Abstract
Local and systemic inflammation often present in chronic obstructive pulmonary disease (COPD). Adipokines are secretory protein mediators by adipose tissue, which have been found to involve in inflammatory responses in many chronic inflammatory diseases. Therefore, we performed this preliminary clinical study to investigate the possible association between 2 adipokines, C1q/tumor necrosis factor-related protein-3 and -5 (CTRP-3 and CTRP-5), with lung function and other markers of inflammation in COPD. Serum CTRP-3 and CTRP-5 levels were measured in 73 COPD patients and 54 health controls, together with lung function and levels of adiponectin, CRP, TNF-α, and MPO in both groups. Pearson's partial correlation was used to analyze the correlations between CTRPs and other serum markers or lung function. Serum CTRP-5 was significantly elevated in COPD patients (0.41 ± 0.35 versus 0.29 ± 0.28 μg/ml, P = 0.01) and correlated inversely with FEV1/FVC ratio in all patients (r = −0.31, P = 0.001). In COPD patients, CTRP-5 was also correlated negatively with FEV1% predicted (r = −0.464, P < 0.001) and had a positive association with CRP levels (r = 0.262, P = 0.04). However, serum CTRP-3 levels were not correlated with measures of lung function or systemic inflammation. In conclusion, circulating CTRP-5 was associated with the severity of airflow obstruction and systemic inflammation in patients with COPD, which suggests that it may be used as a potential novel inflammatory biomarker in COPD. Further studies should be performed to clarify the exact role of CTRP-5 on the pathogenesis and outcomes of COPD.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), characterized as persistent airflow limitation with numerous extrapulmonary complications and comorbidities, remains 1 of the leading causes of morbidity and mortality worldwide.1 Apart from local inflammatory responses in the lungs, patients with COPD often show increased systemic inflammation, which is associated with the pathogenesis of comorbidities present in COPD,2–4 especially for those whose disease is severe or who are experiencing exacerbations. Whether systemic inflammation is the “spill-over” of local inflammation in the lungs or is attributable to some comorbid conditions that affect the lungs remains controversial.3,5 It has been suggested that smoking, tissue hypoxia, lung hyperinflation, and skeletal muscle dysfunction could be possible factors that participate in the pathogenesis of systemic inflammation in COPD.6 Recently, adipose tissue has also been reported to interfere with systemic inflammation in COPD patients. In addition to functioning as the energy storage site, the adipose tissue is an active producer of mediators involved in inflammation, and the so-called adipokines are examples.7
Adipokines are protein mediators secreted by the adipose tissue and they are now known to involve in inflammatory responses in many chronic inflammatory diseases.8,9 Adiponectin is a well-known adipokine that has antidiabetic and anti-inflammatory effects. An increasing number of evidence has demonstrated a significant role of adiponectin in the inflammatory processes in COPD.10 Lately, members of the C1q/tumor necrosis factor-related protein (CTRP) family have been reported to share structural homology with adiponectin, but are expressed in a wide variety of tissues and appear to have diverse functions.11 CTRP-3 is expressed and secreted by adipocytes and monocytes and circulates in human plasma.12,13 Recent data indicate that CTRP-3 is a potent anti-inflammatory adipokine that inhibits proinflammatory pathways induced by lipopolysaccharides (LPS), toll-like receptor (TLR) ligands, and fatty acids in monocytes and adipocytes.14,15 Meanwhile, circulating CTRP-3 showed significant negative correlations with metabolic risk factors, indicating the promising roles in inflammation and metabolism.16 CTRP-5, another CTRP family member, is expressed in adipocytes, myocytes, ciliary epithelium, and retinal pigment.17–21 Similar to adiponectin, CTRP-5 enhances glucose uptake by GLUT4 through inducing phosphorylation of AMP-activated protein kinase.21 Furthermore, CTRP-5 stimulates fatty acid oxidation in rat myocytes by the phosphorylation of acetyl-CoA carboxylase.21
Despite of the findings above, nothing is known whether COPD is also associated with elevated CTRP-3 or CTRP-5 levels. Therefore, a preliminary clinical study was performed to examine whether a possible relationship exist between these adipokines and other markers of inflammation, lung function in COPD patients.
MATERIAL AND METHODS
Subjects and Research Design
The study protocol conforms to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board for Human Studies of West China Hospital of Sichuan University, China. Written consent was obtained from all subjects. COPD patients were recruited from the Outpatient Department of West China Hospital from September 2014 to January 2015, whereas healthy controls, irrespective of smoking habits, were recruited from the hospital's physical examination center. Lung function tests were performed in all control subjects using standardized methods according to the American Thoracic Society guidelines.22 COPD diagnosis was based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy paper,23 and inclusion criteria were: (a) postbronchodilator FEV1/FVC < 70%; (b) reversibility of FVC or FEV1 induced by β-agonist (200 mg salbutamol) < 12% or 200 ml; (c) no exacerbation within the last 12 weeks before recruitment. No COPD patients had received standard COPD treatments, such as inhaled corticosteroids. Patients were excluded if they had a diagnosis or history of asthma and conditions known to affect serum levels of adipokines, including coronary heart disease and metabolic syndrome.
Blood Sampling and Analysis
Subjects were asked to fast overnight from 21:00 the night before, after which venous blood samples were collected and serum was separated immediately and stored at −80 °C until analysis. Levels of CTRP-3 and CTRP-5 were analyzed using an enzyme-linked immunosorbent assay (ELISA; AdipoGen, Incheon, Korea). Adiponectin, CRP, TNF-α, and MPO were analyzed using Human Magnetic Luminex Screening Assay (LXSAHM; R&D Systems China Co., Ltd Shanghai, China) on the Bio-Plex 200 detection platform (Bio-Rad, CA) by the Department of Laboratory Medicine of West China Hospital. All the measurements were carried out strictly according to the manufacturer's instructions. The detection sensitivities were 1 ng/ml for CTRP-3 and CTRP-5, 148 pg/ml for adiponectin, 116 pg/ml for CRP, 26.2 pg/ml for MPO, and 1.2 pg/ml for TNF-α. Technicians performing tests were blinded to the clinical details of the subjects.
Statistical Analysis
All statistical analyses were performed by SPSS 18.0 for Windows (IBM, Chicago, IL). Variables with skewed distributions such as adiponectin, CTRP-3, CTRP-5, CRP, TNF-α, and MPO were log transformed before further analysis. Comparisons of characteristics between groups were performed by unpaired Student's t or Chi-square tests when appropriate. Pearson's partial correlation adjusting for age, BMI, pack-years smoked, and current smoking status was used to analyze the correlations between CTRPs and other serum markers or lung function. All continuous data were presented as mean ± standard deviation (SD), whereas categorical data were presented as frequency and percent. A 2-sided P value <0.05 was considered statistically significant.
RESULTS
Subjects Characteristics
A total of 73 COPD patients and 54 healthy controls were enrolled in this study. All the demographic data and baseline characteristics of the study subjects are summarized in Table 1. There were no differences with respect to the age, sex, or BMI between the COPD patients and the controls. However, as expected, there were significant reductions in FEV1 % predicted and FEV1/FVC ratio in COPD patients compared with healthy controls. Meanwhile, serum levels of adiponectin, CTRP-5, and CRP were significantly elevated in COPD patients compared with healthy controls.
TABLE 1.
Subject Characteristics
Correlations Between CTRPs and Lung Function Parameters
Serum CTRP-5 levels in all patients were correlated inversely with the ratio of FEV1/FVC (r = −0.31, P = 0.001), whereas CTRP-3 showed no such relationship with FEV1/FVC ratio (r = −0.135, P = 0.15). In COPD patients, CTRP-5 levels correlated negatively with FEV1% predicted (r = −0.464, P < 0.001; Figure 1, Table 2). However, no significant correlation was seen between CTRP-3 levels and FEV1% predicted (r = −0.217, P = 0.09; Table 2).
FIGURE 1.
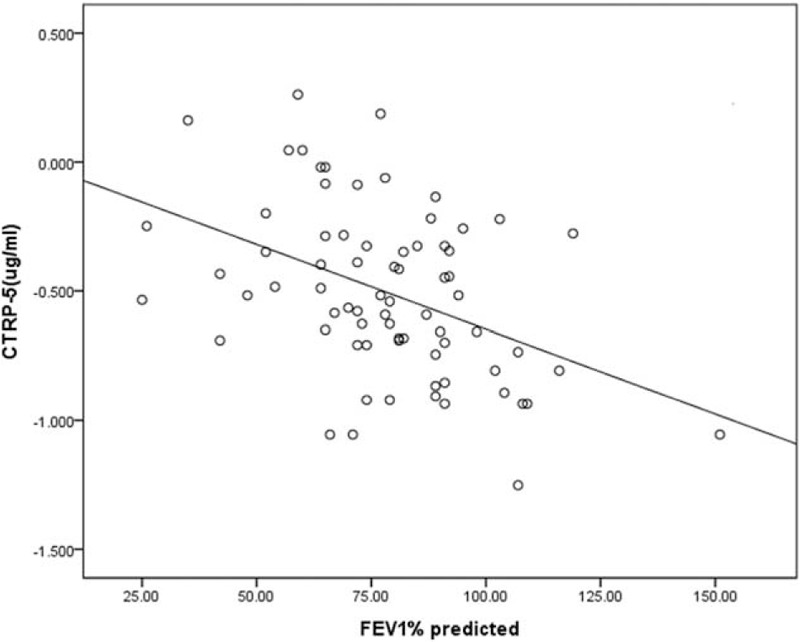
Correlation between serum CTRP-5 levels and lung function (FEV1% predicted) in COPD patients. COPD = chronic obstructive pulmonary disease, CTRP = C1q/tumor necrosis factor related protein, FEV1 = forced expiratory volume in 1 s.
TABLE 2.
Correlations Between CTRPs (CTRP-3 and CTRP-5) and Other Parameters in COPD Patients (n = 73)
Correlations Between CTRPs and Other Serum Parameters
Correlations between CTRPs and other serum parameters in patients with COPD are shown in Table 2. A positive correlation was seen between serum CRTP-5 and CRP (r = 0.262, P = 0.04; Figure 2), whereas CTRP-3 did not correlate with inflammatory mediators CRP, TNF-α, or MPO in COPD patients. In addition, neither CTRP-5 nor CTRP-3 statistically related to adiponectin levels.
FIGURE 2.
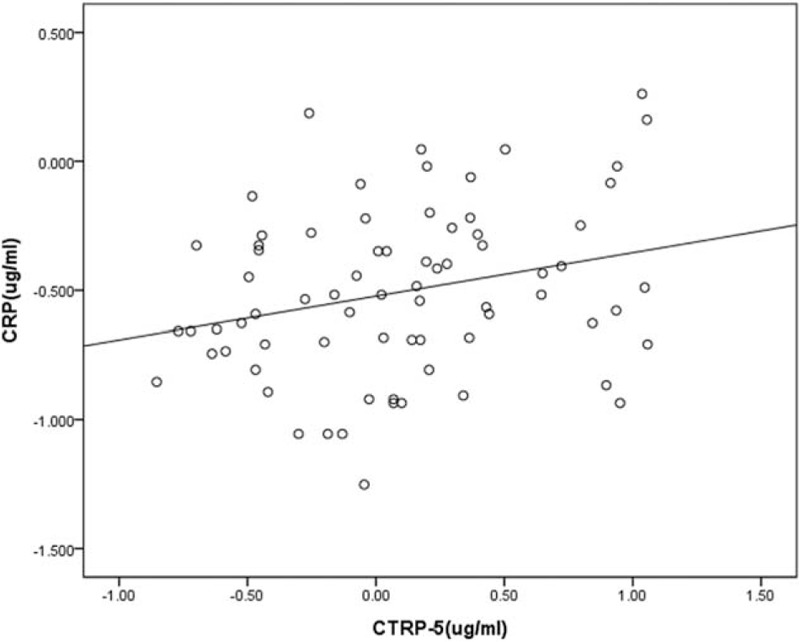
Correlation between serum levels of CTRP-5 and CRP in COPD patients. COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, CTRP = C1q/tumor necrosis factor related protein.
DISCUSSION
The main findings of the present study demonstrate that levels of circulating CTRP-5, but not CTRP-3, have a significant association with lung function decline (measured as FEV1% predicted) in COPD patients and further, correlated positively with systemic inflammation (CRP), which suggests that CTRP-5 may have a role in the inflammatory processes in COPD. To the best of our knowledge, this is the first study to examine correlations between serum CTRPs and indicators of lung function and inflammatory status in COPD.
Adipokines derived from adipocytes/adipose tissue were found to be associated with the chronic low-grade inflammation present in obesity-related metabolic disturbances and inflammatory diseases.24 In recent years, findings from some studies have also linked adipokines to inflammatory lung diseases such as COPD and asthma.25 Particularly, numerous investigations have focused on the role of adiponectin and leptin in these diseases and suggested that low-serum adiponectin and high-serum leptin concentrations predict asthma independent of obesity, whereas high adiponectin and low leptin levels are associated with stable COPD.10,25 However, the results on the associations between adipokines and these lung diseases at the current stage are confusing and frankly paradoxical in places. Besides, novel adipokines such as CTRP-3 and CTRP-5, which are known to be related with inflammatory process, are not covered in previous studies.
In the present study, we observed that serum CTRP-5 levels were negatively associated with FEV1/FVC and FEV1 % predicted in COPD patients, suggesting that CTRP-5 may correlate with the severity of airflow obstruction. It is thought that there are many causes contributing to the airway obstruction in COPD such as contraction of the airway smooth muscle, mucosal edema, small airway fibrosis, and loss of parenchymal support to small airways caused by emphysema.26 The exact role of CTRP-5 in airflow obstruction could not be elucidated, but it would be consistent with numerous lines of indirect evidence linking CTRP-5 with this disease. First, Park SY et al previously reported that depletion of mtDNA resulting in mitochondrial dysfunction leads to increased expression and secretion of CTRP-5 in myocytes.21 As we know, COPD is a chronic lung disease characterized not only by progressive, irreversible airflow obstruction but also by skeletal muscle dysfunction and abnormality of inflammatory responses in lungs and circulation.27,28 Analyses of muscle biopsies showed significant lower mitochondrial oxidative metabolism muscle oxidative phenotype (oxphen) in patients with COPD, whereas in cultured muscle cells, mitochondrial protein content and mitochondrial respiration were reduced on chronic TNF-α stimulation in a nuclear factor kappaB (NF-κB)-dependent manner, which resulted in decreased muscle oxphen.29 Therefore, inflammatory response in COPD may contribute to the increase of CTRP-5 in a pathway related to mitochondrial dysfunction. On the other hand, treatment of myocytes with globular domain of CTRP-5 (gCTRP-5) resulted in increased phosphorylation of AMP-activated protein kinase (AMPK), leading to reduction of ROS accumulation and caspase-3 activation,30 which prevented myocytes from apoptosis. As AMPK activation could effectively decrease airway smooth muscle (ASM) cells proliferation,31 increased levels of circulating CTRP-5 might decrease the contractility of airway smooth muscle and be involved in a negative feedback regulation of airflow obstruction, which is evident in COPD.
Meanwhile, we found that CTRP-5 concentrations in serum correlated with the levels of CRP. CRP is an acute phase protein synthesized predominantly by the hepatocytes in response to tissue damage or inflammation.32 Circulating CRP levels are elevated in COPD patients and are related to the presence of airflow obstruction;33–34 thus it has been regarded as a valid biomarker of systemic inflammation in COPD.35 In our study, elevated serum levels of CRP in COPD patients were observed and associated negatively with FEV1% predicted, which is consistent with previous studies.35–36 Therefore, the positive correlation between CTRP-5 and CRP in COPD patients suggests that CTRP-5 may function as a biomarker of the severity of systematic inflammation in COPD. Interestingly, although serum adiponectin levels were significantly higher in COPD compared to controls, which is in accord with results reported by Carolan37 and Tomoda,38 we have not found any correlation between CRTP-5 and adiponectin in COPD patients, implying that CTRP-5 might correlate with the severity of airflow obstruction and systemic inflammation in COPD independent of adiponectin levels.
In contrast to CTRP-5, we did not find significant associations of serum CTRP-3 with lung function or inflammatory parameters. The underlying mechanisms could not be clarified in the present study, but we could suggest some hypotheses to explain the results. First, the expression sites and biological functions of CTRP family members are diverse. Although both CTRP-3 and CTRP-5 participate in regulation of energy homeostasis and inflammation, they may exert the effects via different pathways. The linkage between lung function decline and CTRP-5 levels in COPD may involve mitochondrial dysfunction of myocytes, in which CTRP-5, but not CTRP-3, has been shown to play an important role.21 Second, the crosstalk between CTRP-3 and CTRP-5 is still unclear. It has been observed by Schmid et al that cellular knockdown of CTRP-3 gene during differentiation upregulates CTRP-5 expression, which leads to a speculation that a counter-regulatory relation may exist between CTRP-3 and CTRP-5 under basal conditions.39 However, under the COPD condition, the balance might be disrupted, where the significant increase of CTRP-5 possibly inhibits the elevation of CTRP-3 levels. Of course, detailed molecular experiments are needed to clarify this question.
There are several limitations in this study. First, the relatively small number of subjects limited our further investigation on how CTRP-5 correlates with lung function and inflammation in different stages of COPD. Since most of our patients presented with relatively mild COPD, it would be necessary to verify our findings in large cohorts of patients with more balanced distribution of disease severity. Second, as it was a cross-sectional study, no causality between parameters investigated could be defined, which implies that more prospective or in vitro studies are needed to elucidate the potential mechanisms under the findings presented in our study. Third, only 3 indicators of systemic inflammation (CRP, TNF-α, and MPO) were analyzed, so the relationship between CTRP-5 and inflammatory responses in COPD needs further investigation. In addition, future studies should also use computed tomography to detect the presence of emphysema, since our results may be biased by the different phenotypes of COPD (“blue bloater” versus “pink puffer”).40
In conclusion, this study demonstrated that circulating levels of CTRP-5 had a significant negative relationship with lung function and correlated positively with CRP concentration. The present results introduce adipokine CTRP-5 as a potential novel inflammatory biomarker in COPD. However, further prospectively-designed and experimental studies should be performed to clarify the influences of CTRP-5 and other novel adipokines on the pathogenesis and outcomes of COPD.
Footnotes
Abbreviations: BMI = body mass index, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, CTRP = C1q/tumor necrosis factor related protein, ELISA = enzyme-linked immunosorbent assay, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, MPO = myeloperoxidase, TNF-α = tumor necrosis factor-α.
Funding: This work was supported by grants from the National Natural Science Foundation of China (Grants 31171103, 81230001, and 81470236 to Fuqiang Wen, 81300032 to Yongchun Shen), grants 2014BAI08B04 and 2012BAI05B02 from National Key Technology Research and Development Program of the Ministry of Science and Technology of China, and National Key Technology Research and Development Program of the Ministry of Science and Technology of China during of the “12th Five-Year Plan” and Projects in the Science and Technology Pillar Program from the Department of science and technology of Sichuan province (2015SZ0151).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365. [DOI] [PubMed] [Google Scholar]
- 2.Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest 2011; 139:165–173. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33:1165–1185. [DOI] [PubMed] [Google Scholar]
- 4.Wouters EF, Reynaert NL, Dentener MA, et al. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease is there a connection? Proc Am Thorac Soc 2009; 6:638–647. [DOI] [PubMed] [Google Scholar]
- 5.Fabbri LM, Luppi F, Beghé B, et al. Complex chronic comorbidities of COPD. Eur Respir J 2008; 311:204–212. [DOI] [PubMed] [Google Scholar]
- 6.Agustí A. Systemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should). Proc Am Thorac Soc 2007; 4:522–525. [DOI] [PubMed] [Google Scholar]
- 7.Bianco A, Mazzarella G, Turchiarelli V, et al. Adiponectin: an attractive marker for metabolic disorders in Chronic Obstructive Pulmonary Disease (COPD). Nutrients 2013; 5:4115–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005; 115:911–919. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation andmetabolic disease. Nat Rev Immunol 2011; 11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali Assad N, Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie 2012; 94:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schäffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab 2012; 23:194–204. [DOI] [PubMed] [Google Scholar]
- 12.Weigert J, Neumeier M, Schäffler A, et al. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett 2005; 579:5565–5570. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann C, Chen N, Obermeier F, et al. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 2011; 17:2462–2471. [DOI] [PubMed] [Google Scholar]
- 14.Compton SA, Cheatham B. CTRP-3: blocking a toll booth to obesity-related inflammation. Endocrinology 2010; 151:5095–5097. [DOI] [PubMed] [Google Scholar]
- 15.Kopp A, Bala M, Buechler C, et al. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010; 151:5267–5278. [DOI] [PubMed] [Google Scholar]
- 16.Yoo HJ, Hwang SY, Hong HC, et al. Implication of progranulin and C1q/TNF-related protein-3 (CTRP-3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS ONE 2013; 8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, et al. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissueselective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 2008; 416:161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia 2005; 48:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayyagari R, Mandal MN, Karoukis AJ, et al. Late-onset macular degeneration and long anterior lens zonules result from a CTRP-5 gene mutation. Invest Invest Ophthalmol Vis Sci 2005; 46:3363–3371. [DOI] [PubMed] [Google Scholar]
- 20.Mandal MN, Vasireddy V, Reddy GB, et al. CTRP-5 is a membrane-associated and secretory protein in the RPE and ciliary body and the S163R mutation of CTRP-5 impairs its secretion. Invest Ophthalmol Vis Sci 2006; 47:5505–5513. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Choi JH, Ryu HS, et al. C1q tumor necrosis factor alpha-related protein isoform 5 is increased in mitochondrial DNA-depleted myocytes and activates AMP-activated protein kinase. J Biol Chem 2009; 284:27780–27789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner RM. Standardization of spirometry: A summary of recommendations from the American Thoracic Society. The 1987 update. Ann Intern Med 1988; 108:217–220. [DOI] [PubMed] [Google Scholar]
- 23.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187:347–365. [DOI] [PubMed] [Google Scholar]
- 24.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6:772–783. [DOI] [PubMed] [Google Scholar]
- 25.Sood A. Obesity, adipokines, and lung disease. J Appl Physiol 2010; 108:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003; 22:672–688. [DOI] [PubMed] [Google Scholar]
- 27.Sin DD, Man SF. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol 2007; 85:141–147. [DOI] [PubMed] [Google Scholar]
- 28.Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remels AH, Gosker HR, Schrauwen P, et al. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J 2010; 24:5052–5062. [DOI] [PubMed] [Google Scholar]
- 30.Yang WM, Lee W. CTRP-5 ameliorates palmitate-induced apoptosis and insulin resistance through activation of AMPK and fatty acid oxidation. Biochem Biophys Res Commun 2014; 452:715–721. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Cao M, Liu Y, et al. PDGF-induced airway smooth muscle proliferation is associated with Human antigen R activation and could be weakened by AMPK activation. Mol Biol Rep 2012; 39:5819–5829. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal R, Zaheer MS, Ahmad Z, et al. The relationship between C-reactive protein and prognostic factors in chronic obstructive pulmonary disease. Multidiscip Respir Med 2013; 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higashimoto Y, Iwata T, Okada M, et al. Serum biomarkers as predictors of lung function decline in chronic obstructive pulmonary disease. Respir Med 2009; 103:1231–1238. [DOI] [PubMed] [Google Scholar]
- 34.Pinto-Plata VM, Mullerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006; 61:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karadag F, Kirdar S, Karul AB, et al. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med 2008; 19:104–108. [DOI] [PubMed] [Google Scholar]
- 36.Dal Negro RW, Visconti M, Micheletto C, et al. Changes in blood ROS, e-NO, and some pro-inflammatory mediators in bronchial secretions following erdosteine or placebo: a controlled study in current smokers with mild COPD. Pulm Pharmacol Ther 2008; 21:304–308. [DOI] [PubMed] [Google Scholar]
- 37.Carolan BJ, Kim YI, Williams AA, et al. The association of adiponectin with computed tomography phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomoda K, Yoshikawa M, Itoh T, et al. Elevated circulating plasma adiponectin in underweight patients with COPD. Chest 2007; 132:135–140. [DOI] [PubMed] [Google Scholar]
- 39.Schmid A, Kopp A, Aslanidis C, et al. Regulation and function of C1Q/TNF-related protein-5 (CTRP-5) in the context of adipocyte biology. Exp Clin Endocrinol Diabetes 2013; 121:310–317. [DOI] [PubMed] [Google Scholar]
- 40.Tulek B, Kivrak AS, Ozbek S, et al. Phenotyping of chronic obstructive pulmonary disease using the modified Bhalla scoring system for high-resolution computed tomography. Can Respir J 2013; 20:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


