Abstract
Background
In older patients with chronic diseases, focusing on subjective, patient-relevant outcomes, such as health-related quality of life (HRQoL), is more pertinent than pursuing clinical or laboratory target values.
Aim
To investigate factors influencing the course of HRQoL in older (aged ≥78 years) primary care patients and to derive non-pharmacological recommendations for improving their quality of life.
Design and setting
A population-based prospective longitudinal observational study featuring data analysis from waves 2 to 5 of the AgeCoDe study, which was conducted in six cities in Germany.
Method
The HRQoL of 1968 patients over the course of 4.5 years was observed. Patients were, on average, aged 82.6 (±3.4) years and their HRQoL was measured using the EQ-5D visual analogue scale in a face-to-face assessment. Fixed-effects regression models were calculated to examine impact of change in potential influencing factors. This method allows unobserved heterogeneity to be controlled.
Results
The course of the participants’ HRQoL declined with increasing age, walking and incident hearing impairment. Increasing the number of physical activities improved the HRQoL. These findings were modified by sex, education level, and depression. Especially in females and patients with rather low education levels, increased physical activity improved the subjects’ HRQoL, while hearing impairment decreased it. Moving to an institution only improved the HRQoL in patients without depression or those with a low level of education (primary education).
Conclusion
Motivating patients to increase their weekly physical activity and to focus on preserving their ability to walk are promising approaches to improving HRQoL in older age. Less-educated patients and those without depression can also benefit from moving into an institution (for example, a care or retirement home).
Keywords: aged ≥80 years, institutionalisation, mobility limitation, nursing homes, primary health care, quality of life, walking
INTRODUCTION
The management of chronic conditions in older patients benefits from focusing on factors influencing subjective, patient-relevant outcomes like health-related quality of life (HRQoL).1 In GP practices in particular, older patients may be demanding when seeking further treatment options to prevent their perceived health statuses from declining, which could lead to overtreatment and potentially harmful polypharmacy without significant benefits. As a result, non-pharmacological strategies are needed to improve or maintain patients’ HRQoL, as well as knowledge about patient-modifiable factors that influence it.
Most studies that investigated HRQoL were designed as cross-sectional studies, such as those by Bowling and colleagues,2 Borglin and colleagues,3 and Layte and colleagues.4 Longitudinal studies are needed to investigate factors influencing HRQoL. Available longitudinal studies are based only on small numbers,5–11 have short follow-up periods of 6–18 months,5,6,8,9,11,12 and/or did not investigate the development of factors influencing HRQoL, but included the factors only at one fixed point in time.13,14 As a result, there are insufficient data to derive practical considerations for GPs on which changes in influencing factors might support patients in maintaining or improving their HRQoL. In light of this, factors affecting the course of patients’ HRQoL over 4.5 years were investigated in a representative cohort of 1968 older (aged ≥78 years) primary care patients.
This study was based on ascertaining the answers to the following questions:
which factors, usually known to GPs, influence HRQoL in older patients?
which patients are at high risk of a decline in their HRQoL?
which non-pharmacological approaches could GPs recommend to their patients to maintain or improve their HRQoL?
How this fits in
To the authors’ knowledge, there are no studies that have investigated the impact of change in factors influencing the course of quality of life in older age. This study showed that the ability to walk has a great impact on maintaining older primary care patients’ health-related quality of life. For certain groups of patients, physical activity, institutionalisation (in a care home or residential home), and hearing aids are further promising approaches. Depending on the patient’s risk profile, clinicians can encourage their patients to make use of appropriate strategies.
METHOD
In this prospective longitudinal observational study, the course of patients’ HRQoL was investigated, based on the data from the population-based cohort of the German Study on Ageing, Cognition, and Dementia in Primary Care Patients (AgeCoDe), established in six German cities (Bonn, Düsseldorf, Hamburg, Leipzig, Mannheim, and Munich).15 The aim was for each study centre to recruit 20 primary care practices with 25 patients. In each practice 50 randomly selected patients from those eligible were invited to participate in the study.
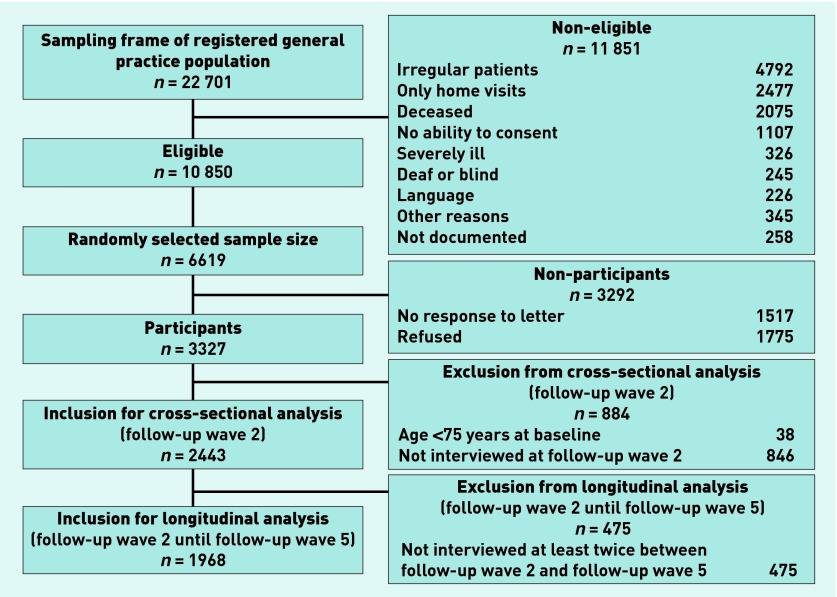
In total, 3327 patients were assessed in baseline assessments in 2003–2004 (Figure 1). The patients were reassessed every 18 months in the follow-up waves 1 to 5. Analyses in this article refer to data from waves 2 to 5 (data assessment 2006–2012) because HRQoL was only assessed from follow-up wave 2 onwards.
Figure 1.
Patient sampling framework.
Patients were recruited by their GPs based on the following inclusion criteria:
≥75 years of age;
absence of dementia; and
at least one GP contact within the previous 12 months.
Exclusion criteria were as follows:
GP consultations by home visit only;
residency in a nursing home;
severe illness deemed fatal within 3 months by the GP;
insufficient proficiency in the German language;
deafness;
blindness;
inability to consent; and
not being a regular patient of the participating practice.
Written, informed consent was obtained from all patients.
In each wave, trained interviewers (physicians or psychologists) assessed patients face-to-face in their homes using standardised assessment instruments, while each patient’s GP filled in a questionnaire regarding their patients’ comorbidities.
Assessment procedures
A variety of tools and strategies were used to classify patients’ characteristics, HRQoL status, and outcomes. These were as follows:
HRQoL: EQ-5D visual analogue scale (EQ-5D VAS),16 a valid scale ranging from 0 to 100 (0 = worst imaginable health state, 100 = best imaginable health state) on which patients were asked to rate their actual, subjective health status;
education level: CASMIN criteria17 (low = primary education [primary school level]; medium = secondary education [up to university-entrance diploma]; high = tertiary education [undergraduate or postgraduate studies];
marital status: married or widowed: re-determined at each assessment;
institutionalisation: defined as living in a nursing home or retirement home (retirement communities were not included);
cognition: Mini-Mental State Examination (MMSE);18
subjective memory impairment: patients were asked the question ‘Do you feel like your memory is becoming worse? (yes/no)’;
instrumental activities of daily living (IADLs): IADL Scale, for example, preparing meals or using the telephone (0 = worst score; 8 = best score);19
depression — Geriatric Depression Scale,20 a 15-item scale scored 0–15, on which a score of ≥5 constitutes having depression;
mobility: patients were asked about their mobility, for example, ‘Do you have trouble walking? (no troubles, aggravated walking, substantial mobility impairment/disability of walking).’
vision and hearing problems: no, mild, or severe/profound impairment;
activity: patients were given a list of physical and cognitive activities (for example, bicycle riding, reading) and asked how often they performed the activities weekly; and
comorbidity: score calculated by adding the number of comorbidities from the GP questionnaire and incorporating the severity level (graded 1 to 4; 1 = light; 2 = medium; 3 = severe; 4 = very severe) of each.
Data analyses
Statistical analyses were performed using Stata (version 13.1). To investigate associations and predictive factors regarding HRQoL, the following were included:
potentially modifiable information known by the GP (for example, physical activity levels); and
unchangeable information, which might help the GP identify patients at risk of decline in HRQoL.
As depression and HRQoL are interrelated constructs,10 depression was controlled for in additional models.
Cross-sectional associations with HRQoL were investigated via linear regression models. The meaningfulness of the cross-sectional analyses was limited as it was not possible to control for unobserved heterogeneity (for example, personality or genetic predisposition) or derive the direction of the influence. Thus, fixed-effects regressions, justified by the Sargan–Hansen test, were applied. In those, the change in predictors as potential influencing factors was considered: each measurement from wave 2 to wave 5 was included in the model.
Data from all patients with personal assessments in follow-up wave 2 were analysed in the cross-sectional analysis. All patients who were personally assessed at least twice in any of the follow-up waves from 2 to 5 were included in the longitudinal analyses. A P-value of P≤0.05 was considered significant.
RESULTS
Sample
Figure 1 displays the sampling framework and the numbers of patients included. Patients were, on average, 82.6 (±3.4) years of age. The sample characteristics at follow-up wave 2 are detailed in Table 1.
Table 1.
Sample characteristics of patients in follow-up wave 2 (n = 2443)
| Characteristic | Mean (SD) | % |
|---|---|---|
| Age, years | 82.6 (3.4) | |
|
| ||
| HRQoL | 65.4 (17.9) | |
|
| ||
| Mini-Mental State Examination | 27.4 (2.9) | |
|
| ||
| Geriatric Depression Scale | 2.5 (2.5) | |
|
| ||
| Sex | ||
| Female | 66.0 | |
| Male | 34.0 | |
|
| ||
| Education levela | ||
| Low | 60.9 | |
| Medium | 27.6 | |
| High | 11.5 | |
|
| ||
| Living in an institution | ||
| Yes | 3.4 | |
| No | 96.6 | |
|
| ||
| Marital status | ||
| Married | 38.4 | |
| Widowed | 50.3 | |
| Other | 11.3 | |
|
| ||
| Subjective memory impairment | ||
| Yes | 60.4 | |
| No | 38.9 | |
| Not specified | 0.7 | |
As measured using CASMIN criteria.15
HRQoL = health-related quality of life. SD = standard deviation.
Cross-sectional analyses
Results of the cross-sectional linear regression (Model 1, Table 2) show that a higher HRQoL was associated with more physical and cognitive activity, and the male sex. Lower HRQoL was associated with an impaired ability to walk, impaired vision, higher comorbidity, and perceived subjective memory impairment. The model explains 25.4% (R2 = 0.254) of the variance. As opposed to subjective memory impairment, objective memory impairment (as measured by the MMSE [β = 0.124; P = 0.54]) did not have a significant association with HRQoL when both variables were exchanged (data not shown).
Table 2.
Model 1: Linear regression model with outcome HRQoL at follow-up wave 2 (cross-sectional analyses, n = 2103)
| Factor | β coefficient | Cluster robust 95% CI |
|---|---|---|
| Instrumental activities of daily living | 0.310 | −0.323 to 0.943 |
| Subjective memory impairment (reference: no subjective memory impairment) | −4.184a | −5.545 to −2.823 |
| Age | 0.0253 | −0.189 to 0.239 |
| Living in an institution (reference: not living in an institution) | 3.102 | −1.427 to 7.630 |
| Widowed (reference: married) | 1.538d | −0.0357 to 3.112 |
| Physical activities (weighted score) | 1.855a | 0.989 to 2.721 |
| Cognitive activities (weighted score) | 1.429b | 0.505 to 2.354 |
| Aggravated walking (reference: no impairment) | −10.71a | −12.20 to −9.208 |
| Substantial mobility impairment/disability of walking (reference: no impairment) | −19.14a | −22.39 to −15.90 |
| Mild visual impairment (reference: no impairment) | −2.215c | −4.321 to −0.110 |
| Severe/profound visual impairment (reference: no impairment) | −3.499c | −6.605 to −0.393 |
| Mild hearing loss (reference: no impairment) | −1.154 | −2.598 to 0.291 |
| Severe/profound hearing loss (reference: no impairment) | −1.418 | −5.836 to 3.001 |
| Comorbidity (weighted score) | −0.239a | −0.372 to −0.106 |
| Sex (reference: females) | 3.034b | 0.814 to 5.255 |
| Secondary education (reference: primary education) | 0.206 | −1.348 to 1.760 |
| Tertiary education (reference: primary education) | 0.237 | −2.044 to 2.519 |
| Constant | 65.18a | 46.55 to 83.81 |
P<0.001.
P<0.01.
P<0.05.
P<0.10.Regional dummies were also included (not shown).
In a second model, depression was also controlled for. As the direction of causality between HRQoL and depression (R = −0.427, P<0.001) is unclear, a simultaneity bias is likely; as such, coefficients are inconsistent. If, however, depression is included in ordinary least squares regression, depression lowers HRQoL (β = −1.952, P<0.001) and there is little change in the other independent variables (data not shown).
Longitudinal analyses
Table 3 displays the results of the longitudinal models describing the course of HRQoL between follow-up waves 2 to 5. Model 2 displays the full sample; models 3 and 4 are stratified by sex and education respectively. As opposed to the cross-sectional results in Table 2, Table 3 displays the influence of change in potential predictive factors.
Table 3.
Fixed-effects regression models with outcome course of HRQoL between follow-up waves 2 to 5 (longitudinal analyses)
| Model 2 | Model 3 | Model 4 | Model 5 | ||||
|---|---|---|---|---|---|---|---|
| HRQoL, full sample | HRQoL, females | HRQoL, males | HRQoL, low education level | HRQoL, medium education level | HRQoL, high education level | HRQoL, no depression in any observation | |
| Factor | β coefficient (cluster robust 95% CI) | β coefficient (cluster robust 95% CI) | β coefficient (cluster robust 95% CI) | β coefficient (cluster robust 95% CI) | β coefficient (cluster robust 95% CI) | β coefficient (cluster robust 95% CI) | β coefficient (cluster robust 95% CI) |
| Increasing activities of daily living | 0.179 (−0.217 to 0.576) | −0.0890 (−0.718 to 0.540) | 0.396 (−0.128 to 0.919) | 0.121 (−0.429 to 0.672) | −0.158 (−0.855 to 0.538) | 1.365b (0.462 to 2.269) | 0.379 (−0.230 to 0.987) |
| Incidence of subjective memory impairment | −1.088d (−2.191 to 0.0148) | −1.069 (−2.516 to 0.379) | −0.718 (−2.417 to 0.981) | −0.838 (−2.268 to 0.591) | −2.418c (−4.542 to −0.295) | 1.763 (−1.104 to 4.631) | −0.817 (−2.238 to 0.605) |
| Increasing age | −0.680a (−0.915 to −0.445) | −0.701a (−1.021 to −0.381) | −0.821a (−1.178 to −0.465) | −0.634a (−0.939 to −0.328) | −0.768a (−1.223 to −0.314) | −0.981b (−1.620 to −0.342) | −0.798a (−1.085 to −0.512) |
| New institutionalisation | 4.091d (−0.135 to 8.316) | 4.201 (−0.853 to 9.255) | 2.510 (−4.586 to 9.605) | 5.660c (0.0337 to 11.29) | 3.499 (−2.667 to 9.664) | −5.942 (−26.17 to 14.28) | 10.26b (3.141 to 17.37) |
| Increasing weekly physical activities (weighted score) | 1.476a (0.757 to 2.194) | 2.380a (1.438 to 3.321) | 0.102 (−1.016 to 1.220) | 1.462b (0.523 to 2.402) | 1.742c (0.267 to 3.218) | 1.229 (−0.449 to 2.906) | 1.011c (0.127 to 1.895) |
| Increasing weekly cognitive activities (weighted score) | 0.469 (−0.350 to 1.287) | 0.290 (−0.802 to 1.381) | 0.743 (−0.449 to 1.935) | 0.965d (−0.168 to 2.097) | −0.638 (−2.107 to 0.832) | 0.666 (−1.345 to 2.677) | 0.204 (−0.807 to 1.215) |
|
Change of status Married to widowed |
1.772 (−0.672 to 4.215) |
0.782 (−2.457 to 4.021) |
3.719c (0.0177 to 7.421) |
3.003d (−0.525 to 6.530) |
−0.463 (−4.208 to 3.283) |
3.435 (−3.081 to 9.951) |
1.595 (−1.340 to 4.530) |
| No impairment of walking to aggravated walking | −4.110a (−5.429 to −2.790) | −3.971a (−5.706 to −2.236) | −4.360a (−6.331 to −2.389) | −3.594a (−5.277 to −1.911) | −5.829a (−8.554 to −3.103) | −2.536 (−6.017 to 0.944) | −4.586a (−6.317 to −2.854) |
| No impairment of walking to substantial mobility impairment/disability of walking | −9.876a (−12.75 to −7.006) | −10.39a (−14.11 to −6.667) | −8.614a (−13.02 to −4.214) | −10.00a (−13.77 to −6.231) | −10.57a (−15.83 to −5.317) | −6.288 (−13.75 to 1.293) | −11.27a (−15.86 to −6.688) |
| No visual impairment to mild visual impairment | −1.262 (−2.771 to 0.248) | −1.254 (−3.128 to 0.620) | −1.090 (−3.669 to 1.489) | −0.382 (−2.395 to 1.631) | −1.963 (−4.480 to 0.554) | −3.824 (−9.075 to 1.427) | −1.367 (−3.416 to 0.683) |
| No visual impairment to severe/profound visual impairment | −2.764 (−6.075 to 0.548) | −2.226 (−6.552 to 2.100) | −3.854 (−8.734 to 1.026) | −2.796 (−6.870 to 1.278) | 0.134 (−5.447 to 5.716) | −6.134 (−18.71 to 6.443) | −5.259c (−9.561 to −0.957) |
| No hearing impairment to mild hearing loss | −1.628c (−3.031 to −0.226) | −1.843c (−3.656 to −0.0294) | −1.119 (−3.290 to 1.053) | −2.075c (−3.919 to −0.230) | −1.844 (−4.517 to 0.829) | 1.361 (−2.202 to 4.924) | −1.465 (−3.418 to 0.488) |
| No hearing impairment to severe/profound hearing loss | −2.553 (−5.646 to 0.541) | −4.001 (−9.304 to 1.302) | −0.991 (−4.567 to 2.586) | −2.551 (−6.594 to 1.492) | −3.233 (−10.03 to 3.565) | 1.720 (−5.080 to 8.520) | 1.969 (−2.137 to 6.075) |
| Increasing comorbidity (weighted score) | −0.0138 (−0.148 to 0.120) | −0.0272 (−0.206 to 0.151) | 0.00931 (−0.191 to 0.210) | −0.0597 (−0.221 to 0.102) | 0.0838 (−0.196 to 0.363) | 0.00611 (−0.348 to 0.361) | −0.0520 (−0.223 to 0.119) |
| Constant | 149.4a (116.0 to 182.9) | 180.2a (151.3 to 209.1) | 142.6a (112.6 to 172.5) | 116.4a (90.56 to 142.2) | 191.8a (151.9 to 231.7) | 137.3a (84.99 to 189.7) | 175.5a (150.2 to 200.9) |
| Observations | 6024 | 3826 | 2198 | 3653 | 1674 | 697 | 2879 |
| Individuals, n | 2159 | 1368 | 791 | 1343 | 578 | 238 | 754 |
| R2 | 0.0689 | 0.0784 | 0.0655 | 0.0642 | 0.0981 | 0.114 | 0.0863 |
P<0.001.
P<0.01.
P<0.05.
P<0.10.Regional dummies were also included (not shown).
In Table 3, Model 2 shows that increasing age, walking impairment, and hearing impairment significantly lower HRQoL in the full sample. Only one factor influenced HRQoL positively in a significant way — namely, increasing weekly physical activity (either doing one activity more often, or adding a different activity). Interestingly, increasing comorbidity, new institutionalisation, or newly arising subjective memory impairment had no significant impact on HRQoL in the full sample. The same is true for objective memory decline measured by the MMSE (β = 0.035; P = 0.83) (data not shown).
Model 3 reveals differences in sex: it is the female patients whose HRQoL is significantly positively affected by physical activity and significantly negatively influenced by mild hearing loss — neither factor significantly affects HRQoL in male patients.
Model 4 depicts that HRQoL in patients with lower education levels is significantly negatively affected by walking impairment, mild hearing loss, and subjective memory impairment, and significantly positively influenced by physical activity. The strongest positive influence for patients with a low education level is moving into an institution, a factor for which the subjective EQ-5D VAS rating went up 5.7 points. Patients with a high level of education were only significantly positively affected by an increased number of IADLs.
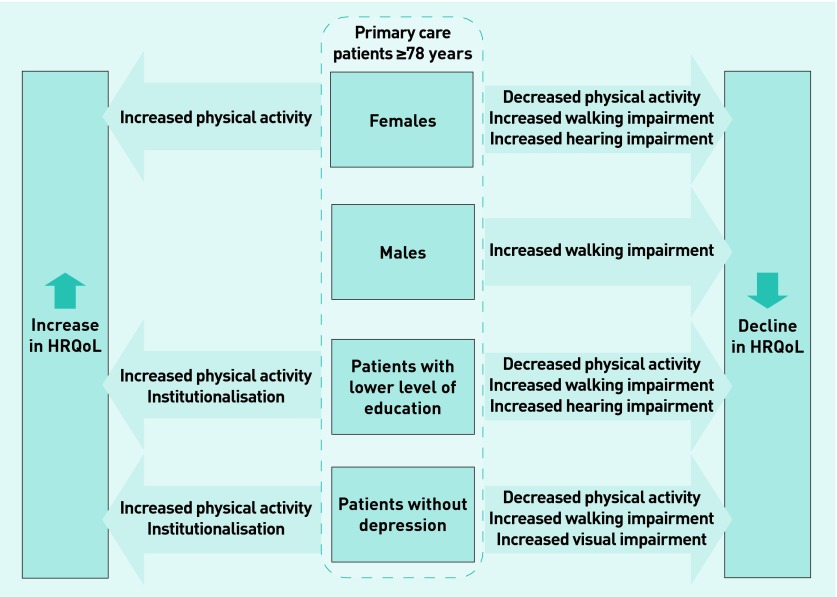
Model 5 displays the longitudinal analyses for individuals who had no depression at any time. As the number of individuals who had depression at each time is too small to be interpreted (n = 42), only the results for patients without depression are shown (Model 5, Table 3). HRQoL in patients without depression rose by 10.3 points on the EQ-5D VAS when they moved to an institution. Additionally, their HRQoL was affected by impaired vision as opposed to impaired hearing, as is the case in the full sample. Figure 2 summarises the factors with significant impact for the different groups.
Figure 2.
Determinants that decrease HRQoL and patient-based approaches that increase HRQoL in subgroups of patients aged ≥78 years. HRQoL = health related quality of life.
DISCUSSION
Summary
Over the course of 4.5 years, patients’ HRQoL significantly declined with increasing age, increasing walking impairment, and incident hearing impairment. A significant positive development was achieved through an increased physical activity score. These findings were modified by sex, education level, and depression; in females and in patients with a low education level in particular, increasing physical activity significantly improved HRQoL, whereas incident hearing impairment significantly decreased it. Moving to an institutional setting (care home or residential home) only strongly improved HRQoL in patients with a low education level and in those without depression.
Strengths and limitations
This is the first study investigating the factors influencing the course of HRQoL in a large representative cohort of 1968 older primary care patients in Germany. Unlike in previous studies, the change in factors that are available through primary care and influence the course of HRQoL were investigated to identify starting points for non-pharmacological interventions. Additionally, HRQoL is a subjective measurement, which might be rated very differently by individual patients due to unobserved time-constant factors, such as optimism. This can influence the independent variables, as well as the dependent variables (omitted variable bias); as such, it is crucial to control for these factors. By using fixed-effects regression analyses it was possible to control for unobserved heterogeneity and receive unbiased estimates (under the assumption of strict exogeneity). In the fixed-effects model, however, variables with little ‘within’ variation should be interpreted with some caution and promising approaches further investigated in randomised controlled trials. Nevertheless, the variation was reasonable in each variable that was investigated.
One limitation of the study is that the results are based on data from follow-up waves 2 to 5, so a selection bias of rather healthy patients — who may have remained in the study longer than less healthy patients — could not be excluded. Finally, due to the overlapping constructs of HRQoL and depression, it is not sensible to include depression in the regression analyses; it was for this reason that a separate model was calculated for patients without depression in the longitudinal analyses.
Comparison with existing literature
In this study an increase in weekly physical activity was found as the key factor to an increase in HRQoL over the course of time in the full sample, whereas walking impairment resulted in a drastically decreased HRQoL. This corresponds to a study which found that general mobility is a significant predictor of HRQoL.9 Likewise, the negative influence of increasing hearing impairment on HRQoL corresponds to earlier findings.13 Vision impairment lost significance from the cross-sectional to the longitudinal analyses model in this study, whereas Fischer and colleagues found that vision had a significant effect on HRQoL.13 The findings presented here are likely to be due to the fact that the researchers controlled for several factors, such as physical activity. Physical activity might be associated with visual impairment, because patients with visual impairment might reduce activity due to a higher risk of, for example, falls. An association of visual impairment with falls21 and with activities of daily living22 has been shown earlier. Therefore, vision impairment might not be significantly associated with HRQoL if controlled for the effect of physical activity.22
Increasing age significantly influenced HRQoL in this study; the same finding has been reached in other studies,11,12 although there are also those that did not find an age influence.9,23 This could be partly explained by the older age of our cohort, as well as the cohort of Zhang and colleagues,11 when compared with the cohort of Davis and colleagues9 (mean age at baseline 69.6 (+/− 3.0)). In old age, each additional year could have a stronger impact on HRQoL, which could be due to increasing frailty.
Factors influencing HRQoL differed in males and females (Figure 2). A mixture of different needs — as found in the study by Borglin and colleagues3 — and lower education levels in females might be reasons for this. Generally, education seems to have more influence on HRQoL than sex.
Increasing comorbidity was associated with HRQoL in the cross-sectional models but lost significance in the longitudinal analyses. The researchers have assumed that increasing functional impairment (caused by comorbidity) affects HRQoL more than increasing comorbidity itself; this corresponds with the interpretations of Blane and colleagues24 and could also explain why comorbidity has been found to be a significant predictor for HRQoL in other longitudinal studies — for example, those by Byles and colleagues7 and Zhang and colleagues11 — especially when the study used a functional comorbidity index.5,9 Although significant cross-sectional associations were detected in this study, objective memory decline, incident subjective memory impairment, and declining cognitive activity were not found to significantly influence the course of HRQoL longitudinally, with the exception of subjective memory impairment in patients with a medium level of education. The results presented here regarding objective memory decline coincide with other longitudinal studies of patients with a similar mean age,6,11 whereas Davis and colleagues found a significant impact in a younger sample (69.6 ± 3.0 years).9 Cognitive abilities above a status of dementia, therefore, do not seem to be key factors that longitudinally influence HRQoL in old age.
The results of this study showed that the HRQoL of older primary care patients who are female or less educated (primary or secondary education) is likely to decline in the subsequent 4.5 years, if they decrease their physical activity. GPs should pay special attention to these patients. Impaired ability to walk is a risk factor for a worsening HRQoL if the patient’s education level is rather low.
Patients with a high level of education may compensate for physical deficits with other abilities that may be more important to them; this could also explain why the increasing number of preserved activities of daily living (as a measure of independence) only have a positive influence on HRQoL in highly educated patients, whereas all physical impairment has no effect. Here, being able to do IADLs rather than actually doing them might influence HRQoL. In other words: physical impairment is less likely to reduce HRQoL as long as independence is preserved.
Generally, highly educated individuals might have a different perception of HRQoL, which could be due to different attitudes, expectations, and coping strategies. This could also explain why only less educated patients and those without depression benefit from living in a care home or residential home: lower expectations, as well as a positive attitude regarding care, might lead to higher satisfaction. An association between expectations and quality of life has already been assumed earlier.2
Implications for practice and research
This study aimed to investigate the non-pharmacological approaches that GPs could advise their older patients to practise in order to maintain or improve their quality of life. The results suggest that patients will benefit most of all when their ability to walk does not worsen over time. Female patients in particular, as well as patients with lower education levels, might benefit from increasing their weekly physical activities such as cycling, taking longer walks, or swimming. More research is needed to investigate which kinds of activities, and in what amounts, are effective. The same patients might also benefit from hearing aids, if mild hearing loss occurs. This has already been shown to improve the psychological components of quality of life.25,26
Patients with a low level of education (primary education) and those without depression, in particular, might benefit from moving into a nursing or retirement home; the perceived HRQoL of all other patients is not significantly negatively affected by institutionalisation (except for depressed patients). Due to small case numbers conclusions cannot be derived for patients with depression. Patients with high education levels might stabilise their HRQoL by maintaining the performance of IADLs.
Promising approaches to improve or maintain HRQoL should be further investigated in randomised controlled trials in order to confirm the findings presented here and to specify the recommendations made.
Acknowledgments
The authors thank all participating patients and their GPs for their collaboration.
Funding
This study/publication is part of the German Research Network on Dementia (KND) and the German Research Network on Degenerative Dementia (KNDD), and was funded by the German Federal Ministry of Education and Research (KND grant reference numbers: 01GI0102, 01GI0420, 01GI0422, 01GI0423, 01GI0429, 01GI0431, 01GI0433, 01GI0434. KNDD grant reference numbers: 01GI0710, 01GI0711, 01GI0712, 01GI0713, 01GI0714, 01GI0715, 01GI0716).
Ethical approval
The study has been approved by the local ethics boards of all participating centres: Ethics Commission of the Medical Association Hamburg (reference numbers: OB/08/02; 2817/2007); Ethics Commission of the University of Bonn (reference numbers: 050/02; 258/07); Medical Ethics Commission II, University of Heidelberg at the University Medical Center of Mannheim (reference numbers: 0226.4/2002; 2007-253E-MA); Ethics Commission at the Medical Center of the University of Leipzig (reference numbers: 143/2002; 309/2007); Ethics Commission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf (reference numbers: 2079/2002; 2999/2008); and Ethics Committee of the TUM School of Medicine, Munich (reference numbers: 713/02; 713/02 E).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Parekh AK, Goodman RA, Gordon C, et al. HHS Interagency Workgroup on Multiple Chronic Conditions Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126(4):460–471. doi: 10.1177/003335491112600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowling A, Banister D, Sutton S, et al. A multidimensional model of the quality of life in older age. Aging Ment Health. 2002;6(4):355–371. doi: 10.1080/1360786021000006983. [DOI] [PubMed] [Google Scholar]
- 3.Borglin G, Jakobsson U, Edberg AK, Hallberg IR. Self-reported health complaints and their prediction of overall and health-related quality of life among elderly people. Int J Nurs Stud. 2005;42(2):147–158. doi: 10.1016/j.ijnurstu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Layte R, Sexton E, Savva G. Quality of life in older age: evidence from an Irish cohort study. J Am Geriatr Soc. 2013;61(Suppl 2):S299–305. doi: 10.1111/jgs.12198. [DOI] [PubMed] [Google Scholar]
- 5.Davis JC, Marra CA, Najafzadeh M, Liu-Ambrose T. The independent contribution of executive functions to health related quality of life in older women. BMC Geriatr. 2010;10:16. doi: 10.1186/1471-2318-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JL, Fiorentino L, Jouldjian S, et al. Sleep quality in residents of assisted living facilities: effect on quality of life, functional status, and depression. J Am Geriatr Soc. 2010;58(5):829–836. doi: 10.1111/j.1532-5415.2010.02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byles JE, D’Este C, Parkinson L, et al. Single index of multimorbidity did not predict multiple outcomes. J Clin Epidemiol. 2005;58(10):997–1005. doi: 10.1016/j.jclinepi.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Stretton CM, Latham NK, Carter KN, et al. Determinants of physical health in frail older people: the importance of self-efficacy. Clin Rehabil. 2006;20(4):357–366. doi: 10.1191/0269215506cr946oa. [DOI] [PubMed] [Google Scholar]
- 9.Davis JC, Marra CA, Liu-Ambrose TY. Falls-related self-efficacy is independently associated with quality-adjusted life years in older women. Age Ageing. 2011;40(3):340–346. doi: 10.1093/ageing/afr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain GH, Lemmon H, Teunisse S, et al. Quality of Life in healthy old age: relationships with childhood IQ, minor psychological symptoms and optimism. Soc Psychiatry Psychiatr Epidemiol. 2003;38(11):632–636. doi: 10.1007/s00127-003-0685-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JX, Walker JD, Wodchis WP, et al. Measuring health status and decline in at-risk seniors residing in the community using the Health Utilities Index Mark 2. Qual Life Res. 2006;15(8):1415–1426. doi: 10.1007/s11136-006-0007-y. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias CP, Manca A, Torgerson DJ. The health-related quality of life and cost implications of falls in elderly women. Osteoporos Int. 2009;20(6):869–878. doi: 10.1007/s00198-008-0753-5. [DOI] [PubMed] [Google Scholar]
- 13.Fischer ME, Cruickshanks KJ, Klein BE, et al. Multiple sensory impairment and quality of life. Ophthalmic Epidemiol. 2009;16(6):346–353. doi: 10.3109/09286580903312236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazell ML, Morris JA, Linehan MF, Frank TL. Temporal change in health-related quality of life: a longitudinal study in general practice 1999–2004. Br J Gen Pract. 2009 doi: 10.3399/bjgp09X472890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luck T, Riedel-Heller SG, Kaduszkiewicz H, et al. Mild cognitive impairment in general practice: age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Dement Geriatr Cogn Disord. 2007;24(4):307–316. doi: 10.1159/000108099. [DOI] [PubMed] [Google Scholar]
- 16.von der Schulenburg J-M, Claes C, Greiner W. The German version of the EuroQol questionnaire. [In German] Zf Gesundheitswiss. 1998;6(1):3–20. [Google Scholar]
- 17.Brauns H, Steinmann S. Educational reform in France, West-Germany and the United Kingdom: updating the CASMIN educational classification. ZUMA-Nachrichten. 1999;44:7–44. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 20.Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter violence. Clin Gerontol. 1986;5(1–2):165–173. [Google Scholar]
- 21.Klein BEK, Moss SE, Klein R, et al. Associations of visual function with physical outcomes and limitations 5 years later in an older population. Ophthalmology. 2003;110(4):644–650. doi: 10.1016/S0161-6420(02)01935-8. [DOI] [PubMed] [Google Scholar]
- 22.West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38(1):72–82. [PubMed] [Google Scholar]
- 23.Franic DM, Jiang JZ. Potentially inappropriate drug use and health-related quality of life in the elderly. Pharmacotherapy. 2006;26(6):768–778. doi: 10.1592/phco.26.6.768. [DOI] [PubMed] [Google Scholar]
- 24.Blane D, Netuveli G, Montgomery SM. Quality of life, health and physiological status and change at older ages. Soc Sci Med. 2008;66(7):1579–1587. doi: 10.1016/j.socscimed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Ciorba A, Bianchini C, Pelucchi S, Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging. 2012;7:159–163. doi: 10.2147/CIA.S26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment: a randomized trial. Ann Intern Med. 1990;113(3):188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]