Abstract
Aims: Angiotensin-converting enzyme 2 (ACE2)–angiotensin (1-7) [Ang (1-7)]-Mas constitutes the vasoprotective axis and is demonstrated to antagonize the vascular pathophysiological effects of the classical renin–angiotensin system. We sought to study the hypothesis that upregulation of ACE2-Ang (1-7) signaling protects endothelial function through reducing oxidative stress that would result in beneficial outcome in diabetes. Results: Ex vivo treatment with Ang (1-7) enhanced endothelium-dependent relaxation (EDR) in renal arteries from diabetic patients. Both Ang (1-7) infusion via osmotic pump (500 ng/kg/min) for 2 weeks and exogenous ACE2 overexpression mediated by adenoviral ACE2 via tail vein injection (109 pfu/mouse) rescued the impaired EDR and flow-mediated dilatation (FMD) in db/db mice. Diminazene aceturate treatment (15 mg/kg/day) activated ACE2, increased the circulating Ang (1-7) level, and augmented EDR and FMD in db/db mouse arteries. In addition, activation of the ACE2-Ang (1-7) axis reduced reactive oxygen species (ROS) overproduction determined by dihydroethidium staining, CM-H2DCFDA fluorescence imaging, and chemiluminescence assay in db/db mouse aortas and also in high-glucose-treated endothelial cells. Pharmacological benefits of ACE2-Ang (1-7) upregulation on endothelial function were confirmed in ACE2 knockout (ACE2 KO) mice both ex vivo and in vitro. Innovation: We elucidate that the ACE2-Ang (1-7)-Mas axis serves as an important signal pathway in endothelial cell protection in diabetic mice, especially in diabetic human arteries. Conclusion: Endogenous ACE2-Ang (1-7) activation or ACE2 overexpression preserves endothelial function in diabetic mice through increasing nitric oxide bioavailability and inhibiting oxidative stress, suggesting the therapeutic potential of ACE2-Ang(1-7) axis activation against diabetic vasculopathy. Antioxid. Redox Signal. 23, 880–892.
Introduction
Diabetes mellitus accelerates atherosclerosis and increases the risks for cardiovascular complications. Although the underlying mechanisms are not fully understood, endothelial dysfunction is implicated in the pathogenesis of diabetic vascular complications (6). The overproduction of reactive oxygen species (ROS) or increased oxidative stress contributes to the impairment of endothelial function in diabetes (16). Angiotensin-converting enzyme 2 (ACE2), a homolog of ACE, is the monocarboxypeptidase in the renin–angiotensin system (RAS). It negatively regulates the RAS by cleaving angiotensin II (Ang II) to generate angiotensin (1-7) [Ang (1-7)], which acts as a vasodilator and vasoprotective peptide through stimulation of its receptor Mas (48, 50, 51, 53). Thus, the ACE2-Ang (1-7)-Mas axis is proposed as a counter-regulator of the ACE-Ang II arm in the cardiovascular system (7) and kidney (37). The balance between Ang II and Ang (1-7) regulates the overall RAS activity, which in turn controls blood pressure, sodium–water homeostasis, and renal function.
Innovation.
The present study provides novel evidence that angiotensin (1-7) [Ang (1-7)] protects endothelial function in renal arteries of diabetic patients and reveals that the upregulation of the angiotensin-converting enzyme 2 (ACE2)-Ang (1-7) axis of the renin–angiotensin system (RAS) by genetic overexpression or pharmacological activation preserves endothelial function through normalizing overproduction of reactive oxygen species in diabetic db/db mice. Thus, the vascular benefits of the ACE2-Ang (1-7)-Mas axis established in the present study highlight the therapeutic potential of drugs that activate this healthy arm of the local RAS in the treatment of diabetic vasculopathy if their safety profile is proven.
Animal studies show that ACE2 overexpression improves endothelial function in hypertension (43), attenuates the progression of atherosclerosis (29, 59), and suppresses the adverse myocardial remodeling (8, 61). In diabetic db/db mice, ACE2-Ang (1-7) upregulation prevents pancreatic beta-cell dysfunction and apoptosis (2) and improves renal function (37). By contrast, ACE2 deficiency leads to or aggravates vascular dysfunction (39, 55), diabetic cardiomyopathy, and nephropathy (38, 47). These studies indicate that the ACE2-Ang (1-7)-Mas axis of the RAS is vasoprotective, probably through reducing Ang II production or acting against Ang II-stimulated oxidative stress and vascular inflammation.
The heart and blood vessels are the main sites for the formation of Ang (1-7) and also the major targets for the action of Ang (1-7). Ang (1-7) infusion preserves cardiac function, coronary perfusion, and aortic endothelial function in rats with coronary ligation-induced heart failure and in diabetic hypertensive rats (1, 28). Ang (1-7) also prevents cardiac remodeling through a direct effect in Ang II-induced hypertensive mice (32) and in 5/6 nephrectomy mice (24). As a vasodilator, Ang (1-7) directly relaxes several vascular beds (3, 41, 42). Ang (1-7)-induced vasodilatation involves an increased production of endothelium-derived relaxing factors (3, 15, 34). Through stimulation of Ang (1-7) receptor Mas, Ang (1-7) exerts counter-regulatory effects in the vasculature and kidney in response to RAS activation or Ang II by suppressing oxidative stress and inflammation (36).
However, the exact role of the local ACE2-Ang (1-7) in the initiation and maintenance of endothelial dysfunction in diabetes, especially in human subjects, is only partially understood. Although adenovirus transduction or human recombinant protein have been employed to overexpress ACE2, targeting to elevate ACE2 activity has rarely been considered in previous studies as an effective therapeutic alternative in the treatment of vascular disease. In the present study, we hypothesize that the upregulation of the ACE2-Ang (1-7) axis by both genetic overexpression and pharmacological activation protects endothelial function in type 2 diabetes.
Results
Ang (1-7) preserves endothelial function in ex vivo human renal arteries
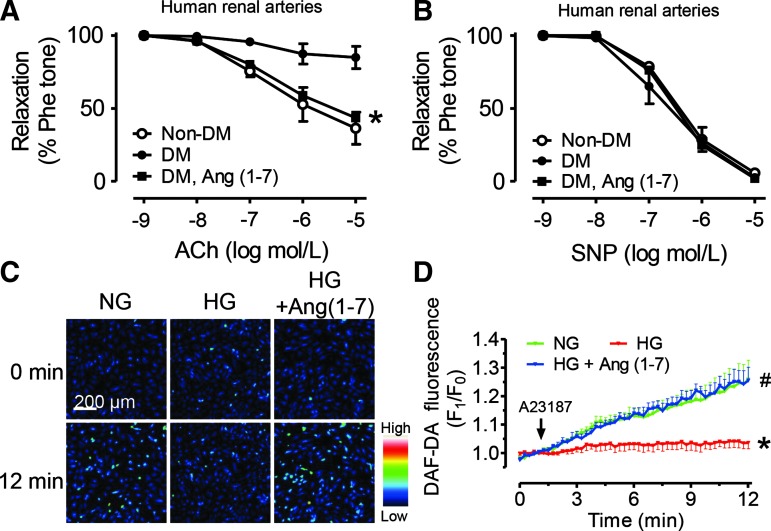
Acetylcholine (ACh)-induced endothelium-dependent relaxation (EDR) was significantly less in renal arteries from diabetic patients than those from nondiabetic subjects (Fig. 1A). By contrast, the arteries from both groups showed identical relaxations induced by the endothelium-independent dilator, sodium nitroprusside (SNP) (Fig. 1B). Renal arteries from diabetic patients were cultured in DMEM containing 1 μM Ang (1-7) for 24 h and this treatment markedly augmented EDR without affecting SNP-induced relaxations (Fig. 1A, B).
FIG. 1.
Angiotensin (1-7) [Ang (1-7)] improves endothelial function in renal arteries from diabetic patients. (A) Acetylcholine (ACh)-induced endothelium-dependent relaxations (EDRs) and (B) sodium nitroprusside (SNP)-induced endothelium-independent relaxations in renal arteries from nondiabetic (Non-DM) subjects and diabetic patients (DM). The effect of ex vivo 24-h exposure to Ang (1-7) (1 μM) on EDR in renal arteries from diabetic patients. Data are mean±SEM from 4 separate human arteries, *p<0.05, versus DM. Nitric oxide (NO) production stimulated by A23187 in human umbilical vein endothelial cells (HUVECs) after different treatments in vitro for 48 h. (C) Representative images and (D) summarized data showing the real-time effect of Ang (1-7) (1 μM) to restore NO production that was suppressed by high glucose (HG: 30 mM) in HUVECs. Data are mean±SEM from five experiments.*p<0.05 versus NG, #p<0.05 versus HG. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In human umbilical vein endothelial cells (HUVECs), stimulation with Ca2+ ionophore, A23187 (0.1 μM), increased DAF-FM diacetate fluorescence intensity, which reflects the level of NO production in normal glucose (NG, 5 mM) condition. Exposure (48 h) to high glucose (HG, 30 mM) inhibited A23187-stimulated NO production, while cotreatment with Ang (1-7) (1 μM) reversed the effect of HG (Fig. 1C, D). These results demonstrate a vasoprotective benefit of Ang (1-7) in human arteries and endothelial cells.
Compared with nondiabetic subjects, diabetic patients had increased Ang II level, decreased Ang (1-7) level, and a lower Ang (1-7)/Ang II ratio in their plasma (Supplementary Fig. S1A–C; Supplementary Data are available online at www.liebertpub.com/ars). However, the plasma ACE2 activity was slightly but insignificantly higher in diabetics (Supplementary Fig. S1D).
Ang (1-7) restores endothelial function in diabetic mice
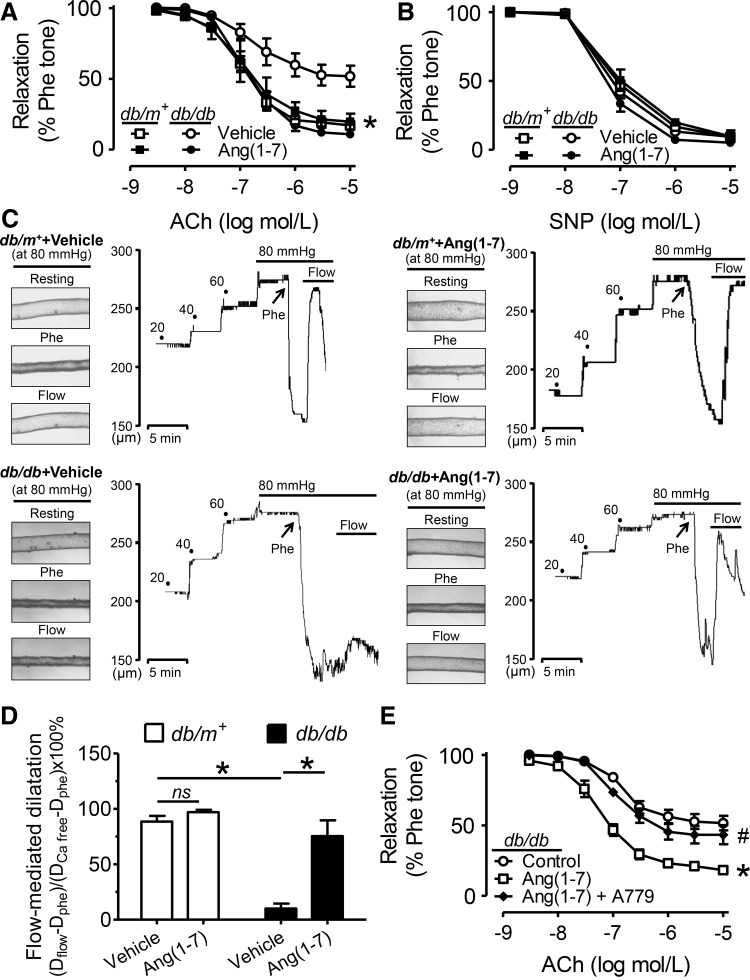
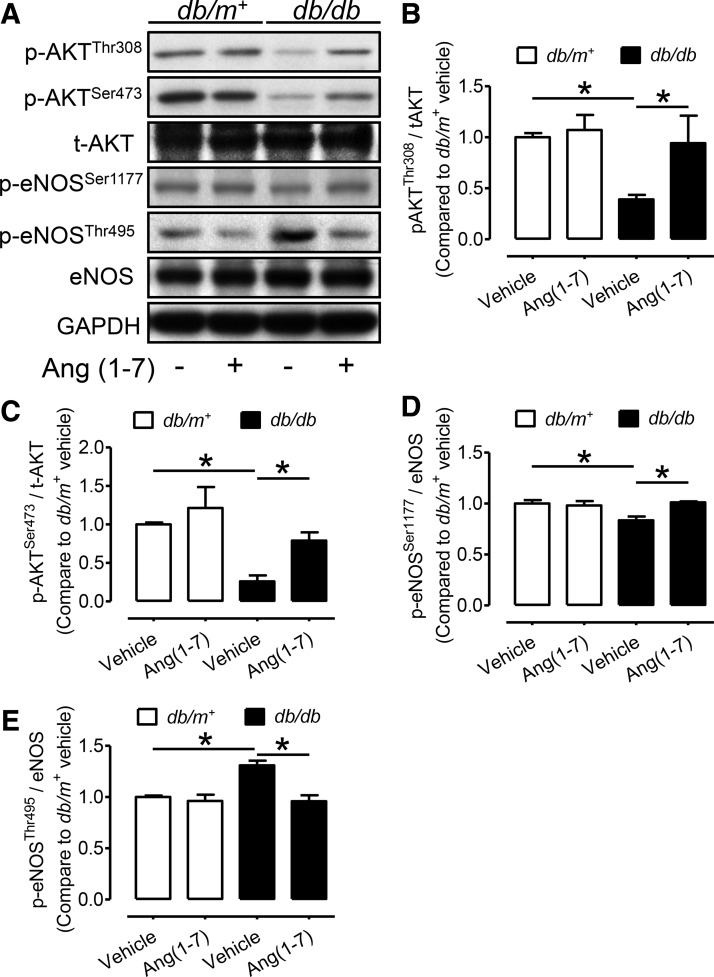
ACh-induced EDR in db/db mouse aortas was significantly less compared with relaxations in db/m+ mouse aortas (Fig. 2A and Supplementary Fig. S2B). Ang (1-7) infusion (500 ng·kg−1·min−1, 2 weeks) via the osmotic minipump increased the plasma Ang (1-7) concentration in db/db mice (Supplementary Fig. S2A) and restored the impaired EDR in aortas from db/db mice without affecting EDR in db/m+ mice (Fig. 2A). SNP-induced relaxations were similar among different groups (Fig. 2B). In addition, flow-mediated dilatation (FMD) in the 2nd-order mesenteric resistance arteries was evoked by creating a pressure difference of 20 mm Hg that equals an initial shear stress of≈15 dynes/cm2. FMD was markedly impaired in db/db mice compared with db/m+ mice and it was restored in arteries from Ang (1-7)-infused db/db mice (Fig. 2C and 2D). Ang (1-7) infusion also normalized the diminished phosphorylations of Akt at Thr308 and Ser473 and of endothelial nitric oxide synthases (eNOS) at Ser1177, but suppressed the phosphorylation of eNOS at the inhibitory site Thr495 in db/db mouse aortas (Fig. 3A–E).
FIG. 2.
Ang (1-7) improves endothelial function in db/db mice. (A) ACh-induced EDRs and (B) SNP-induced endothelium-independent relaxations in aortas from db/m+ and db/db mice with and without Ang (1-7) infusion (500 ng·kg−1·min−1, 2 weeks). (C) Representative traces and (D) summarized data for flow-mediated dilatation (FMD) in the 2nd-order of mesenteric arteries from the four groups of mice. Data are mean±SEM of four to six mice. *p<0.05 versus db/db vehicle. ns, non-significant. (E) EDRs in db/db aortas following a 24-h exposure to Ang (1-7) (1 μM) and Ang (1-7) plus Mas receptor antagonist, A779 (1 μM). Data are mean±SEM of five mice. *p<0.05 versus control, #p<0.05 versus Ang (1-7).
FIG. 3.
The effect of Ang (1-7) on phosphorylation of Akt and eNOS in mouse aortas. The phosphorylation levels of Akt and eNOS in the aortas from db/m+ and db/db mice with or without Ang(1-7) infusion shown by (A) representative blots and (B–E) summarized data. Data are mean±SEM from four individual experiments.*p<0.05.
In ex vivo cultured db/db mouse aortas, a 24-h exposure to Ang (1-7) (1 μM) improved the impaired EDR, and the effect of Ang (1-7) was reversed by the Ang (1-7) antagonist, A779 (1 μM) (Fig. 2E). The restored EDR by Ang (1-7) was abolished by L-NAME or in rings without the endothelium (Supplementary Fig. S3A, B). In db/m+ mouse aortas, HG (30 mM, 48 h)-induced impairment of EDR was reversed by cotreatment with Ang (1-7) and this vascular benefit of Ang (1-7) was antagonized by Mas receptor antagonist, A779 (Supplementary Fig. S3C). A779 alone did not affect EDR in db/m+ mouse aortas (Supplementary Fig. S3D).
Exogenous ACE2 overexpression rescues endothelial function in diabetic mice
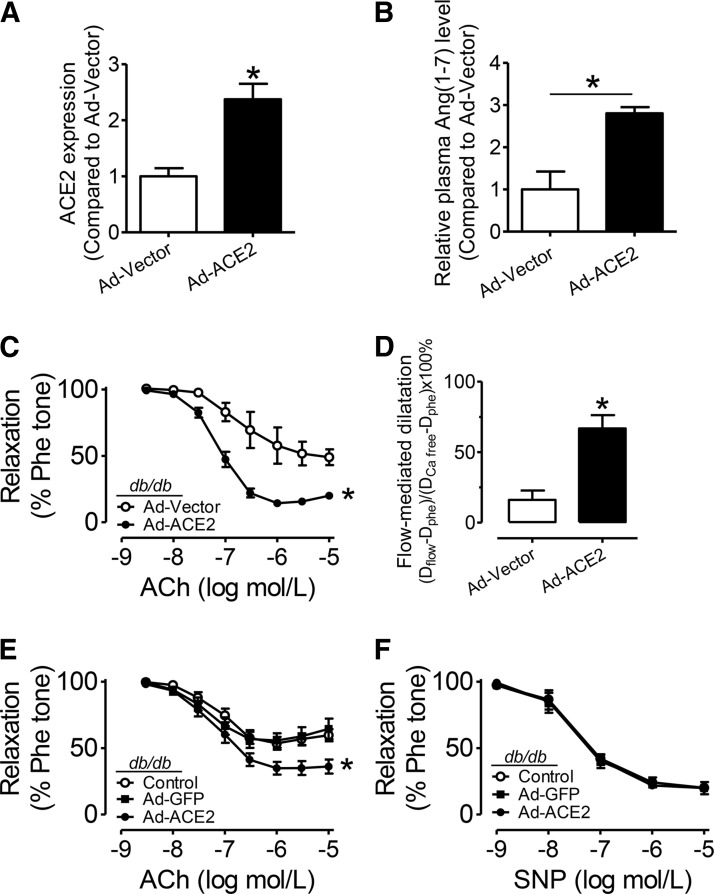
ACE2 catalyzes formation of Ang (1-7). ACE2 overexpression by adenoviral transduction (Ad-ACE2) increased ACE2 content in db/db mouse aortas as shown by qPCR, Western blotting, and endothelium en face immunofluorescence staining (Fig. 4A, Supplementary Figs. S4 and S5A), while the relative activity of aortic ACE2 per unit mass and plasma ACE2 in db/db mice was unaffected by adenoviral transduction (Supplementary Fig. S5B, C). However, the plasma Ang(1-7) level was elevated by Ad-ACE2 transduction in db/db mice (Fig. 4B). In vivo treatment with ACE2 adenovirus, but not vector control, improved EDR in db/db mouse aortas (Fig. 4C). Likewise, FMD in mesenteric arteries was also augmented by ACE2 overexpression (Fig. 4D). In addition, in ex vivo culture of db/db mouse aortas, Ad-ACE2 transduction also enhanced EDR compared with Ad-GFP transduction and nonadenovirus control (Fig. 4E) without affecting SNP-induced relaxations (Fig. 4F).
FIG. 4.
Angiotensin-converting enzyme 2 (ACE2) overexpression rescues endothelial function in db/db mice. (A) Summarized data showing en face immunofluorescence staining of ACE2 expression in endothelial cells of intact mouse aortas from db/db mice with adenoviral ACE2 (Ad-ACE2) or vector (Ad-vector) transduction. (B) Relative Ang(1-7) level in the plasma of db/db mice with Ad-ACE2 and Ad-vector transduction. (C) EDRs in aortas and (D) FMD in mesenteric arteries from Ad-ACE2 or Ad-vector cultured db/db mice. Data are mean±SEM of four to six mice. *p<0.05 versus Ad-vector. (E) EDRs and (F) SNP-induced relaxations in db/db mouse aortas following a 48-h exposure to adenovirus. Data are mean±SEM of five to six mice. *p<0.05 versus Ad-GFP.
Diminazene aceturate increases Ang (1-7) production and protects endothelial function in db/db mice
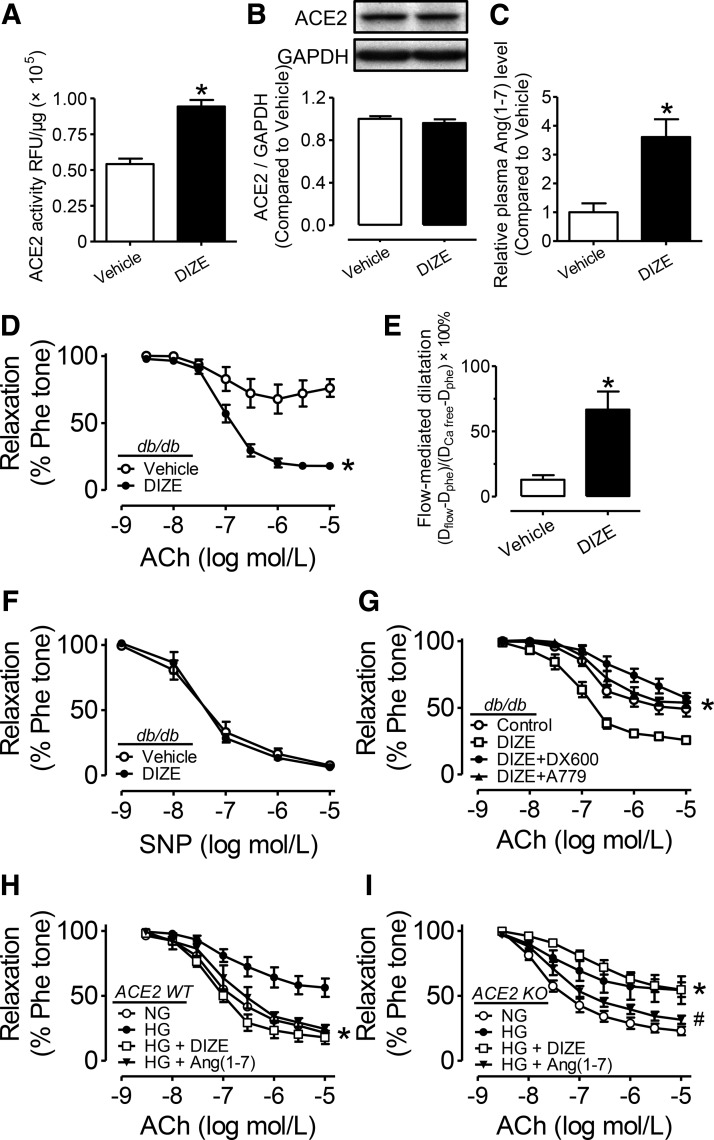
Both expression and activity of aortic ACE2 in the db/db mouse were slightly but insignificantly lower than those in db/m+ mouse aortas (Supplementary Fig. S6A–C). The plasma Ang (1-7) level was smaller in db/db compared with db/m+ mice (Supplementary Fig. S6D). Diminazene aceturate (DIZE) elevated ACE2 activity in HUVECs in a concentration-dependent manner (Supplementary Fig. S7A). Two-week treatment with putative ACE2 activator, DIZE (15 mg/kg/day), to db/db mice increased ACE2 activity in their aortas (Fig. 5A) and plasma (Supplementary Fig. S7B). By contrast, aortic ACE2 expression was unchanged (Fig. 5B). DIZE treatment also significantly elevated the plasma Ang (1-7) level in db/db mice (Fig. 5C) without affecting either glucose tolerance or insulin tolerance (Supplementary Fig. S8).
FIG. 5.
ACE2 activation restores endothelial function in db/db mice. The effect of chronic treatment with diminazene aceturate (DIZE, 15 mg/kg/day, 14 days) on the (A) activity and (B) expression of ACE2 in db/db mouse aortas. RFU, relative fluorescence unit. (C) Plasma Ang (1-7) level in db/db mice with and without DIZE treatment. Data are mean±SEM of five to six mice. *p<0.05 versus vehicle. (D) ACh-induced EDRs and (E) SNP-induced relaxations in aortas and (F) FMD in mesenteric arteries from db/db mice. Data are mean±SEM of six mice. *p<0.05 versus vehicle. (G) EDRs in db/db mouse aortas following a 24-h exposure to DIZE (100 μM), or DIZE plus ACE2 inhibitor, DX600 (1 μM), or DIZE plus Mas receptor antagonist, A779 (1 μM). Data are mean±SEM of five to six mice. *p<0.05 versus DIZE. EDRs in aortas from (H) ACE2 WT or (I) ACE2 KO mice following a 48-h exposure to normal glucose (NG, 5 mM) high glucose (HG, 30 mM), HG plus DIZE (100 μM), and HG plus Ang (1-7) (1 μM). Data are mean±SEM of five mice. (H) *p<0.05 versus HG, (I) *p<0.05 versus NG, #p<0.05 versus HG.
Chronic DIZE treatment restored both the impaired EDR (Fig. 5D) and FMD in db/db mice (Fig. 5E) without affecting SNP-induced relaxations (Fig. 5F). Ex vivo 24-h treatment with DIZE (100 μM) also augmented EDR in db/db mouse aortas (Fig. 5G) and HG (30 mM)-treated aortas of db/m+ mice (Supplementary Fig. S3E). The ex vivo beneficial effect of DIZE was reversed by either ACE2 inhibitor, DX600 (1 μM), or Ang(1-7) antagonist, A779 (1 μM) (Fig. 5G and Supplementary Fig. S3E).
Next, ACE2-deficient mice were employed to confirm the ACE2-mediated effect of DIZE. EDR and plasma Ang (1-7) level were similar between ACE2 wild-type (ACE2 WT) and ACE2 knockout mice (ACE2 KO) (Supplementary Fig. S3F and S6E). In aortas of ACE2 WT mice, HG-impaired EDR was rescued by ex vivo treatment with either DIZE (100 μM) or Ang (1-7) (1 μM) (Fig. 5H). However, in aortas from ACE2 KO mice, HG-induced impairment of EDR was rescued only by Ang (1-7), but not by DIZE (Fig. 5I).
ACE2-Ang (1-7) activation normalizes endothelial ROS overproduction in db/db mice
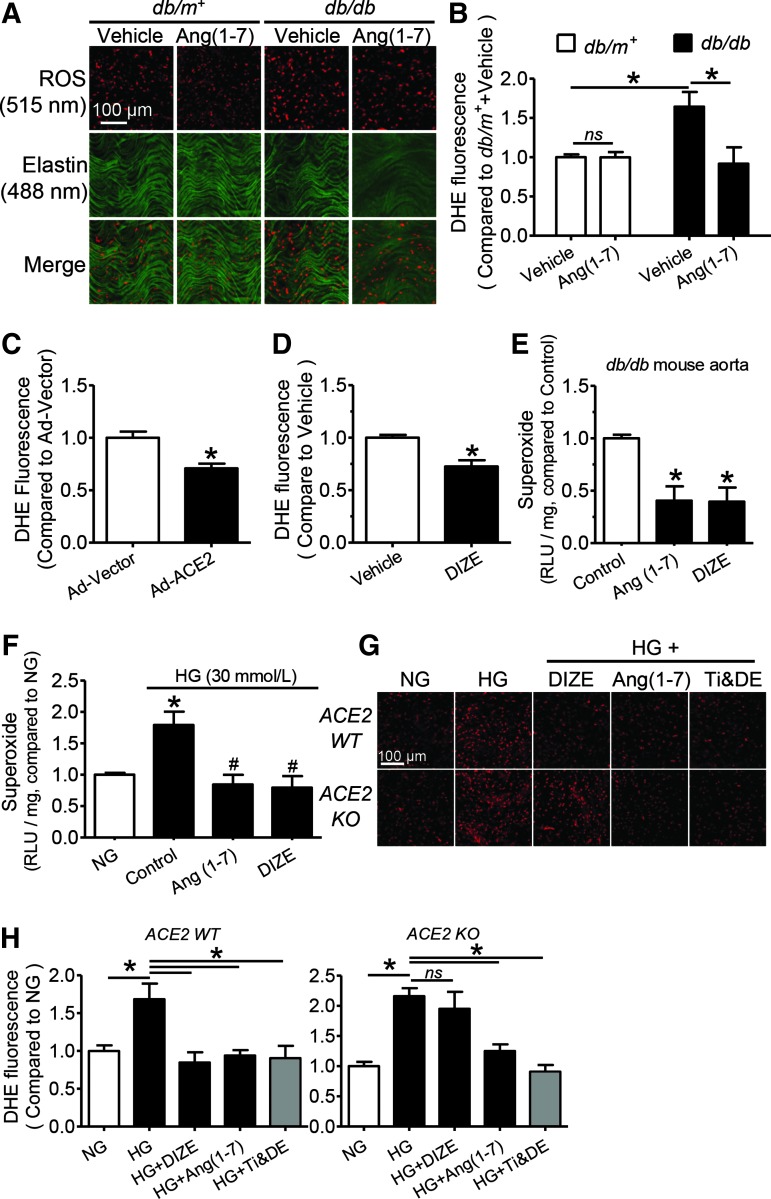
The ROS level indicated by dihydroethidium (DHE) staining was higher in the en face endothelial layer or the vascular wall of aortas from db/db mice compared with those from db/m+ mice (Fig. 6A, B and Supplementary Fig. S9). Two-week Ang (1-7) infusion suppressed the endothelial ROS overgeneration in db/db mouse aortas without affecting the basal ROS level in the endothelium of db/m+ mouse aortas (Fig. 6A, B). Both in vivo Ad-ACE2 transduction and DIZE treatment reduced DHE fluorescence intensity in db/db mouse aortic endothelial cells (MAECs) (Fig. 6C, D and Supplementary Fig. S10).
FIG. 6.
Activation of the ACE2-Ang (1-7) axis reduces reactive oxygen species (ROS) overgeneration in endothelial cells of db/db mice. (A) Representative images and (B) summarized data showing ROS production as detected by dihydroethidium (DHE, 5 μM) staining in endothelium en face of aortas from db/m+ and db/db mice with and without receiving Ang (1-7) infusion. Summarized data showing the relative ROS level in endothelium en face of aortas from db/db mice (C) with Ad-vector or Ad-ACE2 transduction and (D) chronic treatment with vehicle or DIZE. (A high-quality color representation of this figure is available in Supplementary Fig. S8). Data are mean±SEM of five to six mice. *p<0.05. Relative chemiluminescence units (RLUs) showing the superoxide level in (E) db/db mouse aortas after a 24-h exposure to Ang (1-7) (1 μM) or DIZE (100 μM) and in (F) C57BL/6 mouse aortas after a 48-h treatment with high glucose (HG, 30 mM). Data are mean±SEM of four mice. (E) *p<0.05 versus control, (F) *p<0.05 versus NG, #p<0.05 versus HG. (G) Representative images and (H) summarized data of the ROS level in primary cultured mouse aortic endothelial cells from ACE2 WT and ACE2 KO mice following a 48-h exposure to normal glucose (NG, 5 mM), high glucose (HG, 30 mM), HG+DIZE (100 μM), HG+Ang (1-7) (1 μM), and HG plus ROS scavengers, tiron and DETCA (Ti & DE, tiron:1 mM, DETCA: 100 μM). Data are mean±SEM of six experiments, *p<0.05. Red: DHE fluorescence (excitation: 515 nm) in the nucleus; Green: autofluorescence of elastin beneath the endothelium (excitation: 488 nm). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Ex vivo 24-h treatment with either Ang (1-7) (1 μM) or DIZE (100 μM) inhibited the basal content of superoxide anions in db/db mouse aortas (Fig. 6E). In addition, a 48-h exposure to Ang (1-7) or DIZE reversed HG-stimulated superoxide elevation in C57BL/6 mouse aortas (Fig. 6F). Finally, in primary cultured MAECs, HG-triggered ROS overgeneration was inhibited by Ang (1-7) or tiron plus DETCA, while DIZE inhibited ROS overproduction in cells only from ACE2 WT mice, but not those from ACE2 KO mice (Fig. 6G, H). Likewise, HG-induced ROS rise in HUVECs reflected by elevated CM-H2DCFDA fluorescence intensity was inhibited by Ang (1-7) and DIZE, while ACE2 inhibitor, DX600, reversed the effect of DIZE, but not Ang (1-7). Ang (1-7) antagonist, A779, counteracted the ROS-lowering effects of both DIZE and Ang (1-7) in HG-treated HUVECs (Supplementary Fig. S11).
Upregulation of the ACE2-Ang (1-7) axis suppresses HG-induced MAPK phosphorylation and NADPH oxidase expression, while it increases UCP2 expression
The HG-elevated NOX2 expression was reversed by a 48-h cotreatment with DIZE (100 μM), while the expression of p67, p47, and NOX4 was unchanged in HG-exposed HUVECs with or without DIZE treatment (Supplementary Fig. S12). Exposure to HG also increased the phosphorylation of ERK1/2, p38, and JNK, which was reversed by Ang (1-7) (1 μM) in HUVECs (Supplementary Fig. S13). Expression of several antioxidant genes was measured in human aortic endothelial cells (HAECs) transduced for 24 h with Ad-vector or Ad-ACE2. Uncoupling protein-2 (UCP2) was upregulated by ACE2 overexpression (Supplementary Fig. S14A). UCP2 knockdown by adenoviral UCP2 shRNA (Ad-UCP2 shRNA), which was proved by qPCR and Western blot (Supplementary Fig. S14B, C), diminished the inhibitory effect of Ang (1-7) on ROS production in HG-treated HAECs (Supplementary Fig. S15).
Discussion
The present study demonstrates a critical role of activation of the ACE2-Ang (1-7)-Mas axis in protecting endothelial function of db/db diabetic mice. The upregulation of ACE2-Ang (1-7) attenuates oxidative stress and increases the NO bioavailability, thus counteracting the deleterious effect of the local RAS in arteries in diabetic mice. Importantly, ex vivo Ang (1-7) treatment preserves endothelial function in arteries of diabetic patients.
Ang (1-7), as an endogenous heptapeptide, is formed from precursors, Ang I or Ang II, under the catalytic reaction of several peptidases, including carboxypeptidases, ACE and ACE2 (17), and the conversion of Ang II to Ang (1-7) through ACE2 appears to represent the most preferred pathway for Ang (1-7) generation (51). Ang (1-7)-mediated activation of Mas receptor has been reported to counter-regulate Ang II-induced endothelial cell proliferation (44), to inhibit cardiac fibroblast proliferation and cardiac remodeling by negatively modulating extracellular signal-regulated kinase (ERK) (32), and to cause vasorelaxation by releasing NO and prostaglandins (4, 45). Although Ang (1-7) has been assessed for its acute pharmacological action on blood vessels, it is yet to be established whether Ang (1-7) can chronically ameliorate endothelial dysfunction in diabetes. The present study was probably among the first to demonstrate the beneficial effect of Ang (1-7) to augment EDR in ex vivo cultured renal arteries of diabetic patients. This observation in human arteries prompted us to reveal that chronic Ang (1-7) infusion restored the impaired endothelial function in db/db mice, as shown by both improved EDR in aortas and augmented FMD in resistance arteries. We further confirmed the vascular benefit of ex vivo 24-h treatment with Ang (1-7) to rescue EDR in organ culture and also in HG-treated db/m+ mouse aortas. Moreover, the beneficial effect of Ang (1-7) was abolished by A779, a potent antagonist of Mas, thus suggesting that the vasoprotective benefit of Ang (1-7) is most likely through activation of Mas. The improved EDR by chronic Ang (1-7) treatment was also observed in apolipoprotein E-deficient mice (ApoE−/−) (51) and salt-induced hypertensive rats (41). Ang (1-7)-mediated vasodilatation is reportedly mediated through stimulation of Mas, which is coupled to the downstream Akt/eNOS signaling (45). The present study shows that chronic Ang (1-7) treatment increased the basal phosphorylations of Akt and eNOS that were lower in aortas of db/db mice. Therefore, the vasoprotective effect of chronic Ang (1-7) infusion in db/db mice is likely mediated through a G-protein-coupled receptor Mas-mediated PI3K/Akt/eNOS cascade.
ACE2 gene therapy has been initiated experimentally against hypertension, cardiac dysfunction, and renal damage through suppressing the production and downstream signaling of Ang II (10, 26, 59, 61). The present study shows that ACE2 overexpression both in vivo and ex vivo by ACE2 adenovirus transduction rescued the impaired EDRs in aortas and FMD in resistance mesenteric arteries from diabetic mice, which was consistent with earlier studies on ApoE−/− mice and spontaneously hypertensive stroke-prone rats (29, 43). Recently, ACE2 priming by the pharmacological activator, xanthenone (XNT), has been shown to protect the function of endothelial progenitor cells in hypertension and diabetes (5, 11, 18, 23). In the present study, we have used a newly discovered ACE2 activator, DIZE (20, 40, 46), and shown that it enhances ACE2 activity in vitro. Chronic DIZE treatment elevated the activity of local vascular ACE2, subsequently increased the production of Ang (1-7), and augmented both EDRs in conduit aortas and FMD in resistance arteries from db/db mice. Likewise, ex vivo exposure of db/db mouse aortas to DIZE also enhanced EDRs. These effects are probably specific to the ACE2 and Ang (1-7) since the ACE2 inhibitor, DX600, and Ang (1-7) antagonist, A779, reversed the effect. Furthermore, the lack of vasoprotective effect in ACE2 knockout mice confirmed the selectivity of DIZE to activate ACE2.
Endothelial dysfunction results from a decreased bioavailability of NO or/and an increased production of ROS in the vascular wall. The antioxidant defense of the ACE2-Ang (1-7) axis is being just recently examined in several types of cells (14, 25, 33). The present study shows that ROS overgeneration in endothelial cells and aortic vascular wall of db/db mice was suppressed by Ang (1-7) infusion as well as ACE2 activation and overexpression. The elevated NO production is likely due to the antioxidative benefit of the upregulation of the ACE2-Ang (1-7) axis. This was further supported by the observation that DIZE fails to reduce ROS in endothelial cells from ACE2 KO mice. In ACE2 KO mice, Ang (1-7) was still able to inhibit ROS generation. The present results also indicate that ACE2 upregulation and activation may target NADPH oxidases to lower the vascular ROS level in diabetic mice. In fact, the in vitro results show that activation of the ACE2-Ang (1-7) axis suppressed NOX2 expression and attenuated MAPK activation under hyperglycemic conditions, which agrees with a previous report about its beneficial effects on renal proximal tubular cells (12, 52), endothelial progenitor cells (5), and endothelial function (11), Moreover, the downstream antioxidant molecular mechanism initiated by ACE2-Ang (1-7) was investigated. UCP2, which preserves endothelial function in diet-induced obese mice and hypertensive rats (27, 57), can be upregulated by ACE2 overexpression in HAECs. Knockdown of UCP2 attenuated the inhibitory effect of Ang (1-7) on ROS overproduction in HG-treated HAECs, suggesting the possible involvement of UCP2 in Ang (1-7)-mediated antioxidant effects in the vasculature of diabetic mice.
A previous study showed that the ACE2 overexpression in the pancreas improves glucose metabolism through increasing the first-phase insulin secretion without affecting peripheral insulin sensitivity in the early stage of diabetes in db/db mice, while the beneficial effects of ACE2 were absent in 16-week-old db/db mice (2). The present study does not show a metabolic benefit as neither glucose metabolism nor insulin sensitivity was altered in adult db/db mice (>14-week) treated with ACE2 activator. The effect of ACE2 activation on improving glucose metabolism is likely to be related to the pathological stage of diabetes. Nevertheless, whether ACE2 activation improves peripheral insulin resistance is still unknown.
Although ACE2 deficiency induces or exacerbates vascular inflammation, heart failure, atherosclerosis, and renal damage in pathological conditions (19, 35, 38, 39, 54, 55), the EDRs in aortas and the capacity of ROS production in primary cultured mouse endothelial cells and the plasma Ang (1-7) level are not different between ACE2 wild-type and deficient mice on normal chow. Moreover, the pharmacological inhibition of ACE2-Ang (1-7) did not impair EDRs in aortas from normal mice. In the physiological condition, the normal RAS might play a minor role in the control of the endothelial function even if the ACE2-Ang(1-7) axis is inhibited. On the other hand, overactivation of the ACE-Ang II arm of the RAS associated with diabetic situations results in the increased production of Ang II, but the decreased production of Ang (1-7), as shown in both diabetic patients and db/db mice. However, overexpression or activation of the ACE2-Ang (1-7) axis through either genetic or pharmacological approaches is effective to protect against endothelial dysfunction in diabetes through counteracting the exaggerated ACE-Ang II pathway. The post-translational mortification or conformational change of ACE2 by pharmacological treatment can become a potential therapeutic strategy in addition to classic ACE2 gene therapy.
There are still some controversies over replenishing ACE2 as a potential therapeutic target. There are reports that show a link between upregulation of ACE2 or Ang (1-7) to ventricular arrhythmias (9), cardiac fibrosis (31), and renal injury (22). In addition, the expression and activity of ACE2 are found to be higher in the kidney of diabetic mice (49, 58). Although a compensatory increase in ACE2 activity could be proposed under diabetic conditions, more studies are needed to understand the kidney-specific balance between ACE and ACE2 at the post-transcriptional and post-translational levels during the time course of diabetes. Our results on human plasma, however, suggest that the slightly increased ACE2 is probably inadequate to convert Ang II into Ang (1–7). It is probable that functional ACE2 might also depend on the post-translational modification, including conformational change, phosphorylation, and ubiquitination, which are still unclear. Although the present results suggest that pharmacological priming targeting the ACE2-Ang (1-7) axis may be a promising approach to preserve endothelial function in diabetes, the potential tissue-specific side effects certainly need further investigation.
Endothelial dysfunction in diabetes is a chronic pathological process involving not only elevated circulating glucose but also many other inter-related factors such as advanced glycation end products and angiotensin II. The use of 30 mM glucose in both ex vivo and in vitro experiments is simply aimed to support the in vivo vascular benefits of Ang (1-7) and ACE2 activator to preserve endothelial function in diabetic mice. It should be noted that this concentration of glucose is not translatable to the clinical settings in humans.
In summary, the present study establishes the beneficial effect of the ACE2-Ang (1-7)-Mas axis of the RAS to restore endothelial function in renal arteries from diabetic patients and in conduit as well as resistance arteries from diabetic mice. The upregulation and activation of ACE2-Ang (1-7) increase the NO bioavailability, probably as a result of the inhibition of ROS production in endothelial cells. The vascular benefit of the ACE2-Ang (1-7) axis elucidated in the present study highlights the therapeutic potential of drugs that activate this healthy arm of the local RAS in the treatment of diabetic vasculopathy if their safety profile is proven.
Materials and Methods
Human artery specimen
The use of human renal arteries was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee. Human renal arteries were obtained during surgery after informed consent from diabetic patients undergoing nephrectomy at ages between 49–76 years. The diabetic patients had a fasting plasma glucose level of over 7.0 mM (or 126 mg/dl) or 2-h plasma glucose ≥11.1 mM (or 200 mg/dl). The background information of human subjects is presented in Supplementary Table S1.
Animals
All animal experiments were approved by the Animal Experimentation Ethics Committee, Chinese University of Hong Kong (CUHK), and were conducted under institutional guidelines for the humane treatment of laboratory animals. Male C57BL/6J, leptin receptor-deficient db/db mice (homozygous) and db/m+ mice (heterozygous) were supplied by the CUHK Laboratory Animal Center. ACE2 wild-type (ACE2 WT, ACE-2+/+) and knockout (ACE2 KO, ACE-2−/−) mice were generated as described (7). All mice were kept at 22–23°C and 55%±5% humidity with a 12-h light/12-h dark cycle and free access to diet and water.
Metabolic parameters
The insulin tolerance test was performed by measuring blood glucose levels with a commercial glucometer (Ascensia) at 0, 15, 30, 60, and 120 min after injection of insulin (0.5 U/kg; Sigma) to each mouse after 2 h of fasting. For the oral glucose tolerance test, mice were loaded with 10% glucose solution (1.2 g/kg body weight) via oral gavage after 8 h of fasting before blood glucose measurement.
Osmotic minipump infusion
Vehicle or Ang (1-7) (5 mg/ml; Enzo Life Sciences, Inc.) dissolved in phosphate-buffered saline were infused into db/db and db/m+ mice using the osmotic pump (Alzet) with an infusion rate of 500 ng/kg/min for 2 weeks.
Adenoviral construction and transduction in vivo and ex vivo
Human ACE2 cDNA was PCR amplified from hACE2 plasmid (Addgene ID 1786) and ligated into adenoviral shuttle plasmid pAdTrack-CMV (Addgene ID 16405). The shRNA (5′-GCCTGTATGATTCTGTCAAAC-3′) sequence targeting UCP2 mRNA was designed using BLOCK-iT™ RNAi Designer (Life Technologies) and cloned to the pAdtrack-U6 vector. The procedures of plasmid construction, virus package, amplification, and purification followed the previous report (30). Adenovirus was packaged in HEK293A cells and tittered with 1×109 pfu/ml. For the in vivo study, 100 μl (1×109 pfu/ml) of adenoviral ACE2 (Ad-ACE2) or adenoviral vector (Ad-vector) was delivered to db/db mice via tail vein injection and experiments were carried out 7 days after injection. For ex vivo studies, aortas were transduced with Ad-ACE2 or Ad-GFP adenovirus (∼107 pfu) in 10% FBS DMEM for 48 h. UCP2 knockdown efficiency was confirmed by detection of UCP2 mRNA by qPCR and protein by Western blotting.
Drug administration
At the age of 14 weeks, male db/db mice were treated with DIZE, the ACE2 activator dissolved in water, via oral gavage at 15 mg·kg−1 day−1 for 2 weeks (13).
Artery preparation and culture
After mice were sacrificed by CO2 suffocation, thoracic aortas and mesenteric arteries (2nd-order) were dissected out and placed in oxygenated ice-cold Krebs solution containing (in mM) 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1.2 KH2PO4, and 11 D-glucose. Aortas were cut into ring segments ∼1.8 mm long in sterile Krebs solution and incubated in a 10% FBS DMEM supplemented with 1% antibiotic–antimycotic (Invitrogen). Arteries were exposed to HG (30 mM) or normal glucose (NG, 5 mM plus 25 mM mannitol) prepared in DMEM as osmotic control. The chemicals, including DX600 (62337; AnaSpec, Inc.), A779 (H2888; Bachem, Torrance, CA), tiron (D7389; Sigma), and diethyldithiocarbamate (DETCA, D3506; Sigma), were added individually into the culture medium at desired concentrations.
Functional study
Changes of isometric tension of aortas were recorded in a wire myograph (Danish Myo Technology), and FMD was determined in a pressure myograph (60). For FMD study, the vessel diameter was continuously monitored on a light-inverted microscope (Zeiss Axiovert 40 Microscope, model 110P) through a video camera operated on the Myo-View system (Danish Myo Technology). Phenylephrine (5 μM) was used to induce a steady constriction after the artery was stabilized at an intraluminal pressure of 80 mmHg. FMD was then triggered by pressure change that equals ∼15 dynes/cm2 shear stress. At the end of the experiment, a maximum passive dilation was induced by switching to Ca2+-free Krebs solution containing 2 mM EGTA. FMD was expressed as the percentage changes of diameter=[(flow-induced dilation−Phe tone)/(passive dilation−Phe tone)]×100%.
Endothelial cell culture
HUVECs (Lonza) were cultured in endothelial cell growth medium (EGM, CC3024; Lonza) with 10% FBS and antibiotics. HAECs from Invitrogen were cultured in medium 200 (Gibco, M-200-500) supplemented with 2% FBS. Cells from passage 4–8 were used for experiments. Twelve-hour post-transduction with adenoviral vectors, the culture medium was changed with fresh medium. Primary MAECs were isolated, cultured, and identified at passage 2 by positive immunostaining of anti-eNOS and anti-VE-cadherin (60). MAECs at passage 2–4 with 70% confluence were used.
ACE2 activity measurement
The ACE2 activity was determined using an assay kit (AnaSpec, SensoLyte® 390). Mca/Dnp fluorescence resonance energy transfer (FRET) test was performed under the Envision multiple fluorescent reader (Perkin Elmer). The FRET effect was monitored at the endpoint of reagents' reaction and recorded as the relative fluorescence unit (RFU), which was a ratio of fluorescence intensity at excitation/emission=330 nm/390 nm. The ACE2 activity was expressed as the RFU versus protein amount (μg for tissue or cell sample).
Plasma Ang (1-7) and Ang II measurement
Plasma samples were extracted using the Extraction-Free Kit (EIAS) (S-5000; Bachem). Ang (1-7) and Ang II levels were measured by the high-sensitivity peptide enzyme immunoassay kit (Angiotensin I/II (1-7)-EIA Kit: S-1330; Bachem; Angiotensin II EIA Kit: 589301; Cayman). The absorbance was read at 450 nm for Ang (1-7) assay and 414 nm for Ang II assay in a 96-well microtiter plate reader (Bio-rad).
ROS production in arteries and endothelial cells
The amount of intracellular ROS production in the vascular wall and en face endothelium were determined using DHE (Molecular Probes, Invitrogen) (60). For en face DHE staining, after ex vivo culture, aortic rings were incubated with DHE (5 μM) for 15 min in extracellular medium (ECM, in mM: 121 NaCl, 5 NaHCO3, 10 Na-HEPES, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2 CaCl2, 10 glucose; pH=7.4). Rings were washed twice in ECM and cut open longitudinally. Subsequently, the endothelium was placed upside down between two coverslips (1#, Thermo). The fluorescence images were captured on a confocal microscope (FV1000; Olympus) (DHE: excitation: 535 nm, emission: 565–605 nm; autofluorescence of elastin excitation: 488 nm, emission 520–535 nm). Intracellular ROS in MAECs, HAECs, or HUVECs was assayed using DHE (Molecular Probes, Invitrogen, excitation: 535 nm, emission: 565–605 nm) or CM-H2DCFDA (Invitrogen, excitation: 488 nm; emission: 505–525 nm). In addition, the amount of vascular superoxide anion was also determined using the lucigenin-enhanced chemiluminescence method (21).
Western blot analysis
Equal amounts of protein samples were electrophoresed on a 10% SDS-polyacrylamide gel, and then transferred onto an immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore). The membranes were blocked by 2% BSA in 0.05% Tween-20 phosphate-buffered saline (PBST), then incubated overnight at 4°C with primary antibodies, including ACE2 (#3583-1; Epitomics), phospho-AktThr308 (#4056; Cell Signaling Technology), phospho-AktSer473 (#9271; Cell Signaling Technology), Akt1 (#2967, Cell Signaling Technology), phospho-MAPK family members (p-p38, p-Erk1/2, p-SAPK/JNK, #9910 antibody sampler kit; Cell Signaling Technology), Erk1/2 (#9107, Cell Signaling Technology), p38(#9212; Cell Signaling Technology), SAPK/JNK(#9252; Cell Signaling Technology), phospho-eNOSSer1177 (ab51038; Abcam), phospho-eNOSThr495(#9574; Cell Signaling Technology), eNOS (610297; BD Transduction Laboratories), NOX2/gp91phox (ab31092, Abcam), NADPH oxidase 4 (NOX4, ab60940, Abcam), p47phox (sc-14015; Santa Cruz), p67phox (#3923, Cell Signaling Technology), and UCP2 (AF4739; R&D System). The membranes were developed with an enhanced chemiluminescence detection system (ECL reagents; Amersham Pharmacia) and finally exposed to X-ray films.
qPCR analysis
Total RNA was isolated with TRIZOL reagent (Invitrogen) from the aorta, heart, liver, or HAECs. cDNA was synthesized using 1.0 μg RNA as a template with high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative PCR was performed on the ViiATM7 real-time PCR system (Applied Biosystems) with GAPDH used as an internal control. Gene expression was analyzed by the comparative CT method and expressed compared with GAPDH. The sequences of primers used are presented in Supplementary Table S2.
NO detection in endothelial cells
Fluorometric NO measurements were performed on HUVECs as described before (56) using the Olympus FV1000 laser scanning confocal system (Olympus) mounted on an inverted microscope (IX81; Olympus) equipped with 10X objective (NA 0.5). 4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate; Molecular Probes) was used as the NO indicator. Cells were incubated with 1 μM DAF-FM DA in the dark for 15 min and then washed thrice, 5 min each. NO production stimulated by 1 μM A23187 was evaluated by measuring fluorescence intensity excited at 495 nm and emitted at 515 nm. Changes in intracellular NO production were displayed as relative fluorescence intensity F1/F0 (F0=basal fluorescence, F1=fluorescence after A23187 administration).
Statistical analysis
Concentration–response curves were constructed using GraphPad Prism software (Version 5.0). Protein expression was quantified by Quantity One 1-D Analysis software (Version 4.6.2; Bio-Rad) and normalized to GAPDH, and then compared with control. Data are mean±SEM of n separate experiments. Student's t-test or one-way ANOVA, followed by Bonferroni post hoc tests for more than two treatments, were compared. p<0.05 was regarded as significantly different.
Supplementary Material
Abbreviations Used
- ACE2
angiotensin-converting enzyme 2
- ACh
acetylcholine
- Ad-ACE2
adenovirus ACE2
- Ang (1-7)
angiotensin (1-7)
- Ang II
angiotensin II
- DHE
dihydroethidium
- DIZE
diminazene aceturate
- DM
diabetes mellitus
- EDR
endothelium-dependent relaxation
- eNOS
endothelial nitric oxide synthases
- FMD
flow-mediated dilatation
- FRET
fluorescence resonance energy transfer
- HAECs
human aortic endothelial cells
- HG
high glucose
- HUVECs
human umbilical vein endothelial cells
- KO
knockout
- MAECs
mouse aortic endothelial cells
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- Phe
phenylephrine
- RAS
renin–angiotensin system
- RFU
relative fluorescence unit
- RLU
relative chemiluminescence unit
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- UCP2
uncoupling protein-2
- WT
wild-type
Acknowledgments
This study is supported by the Hong Kong Research Grants Council (CUHK2/CRF/12G, 466110, T12/402/13N, CUHK3/CRF/12R), the NIH HL102033 grants, and the Natural Science Foundation of China (91339117, 2012CB517805).
Author Disclosure Statement
There are no competing financial interests.
References
- 1.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, and Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bindom SM, Hans CP, Xia H, Boulares AH, and Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes 59: 2540–2548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosnihan KB, Li P, and Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension 27: 523–528, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, and Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46: 937–942, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Xiao X, Chen S, Zhang C, Chen J, Yi D, Shenoy V, Raizada MK, Zhao B, and Chen Y. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension 61: 681–689, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosentino F. and Luscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol 32 Suppl 3: S54–S61, 1998 [PubMed] [Google Scholar]
- 7.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, and Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Dong B, Yu QT, Dai HY, Gao YY, Zhou ZL, Zhang L, Jiang H, Gao F, Li SY, Zhang YH, Bian HJ, Liu CX, Wang N, Xu H, Pan CM, Song HD, Zhang C, and Zhang Y. Angiotensin-converting enzyme-2 overexpression improves left ventricular remodeling and function in a rat model of diabetic cardiomyopathy. J Am Coll Cardiol 59: 739–747, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, and Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol 35: 1043–1053, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, and Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res 102: 729–736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraga-Silva RA, Costa-Fraga FP, Murca TM, Moraes PL, Martins Lima A, Lautner RQ, Castro CH, Soares CM, Borges CL, Nadu AP, Oliveira ML, Shenoy V, Katovich MJ, Santos RA, Raizada MK, and Ferreira AJ. Angiotensin-converting enzyme 2 activation improves endothelial function. Hypertension 61: 1233–1238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gava E, Samad-Zadeh A, Zimpelmann J, Bahramifarid N, Kitten GT, Santos RA, Touyz RM, and Burns KD. Angiotensin-(1-7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrol Dial Transplant 24: 1766–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gjymishka A, Kulemina LV, Shenoy V, Katovich MJ, Ostrov DA, and Raizada MK. Diminazene Aceturate Is an ACE2 Activator and a Novel Antihypertensive Drug. Faseb J 24: 1032–1033, 2010 [Google Scholar]
- 14.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, and Chappell MC. Angiotensin-(1-7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension 55: 166–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heitsch H, Brovkovych S, Malinski T, and Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension 37: 72–76, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Higashi Y, Noma K, Yoshizumi M, and Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J 73: 411–418, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Iusuf D, Henning RH, van Gilst WH, and Roks AJ. Angiotensin-(1-7): pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol 585: 303–312, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, Kent D, Stitt AW, Thut C, Finney EM, Raizada MK, and Grant MB. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 62: 1258–1269, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin HY, Song B, Oudit GY, Davidge ST, Yu HM, Jiang YY, Gao PJ, Zhu DL, Ning G, Kassiri Z, Penninger JM, and Zhong JC. ACE2 deficiency enhances angiotensin II-mediated aortic profilin-1 expression, inflammation and peroxynitrite production. PLoS One 7: e38502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulemina LV. and Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen 16: 878–885, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Lau YS, Tian XY, Mustafa MR, Murugan D, Liu J, Zhang Y, Lau CW, and Huang Y. Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade. Br J Pharmacol 170: 1190–1198, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lely AT, Hamming I, van Goor H, and Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol 204: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Li G, Xu YL, Ling F, Liu AJ, Wang D, Wang Q, and Liu YL. Angiotensin-converting enzyme 2 activation protects against pulmonary arterial hypertension through improving early endothelial function and mediating cytokines levels. Chin Med J (Engl) 125: 1381–1388, 2012 [PubMed] [Google Scholar]
- 24.Li Y, Wu J, He Q, Shou Z, Zhang P, Pen W, Zhu Y, and Chen J. Angiotensin (1-7) prevent heart dysfunction and left ventricular remodeling caused by renal dysfunction in 5/6 nephrectomy mice. Hypertens Res 32: 369–374, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Lv XH, Li HX, Cao X, Zhang F, Wang L, Yu M, and Yang JK. Angiotensin-(1-7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol 49: 291–299, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Liu CX, Hu Q, Wang Y, Zhang W, Ma ZY, Feng JB, Wang R, Wang XP, Dong B, Gao F, Zhang MX, and Zhang Y. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med 17: 59–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, Xu G, Ng CF, Yao X, Gao Y, and Huang Y. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal 21: 1571–1581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loot AE, Roks AJ, Henning RH, Tio RA, Suurmeijer AJ, Boomsma F, and van Gilst WH. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 105: 1548–1550, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, and Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol 295: H1377–H1384, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, and He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc 2: 1236–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Masson R, Nicklin SA, Craig MA, McBride M, Gilday K, Gregorevic P, Allen JM, Chamberlain JS, Smith G, Graham D, Dominiczak AF, Napoli C, and Baker AH. Onset of experimental severe cardiac fibrosis is mediated by overexpression of Angiotensin-converting enzyme 2. Hypertension 53: 694–700, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, and Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 103: 1319–1326, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Moon JY, Tanimoto M, Gohda T, Hagiwara S, Yamazaki T, Ohara I, Murakoshi M, Aoki T, Ishikawa Y, Lee SH, Jeong KH, Lee TW, Ihm CG, Lim SJ, and Tomino Y. Attenuating effect of angiotensin-(1-7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol 300: F1271–F1282, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Muthalif MM, Benter IF, Uddin MR, Harper JL, and Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther 284: 388–398, 1998 [PubMed] [Google Scholar]
- 35.Niu MJ, Yang JK, Lin SS, Ji XJ, and Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine 34: 56–61, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Ohshima K, Mogi M, Nakaoka H, Iwanami J, Min LJ, Kanno H, Tsukuda K, Chisaka T, Bai HY, Wang XL, Ogimoto A, Higaki J, and Horiuchi M. Possible role of angiotensin-converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin-(1-7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension 63: e53–e59, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, and Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59: 529–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, Lo J, Grant MB, Zhong J, Kassiri Z, and Oudit GY. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res 110: 1322–1335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pena Silva RA, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, and Heistad DD. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 43: 3358–3363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi Y, Zhang J, Cole-Jeffrey CT, Shenoy V, Espejo A, Hanna M, Song C, Pepine CJ, Katovich MJ, and Raizada MK. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension 62: 746–752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raffai G, Durand MJ, and Lombard JH. Acute and chronic angiotensin-(1-7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol 301: H1341–H1352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren Y, Garvin JL, and Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RA, and Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 52: 967–973, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, and Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50: 1093–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, and Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Shenoy V, Gjymishka A, Jarajapu YP, Qi Y, Afzal A, Rigatto K, Ferreira AJ, Fraga-Silva RA, Kearns P, Douglas JY, Agarwal D, Mubarak KK, Bradford C, Kennedy WR, Jun JY, Rathinasabapathy A, Bruce E, Gupta D, Cardounel AJ, Mocco J, Patel JM, Francis J, Grant MB, Katovich MJ, and Raizada MK. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am J Respir Crit Care Med 187: 648–657, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiota A, Yamamoto K, Ohishi M, Tatara Y, Ohnishi M, Maekawa Y, Iwamoto Y, Takeda M, and Rakugi H. Loss of ACE2 accelerates time-dependent glomerular and tubulointerstitial damage in streptozotocin-induced diabetic mice. Hypertens Res 33: 298–307, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Soares de Moura R, Resende AC, Emiliano AF, Tano T, Mendes-Ribeiro AC, Correia ML, and de Carvalho LC. The role of bradykinin, AT2 and angiotensin 1-7 receptors in the EDRF-dependent vasodilator effect of angiotensin II on the isolated mesenteric vascular bed of the rat. Br J Pharmacol 141: 860–866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, and Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 72: 614–623, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Stegbauer J, Oberhauser V, Vonend O, and Rump LC. Angiotensin-(1-7) modulates vascular resistance and sympathetic neurotransmission in kidneys of spontaneously hypertensive rats. Cardiovasc Res 61: 352–359, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Stegbauer J, Potthoff SA, Quack I, Mergia E, Clasen T, Friedrich S, Vonend O, Woznowski M, Konigshausen E, Sellin L, and Rump LC. Chronic treatment with angiotensin-(1-7) improves renal endothelial dysfunction in apolipoproteinE-deficient mice. Br J Pharmacol 163: 974–983, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su Z, Zimpelmann J, and Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69: 2212–2218, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Tallant EA. and Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1-7). Hypertension 42: 574–579, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Thatcher SE, Zhang X, Howatt DA, Lu H, Gurley SB, Daugherty A, and Cassis LA. Angiotensin-converting enzyme 2 deficiency in whole body or bone marrow-derived cells increases atherosclerosis in low-density lipoprotein receptor-/- mice. Arterioscler Thromb Vasc Biol 31: 758–765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, and Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 107: 888–897, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Tian XY, Wong WT, Wang N, Lu Y, Cheang WS, Liu J, Liu L, Liu Y, Lee SS, Chen ZY, Cooke JP, Yao X, and Huang Y. PPARdelta activation protects endothelial function in diabetic mice. Diabetes 61: 3285–3293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian XY, Wong WT, Xu A, Lu Y, Zhang Y, Wang L, Cheang WS, Wang Y, Yao X, and Huang Y. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res 110: 1211–1216, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, and Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Zhao YX, Zhang YH, Zhu L, Deng BP, Zhou ZL, Li SY, Lu XT, Song LL, Lei XM, Tang WB, Wang N, Pan CM, Song HD, Liu CX, Dong B, Zhang Y, and Cao Y. Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions by targeting vascular cells. Proc Natl Acad Sci U S A 107: 15886–15891, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Liu J, Tian XY, Wong WT, Chen Y, Wang L, Luo J, Cheang WS, Lau CW, Kwan KM, Wang N, Yao X, and Huang Y. Inhibition of bone morphogenic protein 4 restores endothelial function in db/db diabetic mice. Arterioscler Thromb Vasc Biol 34: 152–159, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, and Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122: 717–728, 18 p following 728, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.