Abstract
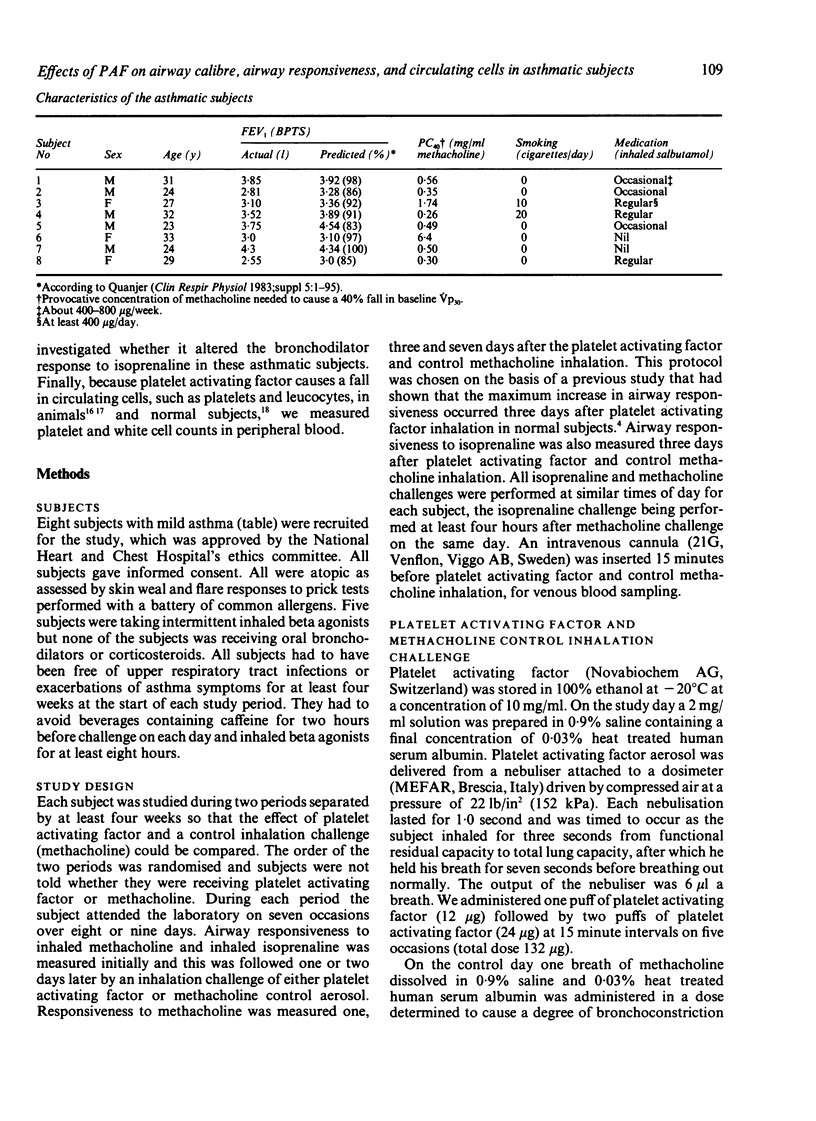
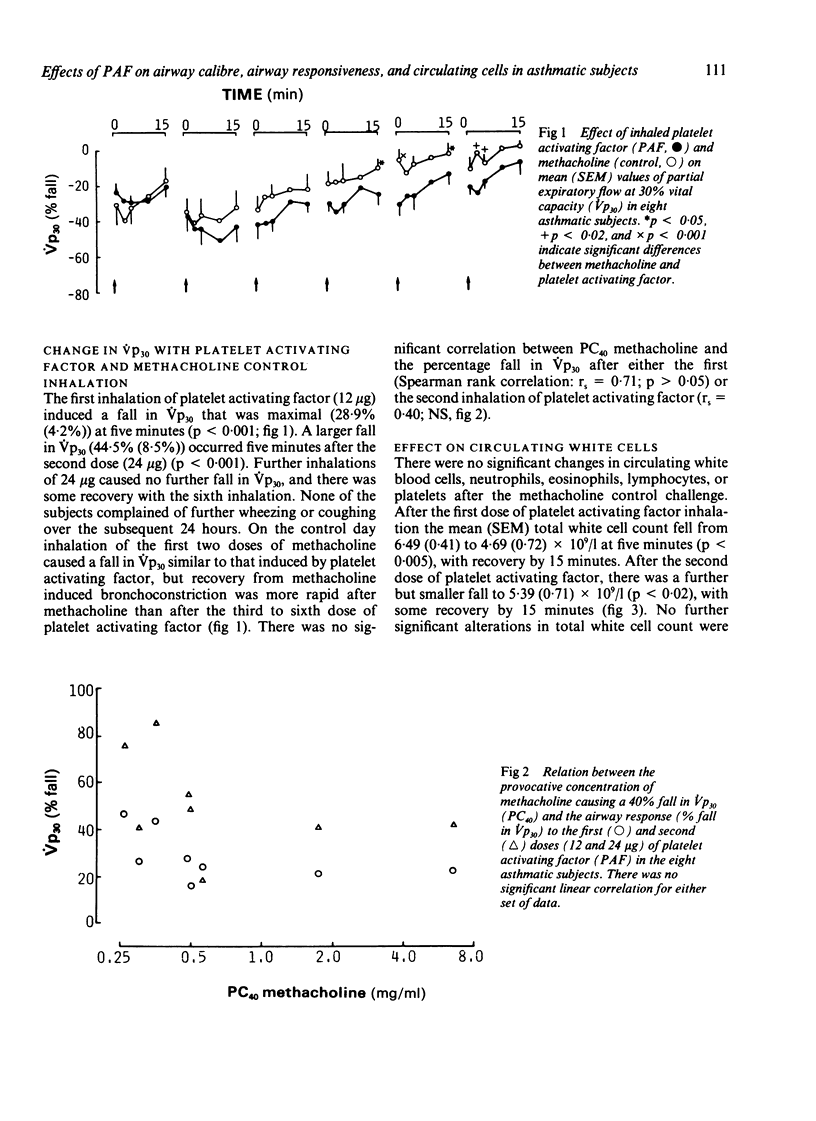
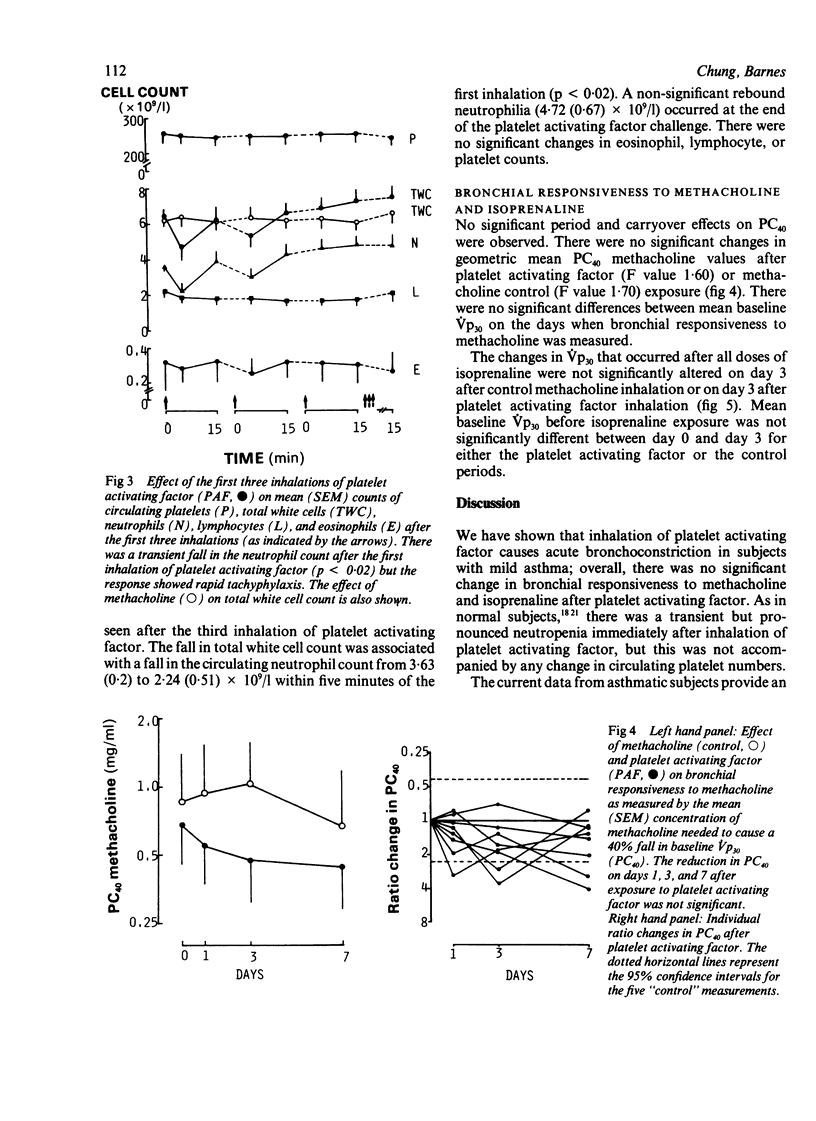
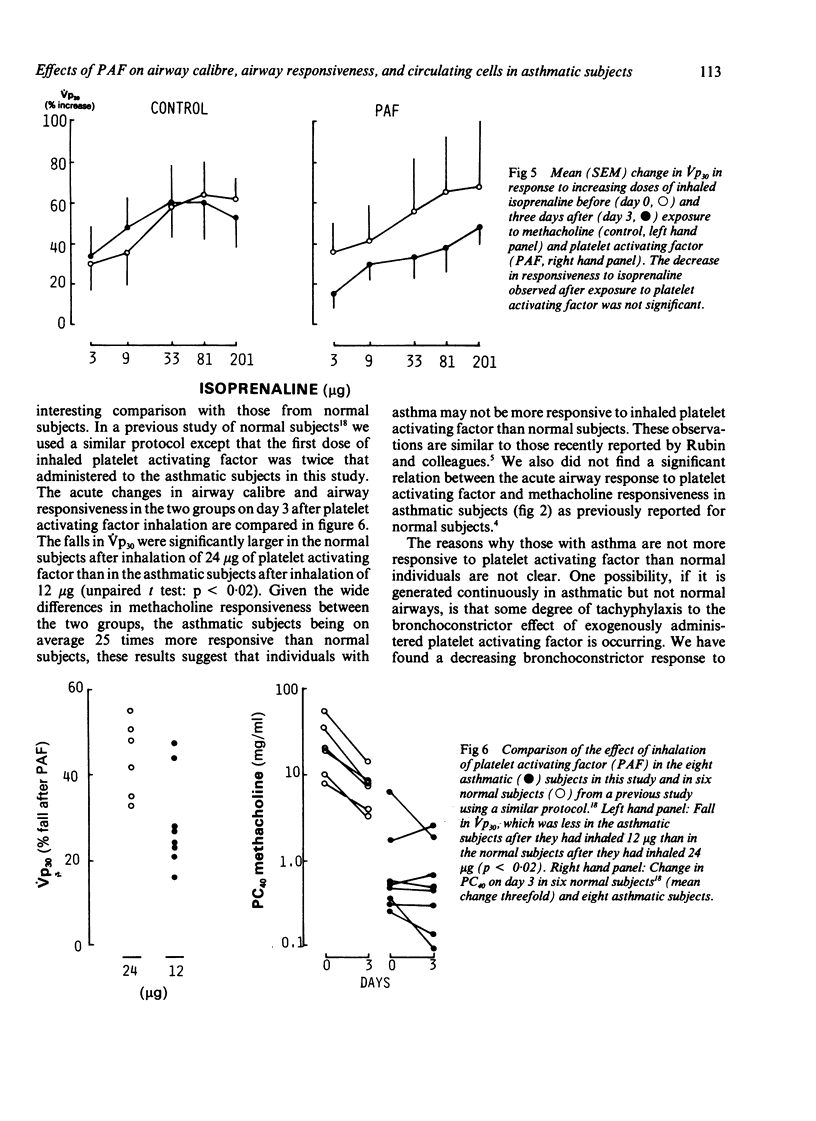
The effects of inhaled platelet activating factor were compared with those of inhaled methacholine (control) on airway calibre, airway responsiveness to methacholine and isoprenaline, and circulating cells in eight subjects with mild, stable asthma. Platelet activating factor was given in six doses at 15 minute intervals and airway response measured as change in partial expiratory flow at 30% of vital capacity (Vp30). Platelet activating factor caused a fall in Vp30, the mean (SEM) maximum percentage fall being 28.9 (4.2) five minutes after the first dose (12 micrograms) and 50.9 (8.0) after the second dose (24 micrograms). Tachyphylaxis occurred, however, with the four subsequent doses of inhaled platelet activating factor. There was transient neutropenia after the first dose, from a mean of 3.6 (0.2) x 10(9) to 2.2 (0.5) x 10(9) neutrophils/l; this response also showed tachyphylaxis with subsequent doses. The mean PC40 (the concentration of methacholine needed to cause a 40% fall in Vp30) was unchanged one, three, and seven days after administration of platelet activating factor. There was no significant correlation between baseline PC40 methacholine and the maximal fall in Vp30 after either the first (12 micrograms) or the second dose (24 micrograms) of platelet activating factor. The control challenge with methacholine produced a degree of bronchoconstriction similar to that of platelet activating factor but was not associated with any significant change in bronchial responsiveness or in circulating cells. The bronchodilator response to inhaled isoprenaline measured three days after inhalation of platelet activating factor and of methacholine was similar after the two challenges. Thus asthmatic subjects who are hyperresponsive to methacholine show a similar bronchoconstrictor response to platelet activating factor, as has been observed in normal subjects; overall this did not cause airway hyperresponsiveness to methacholine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal D. K., Townley R. G. Effect of platelet-activating factor on beta-adrenoceptors in human lung. Biochem Biophys Res Commun. 1987 Feb 27;143(1):1–6. doi: 10.1016/0006-291x(87)90620-6. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Chung K. F., Page C. P. Platelet-activating factor as a mediator of allergic disease. J Allergy Clin Immunol. 1988 May;81(5 Pt 1):919–934. doi: 10.1016/0091-6749(88)90952-9. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Grandordy B. M., Page C. P., Rhoden K. J., Robertson D. N. The effect of platelet activating factor on pulmonary beta-adrenoceptors. Br J Pharmacol. 1987 Apr;90(4):709–715. doi: 10.1111/j.1476-5381.1987.tb11224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman B. W., Lefferts P. L., Snapper J. R. Effect of platelet-activating factor on aerosol histamine responsiveness in awake sheep. Am Rev Respir Dis. 1987 Jun;135(6):1267–1270. doi: 10.1164/arrd.1987.135.6.1267. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Aizawa H., Leikauf G. D., Ueki I. F., Evans T. W., Nadel J. A. Airway hyperresponsiveness induced by platelet-activating factor: role of thromboxane generation. J Pharmacol Exp Ther. 1986 Mar;236(3):580–584. [PubMed] [Google Scholar]
- Chung K. F., Dent G., Barnes P. J. Effects of salbutamol on bronchoconstriction, bronchial hyperresponsiveness, and leucocyte responses induced by platelet activating factor in man. Thorax. 1989 Feb;44(2):102–107. doi: 10.1136/thx.44.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. F., Minette P., McCusker M., Barnes P. J. Ketotifen inhibits the cutaneous but not the airway responses to platelet-activating factor in man. J Allergy Clin Immunol. 1988 Jun;81(6):1192–1198. doi: 10.1016/0091-6749(88)90890-1. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G. The enhancement of inflammatory injury. Am Rev Respir Dis. 1987 Jul;136(1):1–2. doi: 10.1164/ajrccm/136.1.1. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., Rossi M. Neutrophils in normal lungs. Am Rev Respir Dis. 1983 Feb;127(2):S3–S9. doi: 10.1164/arrd.1983.127.2P2.S3. [DOI] [PubMed] [Google Scholar]
- Cuss F. M., Dixon C. M., Barnes P. J. Effects of inhaled platelet activating factor on pulmonary function and bronchial responsiveness in man. Lancet. 1986 Jul 26;2(8500):189–192. doi: 10.1016/s0140-6736(86)92489-x. [DOI] [PubMed] [Google Scholar]
- DUNNILL M. S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960 Jan;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquand S., Touvay C., Randon J., Lagente V., Vilain B., Maridonneau-Parini I., Etienne A., Lefort J., Braquet P., Vargaftig B. B. Interference of BN 52021 (ginkgolide B) with the bronchopulmonary effects of PAF-acether in the guinea-pig. Eur J Pharmacol. 1986 Aug 7;127(1-2):83–95. doi: 10.1016/0014-2999(86)90208-6. [DOI] [PubMed] [Google Scholar]
- Evans T. W., Chung K. F., Rogers D. F., Barnes P. J. Effect of platelet-activating factor on airway vascular permeability: possible mechanisms. J Appl Physiol (1985) 1987 Aug;63(2):479–484. doi: 10.1152/jappl.1987.63.2.479. [DOI] [PubMed] [Google Scholar]
- Frigas E., Gleich G. J. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986 Apr;77(4):527–537. doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- Halonen M., Palmer J. D., Lohman I. C., McManus L. M., Pinckard R. N. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of IgE anaphylaxis in the rabbit. Am Rev Respir Dis. 1980 Dec;122(6):915–924. doi: 10.1164/arrd.1980.122.6.915. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Shoemaker S. A., Canham E. M., Patel M., McMurtry I. F., Morris H. G., Repine J. E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A2. J Clin Invest. 1983 Feb;71(2):351–357. doi: 10.1172/JCI110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henocq E., Vargaftig B. B. Accumulation of eosinophils in response to intracutaneous PAF-acether and allergens in man. Lancet. 1986 Jun 14;1(8494):1378–1379. doi: 10.1016/s0140-6736(86)91683-1. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Lagente V., Lefort J., Randon J., Russo-Marie F., Vargaftig B. B. Desensitization to PAF-induced bronchoconstriction and to activation of alveolar macrophages by repeated inhalations of PAF in the guinea pig. Biochem Biophys Res Commun. 1985 Aug 30;131(1):42–49. doi: 10.1016/0006-291x(85)91767-x. [DOI] [PubMed] [Google Scholar]
- Robertson D. N., Page C. P. Effect of platelet agonists on airway reactivity and intrathoracic platelet accumulation. Br J Pharmacol. 1987 Sep;92(1):105–111. doi: 10.1111/j.1476-5381.1987.tb11301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. J., Moqbel R., Cromwell O., Kay A. B. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986 Dec;78(6):1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]


