Abstract
Lipid phosphate phosphatases (LPPs) are a group of enzymes that belong to a phosphatase/phosphotransferase family. Mammalian LPPs consist of three isoforms: LPP1, LPP2, and LPP3. They share highly conserved catalytic domains and catalyze the dephosphorylation of a variety of lipid phosphates, including phosphatidate, lysophosphatidate (LPA), sphingosine 1-phosphate (S1P), ceramide 1-phosphate, and diacylglycerol pyrophosphate. LPPs are integral membrane proteins, which are localized on plasma membranes with the active site on the outer leaflet. This enables the LPPs to degrade extracellular LPA and S1P, thereby attenuating their effects on the activation of surface receptors. LPP3 also exhibits noncatalytic effects at the cell surface. LPP expression on internal membranes, such as endoplasmic reticulum and Golgi, facilitates the metabolism of internal lipid phosphates, presumably on the luminal surface of these organelles. This action probably explains the signaling effects of the LPPs, which occur downstream of receptor activation. The three isoforms of LPPs show distinct and nonredundant effects in several physiological and pathological processes including embryo development, vascular function, and tumor progression. This review is intended to present an up-to-date understanding of the physiological and pathological consequences of changing the activities of the different LPPs, especially in relation to cell signaling by LPA and S1P.
Keywords: autotaxin, breast cancer, thyroid cancer, cell migration, epidermal growth factor (EGF) receptor, G-protein coupled receptors, lysophosphatidate
Mammalian lipid phosphate phosphatases (LPPs) were first characterized as Mg2+-independent and N-ethylmaleimide-insensitive phosphatidate phosphatases (PAPs) in 1991 (1). These enzymes were called type 2 PAPs (PAP2s) at this time to distinguish them from the type 1 Mg2+-dependent PAPs (PAP1s). PAP1 enzymes exist in the cytosol of cells and they translocate to the endoplasmic reticulum where they produce diacylglycerol (DG) for glycerolipid synthesis (2). It was not until 2006 that the structure of the PAP1 enzymes were identified in yeast and were shown to belong to a family of enzymes called lipins (3). All three mammalian lipins have PAP1 activity and are specific for phosphatidate (PA) as a substrate (4).
By contrast, PAP2 enzymes dephosphorylate a wide variety of lipid phosphates including lysophosphatidate (LPA), sphingosine 1-phosphate (S1P), ceramide 1-phosphate (C1P) (5), DG pyrophosphate (6), and N-oleoylethanolamine phosphate (7). Because of this broad specificity, the PAP2 enzymes were renamed as LPPs (8). cDNA was cloned for mammalian LPP1 in 1996 (9) and LPP3 was identified a year later (10). The identities of genes that encode LPP1 and DG pyrophosphate phosphatase (DPP)1 in Saccharomyces cerevisiae were identified in 1998 (11, 12).
Mammalian LPPs consist of three related proteins named LPP1 (and LPP1a, a splice variant), LPP2, and LPP3, which are encoded by three separate genes PPAP2A, PPAP2C, and PPAP2B, respectively. These enzymes belong to a family of phosphatases and phosphotransferases based on the conserved catalytic domains in their structure (13–17) (Fig. 1). This family includes bacterial acid phosphatases (18), bacterial and yeast DG pyrophosphatases (12, 19), fungal chloroperoxidase (20), yeast dihydrosphingosine/phytosphingosine phosphate phosphatase (21, 22), two specific mammalian S1P phosphatases (23), mammalian glucose 6-phosphatase (24), and presqualene diphosphate phosphatase (25). The functional requirements for the conserved amino acids in the catalytic domains were established for yeast DPP (26) and mammalian LPP1 (27).
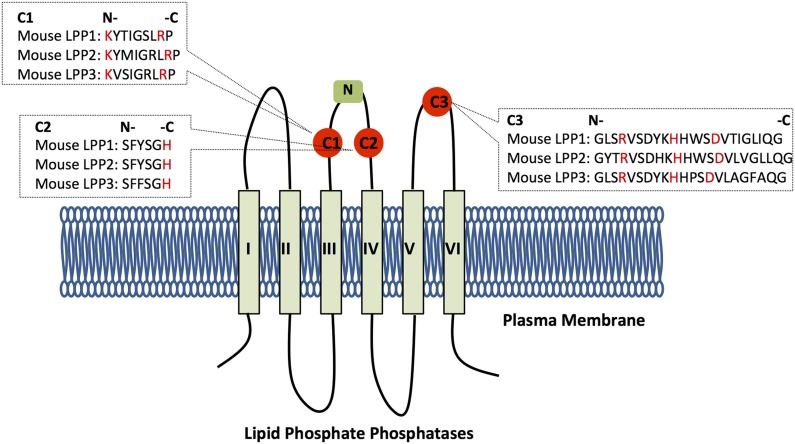
Fig. 1.
Structure of the LPPs. The figure illustrates the structure and orientation of the LPPs in the plasma membrane. The structure of the three conserved catalytic domains (red circles) is shown with the amino acids involved in substrate binding and catalysis highlighted in red. The N-glycosylation site is shown as a green square.
This review is intended to present an up-to-date understanding of the role of the LPPs and especially their functions in various physiological and pathological processes. More information can be obtained by reference to previous reviews on the LPPs and related proteins (28–31).
STRUCTURE AND FUNCTIONS OF THE LPPs
The crystal structure of the LPPs has not yet been solved. However, the orientation of the catalytic site and mechanism of action have been postulated through a combination of computational modeling and the crystallographic structure of a related enzyme, chloroperoxidase (31–33). Chloroperoxidase is a soluble enzyme, and a better understanding of the topology of the LPPs can be gained from the crystal structure of phosphatidylglycerolphosphate phosphatase B from Eshcherichia coli, which, like the LPPs, is an integral membrane protein (34). The LPPs possess six transmembrane α-helices and when located in the plasma membrane, the C- and N-termini face the cytoplasmic side and the three catalytic domains face the extracellular side (Fig. 1) (27). This allows the LPPs to dephosphorylate lipid phosphates outside the cells. Presumably, the active site is expressed on the luminal surface of internal membranes in the cell. The first two conserved catalytic domains, C1 and C2, are localized at the extracellular loop between transmembrane α-helices III and IV (Fig. 1). The external loop contains an N-glycosylation site, which further supports the location of this loop being extracellular (27). The third conserved catalytic domain, C3, is located on the extracellular loop between helices V and VI.
C1 is responsible for substrate recognition, whereas C2 and C3 contain the amino acids required for the phosphotransferase reaction (31). The conserved histidine on C3 acts on the phosphate group as a nucleophile to form a phospho-histidine intermediate (31). The C2 histidine is involved in breaking the phosphate bond to release the dephosphorylated lipid product. The conserved lysine and arginine residues on C1, as well as the arginine on C3, help coordinate the substrate in the active site (14).
LPPs can form homo- and hetero-oligomers, with each subunit capable of functioning independently of the others in the dephosphorylation reactions. These different combinations of oligomeric states could regulate subcellular localization (35). Results from Drosophila melanogaster also show dimerization of Wunen, the homolog of mammalian LPP3, but this is not a requirement for biological function (36).
Dephosphorylation of extracellular LPA by the “ecto-activity” of the LPPs
In order to understand the functions of the LPPs in normal physiology and in pathological conditions, it is necessary to understand how they regulate the turnover of bioactive lipid mediators. A major function of the LPPs is to dephosphorylate extracellular LPA and S1P, which are important regulators of cell division, migration, and survival. LPA is present in extracellular fluids at concentrations of about 100 nM in plasma to 2 μM in serum and other fluids (37, 38). Formation of extracellular LPA is mainly through the hydrolysis of lysophosphatidylcholine (LPC) by autotaxin (ATX) (30), which is a secreted enzyme with lysophospholipase D activity. LPC concentrations in human blood are >200 μM, which provides an abundant supply of LPC for ATX (37). Saturated LPC is produced mainly by lecithin:cholesterol acyltransferase in high density lipoproteins (30). Saturated LPA can also be produced by secretory phospholipase A2 (PLA2), which hydrolyzes PA in microvesicles during inflammation (39) and platelet aggregation (40). However, a large proportion of circulating LPC is polyunsaturated and these LPCs are secreted by hepatocytes and probably by other cells (41, 42). ATX preferentially catalyzes the hydrolysis of mono- and polyunsaturated LPC (42, 43). LPP1 activity is partly responsible for counter-balancing the signaling effects of ATX by degrading circulating LPA.
LPA signals to cells by stimulating at least six G protein-coupled receptors (44, 45). The lack of LPA signaling in ATX knockout mice is embryonically lethal, because ATX and LPA are required for vasculogenesis and neural crest formation in embryos (46, 47). LPA also facilitates wound repair by stimulating platelet aggregation and the migration of fibroblasts, vascular smooth muscle cells, endothelial cells, and keratinocytes into the wounded area (30). LPA mediates lymphocyte extravasation, which is crucial for maintaining immune homeostasis (48, 49). However, in chronically inflamed tissues, high LPA enhances lymphocyte invasion and increases cytokine production in response to repeated micro-injuries and incomplete tissue repair (50–52). LPA signaling plays a major role in neural development and repair (53), neuropathic pain (54), rheumatoid arthritis (55), fertility (38), obesity (56), and cancer (57). All of these actions of LPA could be impacted by LPP action, but this has not yet been investigated fully.
The ecto-activity of LPP1 in vivo has been demonstrated in LPP1 hypomorph mice (PPAP2Atr/tr) (58), which have increased plasma LPA concentrations and a longer half-life for circulating LPA relative to the control mice (12 min versus 3 min). However, it is surprising that the circulating LPA concentrations were not significantly decreased in transgenic mice with LPP1 overexpression (38). Fibroblasts from these transgenic mice responded less to LPA-stimulated migration (59, 60) and showed increased DG accumulation in the cells after stimulation of PA production with phorbol ester (38). These fibroblasts also show 3-fold higher ecto-activity against LPA. Therefore, this unexpected result of circulating LPA in the transgenic mice may suggest a more complex mechanism of LPA regulation in the blood. There was no change in the expressions of LPP2 and LPP3 in the LPP1 hypomorph mice (58). However, it should be noted that the expression level of ATX was not measured in the LPP1 transgenic or LPP1 hypomorph mice. This could be important because ATX expression is controlled partly by negative feedback regulation from LPA (61).
The apparent Km (concentration needed to attain half maximum velocity) for the degradation of exogenous LPA by LPP1 in fibroblasts is about 36 μM (62), which is much higher than physiological concentrations of LPA (100 nM to 2 μM). This indicates that the LPPs can degrade LPA in proportion to its concentration over the physiological and pathological ranges. In addition, extracellular Ca2+ concentrations are about 2 mM and Ca2+ severely decreases LPP1 activity (62). This latter effect could explain some of the differences between results in vitro and in vivo. Salous et al. (63) found that exogenous LPA injected into the circulation of mice is rapidly absorbed by nonparenchymal cells in the liver. Our unpublished results showed the same phenomenon: after injecting [32P]LPA into the circulation of rats, the radioactivity in plasma decreased rapidly and after 2 min, most of the radioactivity accumulated in the liver. Therefore, the liver probably acts as a powerful buffering system to regulate LPA levels in the blood.
Dephosphorylation of extracellular S1P
S1P is the sphingolipid analog of LPA, and it signals through its five G protein-coupled receptors (64). S1P is present in plasma at concentrations from 100 nM to 1 μM (65). Circulatory S1P is bound to albumin, low density lipoproteins, and high density lipoproteins. It can also be carried by erythrocytes (65). S1P is formed inside cells through phosphorylation of sphingosine by sphingosine kinase-1 and -2 (66). Different types of cells, including cancer cells and astrocytes, secrete S1P through the ABC transporters, ABCC1, ABCG2, and ABCA1 (65, 67–69), and by protein spinster homolog-2 (70). S1P is dephosphorylated by two specific S1P phosphatases, which are expressed mainly on the endoplasmic reticulum rather than on the plasma membrane (23). Extracellular S1P is cleared from blood in 15–30 min, and this process depends on a cellular phosphatase activity, but not on S1P-lyase (71). This means that the LPPs should be a major regulator of external S1P concentrations.
Overexpression of LPP1 in HEK293 and human pulmonary artery endothelial cells leads to increased hydrolysis of extracellular S1P (72, 73). The conversion of extracellular S1P to sphingosine by LPP1 facilitates sphingosine uptake, followed by its intracellular conversion to S1P by sphingosine kinase-1 (72). LPP3 is required for hydrolyzing thymic and cerebral S1P to maintain the normal functions of these organs (74, 75). Studies on FTY720 also indicate a role for the LPPs as ecto-enzymes. FTY720 is an analog of sphingosine that is used as an immunomodulatory drug for treating multiple sclerosis (76). FTY720 is converted to FTY720-P by sphingosine kinase-2 (77). Results from cells that overexpress LPP1, -2, and -3 showed that only LPP3 dephosphorylates FTY720-P. LPP3 acted as an ecto-phosphatase in intact cells to control the equilibrium between FTY720 and FTY720-P (77). This result is surprising compared with the broad substrate preference of the LPPs for lipid phosphates (31). Other studies show that LPP1a has the highest activity and affinity for FTY720-P (78). These results suggest that the first extracellular loop, which is different in LPP1a compared with LPP1, is involved in substrate recognition.
Dephosphorylation of other extracellular lipid phosphates
In addition to using extracellular LPA and S1P as substrates, LPPs also degrade extracellular PA and C1P (62). PA and C1P are more hydrophobic than LPA and S1P, and they are not transported to a significant extent by binding to albumin and other proteins in the circulation. PA in the plasma membrane of neutrophils increases endothelial membrane permeability by stimulating intracellular tyrosine kinases (79); this process is suppressed by LPPs, which hydrolyze the extracellular PA. Overexpression of LPPs in HEK293 cells increases the degradation of extracellular PA and the consequent uptake of DG into the cells (80). Exogenously added C1P increases the intracellular concentrations of C1P (81). This is partly explained because the LPPs dephosphorylate exogenous C1P to produce ceramides, which can be phosphorylated back to C1P once they enter the cells. C1P activates cytosolic PLA2 activity, which leads to arachidonate production and increased prostaglandin E2 synthesis in human alveolar epithelial cells (81).
NONCATALYTIC ACTIONS OF LPPs
In addition to its phosphatase activity, human LPP3 interacts with integrins at the plasma membrane. This interaction relies on an arginine-glycine-aspartate (RGD) recognition motif on LPP3, which is located at the second extracellular loop between transmembrane α-helices III and IV. Endothelial cell-to-cell adhesion is promoted by binding of LPP3 to integrins, especially αvβ3 and α5β1 integrins (82). This interaction does not depend on the catalytic LPP activity. Mutation of RGD into arginine-glycine-glutamate (RGE) in human LPP3 can abolish its interaction with integrins. Interestingly, mouse and rat LPP3 contain RGE instead of RGD, but murine LPP3 can also interact with αvβ3 and α5β1 integrins (83). LPP1 possesses arginine-glycine-asparagine (RGN) instead of RGD, and is not able to bind to integrins.
INTRACELLULAR FUNCTIONS OF LPPs
LPPs are also localized on the internal membranes, including the endoplasmic reticulum (84) and Golgi network (10), where, presumably, the catalytic domains face the lumenal sides of these organelles. As such, intracellular LPPs could potentially regulate signal transduction through dephosphorylation of substrates inside cells (Fig. 2). One report shows that overexpression of LPP1 in fibroblasts suppresses the stimulation of cell migration by wls-31, which is a phosphonate analog of LPA that activates LPA1/2 receptors (60). Wls-31 cannot be hydrolyzed by LPPs and therefore the ecto-activity of LPP1 does not contribute to the inhibition. Another report shows LPPs can regulate ERK activation downstream of thrombin signaling (85). Because thrombin is not a LPP substrate, this inhibition in ERK is also not caused by the ecto-activity of LPPs. Results from our group further confirm the intracellular functions of LPPs. MDA-MB-231 cells overexpressing LPP1 also show an inhibition of migration upon wls-31 stimulation, which involves an inhibitory effect on Ca2+-mobilization. LPP1 expression also decreases Ca2+-transients after stimulation of the protease-activated receptor-1. The mechanism of how LPPs affect receptor downstream signaling is not yet known. Because the substrates of LPPs also exist inside cells, degradation of unidentified intracellular substrates may be one of the explanations for the intracellular functions of LPPs (Fig. 2).
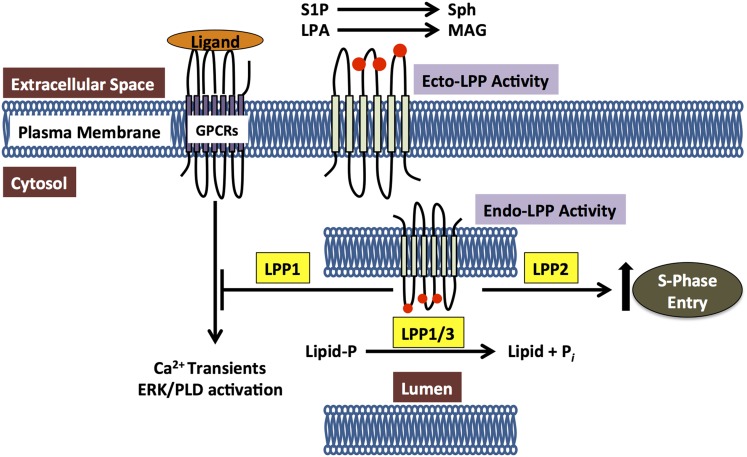
Fig. 2.
Roles of the LPPs in regulating the dephosphorylation of extracellular lipid phosphates at the cell’s surface (ecto-activities) and internal substrates through the intracellular activities. LPPs dephosphorylate extracellular LPA and S1P. LPPs also have intracellular effects, which depend on their catalytic activities. These effects occur downstream of receptor stimulation and they regulate the activations of phospholipase D, ERK, and Ca2+-transients, The lipid targets of these intracellular activities are unknown.
One of the possible substrates for intracellular LPPs is PA, which activates a variety of targets, including Sos, Raf, ERK, mTOR, Akt, and sphingosine kinase-1 (86–88). The LPPs have the potential to convert intracellular PA into DG. DG produced from PA can activate the classical and novel protein kinase Cs and Ras guanyl-releasing protein 1 (29). Increasing LPP1 and LPP2 expression can decrease intracellular PA/DG ratios (89). Overexpression of LPP3 in Swiss 3T3 and HEK293 cells increases the conversion of PA to DG (90). LPP3 depletion decreases the levels of de novo synthesized DG and the Golgi-associated DG content, which impairs protein trafficking in the early secretory pathway (91). Furthermore, LPP2 and LPP3 decrease cell survival by regulating the intracellular levels of PA (92). It should also be noted that a large proportion of intracellular PA is formed by phospholipase D (PLD) activation. PLD produces PA on the cytosolic sides of the membranes, which is opposite to the orientation of the catalytic domains of LPPs. Therefore, PA should not be hydrolyzed unless it can be efficiently transported to the lumenal sides of organelles. Although the evidence listed above strongly suggests a potential role of LPPs in regulating intracellular PA accumulation, it is still not clear how the PA is able to access the intracellular LPPs. Furthermore, increasing the expression of LPP1 in fibroblasts decreases the effects of LPA and platelet-derived growth factor in activating PLD, which provides an alternative explanation of the observed decrease in steady-state PA concentrations (60). Actually, the lipin family of PAPs is now thought to provide the capacity for degrading PA on the cytosolic surface of membranes (93).
LPPs also degrade intracellular C1P and S1P. Both C1P and S1P are involved in inflammation. C1P activates PLA2 to produce arachidonate, and S1P activates cyclooxygenase-2, which converts arachidonate to prostaglandin E2 (81). S1P in the nucleus binds specifically to the histone deacetylases, HDAC1 and HDAC2, and inhibits their enzymatic activities thus activating gene transcription (94). S1P is also an activator of E3 ligase activity and thereby regulates TNF-α signaling (95). In addition, the levels of ceramide and S1P inside cells determine the apoptotic signaling versus cell proliferation (96). These S1P-mediated processes could be regulated by the LPPs or the two specific intracellular S1P phosphatases, which are structurally related to the LPPs.
NONREDUNDANT FUNCTIONS OF THE LPPs
Although the LPPs have similar structural orientation and topology, they have distinct and nonredundant functions, as is evident from various animal models. Mice that overexpress LPP1 show a 50% decrease in birth weight, and they have abnormalities in fur growth with disrupted hair structure and decreased numbers of hair follicles (38). Male mice show decreased fertility with a severe impairment of spermatogenesis. Female mice that overexpress LPP1 also have defects in fertility because implantation of LPP1-overexpressing or wild-type embryos into pseudo-pregnant LPP1 mothers yields a decreased litter size. However, plasma LPA concentrations are not significantly changed by LPP1 expression (38). This may reflect normal variations in circulating LPA among mice, which mask the role of LPP1. By contrast, work with hypomorph mice that expressed little LPP1 activity, except in the brain, did show higher circulating LPA concentrations (58). This, together with the longer half-life of LPA in the circulation, provides evidence for the importance of the ecto-activity of LPP1 in regulating the turnover of circulating LPA. Significantly, these mice showed no obvious phenotypic differences from the wild-type littermates in their anatomy, behavior, and fertility.
LPP2 (PPAP2C) knockout mice (97) are also fertile and viable. The function of LPP2 in mammalian cells is quite different from that of LPP1 and LPP3. Increased expression of catalytically active, but not inactive, LPP2 in synchronized fibroblasts is associated with a premature entry of cells into the S-phase of the cell cycle. This is accompanied by premature expression of cyclin A (98). LPP2 knockdown has the opposite effect of delaying cyclin A expression and S-phase entry. These effects are not observed with LPP1 and LPP3 where over-expression of these two isoforms normally inhibits cell growth and migration (73, 99). Subsequent studies by Flanagan et al. (100) confirmed the effect of LPP2 expression on the progression of cells through S-phase of the cell cycle. These investigators also compared data from a genomic screen between normal and transformed mesenchymal stem cells. PPAP2C/LPP2 was identified as one target whose expression is elevated in numerous carcinomas and sarcomas (100). In contrast to the targeting of LPP1 and LPP2, knockdown of LPP3 (Ppap2C) in mice produces a very severe phenotype resulting in embryo death around E9.5 (101).
Studies on Drosophila also shed light on the differential functions of LPPs in vivo. Drosophila expresses Wunen and Wunen-2, which are homologs of human LPPs with highly conserved phosphatase domains (102). Wunen and Wunen-2 show similar catalytic activity against LPA relative to mammalian LPPs (102). They are required by Drosophila in controlling primordial germ cell migration and maintaining the septate junction paracellular barrier during development of the trachea (102–104). Unlike vertebrate cells, insect cells do not express LPA and S1P receptors. Therefore, these functions of Wunens are independent of these receptors. Furthermore, mutation of Wunens in Drosophila causes impaired migration of primordial germ cells. The death of germ cells can be rescued by expression of human and mouse LPP3, but not human LPP1 or mouse LPP2 (102, 103).
These combined results demonstrate that individual LPPs have distinct functions that cannot be replaced by other family members. The work also shows that the LPPs appear to control cell migration through a signaling system that may be conserved between flies and mammals.
THE ROLE OF LPPs IN PATHOLOGY
This section will concentrate on the LPPs in mammals, while recognizing that the LPPs have a significant role in plant physiology and pathology (105). A significant portion of our knowledge concerning the physiological and pathological functions of the LPPs comes from studies on the functions of the LPPs in the vasculature and cancer.
LPP3 and the vasculature
Studies have already indicated the close relationship between LPP3 and the vascular system. Strong evidence comes from the phenotype of PPAP2B/LPP3 knockout mice. The mice have a deletion of exon 5 in the LPP3 gene that encodes one of its catalytic domains (101). Knockout mice die between E7.5 and E9.5, and do not form a chorio-allantoic placenta and yolk sac vasculature. A subset of embryos show a shortening of the anterior-posterior axis that was similar to that in axin deficiency, a critical regulator of Wnt signaling. Loss of LPP3 activity results in decreased transcription through the β-catenin-mediated T cell transcription factor (TCF), whereas elevated levels of LPP3 produced the opposite effect (101). This action did not appear to depend totally upon the phosphatase activity of LPP3 because a catalytically inactive LPP3 mutant was partially effective in inhibiting TCF/β-catenin transcription in HEK293 cells. Additional support for a role of LPP3 in axis patterning was provided from the observations that ectopic expression of LPP3 in dorsal blastomeres of Xenopus embryos causes a mild ventralizing effect (101). Furthermore, axis duplication by Xwnt8 or Xwnt3a mRNA is inhibited by coinjection of LPP3 mRNA.
Further work was performed using heterozygous embryos carrying a β-galactosidase reporter gene to monitor the expression pattern of LPP3 in different stages (106). The earliest expression of LPP3 in vessels is seen on vascular mesodermal cells of the yolk sac in E8.5 embryos, and then on blood vessel endothelial cells by E10.5. LPP3 is expressed in thalamus ventricular areas at E12.5. This dynamic expression pattern of LPP3 in vasculature could reflect its critical role during vascular development (106). A mouse model with conditional knockout of LPP3 in endothelial and hematopoietic cells shows similar defects in vasculogenesis and embryo death to those in mice with global knockout of LPP3 (107). The function of LPP3 knockout in endothelial cells of mice was revealed using a tamoxifen-inducible Cre-recombination. LPP3 deficiency in endothelial cells increases vascular permeability and enhanced sensitivity of inflammation-induced vascular leak, which is restored by blocking LPA production or signals through LPA receptors (107). LPP3 also attenuates injury-induced proliferation of carotid artery smooth muscle cells. This effect is partially through hydrolyzing LPA, which inhibits the LPA stimulation of smooth muscle cell migration and Rho activation (108). Furthermore, LPP3 is involved in suppressing abnormal accelerated lymphangiogenesis. Knockdown of LPP3 significantly increases capillary formation of human lymphatic endothelial cells (109). A reporter-null allele of PPAP2B/LPP3 was used to make a heterozygous genotype in mice, and this showed that LPP3 is dynamically expressed in limb buds, mammary gland primordia, and heart cushions and valves, among others (106). This suggests that LPP3 plays a key role in regulating multiple signaling pathways during development.
Consistent with data from animal models, genome-wide association studies with a meta-analysis, which includes over 86,000 individuals, identified SNPs in the PPAP2B gene. These represent novel loci, which are strongly associated with coronary artery disease (110). Erbilgin et al. (111) performed expression quantitative locus mapping in normal and pathologically changed vascular tissues to characterize the roles of candidate genes identified from the genome-wide association studies. PPAP2B is one of seven genes, which play a causal role in atherosclerosis. These results support the hypothesis that LPP3 is required for maintaining blood vessel function and LPP3 deficiency increases the risk of coronary disease. It should be noted that some chronic diseases could affect the role of PPAP2B as a predictor. One analysis based on CaTS, power calculator for two stage association studies, did not find any association between PPAP2B and cardiovascular disease in 2,140 rheumatoid arthritis patients (112).
LPA and S1P are two substrates of LPP3 and both play important roles in vascular development. Mice lacking ENPP2, the gene encoding ATX, die in utero at E9.5 with severe defects in the vasculature of the yolk sac and embryo and in neural crest formation (46). These results demonstrate the importance of LPA in tissue remodeling and vasculogenesis. ENPP2−/− embryos have increased expression of vascular endothelial growth factor (VEGF) mRNA, consistent with hypoxic conditions as a consequence of the absence of a functional vascular system (46, 47). The requirement of ATX in vascular development has also been established in zebrafish models (113, 114), demonstrating that LPA or other products derived from ATX activity are required for embryonic blood vessel formation.
S1P increases endothelial cell migration and angiogenesis (64, 115). It does this directly through S1P1 receptors (116), by promoting transactivation of VEGF receptors (117) and by increasing VEGF release (118–121). S1P also shows a critical role in maintaining endothelial barrier integrity by stabilizing tight and adherent junctions between cells (122). Decreases in S1P or S1P receptor antagonists induce vascular leakage (123). It is possible that LPP3 also contributes to vascular function in a phosphatase-independent manner, as illustrated with a catalytically inactive LPP3 mutant, which was partially effective in inhibiting β-catenin-mediated TCF transcription in HEK293 cells (101). This possibility is also supported by a report from Humtsoe et al. (124). In their study, LPP3 stimulated β-catenin/lymphoid enhancer binding factor-1 to induce endothelial cell migration and formation of branching point structures, which regulated endothelial homeostasis. Catalytically inactive LPP3 shows the same effect as the wild-type LPP3 (124).
Role of the ATX-LPA-LPP axis in cancer
LPPs have the potential to regulate tumor progression because LPA signaling is involved at multiple levels in this process. To understand the potential regulatory effects of the LPPs, we will first discuss the relation of ATX activity and LPA production in cancer. Increased ATX activity and LPA signaling are positively correlated to the invasive and metastatic potential of many cancers (57, 125). ATX is among the top 40 most upregulated genes in patients with highly metastatic cancers (126). The importance of LPA in cancer progression was demonstrated because transgenic mice that overexpress ATX, LPA1, LPA2, or LPA3 receptors in mammary cells develop spontaneous metastatic mammary tumors (127). LPA receptors differentially stimulate cell survival and migration through the relative activations of phosphatidylinositol 3-kinase, ERK1/2, mTOR, Ca2+-transients, Rac, Rho, and Ras. LPA increases VEGF production, which stimulates angiogenesis (128), a process necessary for tumor progression. LPA also decreases the expression of the tumor suppressor, p53, thus promoting cancer cell survival and division (129).
High concentrations of LPA are well-documented in the peritoneal ascites fluid and plasma of patients with ovarian cancer (130, 131), and LPA stimulates ovarian tumor growth and metastasis (132). In a colon cancer cell line, LPA increases the synthesis of macrophage migration inhibitory factor, which also promotes tumor growth (133). Women with breast carcinomas that express high levels of LPA3 receptors in epithelial cells, or ATX in stromal cells, have larger tumors, nodal involvement, and higher stage disease (134).
LPA also adversely affects cancer therapies by producing resistance to the cytotoxic effects of Taxol (125, 135, 136), a common first line treatment for breast cancer. This work was confirmed (137) and extended to LPA-induced resistance to carboplatin (138) and radiation-induced cell death (125, 139, 140). LPA also attenuates doxorubicin-induced killing of breast cancer cells. This results partly from the LPA-induced increase in the stability of the transcription factor, Nrf2, which stimulates the anti-oxidant response element to increase the expression of the multidrug resistant transporters, ABCC1 and ABCG2, and anti-oxidant genes (141). The anti-oxidant enzymes decrease the extent of oxidative damage produced by many chemotherapeutic agents. Increased expression of multidrug resistant transporters facilitates the export of several chemotherapeutic agents, including doxorubicin, and also toxic oxidation products, thus contributing to chemo-resistance. ABCC1 and ABCG2 might also facilitate the secretion of S1P, especially if the S1P transporter, spinster homolog-2, is absent (142). This S1P secretion contributes to chemo-resistance (143) and it stimulates angiogenesis, which is required by the growing tumor. Thus LPA and S1P coordinate the tumor growth (125, 139) and the invasion of breast cancer cells into surrounding tissue to form metastases (121). Blocking signaling by LPA (144) or S1P (65, 145) has, therefore, been proposed as an important therapeutic target for the treatment of cancer. We recently showed that inhibition of ATX and LPA formation decreases breast tumor growth and metastasis and increases the efficacy of doxorubicin therapy (141, 146). Blocking the formation of S1P using inhibitors of sphingosine kinase-1 (147), or increasing S1P breakdown through S1P lyase (148) also has therapeutic potential for cancer treatment.
An alternative approach to targeting LPA signaling is to increase LPA and S1P degradation through the LPPs, which could also decrease their abilities to signal downstream of receptor activation in cancer cells. This can be achieved by increasing the low levels of expression of LPP1 and LPP3, which are observed in several types of cancer from the analysis of gene arrays published in (149–151) and accessed through Oncomine™ (152, 153) (Fig. 3). In support of this hypothesis, overexpressing LPP1 in ovarian cancer cell lines increases LPA hydrolysis. This is associated with a marked inhibition of cell proliferation and colony-forming activity and increased apoptosis (154). LPP3 shows similar effects in SKOV3 and OVCAR-3 ovarian cancer cells. LPP3 promotes LPA hydrolysis in the medium and decreases LPA-stimulated colony formation. This effect of LPP3 depends on its ecto-activity and this effect was diminished by using a nonhydrolyzable LPA analog instead of LPA (99). Gonadotropin-releasing hormone increases ecto-LPP expression in ovarian cancer cells and this explains its anti-proliferative effects (155). These studies with cultured cancer cells were validated by measuring tumor growth from SKOV3 ovarian carcinoma cells in nude mice. Cells with overexpressed human LPP3 showed much lower tumor growth (99). The authors ascribed this result to the ecto-phosphatase activities of LPP3 at a time when the effects, which occur downstream of receptor activation, were not widely appreciated.
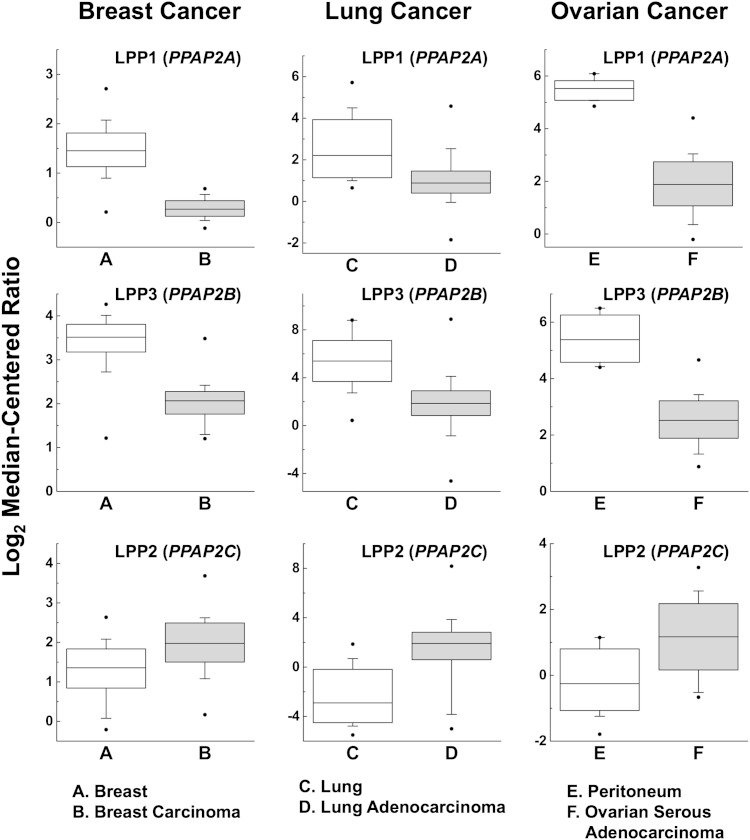
Fig. 3.
Relative expressions of the LPPs in breast, lung, and ovarian cancers. LPP1 and LPP3 expression is decreased in cancers, whereas LPP2 activity is increased. The results for breast (149), lung (150), and ovarian cancers (151) are taken from published gene arrays through the free OncomineTM Research Edition platform (152, 153). LPP expressions in other cancers from other gene arrays can also be obtained from this resource.
We expressed LPP1 and a catalytically inactive mutant, LPP1(R217K), using a doxycycline-inducible promoter in various cancer cells and determined their phenotypes (73). Cells that overexpressed LPP1 show increased hydrolysis of LPA and S1P. Expression of wild-type LPP1, but not the catalytically inactive LPP1, suppresses cell growth in 3D culture and LPA-stimulated cell migration. This latter effect on migration could be related to a decrease in LPA-induced Ca2+-transients and activation of Rho. LPP1 expression also decreased the stimulation of Ca2+-transients and migration by wls-31. This phosphonate analog of LPA, which activates LPA1/2 receptors, cannot be degraded by LPPs. LPP1 expression also attenuated the stimulation Ca2+-transients by the protease-activated receptor-1 peptide, which does not involve LPA receptors. Therefore, the major effects of LPP1 cannot be explained by the degradation of exogenous LPA. Instead, the results put emphasis on the postreceptor signaling effects of LPP1 (Fig. 2).
Zhao et al. (156) also showed that LPP1 expressed in human bronchial epithelial cells inhibits Ca2+-transients and phosphorylation of inhibitory kappa B, followed by NF-κB activation and decreased interleukin-8 production. This work identified a potential role of LPP1 in the regulation of LPA-induced inflammation, which is a major driving stimulus in tumor growth, metastasis, and chemo-resistance (57, 144).
We tested the effects of LPP1 expression in breast and thyroid cancer cells on their abilities to form tumors in mice. Increasing the expression of LPP1 decreased tumor growth and metastasis by up to 80% compared with expression of the inactive LPP1 (R217K) mutant or green fluorescent protein (73). Increasing LPP1 expression in the cancer cells alone did not change the concentrations of LPA in either the tumor or plasma. This work demonstrates in vivo that targeting LPPs could be a beneficial strategy for cancer therapy and that the major effects of LPP1 could be mediated downstream of receptor activation.
Rather than targeting LPP signaling in cancer cells, Nakayama et al. (157) recently examined the contribution of LPP1 expression in the tumor microenvironment to cancer cell seeding. Intraperitoneal injection of syngeneic ovarian cancer cells into LPP1 knockout mice leads to enhanced cancer cell seeding compared with wild-type mice (157). Presumably, higher systemic levels of LPA can explain this result as a consequence of decreased LPA turnover in LPP1 knockout mice compared with wild-type controls (58, 157). Another study showed that mice lacking LPA1 or LPA5 receptors had decreased melanoma-derived lung metastasis compared with wild-type mice following tail-vein injection of B16F10 melanoma cells (158). These experiments demonstrate the importance of LPA signaling for establishing a suitable environment for cancer cell seeding and subsequent growth.
The effects of LPP2 on tumor growth appear to be quite different from LPP1 and LPP3. Flanagan et al. (100) compared the data from a genomic screen between normal and transformed mesenchymal stem cells and identified PPAP2C/LPP2 as a target whose expression is elevated in numerous carcinomas and sarcomas. This increase in LPP2 is seen in breast, lung, and ovarian cancers where the expressions of LPP1 and LPP3 are decreased (Fig. 3). Overexpressing LPP2 leads to premature S-phase entry, and this effect is not seen with LPP1 and LPP3 (98). Knockdown of LPP2 delays entry into S-phase (98) and impairs anchorage-dependent growth (100). This combined work provides preliminary evidence that increasing the low expressions of LPP1 and LPP3 and decreasing the high expression of LPP2 in cancer cells could provide novel targets for cancer therapy.
CONCLUSION
LPPs play an important role in regulating LPA/S1P signaling through the degradation of these bioactive lipids. LPPs are widely involved in many pathophysiological processes, especially in embryo development, vasculogenesis, and tumorigenesis. It is clear that LPP1, -2, and -3 have distinct and nonredundant functions besides their common phosphatase activities in these processes. The different actions of the LPPs may be attributed to their different preference for substrates, different localizations, effects on postreceptor signaling (159), and their noncatalytic functions. The different functions of the LPPs are not completely understood and they warrant further investigation. The development of activators or inhibitors for the LPPs could provide novel therapies for the treatment of vascular diseases and cancers.
Footnotes
Abbreviations:
- ATX
- autotaxin
- C1P
- ceramide 1-phosphate
- DG
- diacylglycerol
- DPP
- diacylglycerol pyrophosphate phosphatase
- LPA
- lysophosphatidic acid, which at physiological pH is in a salt form, i.e., lysophosphatidate
- LPC
- lysophosphatidylcholine
- LPP
- lipid phosphate phosphatases
- PA
- phosphatidate, PAP, phosphatidate phosphatase
- PAP1
- type 1 Mg2+-dependent phosphatidate phosphatase
- PAP2
- type 2 phosphatidate phosphatase
- PLA2
- phospholipase A2
- PLD
- phospholipase D
- S1P
- sphingosine 1-phosphate
- TCF
- T cell transcription factor
- VEGF
- vascular endothelial growth factor
X.T. held a research fellowship from the Canadian Breast Cancer Foundation (CBCF). M.G.K.B. received a Vanier Canada Graduate Scholarship (Government of Canada), a Canadian Institutes of Health Research (CIHR) MD/PhD Studentship, a MD/PhD Studentship from Alberta Innovates-Health Solutions, and a Killam Trust Award. D.N.B. has been supported by grants from CBCF, CIHR with Alberta Cancer Foundation, and Ono Pharmaceuticals Ltd.
REFERENCES
- 1.Jamal Z., Martin A., Gomez-Munoz A., Brindley D. N. 1991. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J. Biol. Chem. 266: 2988–2996. [PubMed] [Google Scholar]
- 2.Kok B. P., Venkatraman G., Capatos D., Brindley D. N. 2012. Unlike two peas in a pod: lipid phosphate phosphatases and phosphatidate phosphatases. Chem. Rev. 112: 5121–5146. [DOI] [PubMed] [Google Scholar]
- 3.Han G. S., Wu W. I., Carman G. M. 2006. The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281: 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donkor J., Sariahmetoglu M., Dewald J., Brindley D. N., Reue K. 2007. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282: 3450–3457. [DOI] [PubMed] [Google Scholar]
- 5.Waggoner D. W., Gomez-Munoz A., Dewald J., Brindley D. N. 1996. Phosphatidate phosphohydrolase catalyzes the hydrolysis of ceramide 1-phosphate, lysophosphatidate, and sphingosine 1-phosphate. J. Biol. Chem. 271: 16506–16509. [DOI] [PubMed] [Google Scholar]
- 6.Dillon D. A., Chen X., Zeimetz G. M., Wu W. I., Waggoner D. W., Dewald J., Brindley D. N., Carman G. M. 1997. Mammalian Mg2+-independent phosphatidate phosphatase (PAP2) displays diacylglycerol pyrophosphate phosphatase activity. J. Biol. Chem. 272: 10361–10366. [DOI] [PubMed] [Google Scholar]
- 7.Hooks S. B., Ragan S. P., Lynch K. R. 1998. Identification of a novel human phosphatidic acid phosphatase type 2 isoform. FEBS Lett. 427: 188–192. [DOI] [PubMed] [Google Scholar]
- 8.Brindley D. N., Waggoner D. W. 1998. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 273: 24281–24284. [DOI] [PubMed] [Google Scholar]
- 9.Kai M., Wada I., Imai S., Sakane F., Kanoh H. 1996. Identification and cDNA cloning of 35-kDa phosphatidic acid phosphatase (type 2) bound to plasma membranes. Polymerase chain reaction amplification of mouse H2O2-inducible hic53 clone yielded the cDNA encoding phosphatidic acid phosphatase. J. Biol. Chem. 271: 18931–18938. [DOI] [PubMed] [Google Scholar]
- 10.Kai M., Wada I., Imai S., Sakane F., Kanoh H. 1997. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J. Biol. Chem. 272: 24572–24578. [DOI] [PubMed] [Google Scholar]
- 11.Toke D. A., Bennett W. L., Oshiro J., Wu W. I., Voelker D. R., Carman G. M. 1998. Isolation and characterization of the Saccharomyces cerevisiae LPP1 gene encoding a Mg2+-independent phosphatidate phosphatase. J. Biol. Chem. 273: 14331–14338. [DOI] [PubMed] [Google Scholar]
- 12.Toke D. A., Bennett W. L., Dillon D. A., Wu W. I., Chen X., Ostrander D. B., Oshiro J., Cremesti A., Voelker D. R., Fischl A. S., et al. 1998. Isolation and characterization of the Saccharomyces cerevisiae DPP1 gene encoding diacylglycerol pyrophosphate phosphatase. J. Biol. Chem. 273: 3278–3284. [DOI] [PubMed] [Google Scholar]
- 13.Hemrika W., Renirie R., Dekker H. L., Bernett P., Wever R. 1997. From phosphatases to vanadium peroxidases: a similar architecture of the active site. Proc. Natl. Acad. Sci. USA. 94: 2145–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuwald A. F. 1997. An unexpected structural relationship between integral membrane phosphatases and soluble haloperoxidases. Protein Sci. 6: 1764–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brindley D. N., English D., Pilquil C., Buri K., Ling Z. C. 2002. Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim. Biophys. Acta. 1582: 33–44. [DOI] [PubMed] [Google Scholar]
- 16.Carman G. M., Han G. S. 2009. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 284: 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stukey J., Carman G. M. 1997. Identification of a novel phosphatase sequence motif. Protein Sci. 6: 469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Icho T., Raetz C. R. 1983. Multiple genes for membrane-bound phosphatases in Escherichia coli and their action on phospholipid precursors. J. Bacteriol. 153: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon D. A., Wu W. I., Riedel B., Wissing J. B., Dowhan W., Carman G. M. 1996. The Escherichia coli pgpB gene encodes for a diacylglycerol pyrophosphate phosphatase activity. J. Biol. Chem. 271: 30548–30553. [DOI] [PubMed] [Google Scholar]
- 20.Simons B. H., Barnett P., Vollenbroek E. G., Dekker H. L., Muijsers A. O., Messerschmidt A., Wever R. 1995. Primary structure and characterization of the vanadium chloroperoxidase from the fungus Curvularia inaequalis. Eur. J. Biochem. 229: 566–574. [DOI] [PubMed] [Google Scholar]
- 21.Mao C., Wadleigh M., Jenkins G. M., Hannun Y. A., Obeid L. M. 1997. Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J. Biol. Chem. 272: 28690–28694. [DOI] [PubMed] [Google Scholar]
- 22.Mandala S. M., Thornton R., Tu Z., Kurtz M. B., Nickels J., Broach J., Menzeleev R., Spiegel S. 1998. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. USA. 95: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandala S. M. 2001. Sphingosine-1-phosphate phosphatases. Prostaglandins Other Lipid Mediat. 64: 143–156. [DOI] [PubMed] [Google Scholar]
- 24.Shelly L. L., Lei K. J., Pan C. J., Sakata S. F., Ruppert S., Schutz G., Chou J. Y. 1993. Isolation of the gene for murine glucose-6-phosphatase, the enzyme deficient in glycogen storage disease type 1A. J. Biol. Chem. 268: 21482–21485. [PubMed] [Google Scholar]
- 25.Fukunaga K., Arita M., Takahashi M., Morris A. J., Pfeffer M., Levy B. D. 2006. Identification and functional characterization of a presqualene diphosphate phosphatase. J. Biol. Chem. 281: 9490–9497. [DOI] [PubMed] [Google Scholar]
- 26.Toke D. A., McClintick M. L., Carman G. M. 1999. Mutagenesis of the phosphatase sequence motif in diacylglycerol pyrophosphate phosphatase from Saccharomyces cerevisiae. Biochemistry. 38: 14606–14613. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q. X., Pilquil C. S., Dewald J., Berthiaume L. G., Brindley D. N. 2000. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its sites of action. Biochem. J. 345: 181–184. [PMC free article] [PubMed] [Google Scholar]
- 28.Kok B. P., Kienesberger P. C., Dyck J. R., Brindley D. N. 2012. Relationship of glucose and oleate metabolism to cardiac function in lipin-1 deficient (fld) mice. J. Lipid Res. 53: 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brindley D. N., Pilquil C. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res. 50(Suppl): S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brindley D. N. 2004. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J. Cell. Biochem. 92: 900–912. [DOI] [PubMed] [Google Scholar]
- 31.Sigal Y. J., McDermott M. I., Morris A. J. 2005. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem. J. 387: 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messerschmidt A., Wever R. 1996. X-ray structure of a vanadium-containing enzyme: chloroperoxidase from the fungus Curvularia inaequalis. Proc. Natl. Acad. Sci. USA. 93: 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messerschmidt A., Prade L., Wever R. 1997. Implications for the catalytic mechanism of the vanadium-containing enzyme chloroperoxidase from the fungus Curvularia inaequalis by X-ray structures of the native and peroxide form. Biol. Chem. 378: 309–315. [DOI] [PubMed] [Google Scholar]
- 34.Fan J., Jiang D., Zhao Y., Liu J., Zhang X. C. 2014. Crystal structure of lipid phosphatase Escherichia coli phosphatidylglycerophosphate phosphatase B. Proc. Natl. Acad. Sci. USA. 111: 7636–7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long J. S., Pyne N. J., Pyne S. 2008. Lipid phosphate phosphatases form homo- and hetero-oligomers: catalytic competency, subcellular distribution and function. Biochem. J. 411: 371–377. [DOI] [PubMed] [Google Scholar]
- 36.Burnett C., Makridou P., Hewlett L., Howard K. 2004. Lipid phosphate phosphatases dimerise, but this interaction is not required for in vivo activity. BMC Biochem. 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moolenaar W. H., van Meeteren L. A., Giepmans B. N. 2004. The ins and outs of lysophosphatidic acid signaling. BioEssays. 26: 870–881. [DOI] [PubMed] [Google Scholar]
- 38.Yue J., Yokoyama K., Balazs L., Baker D. L., Smalley D., Pilquil C., Brindley D. N., Tigyi G. 2004. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell. Signal. 16: 385–399. [DOI] [PubMed] [Google Scholar]
- 39.Fourcade O., Simon M. F., Viode C., Rugani N., Leballe F., Ragab A., Fournie B., Sarda L., Chap H. 1995. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 80: 919–927. [DOI] [PubMed] [Google Scholar]
- 40.Sano T., Baker D., Virag T., Wada A., Yatomi Y., Kobayashi T., Igarashi Y., Tigyi G. 2002. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 277: 21197–21206. [DOI] [PubMed] [Google Scholar]
- 41.Brindley D. N. 1993. Hepatic secretion of lysphosphatidylcholine: a novel transport system for polyunsaturated fatty acids and choline. J. Nutr. Biochem. 4: 442–449. [Google Scholar]
- 42.Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., Fukuzawa K. 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 277: 39436–39442. [DOI] [PubMed] [Google Scholar]
- 43.Morishige J., Touchika K., Tanaka T., Satouchi K., Fukuzawa K., Tokumura A. 2007. Production of bioactive lysophosphatidic acid by lysophospholipase D in hen egg white. Biochim. Biophys. Acta. 1771: 491–499. [DOI] [PubMed] [Google Scholar]
- 44.Tigyi G., Parrill A. L. 2003. Molecular mechanisms of lysophosphatidic acid action. Prog. Lipid Res. 42: 498–526. [DOI] [PubMed] [Google Scholar]
- 45.Rivera R., Chun J. 2008. Biological effects of lysophospholipids. Rev. Physiol. Biochem. Pharmacol. 160: 25–46. [DOI] [PubMed] [Google Scholar]
- 46.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., Arai H. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830. [DOI] [PubMed] [Google Scholar]
- 48.Bai Z., Cai L., Umemoto E., Takeda A., Tohya K., Komai Y., Veeraveedu P. T., Hata E., Sugiura Y., Kubo A., et al. 2013. Constitutive lymphocyte transmigration across the basal lamina of high endothelial venules is regulated by the autotaxin/lysophosphatidic acid axis. J. Immunol. 190: 2036–2048. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Chen Y. C., Krummel M. F., Rosen S. D. 2012. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 189: 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakasaki T., Tanaka T., Okudaira S., Hirosawa M., Umemoto E., Otani K., Jin S., Bai Z., Hayasaka H., Fukui Y., et al. 2008. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am. J. Pathol. 173: 1566–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Meeteren L. A., Moolenaar W. H. 2007. Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid Res. 46: 145–160. [DOI] [PubMed] [Google Scholar]
- 52.Budd D. C., Qian Y. 2013. Development of lysophosphatidic acid pathway modulators as therapies for fibrosis. Future Med. Chem. 5: 1935–1952. [DOI] [PubMed] [Google Scholar]
- 53.Mutoh T., Rivera R., Chun J. 2012. Insights into the pharmacological relevance of lysophospholipid receptors. Br. J. Pharmacol. 165: 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma L., Nagai J., Chun J., Ueda H. 2013. An LPA species (18:1 LPA) plays key roles in the self-amplification of spinal LPA production in the peripheral neuropathic pain model. Mol. Pain. 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourgoin S. G., Zhao C. 2010. Autotaxin and lysophospholipids in rheumatoid arthritis. Curr. Opin. Investig. Drugs. 11: 515–526. [PubMed] [Google Scholar]
- 56.Rancoule C., Dusaulcy R., Treguer K., Gres S., Guigne C., Quilliot D., Valet P., Saulnier-Blache J. S. 2012. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J. Physiol. Biochem. 68: 635–644. [DOI] [PubMed] [Google Scholar]
- 57.Benesch M. G., Ko Y. M., McMullen T. P., Brindley D. N. 2014. Autotaxin in the crosshairs: taking aim at cancer and other inflammatory conditions. FEBS Lett. 588: 2712–2727. [DOI] [PubMed] [Google Scholar]
- 58.Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., Lynch K. R. 2009. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 419: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long J. S., Yokoyama K., Tigyi G., Pyne N. J., Pyne S. 2006. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid- and platelet-derived-growth-factor-induced cell migration. Biochem. J. 394: 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pilquil C., Dewald J., Cherney A., Gorshkova I., Tigyi G., English D., Natarajan V., Brindley D. N. 2006. Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation. J. Biol. Chem. 281: 38418–38429. [DOI] [PubMed] [Google Scholar]
- 61.Benesch M. G. K., Zhao Y. Y., Curtis J. M., McMullen T. P. W., Brindley D. N. 2015. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine-1-phosphate J. Lipid Res. 56: 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jasinska R., Zhang Q. X., Pilquil C., Singh I., Xu J., Dewald J., Dillon D. A., Berthiaume L. G., Carman G. M., Waggoner D. W., et al. 1999. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 340: 677–686. [PMC free article] [PubMed] [Google Scholar]
- 63.Salous A. K., Panchatcharam M., Sunkara M., Mueller P., Dong A., Wang Y., Graf G. A., Smyth S. S., Morris A. J. 2013. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J. Lipid Res. 54: 2775–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.English D., Welch Z., Kovala A. T., Harvey K., Volpert O. V., Brindley D. N., Garcia J. G. 2000. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 14: 2255–2265. [DOI] [PubMed] [Google Scholar]
- 65.Takabe K., Paugh S. W., Milstien S., Spiegel S. 2008. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60: 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maceyka M., Harikumar K. B., Milstien S., Spiegel S. 2012. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A., Okajima F. 2007. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J. Neurochem. 103: 2610–2619. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi N., Nishi T., Hirata T., Kihara A., Sano T., Igarashi Y., Yamaguchi A. 2006. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J. Lipid Res. 47: 614–621. [DOI] [PubMed] [Google Scholar]
- 69.Ulrych T., Bohm A., Polzin A., Daum G., Nusing R. M., Geisslinger G., Hohlfeld T., Schror K., Rauch B. H. 2011. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J. Thromb. Haemost. 9: 790–798. [DOI] [PubMed] [Google Scholar]
- 70.Mendoza A., Breart B., Ramos-Perez W. D., Pitt L. A., Gobert M., Sunkara M., Lafaille J. J., Morris A. J., Schwab S. R. 2012. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Reports. 2: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peest U., Sensken S. C., Andreani P., Hanel P., Van Veldhoven P. P., Graler M. H. 2008. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J. Cell. Biochem. 104: 756–772. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y., Kalari S. K., Usatyuk P. V., Gorshkova I., He D., Watkins T., Brindley D. N., Sun C., Bittman R., Garcia J. G., et al. 2007. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 282: 14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang X., Benesch M. G., Dewald J., Zhao Y. Y., Patwardhan N., Santos W. L., Curtis J. M., McMullen T. P., Brindley D. N. 2014. Lipid phosphate phosphatase-1 expression in cancer cells attenuates tumor growth and metastasis in mice. J. Lipid Res. 55: 2389–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bréart B., Ramos-Perez W. D., Mendoza A., Salous A. K., Gobert M., Huang Y., Adams R. H., Lafaille J. J., Escalante-Alcalde D., Morris A. J., et al. 2011. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.López-Juárez A., Morales-Lázaro S., Sánchez-Sánchez R., Sunkara M., Lomelí H., Velasco I., Morris A. J., Escalante-Alcalde D. 2011. Expression of LPP3 in Bergmann glia is required for proper cerebellar sphingosine-1-phosphate metabolism/signaling and development. Glia. 59: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9: 883–897. [DOI] [PubMed] [Google Scholar]
- 77.Mechtcheriakova D., Wlachos A., Sobanov J., Bornancin F., Zlabinger G., Baumruker T., Billich A. 2007. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 581: 3063–3068. [DOI] [PubMed] [Google Scholar]
- 78.Yamanaka M., Anada Y., Igarashi Y., Kihara A. 2008. A splicing isoform of LPP1, LPP1a, exhibits high phosphatase activity toward FTY720 phosphate. Biochem. Biophys. Res. Commun. 375: 675–679. [DOI] [PubMed] [Google Scholar]
- 79.English D., Cui Y., Siddiqui R., Patterson C., Natarajan V., Brindley D. N., Garcia J. G. 1999. Induction of endothelial monolayer permeability by phosphatidate. J. Cell. Biochem. 75: 105–117. [PubMed] [Google Scholar]
- 80.Roberts R. Z., Morris A. J. 2000. Role of phosphatidic acid phosphatase 2a in uptake of extracellular lipid phosphate mediators. Biochim. Biophys. Acta. 1487: 33–49. [DOI] [PubMed] [Google Scholar]
- 81.Pettus B. J., Kitatani K., Chalfant C. E., Taha T. A., Kawamori T., Bielawski J., Obeid L. M., Hannun Y. A. 2005. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol. Pharmacol. 68: 330–335. [DOI] [PubMed] [Google Scholar]
- 82.Humtsoe J. O., Feng S., Thakker G. D., Yang J., Hong J., Wary K. K. 2003. Regulation of cell-cell interactions by phosphatidic acid phosphatase 2b/VCIP. EMBO J. 22: 1539–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Humtsoe J. O., Bowling R. A., Jr, Feng S., Wary K. K. 2005. Murine lipid phosphate phosphohydrolase-3 acts as a cell-associated integrin ligand. Biochem. Biophys. Res. Commun. 335: 906–919. [DOI] [PubMed] [Google Scholar]
- 84.Barilà D., Plateroti M., Nobili F., Muda A. O., Xie Y., Morimoto T., Perozzi G. 1996. The Dri 42 gene, whose expression is up-regulated during epithelial differentiation, encodes a novel endoplasmic reticulum resident transmembrane protein. J. Biol. Chem. 271: 29928–29936. [DOI] [PubMed] [Google Scholar]
- 85.Pyne S., Kong K. C., Darroch P. I. 2004. Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases. Semin. Cell Dev. Biol. 15: 491–501. [DOI] [PubMed] [Google Scholar]
- 86.Nishioka T., Frohman M. A., Matsuda M., Kiyokawa E. 2010. Heterogeneity of phosphatidic acid levels and distribution at the plasma membrane in living cells as visualized by a Foster resonance energy transfer (FRET) biosensor. J. Biol. Chem. 285: 35979–35987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rizzo M. A., Shome K., Vasudevan C., Stolz D. B., Sung T. C., Frohman M. A., Watkins S. C., Romero G. 1999. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 274: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 88.Andresen B. T., Rizzo M. A., Shome K., Romero G. 2002. The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade. FEBS Lett. 531: 65–68. [DOI] [PubMed] [Google Scholar]
- 89.Alderton F., Rakhit S., Kong K. C., Palmer T., Sambi B., Pyne S., Pyne N. J. 2001. Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J. Biol. Chem. 276: 28578–28585. [DOI] [PubMed] [Google Scholar]
- 90.Sciorra V. A., Morris A. J. 1999. Sequential actions of phospholipase D and phosphatidic acid phosphohydrolase 2b generate diglyceride in mammalian cells. Mol. Biol. Cell. 10: 3863–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gutiérrez-Martínez E., Fernández-Ulibarri I., Lázaro-Diéguez F., Johannes L., Pyne S., Sarri E., Egea G. 2013. Lipid phosphate phosphatase 3 participates in transport carrier formation and protein trafficking in the early secretory pathway. J. Cell Sci. 126: 2641–2655. [DOI] [PubMed] [Google Scholar]
- 92.Long J., Darroch P., Wan K. F., Kong K. C., Ktistakis N., Pyne N. J., Pyne S. 2005. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem. J. 391: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brindley D. N., Pilquil C., Sariahmetoglu M., Reue K. 2009. Phosphatidate degradation: phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim. Biophys. Acta. 1791: 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., et al. 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 325: 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alvarez S. E., Harikumar K. B., Hait N. C., Allegood J., Strub G. M., Kim E. Y., Maceyka M., Jiang H., Luo C., Kordula T., et al. 2010. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 465: 1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maceyka M., Payne S. G., Milstien S., Spiegel S. 2002. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta. 1585: 193–201. [DOI] [PubMed] [Google Scholar]
- 97.Zhang N., Sundberg J. P., Gridley T. 2000. Mice mutant for Ppap2c, a homolog of the germ cell migration regulator wunen, are viable and fertile. Genesis. 27: 137–140. [DOI] [PubMed] [Google Scholar]
- 98.Morris K. E., Schang L. M., Brindley D. N. 2006. Lipid phosphate phosphatase-2 activity regulates S-phase entry of the cell cycle in Rat2 fibroblasts. J. Biol. Chem. 281: 9297–9306. [DOI] [PubMed] [Google Scholar]
- 99.Tanyi J. L., Morris A. J., Wolf J. K., Fang X., Hasegawa Y., Lapushin R., Auersperg N., Sigal Y. J., Newman R. A., Felix E. A., et al. 2003. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 63: 1073–1082. [PubMed] [Google Scholar]
- 100.Flanagan J. M., Funes J. M., Henderson S., Wild L., Carey N., Boshoff C. 2009. Genomics screen in transformed stem cells reveals RNASEH2A, PPAP2C, and ADARB1 as putative anticancer drug targets. Mol. Cancer Ther. 8: 249–260. [DOI] [PubMed] [Google Scholar]
- 101.Escalante-Alcalde D., Hernandez L., Le Stunff H., Maeda R., Lee H. S., Jr Gang C., Sciorra V. A., Daar I., Spiegel S., Morris A. J., et al. 2003. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 130: 4623–4637. [DOI] [PubMed] [Google Scholar]
- 102.Burnett C., Howard K. 2003. Fly and mammalian lipid phosphate phosphatase isoforms differ in activity both in vitro and in vivo. EMBO Rep. 4: 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ile K. E., Tripathy R., Goldfinger V., Renault A. D. 2012. Wunen, a Drosophila lipid phosphate phosphatase, is required for septate junction-mediated barrier function. Development. 139: 2535–2546. [DOI] [PubMed] [Google Scholar]
- 104.Renault A. D., Kunwar P. S., Lehmann R. 2010. Lipid phosphate phosphatase activity regulates dispersal and bilateral sorting of embryonic germ cells in Drosophila. Development. 137: 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pierrugues O., Brutesco C., Oshiro J., Gouy M., Deveaux Y., Carman G. M., Thuriaux P., Kazmaier M. 2001. Lipid phosphate phosphatases in Arabidopsis. Regulation of the AtLPP1 gene in response to stress. J. Biol. Chem. 276: 20300–20308. [DOI] [PubMed] [Google Scholar]
- 106.Escalante-Alcalde D., Morales S. L., Stewart C. L. 2009. Generation of a reporter-null allele of Ppap2b/Lpp3 and its expression during embryogenesis. Int. J. Dev. Biol. 53: 139–147. [DOI] [PubMed] [Google Scholar]
- 107.Panchatcharam M., Salous A. K., Brandon J., Miriyala S., Wheeler J., Patil P., Sunkara M., Morris A. J., Escalante-Alcalde D., Smyth S. S. 2014. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol. 34: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panchatcharam M., Miriyala S., Salous A., Wheeler J., Dong A., Mueller P., Sunkara M., Escalante-Alcalde D., Morris A. J., Smyth S. S. 2013. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 33: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Senda K., Koizumi K., Prangsaengtong O., Minami T., Suzuki S., Takasaki I., Tabuchi Y., Sakurai H., Doki Y., Misaki T., et al. 2009. Inducible capillary formation in lymphatic endothelial cells by blocking lipid phosphate phosphatase-3 activity. Lymphat. Res. Biol. 7: 69–74. [DOI] [PubMed] [Google Scholar]
- 110.Schunkert H., König I. R., Kathiresan S., Reilly M. P., Assimes T. L., Holm H., Preuss M., Stewart A. F., Barbalic M., Gieger C., et al. 2011. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erbilgin A., Civelek M., Romanoski C. E., Pan C., Hagopian R., Berliner J. A., Lusis A. J. 2013. Identification of CAD candidate genes in GWAS loci and their expression in vascular cells. J. Lipid Res. 54: 1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.López-Mejías R., Genre F., Garcia-Bermúdez M., Ubilla B., Castañeda S., Llorca J., González-Juanatey C., Corrales A., Miranda-Filloy J. A., Pina T., et al. 2014. Lack of association between ABO, PPAP2B, ADAMST7, PIK3CG, and EDNRA and carotid intima-media thickness, carotid plaques, and cardiovascular disease in patients with rheumatoid arthritis. Mediators Inflamm. 2014: 756279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yukiura H., Hama K., Nakanaga K., Tanaka M., Asaoka Y., Okudaira S., Arima N., Inoue A., Hashimoto T., Arai H., et al. 2011. Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J. Biol. Chem. 286: 43972–43983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moolenaar W. H., Houben A. J., Lee S. J., van Meeteren L. A. 2013. Autotaxin in embryonic development. Biochim. Biophys. Acta. 1831: 13–19. [DOI] [PubMed] [Google Scholar]
- 115.English D., Kovala A. T., Welch Z., Harvey K. A., Siddiqui R. A., Brindley D. N., Garcia J. G. 1999. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J. Hematother. Stem Cell Res. 8: 627–634. [DOI] [PubMed] [Google Scholar]
- 116.English D., Brindley D. N., Spiegel S., Garcia J. G. 2002. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim. Biophys. Acta. 1582: 228–239. [DOI] [PubMed] [Google Scholar]
- 117.Tanimoto T., Jin Z. G., Berk B. C. 2002. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J. Biol. Chem. 277: 42997–43001. [DOI] [PubMed] [Google Scholar]
- 118.Heo K., Park K. A., Kim Y. H., Kim S. H., Oh Y. S., Kim I. H., Ryu S. H., Suh P. G. 2009. Sphingosine 1-phosphate induces vascular endothelial growth factor expression in endothelial cells. BMB Rep. 42: 685–690. [DOI] [PubMed] [Google Scholar]
- 119.Sun H. Y., Wei S. P., Xu R. C., Xu P. X., Zhang W. C. 2010. Sphingosine-1-phosphate induces human endothelial VEGF and MMP-2 production via transcription factor ZNF580: novel insights into angiogenesis. Biochem. Biophys. Res. Commun. 395: 361–366. [DOI] [PubMed] [Google Scholar]
- 120.Balthasar S., Bergelin N., Lof C., Vainio M., Andersson S., Tornquist K. 2008. Interactions between sphingosine-1-phosphate and vascular endothelial growth factor signalling in ML-1 follicular thyroid carcinoma cells. Endocr. Relat. Cancer. 15: 521–534. [DOI] [PubMed] [Google Scholar]
- 121.Boucharaba A., Guillet B., Menaa F., Hneino M., van Wijnen A. J., Clezardin P., Peyruchaud O. 2009. Bioactive lipids lysophosphatidic acid and sphingosine 1-phosphate mediate breast cancer cell biological functions through distinct mechanisms. Oncol. Res. 18: 173–184. [Erratum. 2009. Oncol. Res. 18: 357.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerage D., Brindley D. N., Hemmings D. G. 2014. Review: novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta. 35(Suppl): S86–S92. [DOI] [PubMed] [Google Scholar]
- 123.Sanchez T., Estrada-Hernandez T., Paik J. H., Wu M. T., Venkataraman K., Brinkmann V., Claffey K., Hla T. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278: 47281–47290. [DOI] [PubMed] [Google Scholar]
- 124.Humtsoe J. O., Liu M., Malik A. B., Wary K. K. 2010. Lipid phosphate phosphatase 3 stabilization of beta-catenin induces endothelial cell migration and formation of branching point structures. Mol. Cell. Biol. 30: 1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samadi N., Bekele R., Capatos D., Venkatraman G., Sariahmetoglu M., Brindley D. N. 2011. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie. 93: 61–70. [DOI] [PubMed] [Google Scholar]
- 126.Euer N., Schwirzke M., Evtimova V., Burtscher H., Jarsch M., Tarin D., Weidle U. H. 2002. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 22: 733–740. [PubMed] [Google Scholar]
- 127.Liu S., Umezu-Goto M., Murph M., Lu Y., Liu W., Zhang F., Yu S., Stephens L. C., Cui X., Murrow G., et al. 2009. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 15: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.So J., Wang F. Q., Navari J., Schreher J., Fishman D. A. 2005. LPA-induced epithelial ovarian cancer (EOC) in vitro invasion and migration are mediated by VEGF receptor-2 (VEGF-R2). Gynecol. Oncol. 97: 870–878. [DOI] [PubMed] [Google Scholar]
- 129.Murph M. M., Hurst-Kennedy J., Newton V., Brindley D. N., Radhakrishna H. 2007. Lysophosphatidic acid decreases the nuclear localization and cellular abundance of the p53 tumor suppressor in A549 lung carcinoma cells. Mol. Cancer Res. 5: 1201–1211. [DOI] [PubMed] [Google Scholar]
- 130.Fang X., Schummer M., Mao M., Yu S., Tabassam F. H., Swaby R., Hasegawa Y., Tanyi J. L., LaPushin R., Eder A., et al. 2002. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta. 1582: 257–264. [DOI] [PubMed] [Google Scholar]
- 131.Baker D. L., Morrison P., Miller B., Riely C. A., Tolley B., Westermann A. M., Bonfrer J. M., Bais E., Moolenaar W. H., Tigyi G. 2002. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 287: 3081–3082. [DOI] [PubMed] [Google Scholar]
- 132.Li H., Wang D., Zhang H., Kirmani K., Zhao Z., Steinmetz R., Xu Y. 2009. Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol. Cancer Ther. 8: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 133.Sun B., Nishihira J., Suzuki M., Fukushima N., Ishibashi T., Kondo M., Sato Y., Todo S. 2003. Induction of macrophage migration inhibitory factor by lysophosphatidic acid: relevance to tumor growth and angiogenesis. Int. J. Mol. Med. 12: 633–641. [PubMed] [Google Scholar]
- 134.Popnikolov N. K., Dalwadi B. H., Thomas J. D., Johannes G. J., Imagawa W. T. 2012. Association of autotaxin and lysophosphatidic acid receptor 3 with aggressiveness of human breast carcinoma. Tumour Biol. 33: 2237–2243. [DOI] [PubMed] [Google Scholar]
- 135.Samadi N., Gaetano C., Goping I. S., Brindley D. N. 2009. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene. 28: 1028–1039. [DOI] [PubMed] [Google Scholar]
- 136.Samadi N., Bekele R. T., Goping I. S., Schang L. M., Brindley D. N. 2011. Lysophosphatidate induces chemo-resistance by releasing breast cancer cells from Taxol-induced mitotic arrest. PLoS ONE. 6: e20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang R., Wang J., Ma S., Huang Z., Zhang G. 2011. Requirement of Osteopontin in the migration and protection against Taxol-induced apoptosis via the ATX-LPA axis in SGC7901 cells. BMC Cell Biol. 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vidot S., Witham J., Agarwal R., Greenhough S., Bamrah H. S., Tigyi G. J., Kaye S. B., Richardson A. 2010. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell. Signal. 22: 926–935. [DOI] [PubMed] [Google Scholar]
- 139.Bekele R. T., Brindley D. N. 2012. Role of autotaxin and lysophosphatidate in cancer progression and resistance to chemotherapy and radiotherapy. Clin. Lipidol. 7: 313–328. [Google Scholar]
- 140.Deng W., Shuyu E., Tsukahara R., Valentine W. J., Durgam G., Gududuru V., Balazs L., Manickam V., Arsura M., VanMiddlesworth L., et al. 2007. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 132: 1834–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Venkatraman G., Benesch M. G., Tang X., Dewald J., McMullen T. P., Brindley D. N. 2015. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment. FASEB J. 29: 772–785. [DOI] [PubMed] [Google Scholar]
- 142.Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J., et al. 2012. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sukocheva O., Wang L., Verrier E., Vadas M. A., Xia P. 2009. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 150: 4484–4492. [DOI] [PubMed] [Google Scholar]
- 144.Brindley D. N., Lin F. T., Tigyi G. J. 2013. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta. 1831: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pyne N. J., Pyne S. 2010. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer. 10: 489–503. [DOI] [PubMed] [Google Scholar]
- 146.Benesch M. G., Tang X., Maeda T., Ohhata A., Zhao Y. Y., Kok B. P., Dewald J., Hitt M., Curtis J. M., McMullen T. P., et al. 2014. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 28: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 147.Kapitonov D., Allegood J. C., Mitchell C., Hait N. C., Almenara J. A., Adams J. K., Zipkin R. E., Dent P., Kordula T., Milstien S., et al. 2009. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 69: 6915–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leong W. I., Saba J. D. 2010. S1P metabolism in cancer and other pathological conditions. Biochimie. 92: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Curtis C., Shah S. P., Chin S. F., Turashvili G., Rueda O. M., Dunning M. J., Speed D., Lynch A. G., Samarajiwa S., Yuan Y., et al. 2012. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 486: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bhattacharjee A., Richards W. G., Staunton J., Li C., Monti S., Vasa P., Ladd C., Beheshti J., Bueno R., Gillette M., et al. 2001. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl. Acad. Sci. USA. 98: 13790–13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshihara K., Tajima A., Komata D., Yamamoto T., Kodama S., Fujiwara H., Suzuki M., Onishi Y., Hatae M., Sueyoshi K., et al. 2009. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates ZEB2 in tumor progression and prognosis. Cancer Sci. 100: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rhodes D. R., Yu J., Shanker K., Deshpande N., Varambally R., Ghosh D., Barrette T., Pandey A., Chinnaiyan A. M. 2004. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rhodes D. R., Kalyana-Sundaram S., Mahavisno V., Varambally R., Yu J., Briggs B. B., Barrette T. R., Anstet M. J., Kincead-Beal C., Kulkarni P., et al. 2007. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 9: 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tanyi J. L., Hasegawa Y., Lapushin R., Morris A. J., Wolf J. K., Berchuck A., Lu K., Smith D. I., Kalli K., Hartmann L. C., et al. 2003. Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer. Clin. Cancer Res. 9: 3534–3545. [PubMed] [Google Scholar]
- 155.Imai A., Furui T., Tamaya T., Mills G. B. 2000. A gonadotropin-releasing hormone-responsive phosphatase hydrolyses lysophosphatidic acid within the plasma membrane of ovarian cancer cells. J. Clin. Endocrinol. Metab. 85: 3370–3375. [DOI] [PubMed] [Google Scholar]
- 156.Zhao Y., Usatyuk P. V., Cummings R., Saatian B., He D., Watkins T., Morris A., Spannhake E. W., Brindley D. N., Natarajan V. 2005. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem. J. 385: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakayama J., Raines T. A., Lynch K. R., Slack-Davis J. K. 2015. Decreased peritoneal ovarian cancer growth in mice lacking expression of lipid phosphate phosphohydrolase 1. PLoS ONE. 10: e0120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee S. C., Fujiwara Y., Liu J., Yue J., Shimizu Y., Norman D. D., Wang Y., Tsukahara R., Szabo E., Patil R., et al. 2015. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis. Mol. Cancer Res. 13: 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kai M., Sakane F., Jia Y. J., Imai S., Yasuda S., Kanoh H. 2006. Lipid phosphate phosphatases 1 and 3 are localized in distinct lipid rafts. J. Biochem. 140: 677–686. [DOI] [PubMed] [Google Scholar]