. 10.1111/nph.12874 [DOI] [PubMed] [Google Scholar]
- 5. Wheeler MJ, Vatovec S, Franklin-Tong VE. The pollen S-determinant in Papaver: comparisons with known plant receptors and protein ligand partners. J Exp Bot. 2010; 61: 2015–2025. 10.1093/jxb/erp383 [DOI] [PubMed] [Google Scholar]
- 6. Luu DT, Qin X, Morse D, Cappadocia M. S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature. 2000; 407: 649–651. 10.1038/35036623 [DOI] [PubMed] [Google Scholar]
- 7. Lai Z, Ma WS, Han B, Liang LZ, Zhang YS, Hong GF, et al. An F-box gene linked to the self-incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol Biol. 2002; 50: 29–42. 10.1023/A:1016050018779 [DOI] [PubMed] [Google Scholar]
- 8. Sassa H, Kakui H, Minamikawa M. Pollen-expressed F-box gene family and mechanism of S-RNase-based gametophytic self-incompatibility (GSI) in Rosaceae. Sex Plant Reprod. 2009; 23(1): 39–43. 10.1007/s00497-009-0111-6 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Wang X, McCubbin AG, Kao T. Genetic mapping and molecular characterization of the self-incompatibility S-locus in Petunia inflata . Plant Mol Biol. 2003; 53: 565–580. 10.1023/B:PLAN.0000019068.00034.09 [DOI] [PubMed] [Google Scholar]
- 10. Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, Vasquez-Santana S, et al. Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana . Nature 2006; 439: 805–810. 10.1038/nature04491 [DOI] [PubMed] [Google Scholar]
- 11. Thomas SG, Franklin-Tong VE. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 2004; 429: 305–309. 10.1038/nature02540 [DOI] [PubMed] [Google Scholar]
- 12. Wheeler MJ, de Graaf BH, Hadjiosif N, Perry RM, Poulter NS, Osman K, et al. Identification of the pollen self-incompatibility determinant in Papaver rhoeas . Nature 2009; 459: 992–995. 10.1038/nature08027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea . Proc Natl Acad Sci USA 1991; 88: 8816–8820. 10.1073/pnas.88.19.8816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe M, Suzuki G, Takayama S (2008) Milestones identifying self-incompatibility genes in Brassica species: from old stories to new findings In: Franklin-Tong VE, ed. Self-incompatibility in flowering plants—evolution, diversity and mechanisms. Berlin, Germany: Springer. [Google Scholar]
- 15. Schopfer CR, Nasrallah ME, Nasrallah JB. The male determinant of self-incompatibility in Brassica . Science 1999; 286: 1697–1700. 10.1126/science.286.5445.1697 [DOI] [PubMed] [Google Scholar]
- 16. Takasaki T. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 2000; 403: 913–916. 10.1038/35002628 [DOI] [PubMed] [Google Scholar]
- 17. Giranton JL, Dumas C, Cock JM, Gaude T. The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proc Natl Acad Sci USA 2000; 97: 3759–3764. 10.1073/pnas.070025097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murase K, Shiba H, Iwano M, Che FS, Watanabe M, Isogai A, et al. A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science 2004; 303: 1516–1519. 10.1126/science.1093586 [DOI] [PubMed] [Google Scholar]
- 19. Kakita M, Murase K, Iwano M, Matsumoto T, Watanabe M, Shiba H, et al. Two distinct forms of M-locus protein kinase localize to the plasma membrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa . Plant Cell 2007; 19: 3961–3973. 10.1105/tpc.106.049999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sage TL, Sampson FB. Evidence for ovarian self-incompatibility as a cause of self-sterility in the primitive woody angiosperm, Pseudowintera axillaris (Winteraceae). Ann Bot. 2003; 91: 1–10. 10.1093/aob/mcg085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seavey SR, Bawa KS. Late-acting self-incompatibility in angiosperms. Bot Rev. 1986; 52: 195–219. 10.1007/BF02861001 [DOI] [Google Scholar]
- 22. Cope FW. The mechanism of pollen incompatibility in Theobroma cacao L. Heredity 1962; 17: 157–182. [Google Scholar]
- 23. Lipow SR, Wyatt R. Single gene control of postzygotic self-Incompatibility in poke milkweed, Asclepias exaltata L. Genetics 2000; 154: 893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sage TL, Bertin R, Williams G (1994) Ovarian and other late acting self-incompatibility In Williams EB, Knox RB, Clarke AE [eds.], Genetic control of self-incompatibility and reproductive development in flowering plants. Kluwer, Dordrecht, Netherlands. [Google Scholar]
- 25. Knox RB, Kenrick J (1983) Polyad function in relation to the breeding system of Acacia In: Mulcahy DL, Ottaviano E (eds) Pollen: Biology and implications for plant breeding. Elsevier Biomedical, New York. [Google Scholar]
- 26. Sage TL, Williams EG. Structure, ultrastructure and histochemistry of the pollen tube pathway in the milkweed Asclepias exaltata . Sex Plant Reprod. 1995; 8: 257–265. [Google Scholar]
- 27. Zhou QY, Liu GS. The embryology of Xanthoceras and its phylogenetic implications. Plant Syst Evol. 2012; 298: 457–468. 10.1007/s00606-011-0558-4 [DOI] [Google Scholar]
- 28. Iyer MK, Chinnaiyan AM. RNA-Seq unleashed. Nat Biotech. 2011; 29: 599–600. 10.1038/nbt.1915 [DOI] [PubMed] [Google Scholar]
- 29. Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang ZJ, et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006; 34: W293–W297. 10.1093/nar/gkl031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du Z, Zhou X, Ling Y, Zhang Z, Su Z. AgriGO: a GO analysis tool kit for the agricultural community. Nucleic Acids Res. 2010; 38: W64–W70. 10.1093/nar/gkq310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007; 35: W182–W185. 10.1093/nar/gkm321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005; 21: 3787–3793. 10.1093/bioinformatics/bti430 [DOI] [PubMed] [Google Scholar]
- 33. Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997; 7: 986–995. [DOI] [PubMed] [Google Scholar]
- 34. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001; 29: 1165–1188. [Google Scholar]
- 35. Gibbs PE, Bianchi M . Does late-acting self-incompatibility (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa . Ann Bot. 1999; 84: 449–457. 10.1006/anbo.1999.0933 [DOI] [Google Scholar]
- 36. Gibbs PE, Bianchi M. Does late-acting self-incompatibility (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa . Ann Bot. 1999; 84: 449–457. 10.1006/anbo.1999.0933 [DOI] [Google Scholar]
- 37. Bittencourt NS, Gibbs PE, Semir J. Histological study of post-pollination events in Sapthodea campanulata Beauv. (Bignoniaceae), a species with late-acting self-incompatibility. Ann Bot. 2003; 91: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bittencourt NS, Semir J. Late-acting self-incompatibility and other breeding systems in Tabebuia (Bignoniaceae). Int J Plant Sci. 2005; 166: 493–506. 10.1086/428758 [DOI] [Google Scholar]
- 39. LaDoux T, Friar EA. Late-acting self-incompatibility in Ipomopsis tenuifoloia (Gray) V. Grant (Polemoniaceae). Int J Plant Sci. 2006; 167: 463–471. 10.1086/500985 [DOI] [Google Scholar]
- 40. Pound LM, Patterson B, Wallwork MBA, Potts BM, Sedgley M. Pollen competition does not affect the success of self pollination in Eucalyptus globules (Myrtaceae). Austral J Bot. 2003; 51: 189–195. 10.1071/BT02082 [DOI] [Google Scholar]
- 41. Sage TL, Price MV, Waser NM. Self-sterility in Ipomopsis aggregate (Polemoniaceae) is due to prezygotic ovule degeneration. Amer J Bot. 2006; 93: 254–262. 10.3732/ajb.93.2.254 [DOI] [PubMed] [Google Scholar]
- 42. Vaughton G, Ramsey M, Johnson SD. Pollination and late acting self-incompatibility in Cyrtanthus breviflorus (Amaryllidaceae): implications for seed production. Ann Bot. 2010; 106: 547–555. 10.1093/aob/mcq149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Finatto T, Santos KLD, Steiner N, Bizzocchi L, Holderbaum DF, Ducroquet JPHJ, et al. Late-acting self-incompatibility in Acca sellowiana (Myrtaceae). Austral J Bot. 2011; 59: 53–60. 10.1071/BT10152 [DOI] [Google Scholar]
- 44. Ford CS, Wilkinson MJ. Confocal observations of late-acting self-incompatibility in Theobroma cacao L. Sex Plant Reprod. 2012; 25: 169–183. 10.1007/s00497-012-0188-1 [DOI] [PubMed] [Google Scholar]
- 45. De Nettancourt D. Incompatibility in angiosperms. Sex Plant Reprod. 1997; 10: 185–199. 10.1007/s004970050087 [DOI] [Google Scholar]
- 46. Gandolphi G, Bittencourt NS. Breeding system of the White Trumpet Tree—Tabebuia roseo-alba (Ridley) Sandwith (Bignoniaceae). Act Bot Brasil. 2010; 24: 840–851. 10.1590/S0102-33062010000300026 [DOI] [Google Scholar]
- 47. Sears ER. Cytological phenomena connected with self-sterility in the flowering plants. Genetics 1937; 22: 130–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sage TL, Strumas F, Cole B, Barrett SCH. Differential ovule development following self- and cross-pollination: the basis of self-sterility in Narcissus triandrus (Amaryllidaceae). Amer J Bot. 1999; 86: 855–870. 10.2307/2656706 [DOI] [PubMed] [Google Scholar]
- 49. Pimienta E, Polito VS. Embryo sac development in almond (Prunus dulcis [Mill.] D.A. Webb) as affected by cross, self, and nonpollination. Ann Bot. 1983; 71: 469–479. [Google Scholar]
- 50. O’Neill S. Pollination regulation of flower development. Ann Rev Plant Physiol. 1997; 48: 547–574. 10.1146/annurev.arplant.48.1.547 [DOI] [PubMed] [Google Scholar]
- 51. Huber SC. Exploring the role of protein phosphorylation in plants: from signaling to metabolism. Biochem Soc Transact. 2007; 35: 28–32. [DOI] [PubMed] [Google Scholar]
- 52. Giranton JL, Dumas C, Cock JM, Gaude T. The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proc Natl Acad Sci USA 2000; 97: 3759–3764. 10.1073/pnas.070025097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Graaf BH, Rudd JJ, Wheeler MJ, Perry RM, Bell EM, Osman K, et al. Self-incompatibility in Papaver targets soluble inorganic pyrophosphatases in pollen. Nature 2006; 444: 490–493. 10.1038/nature05311 [DOI] [PubMed] [Google Scholar]
- 54. Rudd JJ, Franklin FC, Lord JM, Franklin-Tong VE. Increased phosphorylation of a 26-kD pollen protein is induced by the self-incompatibility response in Papaver rhoeas . Plant Cell 1996; 8: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rudd JJ, Osman K, Franklin FC, Franklin-Tong VE. Activation of a putative MAP kinase in pollen is stimulated by the self-incompatibility (SI) response. FEBS Let. 2003; 547: 223–227. 10.1016/S0014-5793(03)00710-5 [DOI] [PubMed] [Google Scholar]
- 56. Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature 2001; 410: 37–40. 10.1038/35065000 [DOI] [PubMed] [Google Scholar]
- 57. Kunz C, Chang A, Faure JD, Clarke AE, Polya GM, Anderson MA. Phosphorylation of style S-RNases by Ca2+ -dependent protein kinases from pollen tubes. Sex Plant Reprod. 1996; 9: 25–34. [Google Scholar]
- 58. Anil VS, Rao KS. Calcium-mediated signal transduction in plants: A perspective on the role of Ca2+ and CDPKs during early plant development. J Plant Physiol. 2001; 158: 1237–1256. 10.1078/0176-1617-00550 [DOI] [Google Scholar]
- 59. Luan S, Kudla J, Rodriguez-Concepcionc M, Yalovsky S, Gru-issem W. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 2002; 54: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang T, Poovaiah BW. Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA 2002; 99: 4097–4102. 10.1073/pnas.052564899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim KN, Cheong Y, Pandey G, Grant J, Luan S. CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 2003; 15: 411–423. 10.1105/tpc.006858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pandey G, Cheong YH, Kim KN, Kudla J, Luan S. The calcium sensor calcineurin B-like 9 modulates abscisic acid sensitivity and biosynthesis in Arabidopsis. Plant Cell 2004; 16: 1912–1924. 10.1105/tpc.021311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riechmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000; 3: 423–434. 10.1016/S1369-5266(00)00107-2 [DOI] [PubMed] [Google Scholar]
- 64. Ito M. Conservation and diversification of three-repeat Myb transcription factors in plants. J Plant Res. 2005; 118: 61–69. 10.1007/s10265-005-0192-8 [DOI] [PubMed] [Google Scholar]
- 65. Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trend Plant Sci. 2010; 15: 573–581. 10.1016/j.tplants.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 66. Gonzalez A, Mendenhall J, Huo Y, Lloyd A. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev Biol. 2009; 325: 412–421. 10.1016/j.ydbio.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 67. Zhong R, Lee C, Zhou J, McCarthy RL, Ye ZH. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008; 20: 2763–2782. 10.1105/tpc.108.061325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou J, Lee C, Zhong R, Ye ZH. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009; 21: 248–266. 10.1105/tpc.108.063321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Feng C, Andreasson E, Maslak A, Mock HP, Mattsson O, Mundy J. Arabidopsis MYB68 in development and responses to environmental cues. Plant Sci. 2004; 167: 1099–1107. 10.1016/j.plantsci.2004.06.014 [DOI] [Google Scholar]
- 70. Morse AM, Whetten RW, Dubos C, Campbell MM. Post-translational modification of an R2R3-MYB transcription factor by a MAP kinase during xylem development. New phytol. 2009; 183: 1001–1013. 10.1111/j.1469-8137.2009.02900.x [DOI] [PubMed] [Google Scholar]
- 71. Elleman CJ, Dickinson HG. Commonalities between pollen/stigma and host/pathogen interactions: calcium accumulation during stigmatic penetration by Brassica oleracea pollen tubes. Sex Plant Reprod. 1999; 12: 194–202. 10.1007/s004970050192 [DOI] [Google Scholar]
- 72. Schuler MA, Werck-Reichhart D. Functional genomics of P450s. Annu Rev Plant Biol. 2003; 54: 629–667. 10.1146/annurev.arplant.54.031902.134840 [DOI] [PubMed] [Google Scholar]
- 73. Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, et al. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993; 12: 1–51. 10.1089/dna.1993.12.1 [DOI] [PubMed] [Google Scholar]
- 74. Chapple C. Molecular-genetic analysis of plant cytochrome P450-dependent monooxygenases. Annu Rev Plant Biol. 1998; 49: 311–343. 10.1146/annurev.arplant.49.1.311 [DOI] [PubMed] [Google Scholar]
- 75. Nelson DR, Ming R, Alam M, Schuler MA. Comparison of cytochrome P450 genes from six plant genomes. Trop Plant Biol. 2008; 1: 216–235. 10.1007/s12042-008-9022-1 [DOI] [Google Scholar]
- 76. Nelson DR. The cytochrome P450 homepage. Human Genom. 2009; 4: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee TS. Reverse conservation analysis reveals the specificity determining residues of cytochrome P450 family 2 (CYP 2). Evol Bioinform. 2008; 4: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang H, Tompkins LM. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab. 2008; 9: 598–610. 10.2174/138920008785821710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.






