Abstract
Neuroinflammation is emerging as an important pathway involved in Parkinson's disease (PD) pathogenesis. Herein, we investigated the effect of 4 top PD-associated genetic variants in Caucasians listed on the top risk loci identified by meta-analysis of genome wide-association studies in PDGene database (http://www.pdgene.org/top_results), including serine threonine kinase 39 (STK39) rs1955337, bone marrow stromal cell antigen 1 (BST1) rs11724635, major histocompatibility complex, class II, DQ beta 1 (HLA-DQB1) rs9275326, and signal peptide peptidase-like 2B (SPPL2B) rs62120679, by genotyping 596 Han-Chinese patients with PD and 597 age-matched control subjects. Compared with subjects with STK39 rs1955337 GG genotype, those with TT genotype had a 1.64-fold increased risk of PD (95% confidence interval: 1.13–2.39, P = 0.010). The recessive model also demonstrated an increased PD risk in TT genotype (odds ratio: 1.59, 95% confidence interval: 1.12–2.27) compared with the other genotypes (GT + GG). PD patients demonstrate a similar genotypic and allelic frequency in BST1 rs11724635, HLA-DQB1 rs9275326, and SPPL2B rs62120679 compared with controls. These findings suggested that the STK39 rs1955337 TT genotype is a risk factor for Han-Chinese patients with PD in Taiwan. The ethnic discrepancies of the other 3 genetic variants may indicate a distinct genetic background of neuroinflammation between PD patients in Han-Chinese and Caucasians.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
INTRODUCTION
Parkinson's disease (PD) is one of the most common neurodegenerative disorders, involving progressive loss of the nigro-striatal dopaminergic neurons. In addition, the pathogenesis of PD is associated with the presence of eosinophilic cytoplasmic inclusion bodies (Lewy bodies) with enrichment of α-synuclein in the ventral midbrain.1 Alpha-synuclein (SNCA), parkin (PARK2), PTEN-induced putative kinase 1 (PINK1), DJ-1, leucine-rich repeat kinase 2 (LRRK2) ATPase type 13A2 (ATP13A2), vacuolar protein sorting-associated protein 35 (VPS35), eukaryotic translation initiation factor 4 gamma, 1 (EIF4G1), synaptojanin-1 (SYNJ1), dnaJ (Hsp40) homolog, subfamily C, member 6 (DNAJC6), and dnaJ (Hsp40) homolog, subfamily C, member 13 (DNAJC13) have been identified to be the causative genes for familiar and early-onset PD (EOPD).2 Owing to the discovery of these causative genes, several pathogenic pathways were identified, which includes accumulation of aberrant or misfolded proteins, mitochondrial dysfunction, increased oxidative stress, impaired ubiquitin-proteasome function, failure of autophagy-lysosome and mitophagy, and deficits in synaptic exocytosis and endocytosis and endosomal trafficking.2 Genome-wide association studies (GWAS) also have identified novel genetic associations with PD and these genes are linked to the previously known pathogenic pathways and other less recognized pathways such as neurotransmission, vascular pathology, transcriptional dysregulation, inflammation, as well as immune dysfunction.3
Neuroinflammation and immune dysfunction is emerging as an important pathway involved in PD pathogenesis.4–6 A pathway-based analysis of the data from 2 independent GWAS has shown that genes involved in the “regulation of leucocyte/lymphocyte activity” and “cytokine-mediated signaling” confer an increased susceptibility to PD.7 Interestingly, epidemiological studies have also shown decreased risk of PD in people who take nonaspirin, nonsteroidal anti-inflammatory drugs.8 Several genetic variants involved in inflammatory pathways, such as serine threonine kinase 39 (STK39) rs1955337, BST1 rs11724635, major histocompatibility complex, class II, DQ beta 1 (HLA-DQB1) rs9275326, and signal peptide peptidase-like 2B (SPPL2B) rs62120679 were listed on the top risk loci by meta-analysis of GWAS in Caucasian.9,10 Although a number of genetic studies have shown the association between genetic variants involved in the inflammatory pathways and PD in Caucasian,11 these genetic associations in Han-Chinese are limitedly revealed. To provide more evidence for inflammatory gene loci contributing to PD across different populations, we conducted a case-control study by examining the genotypic and allelic frequencies of STK39 rs1955337, BST1 rs11724635, HLA-DQB1 rs9275326, and SPPL2B rs62120679 in 596 Taiwanese PD patients and 597 control subjects.
SUBJECTS AND METHODS
Ethics Statement
This study was performed under a protocol approved by the institutional review boards of Chang Gung Memorial Hospital (ethical license No: 102-5614A3) and all examinations were performed after obtaining written informed consents.
Patient Population
Patients with PD were enrolled from the neurological clinics of Chang Gung Memorial Hospital-Linkou Medical Center. Two neurologists specialized in movement disorders (Y-RW and C-MC) confirmed the diagnosis of PD according to the UK PD Society Brain Bank clinical diagnostic criteria.12 We also recruited age, sex, and ethnic-matched unrelated healthy individuals as controls.
Genetic Analysis
Four genetic loci (STK39 rs1955337, BST1 rs11724635, HLA-DQB1 rs9275326, and SPPL2B rs62120679) involved in inflammatory pathways were selected from the top 20 risk loci identified by meta-analysis in PDGene database (http://www.pdgene.org/top_results). The single-nucleotide polymorphism (SNP) genotyping was performed by Agena MassARRAY platform with iPLEX gold chemistry SNP, San Diego, CA). By following the manufacture guide, the specific PCR primer and extension primer sequences (Table 1) were designed with Assay Designer software package (v.4.0). One microliter of Genomic DNA sample (10 ng/μL) was applied to mutiplex PCR reaction in 5 μL containing 1 unit of Taq polymerase, 500 nmol of each PCR primer mix, and 2.5 mM of each dNTP (Agena, PCR accessory and enzyme kit). Thermocycling was at 94°C for 4 min followed by 45 cycles of 94°C for 20 s, 56°C for 30 s, and 72°C for 1 min, than 72°C for 3 min. Unincorporated dNTPs were deactivated using 0.3 U of shrimp alkaline phosphatase. The single base extension reaction was using iPLEX enzyme, terminator mix, and extension primer mix followed by 94°C for 30 s followed by 40 cycles of 94°C for 5 s, and 5 inner cycle of 56°C for 5 s and 80°C for 5 s, than 72°C for 3 min Agena, iPLEX gold kit). After the addition of a cation exchange resin to remove residual salt from the reactions, 7 nL of the purified primer extension reaction was loaded onto a matrix pad of a SpectroCHIP (Agena). SpectroCHIPs were analyzed using a MassARRAY Analyzer 4, and the calling by clustering analysis with TYPER 4.0 software.
TABLE 1.
Sequences of Primers Used in This Study
Statistical Analysis
The genotypes of all variants in the PD patients and the controls did not deviate from the Hardy Weinberg equilibrium. We used χ2 test to compare allele and genotypic frequencies between the PD patients and the controls. To account for the multiple comparisons, the 2-tailed P values <0.0125 were judged to be statistically significant using Bonferroni correction. For the recessive model, we had a power greater than 0.8 to detect association when the odds ratio was greater than 1.5.
RESULTS
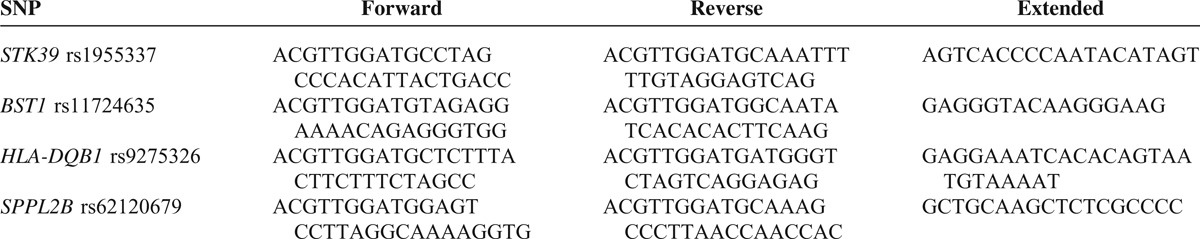
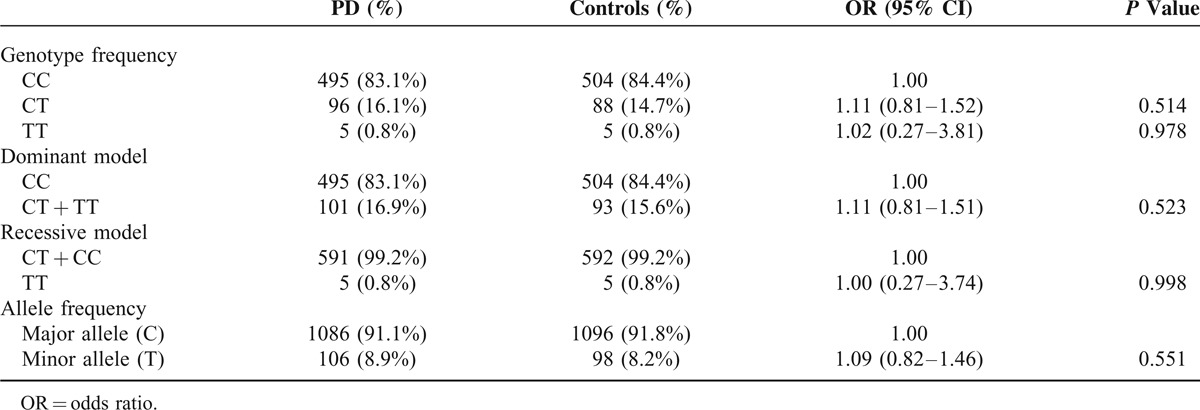
A total of 1193 subjects, including 596 PD patients (female/male: 272/324) with the current age of 68.68 ± 10.82 years, and 597 normal controls (female/male: 315/282), were recruited. Only 1 proband with familial PD in the same family was included. The mean age at onset of PD symptoms was 62.8 ± 11.1 years (range 19–93). The mean age of recruitment of controls was 60.0 ± 12.7 years (range 17–89). STK39 rs1955337 TT genotype showed a higher prevalence in PD than GG genotype did (odds ratio [OR] = 1.64, 95% CI: 1.13–2.39, P = 0.010, Table 2). This finding was also present in the recessive model with statistical significance (OR = 1.59, 95% CI: 1.12–2.27, P = 0.010). The T allele showed a greater frequency in PD than the C allele did on the borderline of statistical significance (OR = 1.21, 95% CI: 1.02–1.43, P = 0.030). The frequencies of BST1 rs11724635, HLA-DQB1 rs9275326, and SPPL2B rs62120679 genotypes and alleles were similar in both PD patients and controls (Tables 3–5).
TABLE 2.
Genotype and Allele Frequency of Polymorphism STK39 rs1955337 Among Parkinson's Disease (PD) and Controls in Taiwan
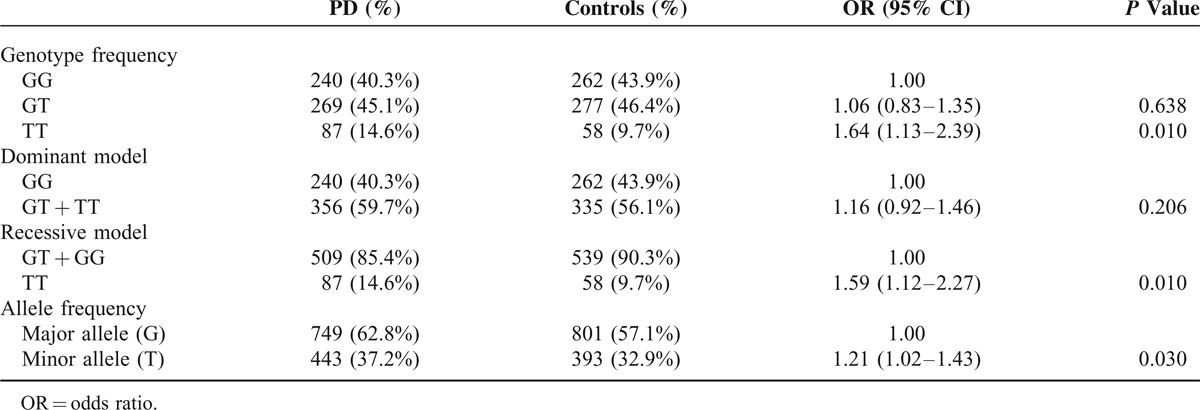
TABLE 3.
Genotype and Allele Frequency of Polymorphism BST1 rs11724635 Among Parkinson's Disease (PD) and Controls in Taiwan
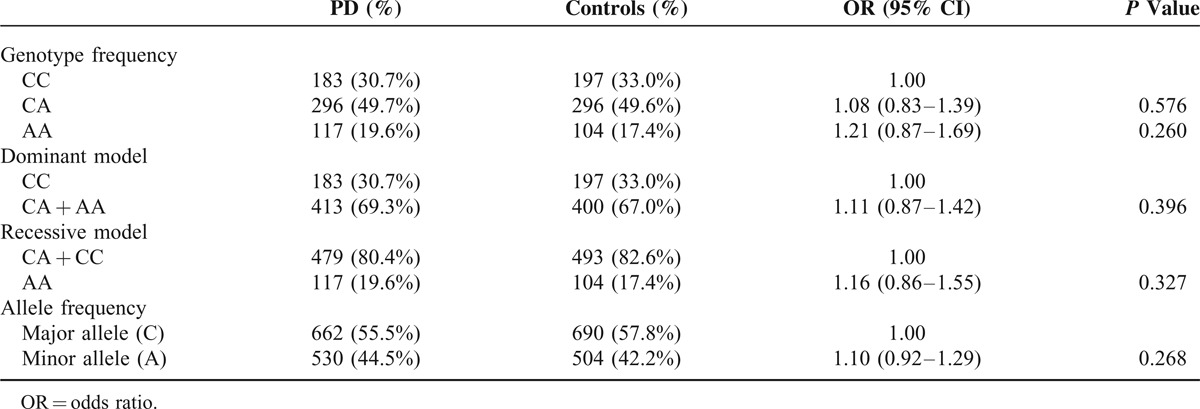
TABLE 5.
Genotype and Allele Frequency of Polymorphism SPPL2B rs62120679 Among Parkinson's Disease (PD) and Controls in Taiwan
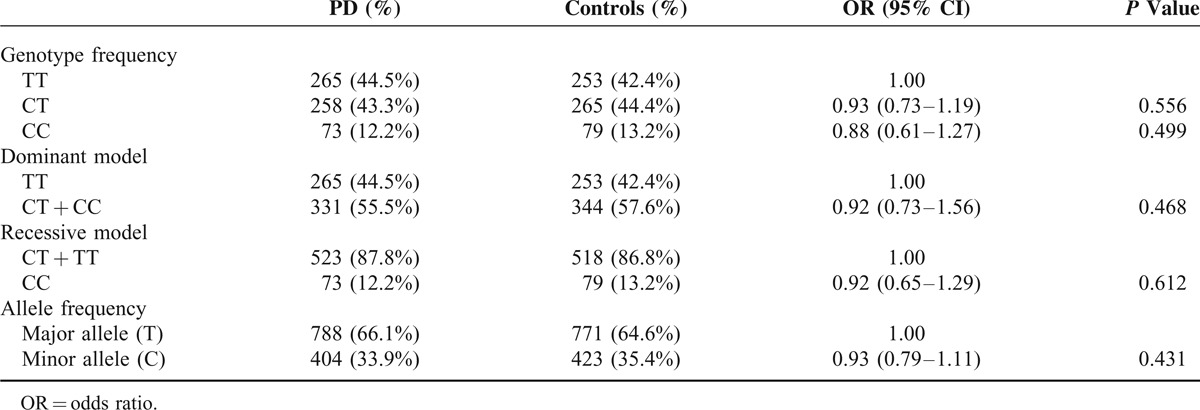
TABLE 4.
Genotype and Allele Frequency of Polymorphism HLA-DQB1 rs9275326 Among Parkinson's Disease (PD) and Controls in Taiwan
DISCUSSION
The present study shows that the STK39 rs1955337 affects risk of PD in Taiwanese population, while BST1 rs11724635, HLA-DQB1 rs9275326, and SPPL2B rs62120679 demonstrate absent association with PD. It is worthy to note that the minor allele frequency of STK39 rs1955337 in controls of this study (32.9%) is compatible with that (31.7%) in Chinese + Japanese dataset from 1000 Genome but very different from that (8.3%) in Northern Europeans from Utah dataset from 1000 Genome (http://www.1000genomes.org/home). Most of these results are not consistent with those of Caucasian cohorts, suggesting the differential effect of neuroinflammatory gene loci on PD risk between Eastern and Western populations.
The STK39 encodes a serine/threonine kinase (STE20/SPS1-related proline–alanine-rich protein kinase) with roles in stress and inflammatory pathways.13,14 The PD-associated loci in STK39, such as rs1955337, rs2102808, and rs3754775, have been reported in the studies from Caucasian and Ashkenazi Jewish populations.9,15,16 However, the associations in Han-Chinese have not been consistently replicated. Li et al demonstrated a negative result for the association between STK39 rs2102808 and PD susceptibility in Han-Chinese.17 Again, a study by Wang et al found no significant difference in allele and genotype frequencies of STK39 rs2102808 and rs3754775 between PD patients and control group in Han-Chinese population.18 Our study, for the first time, demonstrates the association of rs1955337 with PD susceptibility in Han-Chinese, which is consistent with the finding in Caucasian populations. Recently, by next-generation sequencing, a new risk loci 352G >T in STK39 was found to be associated with sporadic PD patients in China.19 These associations of STK39 variants with PD may suggest a role of inflammation and stress in PD pathogenesis, whereas the functional consequence of the STK39 variants should be further investigated.
BST1 encodes a stromal cell line-derived glycosylphosphatidylinositol-anchored glycoprotein that facilitates pre-B-cell growth.20BST1 rs11724635 is associated with PD risk in Caucasian populations.21 A study from China reported that the minor allele A conferred a weak signal of developing PD.22 However, this association was not recapitulated in Japanese and Han-Chinese in Taiwan.23,24 Belonging to the HLA class II beta chain paralogs, HLA-DQB1 plays a central role in the immune system by presenting peptides derived from extracellular proteins.25 The association of HLA-DQB1 rs9275326 T allele with PD risk reduction has been recently shown by Nall's meta-analysis of GWAS in Caucasians.9 Our study, for the first time, tested this association in Han-Chinese and the result was negative. SPPL2B encodes a GXGD family of aspartic proteases, which triggers cytokine expression by cleaving transmembrane domain of tumor necrosis factor α.26SPPL2B rs62120679 C allele has been identified as a significant risk variant for PD in Caucasians.9 Here, we found this association may not be present in Han-Chinese. All of these results demonstrate the potential ethnic divergence of these genetic variants, suggesting a distinct genetic background of neuroinflammation between Han-Chinese and Caucasian PD patients.
Our study provides important genetic information about PD patients in Han-Chinese and the ethnic-specific effect of the genes associated with PD. However, these genetic analyses do not clarify the epigenetic effect and gene–gene interaction, as well as the association of other SNPs within the tested genes and PD. The potential impact of environmental factors on development of PD in individuals carrying these genetic variants still needs to be clarified. For example, Chen et al found BST1 rs11724635 interacted with well water drinking to increase the risk of PD.23 Nevertheless, our finding indicates the potential of STK39 rs1955337 affects the risk in developing PD and suggests ethnic discrepancies in the other 3 PD-associated genetic variants in Han-Chinese. More large series of case-control studies of genotyping STK39 variants in the PD patients of other Asian populations, and functional approaches to study their potential neuroinflammatory mechanisms for PD are warranted.
Acknowledgment
This study was sponsored by Chang Gung Memorial Hospital, Taipei, Taiwan (CMRPG3D005, CMRPG3E142, CMRPG391183), and National Science Council, Executive Yuan, Taiwan (102-2314-B-182A-087-MY3, NSC102-2314-B-182A-113 -MY2).
Footnotes
Abbreviations: ATP13A2 = ATPase type 13A2, BST1 = bone marrow stromal cell antigen 1, DNAJC13 = dnaJ (Hsp40) homolog, subfamily C, member 13, DNAJC6 = dnaJ (Hsp40) homolog, subfamily C, member 6, EIF4G1 = eukaryotic translation initiation factor 4 gamma, 1, EOPD = early-onset Parkinson's disease, GWAS = genome-wide association studies, HLA-DQB1 = major histocompatibility complex, class II, DQ beta 1, LRRK2 = leucine-rich repeat kinase 2, PARK2 = parkin, PD = Parkinson's disease, PINK1 = PTEN-induced putative kinase 1, SNCA = alpha-synuclein, SNP = single-nucleotide polymorphism, SPP2B = signal peptide peptidase-like 2B, STK39 = serine threonine kinase 39, SYNJ1 = synaptojanin-1, VPS35 = vacuolar protein sorting-associated protein 35.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Halbach OvBu, Schober A, Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson's disease. Prog Neurobiol 2004; 73:151–177. [DOI] [PubMed] [Google Scholar]
- 2.Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol 2013; 9:445–454. [DOI] [PubMed] [Google Scholar]
- 3.Ramanan VK, Saykin AJ. Pathways to neurodegeneration: mechanistic insights from GWAS in Alzheimer's disease, Parkinson's disease, and related disorders. Am J Neurodegener Dis 2013; 2:145–175. [PMC free article] [PubMed] [Google Scholar]
- 4.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson's disease. Biomed Res Int 2014; 2014:308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J, Fu Q, Cheng L, et al. Inflammatory response in Parkinson's disease (review). Mol Med Rep 2014; 10:2223–2233. [DOI] [PubMed] [Google Scholar]
- 6.Vivekanantham S, Shah S, Dewji R, et al. Neuroinflammation in Parkinson's disease: role in neurodegeneration and tissue repair. Int J Neurosci 2015; 125:717–725. [DOI] [PubMed] [Google Scholar]
- 7.Holmans P, Moskvina V, Jones L, et al. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson's disease. Hum Mol Genet 2013; 22:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol 2005; 58:963–967. [DOI] [PubMed] [Google Scholar]
- 9.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet 2014; 46:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lill CM, Roehr JT, McQueen MB, et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson's disease genetics: the PDGene database. PLoS Genet 2012; 8:e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Navarro H, Jimenez-Jimenez FJ, Garcia-Martin E, et al. Genomic and pharmacogenomic biomarkers of Parkinson's disease. Curr Drug Metab 2014; 15:129–181. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston AM, Naselli G, Gonez LJ, et al. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene 2000; 19:4290–4297. [DOI] [PubMed] [Google Scholar]
- 14.Yan Y, Laroui H, Ingersoll SA, et al. Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice. J Immunol 2011; 187:1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Cheng R, Verbitsky M, et al. Genome-wide association study identifies candidate genes for Parkinson's disease in an Ashkenazi Jewish population. BMC Med Genet 2011; 12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nalls MA, Plagnol V, et al. International Parkinson Disease Genomics C. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet 2011; 377:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li NN, Tan EK, Chang XL, et al. Genetic association study between STK39 and CCDC62/HIP1R and Parkinson's disease. PLoS One 2013; 8:e79211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YQ, Tang BS, Yu RL, et al. Association analysis of STK39, MCCC1/LAMP3 and sporadic PD in the Chinese Han population. Neurosci Lett 2014; 566:206–209. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Lin Q, Huang W, et al. Target gene capture sequencing in chinese population of sporadic Parkinson disease. Medicine 2015; 94:e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaisho T, Ishikawa J, Oritani K, et al. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc Natl Acad Sci U S A 1994; 91:5325–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma M, Ioannidis JP, Aasly JO, et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology 2012; 79:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Xiao Q, Wang Y, et al. Analysis of genome-wide association study-linked loci in Parkinson's disease of Mainland China. Mov Disord 2013; 28:1892–1895. [DOI] [PubMed] [Google Scholar]
- 23.Chen ML, Lin CH, Lee MJ, et al. BST1 rs11724635 interacts with environmental factors to increase the risk of Parkinson's disease in a Taiwanese population. Parkinsonism Relat Disord 2014; 20:280–283. [DOI] [PubMed] [Google Scholar]
- 24.Miyake Y, Tanaka K, Fukushima W, et al. Lack of association between BST1 polymorphisms and sporadic Parkinson's disease in a Japanese population. J Neurol Sci 2012; 323:162–166. [DOI] [PubMed] [Google Scholar]
- 25.Veijola R, Reijonen H, Vahasalo P, et al. HLA-DQB1-defined genetic susceptibility, beta cell autoimmunity, and metabolic characteristics in familial and nonfamilial insulin-dependent diabetes mellitus. Childhood Diabetes in Finland (DiMe) Study Group. J Clin Invest 1996; 98:2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedmann E, Hauben E, Maylandt K, et al. SPPL2a and SPPL2b promote intramembrane proteolysis of TNF( in activated dendritic cells to trigger IL-12 production. Nat Cell Biol 2006; 8:843–848. [DOI] [PubMed] [Google Scholar]


