Abstract
Background
Little is known about the effect of weight loss/gain on kidney function. Analyses are complicated by uncertainty about optimal body surface indexing strategies for measured glomerular filtration rate (mGFR).
Methods
Using data from the African-American Study of Kidney Disease and Hypertension (AASK), we determined the association of change in weight with three different estimates of change in kidney function: (i) unindexed mGFR estimated by renal clearance of iodine-125-iothalamate, (ii) mGFR indexed to concurrently measured BSA and (iii) GFR estimated from serum creatinine (eGFR). All models were adjusted for baseline weight, time, randomization group and time-varying diuretic use. We also examined whether these relationships were consistent across a number of subgroups, including tertiles of baseline 24-h urine sodium excretion.
Results
In 1094 participants followed over an average of 3.6 years, a 5-kg weight gain was associated with a 1.10 mL/min/1.73 m2 (95% CI: 0.87 to 1.33; P < 0.001) increase in unindexed mGFR. There was no association between weight change and mGFR indexed for concurrent BSA (per 5 kg weight gain, 0.21; 95% CI: −0.02 to 0.44; P = 0.1) or between weight change and eGFR (−0.09; 95% CI: −0.32 to 0.14; P = 0.4). The effect of weight change on unindexed mGFR was less pronounced in individuals with higher baseline sodium excretion (P = 0.08 for interaction).
Conclusion
The association between weight change and kidney function varies depending on the method of assessment. Future clinical trials should examine the effect of intentional weight change on measured GFR or filtration markers robust to changes in muscle mass.
Keywords: body surface area, glomerular filtration rate, indexing, obesity, weight
INTRODUCTION
Over the last few decades, the prevalence of obesity has increased dramatically, now affecting approximately one in three adults in the USA and one in five adults in the developed world [1]. Obesity increases the risk for a number of health conditions including hypertension, diabetes and chronic kidney disease (CKD) [2–5]. Obese persons have a markedly higher risk of end-stage renal disease (ESRD) [6]. Obesity-related glomerulopathy characterized by proteinuria, glomerulomegaly and foot process effacement has been well described [7]. Although weight loss has been shown to reduce proteinuria [8–13], the effects of weight loss or gain on glomerular filtration rate (GFR) have been less studied [13–15]. Accurate assessment of GFR is important, both to evaluate the effect of weight loss or gain on kidney function as well as to inform drug dosing and patient counseling. However, optimal methods of measuring kidney function in the setting of obesity [16] or longitudinally in the setting of weight change are uncertain.
In the direct assessment of kidney function, it is standard practice [and recommended in the current Kidney Disease Improving Global Outcomes (KDIGO) CKD evaluation guidelines] to index measured glomerular filtration rate (mGFR) to BSA [17]. The rationale for BSA indexing arises from the observation that kidney function across mammalian species is directly proportional to body size [18]. Thus, indexing of GFR by BSA allows for comparable estimates of GFR across different body sizes in a population. However, controversy exists about the appropriateness of BSA indexing in persons at the extremes of body size, and in longitudinal analyses, where indexing for concurrently measured BSA might introduce bias since BSA changes as weight changes [19].
Few studies have examined the longitudinal association between kidney function and weight change using multiple methods of kidney function assessment, particularly in patients with CKD. Thus, we evaluated this association among participants from the African-American Study of Kidney Disease and Hypertension (AASK), assessing kidney function by three different methods: (i) ‘unindexed mGFR’, where change in mGFR assessed by renal clearance of iodine-125-iothalamate was presented as the raw change from initial mGFR, (ii) mGFR indexed to concurrently measured BSA, and (iii) eGFR estimated from serum creatinine using the AASK equation [20].
MATERIALS AND METHODS
Study population
The AASK study was a multicenter randomized clinical trial of 1094 African-American adults with mGFR of 20–65 mL/min/1.73 m2 [21]. Participants were randomly assigned to one of two BP goals: a usual mean arterial pressure (MAP) goal of 102 to 107 mmHg or to a lower MAP goal of <92 mmHg, and to treatment with one of three anti-hypertensive drugs (metoprolol, ramipril or amlodipine). Participants were self-identified African-American adults with hypertension and no other identified causes of renal insufficiency. Exclusion criteria were diastolic BP <95 mmHg, known history of diabetes, urinary protein/creatinine ratio >2.5 g/g, accelerated or malignant hypertension within 6 months, secondary hypertension, evidence of non-BP-related causes of renal disease, serious systemic disease, clinical congestive heart failure or specific indication or contraindication to a study drug or procedure. Participant enrollment began in February 1995 and ended in September 1998.
Assessment of anthropomorphic data and kidney function
Body surface area was calculated using the DuBois formula [22]: , and body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared [23]. Measurement of height was recorded at baseline; mGFR, serum creatinine, and weight were obtained at baseline, follow-up months 3 and 6 and then every 6 months during the trial. GFR was directly measured by renal clearance of iodine-125-iothalamate (mGFR). In brief, participants were instructed to ingest ∼5 mL/kg of body weight of water prior to arrival, and another 10 mL/kg after arrival to the clinic. Next, participants ingested two drops of supersaturated potassium iodide to block thyroidal uptake of 125I-iothalamate, and then received a subcutaneous injection of 35 uCi of 125I-iothalamate in the subdeltoid area. Four 30-min urine collections were performed with blood samples taken at the end of each period. GFR for each 30-min period was calculated using the logarithmic mean of the plasma 125I-iothalamate counts compared with urine counts during each period. The mean of four periods was used to calculate GFR in the vast majority of participants. For the present study, change in mGFR was represented two ways: ‘unindexed GFR’, where change in mGFR was presented as the raw change from initial mGFR (scaled to baseline BSA for comparability to change in indexed mGFR and eGFR), and GFR indexed by concurrently measured BSA. Serum creatinine was measured using a kinetic alkaline picrate assay (Jaffe method). Estimated GFR (eGFR) was calculated using the AASK equation: [20]. Units for both measured GFR and eGFR are expressed as mL/min/1.73 m2.
Statistical analysis
For comparison of baseline characteristics, annual slope of weight change was determined for each individual using ordinary least squares regression with the first 2 years of weight data so as to minimize the effect of survival on category of weight change. Means and proportions of baseline characteristics were compared across quintiles of weight change using analysis of variance without adjustment for other baseline variables. For analyses of change in kidney function as a function of change in weight, data from all participants during the full follow-up period was used. Mixed-effects models were adjusted for time, baseline weight, time-varying diuretic use (yes/no), randomization group and an interaction term for randomization group with time (before and after 3 months), allowing the rate of eGFR decline to vary by baseline weight, using a robust covariance structure. An interaction of time and change in weight with change in GFR was tested but not statistically significant. Since the relationship between change in weight and change in GFR did not differ by time, the relationship between weight change and change in GFR was modeled by aggregating data from all time-points during the AASK trial. Weight change was modeled continuously and also categorically, comparing quintiles of weight change to the middle quintile. Continuous analyses were repeated using log-transformed mGFR and eGFR to express change in GFR on a percentage basis. Analyses were performed for each method of kidney function assessment: (i) unindexed mGFR (scaled by baseline BSA); (ii) mGFR indexed for concurrent BSA and (iii) eGFR using the AASK equation. By scaling the unindexed mGFR to baseline BSA, each of the three measures of kidney function will be expressed in the same units, mL/min/1.73 m2. Such an approach will facilitate direct comparisons of results across the three measures.
We conducted subgroup analyses by baseline BMI categories (<25 kg/m2, 25–29.99, 30–34.99, ≥35), and body shape categories. No measures of central obesity were collected; thus, body shape categories were categorized as heavier/taller (above median weight/above median height), heavier/shorter (above median weight/below median height), lighter/taller (below median weight/above median height) and lighter/shorter (below median weight/below median height). Because changes in weight could be due to changes in total body water, subgroup analyses were done by history of heart disease (yes/no), tertiles of baseline 24-h urine sodium excretion, and in a subgroup of patients who reported no diuretic use throughout the trial. Importantly, AASK excluded patients with clinical congestive heart failure, so the stratification by baseline heart disease does not contain participants with prevalent heart failure. Additional subgroup analyses were done by gender, proteinuria status [protein/creatinine ratio (PCR) >1.0, 0.5–1.0 and <0.5 g/d] and baseline GFR (≥45 mL/min/1.73 m2 versus <45 mL/min/1.73 m2). Each subgroup was also formally tested for an interaction with change in weight by adding relevant product terms to the mixed models.
For the analyses evaluating the relationship between weight change and GFR, we performed the following sensitivity analyses: (i) adjusting for time-varying 24-h urine urea nitrogen excretion to account for protein intake as a confounder; (ii) using unindexed mGFR, not scaled to baseline BSA and (iii) adjusting analyses with eGFR as the dependent variable for time-varying 24-h urine creatinine to examine whether changes in muscle mass could account for observed differences between eGFR and mGFR trajectories with weight change.
Finally, to understand the magnitude by which findings could differ depending on method of kidney function assessment, we evaluated the difference between each pair of GFR estimates [(mGFR/concurrent BSA − unindexed mGFR), (eGFR − unindexed mGFR) and (eGFR − mGFR/concurrent BSA)] with change in weight using the same model as described above.
All analyses were performed using Stata version 13.0 (College Station, TX). P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics by weight change trajectory over initial 2 years
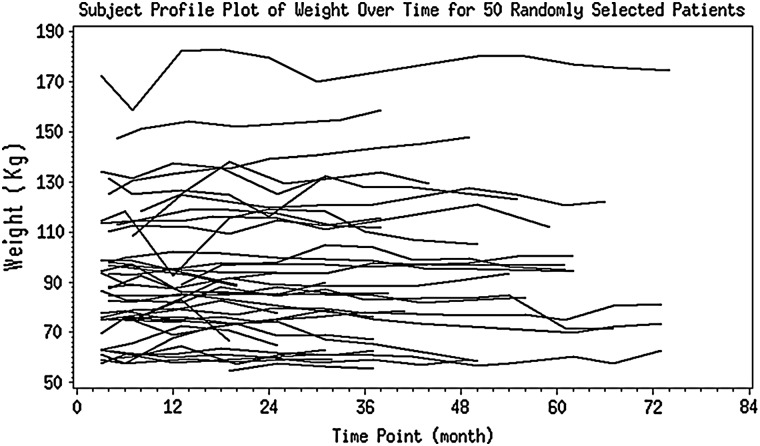
Follow-up weight measurements within the first 2 years were available in 1045 of the 1094 participants. Median weight change over the first 2 years was +0.9 kg/year (IQR −1.4 to 3.2 kg/year). There was substantial variation in the degree of weight change over the first 2 years as median weight change in the highest quintile was +6.4 kg/year compared with −3.4 kg/year in the lowest quintile (Table 1). Figure 1 shows the weight trajectories for 50 randomly selected individuals. There were significant differences in baseline age, weight, BMI, BSA, diastolic blood pressure, mGFR and prevalence of proteinuria between quintiles of weight change (P < 0.05 for all analysis of variance comparisons). Individuals with the largest weight gain and largest weight loss tended to have higher baseline weight, BMI, diastolic blood pressure and prevalence of proteinuria than the more stable weight change groups. Individuals with the largest weight gains tended to be younger and have higher BSA, whereas individuals with the largest weight loss tended to have lower baseline mGFR and eGFR. There were no significant differences in randomization group, baseline diuretic use or history of heart disease among the different weight change categories.
Table 1.
Baseline characteristics by weight change slope over initial 2 years
| N | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P valuea |
|---|---|---|---|---|---|---|
| 209 | 209 | 209 | 209 | 209 | ||
| Weight change slope over first 2 years (kg/year) | −3.4 (−4.9 to −2.5) | −0.9 (−1.4 to −0.3) | 0.9 (0.6 to 1.3) | 2.8 (2.2 to 3.2) | 6.4 (5.0 to 8.8) | |
| Total weight change over entire study | −4.5 (−8.2 to −1.8) | −0.5 (−2.3 to 2.3) | 0.9 (−1.8 to 3.6) | 3.6 (0.9 to 7.3) | 9.1 (3.2 to 14.5) | |
| Total study follow-up time | 3.2 (2.1 to 4.6) | 4.0 (3.0 to 5.0) | 4.0 (3.1 to 5.0) | 3.7 (3.0 to 5.0) | 4.1 (2.7 to 5.0) | 0.002 |
| Age (years) | 55.4 (10.5) | 55.4 (10.8) | 56.7 (10.2) | 53.6 (10.8) | 51.7 (10.6) | <0.001 |
| Female (%) | 41.6 | 35.9 | 39.2 | 40.2 | 37.3 | 0.8 |
| <High school education (%) | 40.2 | 48.8 | 40.2 | 36.8 | 38.8 | 0.1 |
| Current smoker (%) | 31.1 | 31.6 | 30.6 | 23.4 | 31.1 | 0.3 |
| History of heart disease (%) | 53.1 | 49.3 | 54.1 | 46.9 | 54.5 | 0.4 |
| Height (cm) | 170.4 (10.2) | 171.5 (9.9) | 170.2 (9.5) | 170.5 (10.0) | 172.5 (10.7) | 0.1 |
| Weight (kg) | 90.9 (22.4) | 87.6 (19.0) | 85.1 (18.5) | 88.7 (18.9) | 94.7 (22.7) | <0.001 |
| BMI (kg/m2) | 31.3 (7.1) | 29.8 (6.3) | 29.4 (6.1) | 30.6 (6.4) | 31.7 (6.6) | 0.001 |
| BSA (m2) | 2.01 (0.26) | 1.99 (0.23) | 1.96 (0.22) | 2.00 (0.23) | 2.08 (0.27) | <0.001 |
| SBP (mmHg) | 152.6 (24.5) | 150.7 (22.8) | 148.4 (23.4) | 148.5 (23.5) | 149.8 (23.1) | 0.3 |
| DBP (mmHg) | 96.8 (14.5) | 94.6 (13.9) | 93.3 (13.6) | 95.1 (13.8) | 97.3 (14.6) | 0.02 |
| mGFR (mL/min/1.73 m2) | 44.2 (14.3) | 46.8 (14.2) | 47.8 (13.1) | 47.9 (13.4) | 46.2 (12.6) | 0.04 |
| Protein/creatinine ratio >0.22 (%) | 40.7 | 33.0 | 20.6 | 27.3 | 37.8 | <0.001 |
| Randomized to low BP goal (%) | 48.3 | 47.8 | 55.0 | 50.2 | 48.8 | 0.6 |
| Randomized drug (%) | ||||||
| Ramipril | 43.5 | 40.7 | 42.6 | 36.4 | 34.5 | 0.7 |
| Metoprolol | 34.5 | 38.8 | 39.7 | 43.5 | 47.4 | |
| Amlodipine | 22.0 | 20.6 | 17.7 | 20.1 | 18.2 | |
| Baseline diuretic use (%) | 66.5 | 61.7 | 62.9 | 64.9 | 64.7 | 0.9 |
Annual slope of weight change was determined for each individual using ordinary least squares regression with first 2 years of weight data to minimize the effect of survival on category of weight change. Weight change slope, total weight change and total study follow-up time are reported as median (IQR). Otherwise, results are presented as mean (SD) or percentages.
aMeans and proportions of baseline characteristics compared using ANOVA.
FIGURE 1:
Weight trajectories of 50 randomly selected patients.
Association between change in weight and mGFR
Over a median follow-up time of 3.6 years, a direct, linear association was observed between change in weight and change in unindexed mGFR. Every 5 kg increase in weight was associated with a 1.10 mL/min/1.73 m2 (95% CI: 0.87 to 1.33; P < 0.001) increase in unindexed mGFR (Table 2). In contrast, the association was greatly attenuated and no longer significant between weight change and mGFR indexed for concurrent BSA (0.21 mL/min1.73 m2; 95% CI: −0.02 to 0.44; P = 0.1).
Table 2.
Relationship of change in GFR measures with change in weight (per 5 kg)
| GFR measure | Mean (95% CI) change in GFR per 5 kg change in weight (mL/min/1.73 m2) | P value |
|---|---|---|
| Unindexed mGFR (scaled to baseline BSA: mL/min/1.73 m2) | 1.10 (0.87 to 1.33) | <0.001 |
| mGFR/concurrent BSA (mL/min/1.73 m2) | 0.21 (−0.02 to 0.44) | 0.1 |
| eGFR (mL/min/1.73 m2) | −0.09 (−0.32 to 0.14) | 0.4 |
Because the relationship between change in weight and change in GFR did not differ by time, data from all time-points were aggregated in mixed-effects models adjusted for time, baseline weight, time-varying diuretic use, randomization group and an interaction term for randomization group with time (before and after 3 months).
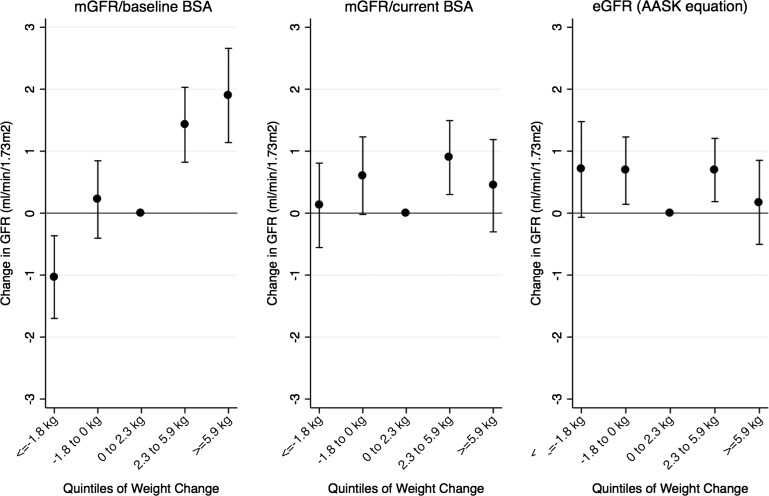
Figure 2 shows the relationship between quintiles of weight change and change in GFR measures. Ranges of weight change in the lowest (Q1) to highest quintile (Q5) were ≤ −1.8 kg, −1.8 to 0 kg, 0 to +2.3 kg, +2.3 to +5.9 g and ≥ +5.9 kg. Compared with Q3, Q1 was associated with a decrease in unindexed mGFR of −1.03 (95% CI: −1.70 to −0.37; P = 0.002), and Q2 was not significantly different (0.22, 95% CI: −0.40 to 0.84, P = 0.5). Q4 and Q5 were associated with increases of 1.43 (95% CI: 0.82 to 2.03; P < 0.001) and 1.90 (95% CI: 1.14 to 2.66; P < 0.001) (Figure 2, left panel). These estimates were attenuated when mGFR was indexed for concurrent BSA (Figure 2, middle panel).
FIGURE 2:
Association between categories of weight change and GFR measures. Because the relationship between change in weight and change in GFR did not differ by time, data from all time-points were aggregated in mixed-effects models adjusted for time, baseline weight, time-varying diuretic use, randomization group, and an interaction term for randomization group with time (before and after 3 months). Quintiles of weight gain/loss were compared with the middle quintile (0–2.3 kg).
Similarly, when evaluated using log-transformed mGFR as the outcome, a 5 kg increase in weight was associated with a 3.23% (95% CI: 2.60 to 3.86%, P < 0.001) increase in unindexed mGFR. This association was attenuated though significant when mGFR was indexed to concurrent BSA (per 5 kg weight gain, 1.08% (95% CI: 0.46 to 1.70%, P = 0.001).
Association between change in weight and eGFR
There was no significant association between change in weight and change in eGFR in continuous models (per 5 kg weight gain, −0.09 mL/min/1.73 m2, 95% CI: −0.32 to 0.14; P = 0.4) (Table 2), or in categorical analyses (Figure 2, right panel). Likewise, when using log-transformed eGFR as the outcome, there was no significant association between weight change and eGFR [per 5 kg weight gain, 0.29% (95% CI: −0.31 to 0.89%, P = 0.3)].
Subgroup and sensitivity analyses
In subgroup analyses, results were similar across groups stratified by gender, baseline BMI, body shape, urine PCR and history of heart disease (Table 3). For baseline 24-h urine sodium excretion, a significant interaction was detected; weight gain was associated with a more negative change in unindexed mGFR and eGFR in those with higher baseline 24-h urine sodium excretion (P = 0.08 for interaction on mGFR and 0.09 for interaction on eGFR). For instance, individuals in the lowest tertile of baseline 24-h urine sodium excretion had a 1.37 mL/min/1.73 m2 (95% CI: 0.96 to 1.77; P < 0.001) increase in unindexed mGFR whereas individuals in the highest tertile of baseline 24-h urine sodium excretion had a 0.87 (95% CI: 0.49 to 1.25; P < 0.001) increase in unindexed mGFR. In individuals in the highest tertile of baseline 24-h urine sodium excretion, a 5-kg weight gain was associated with a −0.38 (95% CI: −0.73 to −0.02; P = 0.04) change in eGFR. When stratified by diuretic use (ever versus never), no significant interaction was detected. However, in participants who never used diuretics (n = 93), a 5-kg weight change was associated with a −0.92 (95% CI: −1.83 to −0.01; P = 0.05) change in eGFR (Table 3). In sensitivity analyses, additional adjustment for time-varying 24-h urine urea nitrogen to assess for protein intake as a confounder had little effect on the mGFR or eGFR analyses [per 5 kg weight gain, 1.05 mL/min/1.73 m2 (0.82 to 1.29, P < 0.001) change in unindexed mGFR, 0.16 (−0.07 to 0.39, P = 0.2) change in mGFR indexed to concurrent BSA and −0.11 (−0.33 to 0.12, P = 0.3) change in eGFR]. Adjusting for time-varying 24-h urine creatinine as a surrogate for muscle mass had little effect on eGFR analyses (per 5 kg weight gain, −0.09 (−0.31 to 0.14, P = 0.4).
Table 3.
Subgroup analyses examining relationship between change in weight (per 5 kg) with changes in GFR measures
| Subgroupsa | Unindexed mGFR (scaled to baseline BSA) |
mGFR/concurrent BSA |
eGFR |
|||
|---|---|---|---|---|---|---|
| Estimate (95% CI) (mL/min/1.73 m2) | P value | Estimate (95% CI) (mL/min/1.73 m2) | P value | Estimate (95% CI) (mL/min/1.73 m2) | P value | |
| Gender | ||||||
| Male (n = 669) | 1.01 (0.70 to 1.31) | <0.001 | 0.15 (−0.15 to 0.45) | 0.3 | −0.18 (−0.46 to 0.10) | 0.2 |
| Female (n = 425) | 1.27 (0.93 to 1.62) | <0.001 | 0.31 (−0.04 to 0.66) | 0.08 | 0.08 (−0.31 to 0.47) | 0.9 |
| Baseline BMI groups | ||||||
| BMI <25 (n = 222) | 1.01 (0.31 to 1.71) | 0.01 | −0.16 (−0.85 to 0.53) | 0.7 | −0.34 (−1.08 to 0.39) | 0.4 |
| BMI 25–29.99 (n = 362) | 1.22 (0.72 to 1.72) | <0.001 | 0.16 (−0.36 to 0.68) | 0.6 | 0.05 (−0.41 to 0.51) | 0.8 |
| BMI 30–34.99 (n = 266) | 1.06 (0.62 to 1.50) | <0.001 | 0.12 (−0.32 to 0.56) | 0.6 | −0.33 (−0.79 to 0.13) | 0.2 |
| BMI ≥35 kg/m2 (n = 244) | 1.05 (0.69 to 1.40) | <0.001 | 0.36 (0.01 to 0.72) | 0.05 | 0.02 (−0.30 to 0.34) | 0.9 |
| Baseline body shape groups | ||||||
| Wt ≤87.1 kg, ht ≤1.72 m (n = 359) | 1.28 (0.81 to 1.975) | <0.001 | 0.06 (−0.42 to 0.54) | 0.8 | −0.01 (−0.59 to 0.56) | 1.0 |
| Wt ≤87.1 kg, ht >1.72 m (n = 201) | 0.96 (0.29 to 1.63) | 0.01 | −0.04 (−0.73 to 0.64) | 0.9 | −0.46 (−1.07 to 0.16) | 0.1 |
| Wt >87.1 kg, ht ≤1.72 m (n = 207) | 1.16 (0.74 to 1.58) | <0.001 | 0.31 (−0.10 to 0.72) | 0.1 | −0.01 (−0.38 to 0.37) | 1.0 |
| Wt >87.1 kg, ht >1.72 m (n = 327) | 1.00 (0.55 to 1.45) | <0.001 | 0.24 (−0.16 to 0.63) | 0.2 | −0.14 (−0.50 to 0.22) | 0.4 |
| Urine protein/creatinine ratio (PCR) | ||||||
| PCR <0.5 (n = 866) | 1.05 (0.78 to 1.33) | <0.001 | 0.08 (−0.19 to 0.35) | 0.5 | −0.15 (−0.41 to 0.11) | 0.3 |
| PCR 0.5–0.99 (n = 97) | 1.13 (0.58 to 1.67) | <0.001 | 0.48 (−0.06 to 1.02) | 0.08 | 0.12 (−0.47 to 0.72) | 0.7 |
| PCR ≥1.0 (n = 127) | 0.96 (0.46 to 1.46) | <0.001 | 0.37 (−0.14 to 0.88) | 0.2 | −0.68 (−1.14 to −0.22) | 0.004 |
| History of heart disease | ||||||
| No (n = 530) | 1.09 (0.78 to 1.40) | <0.001 | 0.23 (−0.07 to 0.54) | 0.1 | −0.04 (−0.32 to 0.25) | 0.8 |
| Yes (n = 564) | 1.11 (0.77 to 1.46) | <0.001 | 0.18 (−0.17 to 0.53) | 0.3 | −0.08 (−0.43 to 0.26) | 0.6 |
| Baseline 24-h urine Na excretiona | ||||||
| <2.74 g/day (n = 377) | 1.37 (0.96 to 1.77) | <0.001 | 0.45 (0.06 to 0.84) | 0.02 | 0.19 (−0.23 to 0.62) | 0.4 |
| 2.74–4.24 g/day (n = 362) | 1.17 (0.73 to 1.61) | <0.001 | 0.25 (−0.21 to 0.70) | 0.3 | 0.05 (−0.35 to 0.45) | 0.8 |
| ≥4.24 g/day (n = 354) | 0.87 (0.49 to 1.25) | <0.001 | 0.02 (−0.36 to 0.41) | 0.9 | −0.38 (−0.73 to −0.02) | 0.04 |
| Diuretic use throughout trial | ||||||
| Never (n = 93) | 0.89 (−0.25 to 2.03) | 0.1 | −0.30 (−1.48 to 0.88) | 0.6 | −0.92 (−1.83 to −0.01) | 0.05 |
| Ever (n = 999) | 1.09 (0.85 to 1.32) | <0.001 | 0.21 (−0.29 to 0.44) | 0.09 | −0.07 (−0.30 to 0.16) | 0.6 |
| Baseline GFR | ||||||
| <45 mL/min/1.73 m2 (n = 475) | 1.01 (0.69 to 1.34) | <0.001 | 0.33 (0.02 to 0.64) | 0.04 | −0.05 (−0.35 to 0.26) | 0.8 |
| ≥45 mL/min/1.73 m2 (n = 619) | 1.14 (0.82 to 1.47) | <0.001 | 0.10 (−0.23 to 0.43) | 0.5 | −0.18 (−0.48 to 0.12) | 0.2 |
Because the relationship between change in weight and change in GFR did not differ by time, data from all time-points were aggregated in mixed-effects models adjusted for time, baseline weight, time-varying diuretic use, randomization group and an interaction term for randomization group with time (before and after 3 months).
aAssociation between weight change and unindexed mGFR and eGFR differed by baseline 24-h urine sodium excretion (P = 0.08 for interaction on mGFR and P = 0.09 for interaction on eGFR).
Differences between the three methods of GFR assessment in evaluating the relationship between weight change and kidney function
Using unindexed mGFR as the reference, mGFR/concurrent BSA underestimated the association between weight change and kidney function (per 5 kg weight gain, −0.89 mL/min/1.73 m2, 95% CI: −0.94 to −0.83, P < 0.001). Analyses using eGFR underestimated the association between weight change and kidney function whether using unindexed mGFR (scaled to baseline BSA) as the reference (per 5 kg weight gain, −1.12 mL/min/1.73 m2, 95% CI: −1.33 to −0.91, P < 0.001), or using mGFR adjusted for concurrent BSA as the reference (per 5 kg weight gain, −0.26 mL/min/1.73 m2, 95% CI: −0.46 to −0.05; P = 0.02) (Table 4).
Table 4.
Differences between the three methods of GFR assessment in evaluating the relationship between weight change and kidney function
| Difference in estimate (95% CI) (mL/min/1.73 m2) | P value | |
|---|---|---|
| mGFR/concurrent BSA − unindexed mGFR (scaled to baseline BSA) | −0.89 (−0.94 to −0.83) | <0.001 |
| eGFR − unindexed mGFR (scaled to baseline BSA) | −1.12 (−1.33 to −0.91) | <0.001 |
| eGFR − mGFR/concurrent BSA | −0.26 (−0.46 to −0.05) | 0.02 |
To understand the magnitude by which findings could differ depending on method for kidney function assessment, we evaluated the difference between each pair of GFR with change in weight in mixed-effects models adjusted for time, baseline weight, time-varying diuretic use, randomization group and an interaction term for randomization group with time (before and after 3 months).
DISCUSSION
In this study of African-Americans with CKD attributed to hypertension, measured GFR increased with weight gain, although this relationship was attenuated and not statistically significant when GFR was indexed for concurrent BSA. Whether compared with unindexed mGFR or mGFR indexed to concurrent BSA, eGFR had a significantly smaller association with weight change. Furthermore, the direction of the association was reversed in some of the subgroup analyses demonstrating how analyses using eGFR can differ significantly from mGFR in the setting of weight fluctuation. This implies that studies evaluating the impact of weight loss on kidney function should use either directly measured GFR or other markers of filtration that are unrelated to muscle mass.
The attenuation of the longitudinal relationship between weight change and mGFR after adjustment for concurrent BSA is consistent with observations in cross-sectional studies [19, 24–26]. These findings should not be surprising since BSA increases as weight increases, resulting in lower indexed mGFR than unindexed mGFR values. For example, in a study of 301 non-diabetic participants of East African descent, individuals who were overweight and obese had higher unindexed mGFR, filtration fraction, and higher prevalence of glomerular hyperfiltration (defined as GFR ≥140 mL/min in this study) than normal-weight individuals [24]. These differences were attenuated and no longer significant when mGFR was indexed for BSA. Similar findings were observed in a study of 315 healthy individuals in the Netherlands [26], where higher BMI was associated with higher filtration fraction and higher unindexed mGFR. Again, indexing for BSA attenuated the association between BMI and mGFR to non-significance.
Although our study focused on persons with CKD, the direction of association between increased weight and increased mGFR may support the concept of ‘obesity-associated glomerular hyperfiltration.’ No universal definition for hyperfiltration exists, and the mechanisms behind this relationship are unknown [27]. Although usually described in the setting of normal kidney function, hyperfiltration can theoretically occur in the setting of globally decreased kidney function as well. In patients with CKD who may have experienced significant nephron loss, hyperfiltration may occur at the single nephron level. Experimental studies suggest that compensatory glomerular hyperfiltration leads to increased glomerular capillary pressure and subsequent injury [28]. This relationship between body mass and unindexed mGFR is also supported by studies of patients undergoing bariatric surgery. Unindexed mGFR has been shown to decrease after bariatric surgery whereas mGFR/concurrent BSA does not change 1 year after bariatric surgery in patients with baseline normal or supranormal kidney function [13, 29]. Others have reported cases of nephrotic-range proteinuria resolving after bariatric surgery [30, 31]. Few studies have examined the effects of intentional weight loss in patients with CKD and have been limited by small sample size or use of creatinine-based eGFR [32, 33].
Although we show that indexing for BSA affects the interpretation of the relationships between weight and mGFR, it remains unclear whether using indexed mGFR is more appropriate than using unindexed mGFR. In general, the impact of indexing is likely small for most individuals. However, there may be some situations where exact knowledge of kidney function may be important, particularly when renally cleared medications with narrow therapeutic windows are prescribed (e.g. cisplatin). One approach to examine the optimal strategy for indexing could be to compare the longitudinal relationship between metabolic burden (i.e. urea nitrogen, phosphate) with GFR (unindexed and indexed for BSA) [34]. More research on the implications of BSA indexing is needed, particularly in patients at the extremes of body mass or in the setting of massive weight change.
This study has several strengths, including a large cohort of over 1000 individuals followed for nearly 4 years with both directly measured and estimated GFR assessed at several time-points. Our study also has limitations. The analysis is observational; as such, cause-and-effect cannot be determined. Secondly, the reasons for weight change are unknown, and we are unable to determine whether changes in weight reflect changes in fat mass, fat-free mass or total body water. Indeed, the effect of weight change on unindexed mGFR varied by baseline 24-h urine sodium excretion, suggesting that some of the observed association may be driven by changes in total body water. Weight loss/gain could have been intentional or unintentional, and weight loss may occur due to progression of CKD. Thirdly, other factors may confound the relationship between weight and kidney function, driving both changes in weight and GFR simultaneously. For example, decreased protein intake could result in decreased weight and acutely decreased GFR though results were unchanged after adjusting for 24-h urine urea nitrogen. The AASK study did not collect measures of central obesity, which may promote renal injury more strongly than general obesity [26]. Other novel filtration markers such as cystatin C, beta-trace protein or b2-microglobulin may be less influenced by factors associated with weight loss/gain than creatinine but were unavailable in follow-up visits and thus not examined.
In conclusion, we demonstrate that weight gain is associated with increased unindexed mGFR, but no change in mGFR adjusted for concurrently measured BSA or eGFR. Future clinical trials should carefully assess anthropometrics, and examine the effect of intentional weight loss using directly measured GFR or alternative filtration markers not affected by muscle mass.
ACKNOWLEDGEMENTS
A.C. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant T32DK007732). T.H.G. was supported by the NIDDK (grant 1R01DK90046). AASK was supported by grants to each clinical center and the coordinating center from the National Institute of Diabetes and Digestive and Kidney Diseases. In addition, AASK was supported by the Office of Research in Minority Health [now the National Center on Minority Health and Health Disparities (NCMHD)] and the following institutional grants from the National Institutes of Health: M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887 and DK 2818-02. Additional support was provided by MCRDP K12RR023250 King Pharmaceuticals provided monetary support and antihypertensive medications to each clinical center. Pfizer Inc., AstraZeneca Pharmaceuticals, Glaxo Smith Kline, Forest Laboratories, Pharmacia and Upjohn also donated antihypertensive medications. A poster presentation of this work was presented at ASN Kidney Week November 2013.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.Ng M, Fleming T, Robinson M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Larson MG, Leip EP et al. Predictors of new-onset kidney disease in a community-based population. JAMA 2004; 291: 844–850 [DOI] [PubMed] [Google Scholar]
- 3.Chang A, Van Horn L, Jacobs DR Jr et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis 2013; 62: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer H, Luke A, Bidani A et al. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 2005; 46: 587–594 [DOI] [PubMed] [Google Scholar]
- 5.Kramer H, Luke A. Obesity and kidney disease: a big dilemma. Curr Opin Nephrol Hypertens 2007; 16: 237–241 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, McCulloch CE, Iribarren C et al. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21–28 [DOI] [PubMed] [Google Scholar]
- 7.Kambham N, Markowitz GS, Valeri AM et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498–1509 [DOI] [PubMed] [Google Scholar]
- 8.Morales E, Valero MA, Leon M et al. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 2003; 41: 319–327 [DOI] [PubMed] [Google Scholar]
- 9.Afshinnia F, Wilt TJ, Duval S et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 2010; 25: 1173–1183 [DOI] [PubMed] [Google Scholar]
- 10.Chang A, Batch BC, McGuire HL et al. Association of a reduction in central obesity and phosphorus intake with changes in urinary albumin excretion: the PREMIER study. Am J Kidney Dis 2013; 62: 900–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarevic G, Antic S, Vlahovic P et al. Effects of aerobic exercise on microalbuminuria and enzymuria in type 2 diabetic patients. Ren Fail 2007; 29: 199–205 [DOI] [PubMed] [Google Scholar]
- 12.Saiki A, Nagayama D, Ohhira M et al. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes (Lond) 2005; 29: 1115–1120 [DOI] [PubMed] [Google Scholar]
- 13.Chagnac A, Weinstein T, Herman M et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 2003; 14: 1480–1486 [DOI] [PubMed] [Google Scholar]
- 14.Ryu S, Chang Y, Woo HY et al. Changes in body weight predict CKD in healthy men. J Am Soc Nephrol 2008; 19: 1798–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh SW, Ahn SY, Jianwei X et al. Relationship between changes in body fat and a decline of renal function in the elderly. PLoS One 2014; 9: e84052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fotheringham J, Weatherley N, Kawar B et al. The body composition and excretory burden of lean, obese, and severely obese individuals has implications for the assessment of chronic kidney disease. Kidney Int 2014; 86: 1221–1228 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 18.Holt JP, Rhode EA. Similarity of renal glomerular hemodynamics in mammals. Am Heart J 1976; 92: 465–472 [DOI] [PubMed] [Google Scholar]
- 19.Delanaye P, Radermecker RP, Rorive M et al. Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol Dial Transplant 2005; 20: 2024–2028 [DOI] [PubMed] [Google Scholar]
- 20.Lewis J, Agodoa L, Cheek D et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 2001; 38: 744–753 [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Middleton J, Miller ER III et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol 2003; 14(7 Suppl 2):S166–S172 [DOI] [PubMed] [Google Scholar]
- 22.Du Bois D, Du Bois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 17: 863–871 [PubMed] [Google Scholar]
- 23.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894.i–xii:1–253 [PubMed] [Google Scholar]
- 24.Wuerzner G, Pruijm M, Maillard M et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis 2010; 56: 303–312 [DOI] [PubMed] [Google Scholar]
- 25.Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension 1995; 26: 610–615 [DOI] [PubMed] [Google Scholar]
- 26.Kwakernaak AJ, Zelle DM, Bakker SJ et al. Central body fat distribution associates with unfavorable renal hemodynamics independent of body mass index. J Am Soc Nephrol 2013; 24: 987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helal I, Fick-Brosnahan GM, Reed-Gitomer B et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012; 8: 293–300 [DOI] [PubMed] [Google Scholar]
- 28.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996; 49: 1774–1777 [DOI] [PubMed] [Google Scholar]
- 29.Friedman AN, Moe S, Fadel WF et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol 2014; 39: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huan Y, Tomaszewski JE, Cohen DL. Resolution of nephrotic syndrome after successful bariatric surgery in patient with biopsy-proven FSGS. Clin Nephrol 2009; 71: 69–73 [DOI] [PubMed] [Google Scholar]
- 31.Fowler SM, Kon V, Ma L et al. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol 2009; 24: 851–855 [DOI] [PubMed] [Google Scholar]
- 32.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant 2013; 28(Suppl 4): iv82–iv98 [DOI] [PubMed] [Google Scholar]
- 33.Navaneethan SD, Yehnert H. Bariatric surgery and progression of chronic kidney disease. Surg Obes Relat Dis 2009; 5: 662–665 [DOI] [PubMed] [Google Scholar]
- 34.Ellam T, Fotheringham J, Kawar B. Differential scaling of glomerular filtration rate and ingested metabolic burden: implications for gender differences in chronic kidney disease outcomes. Nephrol Dial Transplant 2014; 29: 1186–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]