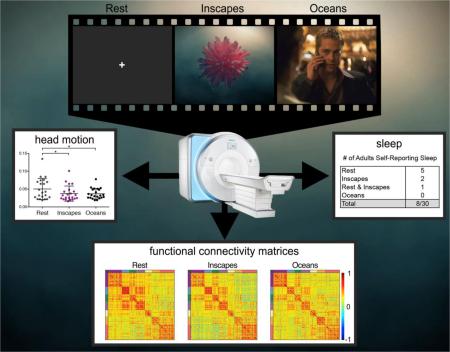
Abstract
The examination of functional connectivity in fMRI data collected during task-free “rest” has provided a powerful tool for studying functional brain organization. Limitations of this approach include susceptibility to head motion artifacts and participant drowsiness or sleep. These issues are especially relevant when studying young children or clinical populations. Here we introduce a movie paradigm, Inscapes, that features abstract shapes without a narrative or scene-cuts. The movie was designed to provide enough stimulation to improve compliance related to motion and wakefulness while minimizing cognitive load during the collection of functional imaging data. We compare Inscapes to eyes-open rest and to age-appropriate movie clips in healthy adults (Ocean's Eleven, n = 22) and a pilot sample of typically developing children ages 3-7 (Fantasia, n = 13). Head motion was significantly lower during both movies relative to rest for both groups. In adults, movies decreased the number of participants who self-reported sleep. Intersubject correlations, used to quantify synchronized, task-evoked activity across movie and rest conditions in adults, involved less cortex during Inscapes than Ocean's Eleven. To evaluate the effect of movie-watching on intrinsic functional connectivity networks, we examined mean functional connectivity using both whole-brain functional parcellation and network-based approaches. Both inter- and intra-network metrics were more similar between Inscapes and Rest than between Ocean's Eleven and Rest, particularly in comparisons involving the default network. When comparing movies to Rest, the mean functional connectivity of somatomotor, visual and ventral attention networks differed significantly across various analyses. We conclude that low-demand movies like Inscapes may represent a useful intermediate condition between task-free rest and typical narrative movies while still improving participant compliance. Inscapes is publicly available for download at headspacestudios.org/inscapes.
Keywords: naturalistic, resting state, default network, fMRI, head motion, movies
Graphical Abstract
1. Introduction
1.1 Challenges of scanning in the absence of a task
Functional connectivity (FC) analyses of fMRI data identify spatially separate brain regions that exhibit correlated blood-oxygen level dependent (BOLD) signal time courses (Biswal et al., 1995). Such data are frequently collected in the absence of a typical task, often called “rest” or “resting state fMRI” (R-fMRI). Subjects are usually asked to lay still and to remain awake while keeping their eyes open for 6-10 minutes, often with a fixation cross displayed on a screen to provide something central at which to look.
A major limitation of this approach is that even small head movements can produce systematic artifacts in FC measures (Power et al., 2012, Van Dijk et al., 2012, Satterthwaite et al., 2013, Power et al., 2015). Head movement is a particular problem when studying awake children, especially those below the age of seven, as indicated by the fact that the “developmental” curves or trajectories we currently have describing FC in awake participants start at age seven (Dosenbach et al., 2010, Vogel et al., 2010, Alexander-Bloch et al., 2013, Dennis and Thompson, 2014). Movement is also problematic when studying individuals with psychiatric or neurological disorders who find staying still in the absence of a formal task to be a challenging task in and of itself.
A second drawback to the resting state approach is the tendency of many participants to fall asleep (Tagliazucchi and Laufs, 2014). Sleep can alter FC patterns (Horovitz et al., 2008, Horovitz et al., 2009, Boly et al., 2012, Spoormaker et al., 2012), which is problematic when subjects are presumed to be awake. Variable onset of sleep can confound studies of healthy adults but is of particular concern in studies of geriatric patients; patients taking sedating medications, such as atypical antipsychotics or some antidepressants; and sleep-deprived populations such as young parents, adolescents and young adults.
1.2 Movies and compliance
In an attempt to improve compliance when collecting data for FC analyses, we developed a movie called Inscapes that could be used with young children and clinical populations, providing a practical way to improve compliance that would be easy to disseminate. Movies have been shown to positively affect compliance during MRI scanning. In clinical settings, movies are routinely shown to children undergoing MRI for anatomical studies to help them stay sufficiently still to avoid the need for sedation or anesthesia (Khan et al., 2007, Raschle et al., 2009). Similarly, it is common practice in pediatric fMRI research to show cartoons during structural sequences. In children ages 4-10, Cantlon and Li report lower head movement during movie clips relative to a task condition (Cantlon and Li, 2013). Movies have also been used to facilitate long periods of data collection in healthy adults, such as 55 minutes continuously (Sabuncu et al., 2010, Conroy et al., 2013) and 10 5-minute runs repeated at 10 separate sessions (Anderson et al., 2011).
1.3 Movies and fMRI measures
A second and competing goal of Inscapes was to minimize the cognitive processing evoked by the paradigm. Because we are interested in studying development, we also wanted to avoid some of the developmental confounds inherent to typical movies and tasks that rely on verbal or spatial processing, social inference, or general task performance, as these competencies can vary widely at different developmental stages. Consequently, we created a nonsocial, nonverbal movie that features abstract shapes and is without scene-cuts or camera-based perspective changes.
Movies as fMRI stimuli have been studied extensively (for reviews see Spiers and Maguire, 2007, and Hasson et al., 2010). A consistent finding is that movies evoke time-locked responses that are shared across subjects. These intersubject correlations (ISCs) occur when the BOLD signal time course from voxel A in subject A correlates with the time-course from voxel A in subject B (Hasson et al., 2004). ISCs have been quantified in animals (Haider et al., 2010, Mantini et al., 2012a, Mantini et al., 2012b) and humans (Bartels and Zeki, 2004, 2005, Hasson et al., 2008, Wilson et al., 2008, Kauppi et al., 2010, Pajula et al., 2012), and are reliable across multiple viewings of the same movie (Hasson et al., 2009, Hasson et al., 2010). ISCs have been studied most extensively in the visual cortex, and the extent of ISCs evoked by a movie depends on the content and nature of each movie. In the absence of a time-locked stimulus (i.e., during rest), no ISCs exist, while during rich, complex movies, ISCs can extend throughout the brain.
A number of researchers have studied the modulation of intrinsic functional connectivity by task-related activity (Fransson, 2006, Calhoun et al., 2008, Lv et al., 2013, Mennes et al., 2013, Li et al., 2015a, Li et al., 2015b). Some of this work has specifically investigated the effects of movie-watching on spontaneous neural activity and FC. In visual cortex, Fiser et al. used implanted electrodes in ferrets to study neural responses during a movie (Tomorrow Never Dies) and “dark” rest (Fiser et al., 2004). They demonstrated that even complex visual stimulation did not significantly alter the basic correlational structure of spontaneous activity in the visual cortex. In macaques, Moeller et al. showed that independent component analyses (ICA) of fMRI data acquired during movie-watching, rest and various visual task conditions revealed FC networks that were highly similar across conditions (Moeller et al., 2009). Compared with changes induced by different anesthetic states, the changes induced by movie-watching conditions relative to rest were small. In humans, Golland and colleagues investigated which brain regions did and did not exhibit strong FC during movie-watching (Golland et al. 2007). They selected the brain region that showed the lowest intrasubject correlations across repeated viewings of the same movies as a seed for functional connectivity analyses, and found that the regions that did not demonstrate intrasubject correlations demonstrated strong functional connectivity. Overlaying their “extrinsic” (intrasubject correlations) and “intrinsic” (FC) maps resulted in almost full anatomical coverage of the posterior cortex. Finally, Betti et al. used magnetoencephalography (MEG) and fMRI to compare movies and resting state conditions (Betti et al., 2013). Data from 12 adult subjects who watched 5-minute clips from the movie “The Good, the Bad and the Ugly” show that movie-watching decreased FC within visual and dorsal attention networks compared to Rest. The power of MEG data frequencies differed between the movie and rest, but the spatial topography of networks was preserved across conditions in both fMRI and MEG data.
Overall, these studies suggest that while movies may modulate aspects of FC, other characteristics of FC patterns are preserved and can be measured during movie-watching. This observation fits with recent discussions about the nature of different acquisition states for fcMRI such as “rest”, task and movie-watching. Buckner et al. write that a portion of the patterns observed under any acquisition state (including rest and task) arise from “invariant constraints” that include anatomic connectivity, while the other portion arises from “dynamic properties” evoked by the task elements of the state (Buckner et al., 2013). By creating a movie that avoids social narrative and is nonverbal, slow moving and abstract, we attempted to shift these proportions in a novel, arbitrary way. We hypothesized that relative to typical movie paradigms, a “low-demand” non-narrative movie would decrease task-evoked neural activity and increase our ability to capture intrinsic (i.e., non-evoked) FC relationships. At the same time, we hypothesized that relative to rest, our paradigm would provide a more constrained state that would improve movement and wakefulness, with implications for improved data collection in populations such as children and those with psychiatric disorders.
1.4 Approach to characterizing the novel paradigm
In the present study, we examined patterns of FC and ISCs during two different movie conditions (one conventional, socially rich movie and Inscapes, the abstract, nonverbal movie) and eyes-open rest. We used ISCs to index the extent of synchronized evoked activity across subjects and across conditions. We used whole-brain and network-level measures of functional connectivity to characterize patterns of intrinsic FC. Overall, this study aims to introduce the novel paradigm, to quantify compliance regarding head motion and sleep, and to characterize basic patterns of FC and ISCs observed while subjects watch the novel movie.
Our corresponding hypotheses were: i) all movie conditions tested will be associated with lower head movement than the Rest condition, and children will show a greater improvement in head motion during movies compared to Rest than adults; ii) Inscapes will evoke weaker and less extensive ISCs than a conventional movie, but more ISCs than Rest; iii) Inscapes will be associated with patterns of FC that more closely resemble those obtained during Rest than during a conventional movie.
2. Materials and Methods
2.1 Inscapes design and production
Visuals
Inscapes is a computer-generated (CG) animation, produced mainly using Cinema 4D software (MAXON Computer Inc.) by visual artist Tobias S. Hoffmann. The frame rate is 25 frames per second, and the resolution is 1024 × 800 pixels with a square pixel aspect ratio. The color format is YUV 4:2:0 (see Supplementary Table 1 for more details). Inscapes can be viewed online at headspacestudios.org/inscapes.
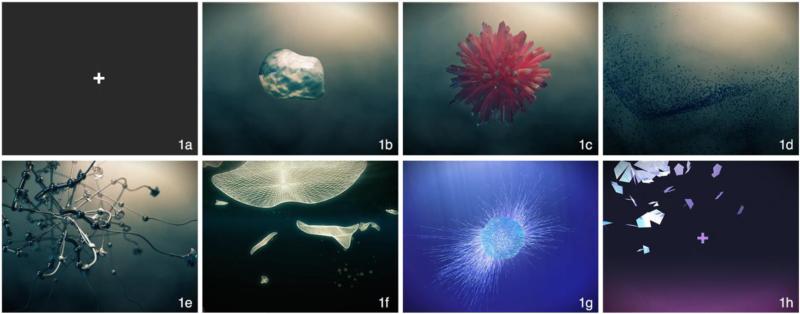
Unique, technological-looking, three-dimensional graphics were used to enhance novelty and to blend stylistically with high-tech MRI environments. Motion as a visual feature is engaging even to infants and is a central visual feature throughout the movie. The visual forms are abstract and nonsocial, and the film is nonverbal. Figure 1 depicts images from the main scenes, and Figure 2 provides a movie timeline.
Figure 1. Images from the movie.
Inscapes features a series of technological-looking, abstract shapes. The movie is nonverbal, avoids explicit social references, has no direct story or narrative, and does not use scene-cuts. It can be viewed at headspacestudios.org/inscapes.
Figure 2. Timeline of the movie.
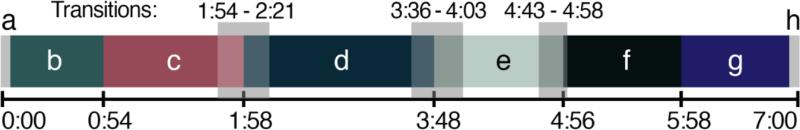
Letters correspond to scene images in Figure 1. The 7-minute movie comprises four main scenes (c-f), with introductory and finale scenes (b and g). The movie begins and ends with five seconds during which a fixation cross is presented (a and h). Transparent boxes indicate transitional periods during which scenes are switching.
To impart a sense of participant stillness, a fixed-camera perspective was used. Scenes were created to be as long and homogenous as possible without becoming boring or repetitive. Scene cuts in film dramatically affect attention and eye-gaze patterns (Smith, 2013), so each scene in Inscapes gradually evolves into the next without any scene cuts. We used a limited color palette, and we calculated mean luminance per frame and altered the coloration in the scenes with the highest and lowest mean luminance values to attenuate the most extreme changes.
Audio
The movie soundtrack features a piano score composed by Jodi S. Vander Woude in an uncompressed, stereophonic format, sampled at a rate of 48 kilohertz with both left and right channels. The score was recorded using a Steinway concert grand piano. Priority in the mix down was for even sound distribution for use with stereo headphones. Additionally, a minimal layer of sound design and sound effects was added to increase audiovisual synchrony.
The score for the movie was created to work well with the audible frequency produced by a Siemens Trio scanner during echo-planar imaging (EPI), which for our sequence is a pitch of 987.767 Hz (B5 on the piano). This was meant to musicalize the noise produced during scanning as much as possible. The composition is based on the pentatonic scale, a musical structure common to traditional music throughout most of the world including Africa, Asia and Europe, which sounds familiar to most listeners. The composer was tasked with creating a homogenous mood throughout the piece. This was achieved in part by maintaining a slow and constant tempo (48 beats per minute), using repetitive musical patterns, and limiting pitch content to five transpositions. Statistically extracted time courses that graphically depict visual and audio features from Inscapes and comparison movies are available in Figures 1s and 2s in Supplemental Materials.
2.2 Pediatric head movement study
Participants
Fifteen healthy children aged 3-7 years were recruited from the community. Two participants declined to enter the MRI simulator, leaving n = 13 (7 males, mean age = 5.4 ± 1.6 years). Exclusion criteria included centrally-acting medications, history of major head trauma, and significant vision or hearing impairment. Written consent was provided by each participant's parent, and participants were compensated for their participation. This study was approved by the Human Investigations Committee at Yale University School of Medicine.
Procedure
Participants were fit with a motion sensor headband (MoTrak System 1.0, Psychology Software Tools, Inc.) and then helped onto the MRI simulator. A blanket was tucked around the body, but head movement was not physically restricted. A mock head coil with an attached mirror facilitated viewing of the monitor at the back of the gantry. Stimuli were presented with MoTrak software. Sound-reducing, stereophonic headphones enabled participants to hear the movie soundtracks, and an audio recording of an echo-planar imaging sequence was played on the MRI simulator speakers during all conditions. Three 7-minute conditions included Rest (white fixation cross centered on a dark grey background), Inscapes, and the Sorcerer's Apprentice scene from Fantasia (Walt Disney Productions, 1940, directed by James Algar). Our convention when using the title of the film to name an experimental condition is to use the title in non-italicized form, so conditions here are called Inscapes, Fantasia and Rest. Participants were asked to relax and watch the movies, and to stay as still as possible. Condition order was counterbalanced. Participants knew that the headband was measuring head motion, but no practice or feedback was provided other than telling each participant that they were doing a great job (regardless of performance).
Data Processing
Head movement data were collected in MoTrak at 10 samples per second, yielding approximately 4200 data points per condition per participant. At each point, motion was encoded as deviation from the participant's starting position in three translational axes (x, y, z) and three rotational axes (pitch = α, roll = β, yaw = γ), yielding a six-dimensional time series. Frame-wise displacement (FD), a summary value of moment-to-moment motion between each point, was calculated from these six measurements using the formula, FDi = |Δdix| + |Δdiy| + |Δdiz| + |Δαi| + |Δβi| + |Δγi|, where Δdix = d(i − 1)x – dix, and similarly for the other rigid body parameters [dix diy diz αi βi γi] (Power et al., 2012). Rotational measures were converted from degrees to millimeters of movement on the surface of the head, assuming a spherical cranium. Due to the variability in head circumference in young children, we used measured head circumference to calculate each subject's approximate spherical radius. To facilitate comparison of this high sample-rate with in-scanner motion data (TR = 2500ms), the calculated one-dimensional FD time course was then segmented into 2500ms epochs and averaged (25-sample bins). A motion spike was defined as a change in FD greater than 0.15mm per bin, and the number of spikes per participant per condition was tabulated.
2.3 Adult fMRI study
Participants. Thirty healthy right-handed adults were recruited from the community as part of an ongoing fMRI study (18 males, mean age = 24.1 ± 4.4 years). Participants who reported falling asleep in the scanner at either session were excluded from all analyses, leaving n = 22 (12 males, mean age 24.3 ± 5.1). Exclusion criteria included history of concussion within the past 10 years, neurological or psychiatric diagnoses, less than an average of six hours of sleep per night, current use of centrally-acting medications, heavy alcohol use, any illicit drug use in the past 6 months, cardiovascular disease, and significant hearing or visual impairment. All participants gave written informed consent and were compensated for their participation. The study was approved by the Human Investigations Committee at Yale University School of Medicine.
Procedure
Imaging was performed on a Siemens Trio 3-Tesla scanner with a 32-channel head coil. Standard structural images used an MP-RAGE sequence (TR = 1900ms, TE = 2.52ms, TI = 900ms, flip angle = 9°) yielding 1mm3 voxel size. Functional data were collected using a single shot echo planar imaging sequence (TR = 2500ms, TE = 30ms, flip angle = 80°, voxel size = 3mm3) across 38 slices in the same location as the inplane anatomical scans. All participants completed 3 functional scans during which stimuli were presented via E-Prime software, version 2.0 (Psychology Software Tools, Pittsburgh, PA). Images were back-projected onto a screen at the end of the gantry, which participants viewed via a mirror mounted above their eyes on the head coil. Sound-reducing, stereophonic headphones over protective earplugs enabled participants to hear the movie soundtracks. Three 7 minute and 20 second conditions included Inscapes, a clip from the movie Ocean's Eleven (Warner Brothers, 2001, directed by Steven Soderbergh) referred to here as Oceans, and Rest (static fixation cross on a grey background). The order of conditions was counter-balanced across participants. Each condition started and ended with 10 seconds of fixation; only the middle 7 minutes were utilized for all analyses. Participants were asked to watch the screen and to stay as still as possible during each condition. Foam wedges were fitted around the participant's head for comfort and to decrease movement.
Data Processing
Standard data preprocessing was performed, including motion realignment, despiking, grand-mean scaling, and linear and quadratic trend removal. Nuisance signal regression utilized COMPCOR (Behzadi et al. 2007), followed by spatial smoothing (Gaussian kernel 6mm FWHM) and temporal filtering (0.009-0.1Hz). Data were transformed into Montreal Neurological Institute (MNI) space using ANTs (Advanced Normalization Tools).
Movement
Motion was evaluated using an algorithm that quantifies the relative framewise displacement (FD) between each volume of functional data (Jenkinson et al., 2002). A composite, linear transformation is performed to best fit each volume to the one that came before it. The root-mean square of this (FDRMS) is strongly correlated with voxel-specific measures of head movement and with the Powers FD calculation used with the MoTrak data in the pediatric study (Yan et al., 2013). We defined a “spike” in head movement as FDRMS > 0.15mm, and counted the number of spikes per subject per condition.
Intersubject Correlations
Following an approach developed by Hasson et al., the time course of voxel A in subject A was correlated with voxel A in subject B, in subject C, and so on for all subjects (Hasson et al., 2004). The resulting Pearson's correlation scores were then Fisher z-transformed and averaged at each voxel. Significant group-level intersubject correlations (ISCs) were identified using a one-sample t-test, corrected for multiple comparisons using Gaussian Random Field theory (Z < 2.3, cluster significance p= 0.05, corrected).
Whole-Brain Functional Connectivity
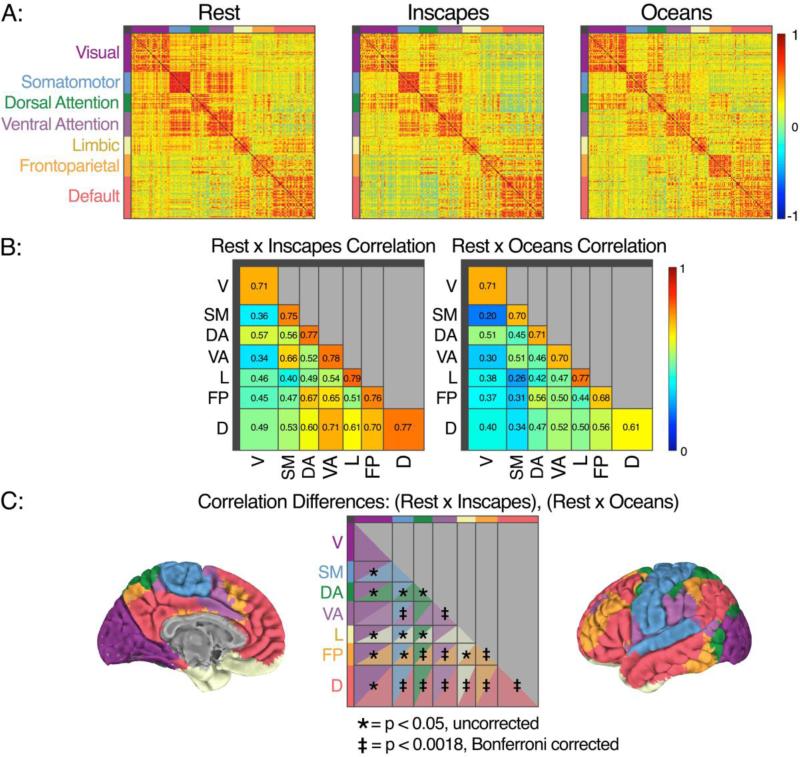
For functional connectivity (FC) analyses, we used a functional parcellation scheme comprising 200 regions of interest (ROIs) (Craddock et al., 2012). For each subject, we extracted the mean time series of each ROI and then computed the Pearson's correlation between all ROI pairs to produce a 200×200 whole-brain connectivity matrix for each condition. Correlation coefficients were Fisher z-transformed, averaged across subjects, and then reverted to r-values to produce group-level correlation matrices. To qualitatively assess similarities across conditions in terms of the correlations within and between large-scale functional networks, we arranged the ROIs on the matrix according to network membership using the 7-network scheme (visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal and default networks; see Figure 6), as defined by Yeo and colleagues (Yeo et al., 2011). This 7-network scheme allows us to assess the effect of condition on FC within each of the 7 large-scale functional networks (i.e. intra-network FC) as well as between each pair of networks (21 inter-network pairings).
Figure 6. Cross-condition correlations in mean functional connectivity using functional parcellation, healthy adults, n = 22.
Using a parcellation scheme of 200 functional ROIs (Craddock et al. 2012), the time-series of BOLD signal change in each ROI was extracted and Pearson's correlations were computed between all ROI pairs. Correlation coefficients were Fischer z-transformed, averaged across subjects, and reverted to r-values to produce group-level correlation matrices (Row A). For visualization purposes, these ROIs are arranged on the matrix according to network membership using a 7-network system (Yeo et al., 2011, see brain pictures in Row C for anatomical depiction of these networks, and the text at top left to indicate which color denotes which network in Rows A and C). To assess similarity, for each subject and for each of the 28 intra- and internetwork groupings of ROIs, we computed the correlation between conditions (Rest, Inscapes and Rest, Oceans) across ROIs within a grouping. These correlations (reverted r-values as above) are shown in Row B. Both Inscapes and Oceans demonstrate moderate to strong correlations with Rest in multiple groupings. Row C shows the results of a statistical comparison of the subject-level correlations from Row B, which demonstrated that the correlation with Rest is significantly higher for Inscapes than for Oceans for 11 of 28 comparisons when corrected for multiple comparisons. In particular, intra- and inter-network FC for the default network is signficantly more correlated with Rest during Inscapes than during Oceans.
Next, we quantitatively assessed similarities between Rest and Inscapes and between Rest and Oceans using this 7-network scheme. For each subject, and for each of the 28 intra- and inter-network groupings of ROIs, we computed the correlation between conditions across ROIs within a grouping. For example, the default network grouping contains 41 ROIs. For each subject, we computed the Fisher z-transformed Pearson correlation between the 41 FC values belonging to the default network at Rest and the 41 values belonging to the default network during Inscapes. We then computed the mean Fisher z-transformed correlation across subjects, and reverse-transformed the averaged values to r-values. This analysis tells us, on average, how similar the conditions are within subjects.
Finally, to quantitatively evaluate differences in FC, we performed a series of repeated-measures ANOVAs that tested the effect of condition (Inscapes, Oceans, Rest) on the subject-level mean Fischer z-transformed r-values quantifying intra- and inter-network FC. Post hoc paired t-tests were conducted where there was a main effect of condition.
Mean Network Connectivity
To quantify a measure of mean connectivity within intrinsic connectivity networks for each of the three conditions based on voxel-wise measures, we performed a dual regression analysis using FSL (Filippini et al., 2009), again applying the Yeo et al. 7-network scheme (Yeo et al., 2011). First, for each subject, a best-fit mean time course was calculated for each of the 7 networks using spatial regression. These time courses were then simultaneously regressed on each subject's preprocessed data to produce 7 functional connectivity maps. Finally, we extracted the mean functional connectivity by averaging across regression coefficients falling within each network (using the respective Yeo network masks) for each subject.
3. Results
3.1Compliance
Pediatric Study
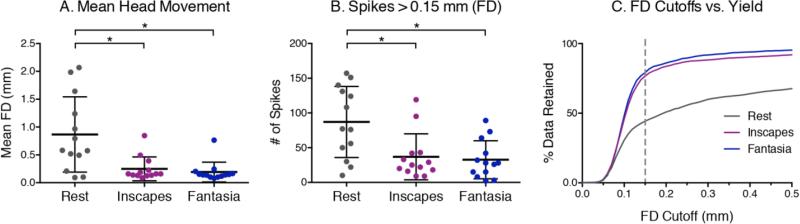
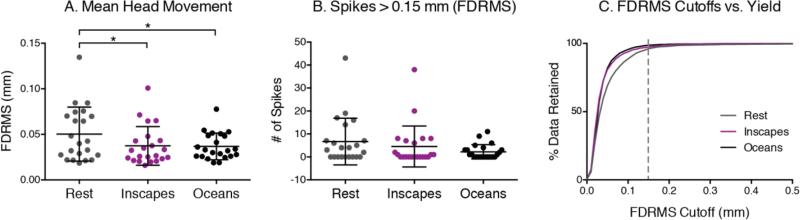
No pediatric participants reported falling asleep or were observed to fall asleep. Mean FD in the pediatric group was significantly lower for both movie conditions than for Rest (repeated measures ANOVA, F (2,12) = 10.10, p < 0.005, post hoc two-tailed t-test, Inscapes p < 0.01, Fantasia p < 0.01), as was the number of spikes (repeated measures ANOVA, F (2,12) = 17.45, p < 0.001, post hoc two-tailed t-tests, Inscapes p < 0.0005, Fantasia p < 0.005) (Figure 3). At the subject level, 11 of 13 participants had higher mean FD during Rest than during the movies. Inscapes and Fantasia were not significantly different from each other for either outcome. Figure 5c shows the trade-off in data yield at different movement thresholds. For example, at a cut-off of FD = 0.15mm, 44% of the data is usable in Rest, 77% in Inscapes, and 79% in Fantasia. We also tested for and found no effects of condition presentation order on head movement.
Figure 3. Pediatric head motion, n = 13 (7 males, mean age = 5.4 ± 1.6 years).
Head motion was measured in an MRI simulator. (A) Framewise displacement (FD) was calculated using an algorithm that quantifies relative displacement between each data point (Power et al., 2012). (B) A motion “spike” is a 2500ms time period with mean FD > 0.15mm. Both Inscapes and Fantasia were associated with significantly lower mean FD and lower number of spikes compared to Rest (p < 0.001). (C) The relationship between data yield and different motion cutoffs across the three conditions is shown. At a cut-off of 0.15 mm for each data point, 44% of the data can be kept from Rest, 77% from Inscapes and 79% from Fantasia.
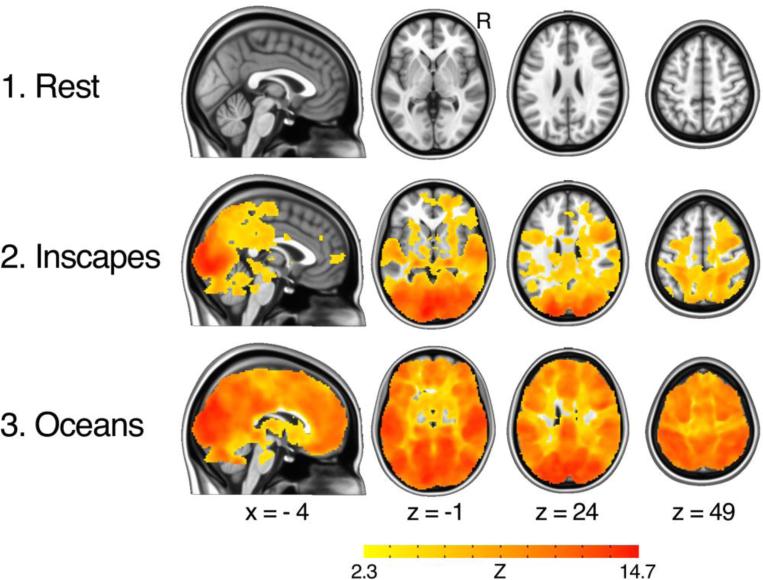
Figure 5. Intersubject correlations (ISCs), n = 22, healthy adults (12 adults, mean age = 24.3 ± 5.1 years).
This analysis utilizes the approach developed by Hasson et al. that maps correlations between voxel A in subject A and voxel A in subject B (Hasson et al. 2004). This approach identifies regions that exhibit synchronized activity across subjects. As hypothesized, Inscapes exhibits an intermediate level of ISCs with more widespread and stronger ISCs than Rest and spatially more limited and weaker correlations than Oceans. Correction for multiple comparisons was performed using Gaussian Random Field theory (Z < 2.3, cluster significance p = 0.05, corrected).
Adult Study
Of 30 adult participants, 8 participants reported falling asleep: 5 during Rest, 2 during Inscapes, none during Oceans, and 1 during both Rest and Inscapes (Fischer's exact p = 0.16 for Inscapes and p = 0.01 for Oceans relative to Rest; p = 0.12 for Inscapes vs. Oceans). These 8 participants were removed from all subsequent analyses. Measures of head movement in the remaining 22 participants were significantly lower for Inscapes when compared to Rest for mean FD but not for number of motion spikes (Figure 4). Inscapes did not differ significantly from Oceans on either measure. When examined at the subject level, 11 of 22 subjects exhibited the group mean pattern, i.e., higher mean FD during Rest compared to both movie conditions. These 11 subjects had a significantly higher mean FD during Rest than the subjects who exhibited a mixed pattern (e.g., higher FD during one of the movie conditions than during Rest), suggesting that the subjects who moved the most during Rest exhibited the greatest reductions in mean FD during movie conditions. This association is supported by the positive correlations between mean FD during Rest and the difference in FD between Rest and Inscapes (r = 0.71, p < 0.0005) and between mean FD during Rest and the difference in FD between Rest and Oceans (r = 0.87, p < 0.0001). Tests for order effect again revealed no effects of condition presentation order on head movement.
Figure 4. Head motion outcomes, n = 22, healthy adults (12 males, mean age = 24.3 ± 5.1 years).
(A) Head motion was evaluated using an algorithm that quantifies the root mean square (RMS) of the relative framewise displacement (FD) between each volume of functional data (Jenkinson et al, 2002). Both Inscapes and Oceans were associated with significantly lower FDRMS than Rest (p < 0.05). (B) A motion “spike” is a volume with FDRMS > 0.15mm, and no significant differences were found across conditions in this sample. (C) At a threshold of FDRMS 0.15 mm for each data point, 95% of the data can be retained from Rest, 97% from Inscapes and 99% from Oceans. At a threshold of 0.10mm, both movies become significantly better than Rest (p<0.05) with regards to percent data retained.
3.2 Functional correlations across conditions
Intersubject Correlations (Figure 5)
Strong ISCs throughout the brain were observed for Oceans, while no significant ISCs were observed for Rest. For Inscapes, ISCs covered the posterior cortex, with the strongest ISCs present in occipital cortex and temporal poles. Areas of the brain lacking significant ISCs during Inscapes included prefrontal cortex and some parietal regions bilaterally. Overall, the strength and spatial extent of intersubject correlations followed the pattern Oceans > Inscapes > Rest.
Whole-brain Functional Connectivity (Figures 6 and 7)
Figure 7. Differences in mean functional connectivity (FC) across conditions, healthy adults, n = 22.
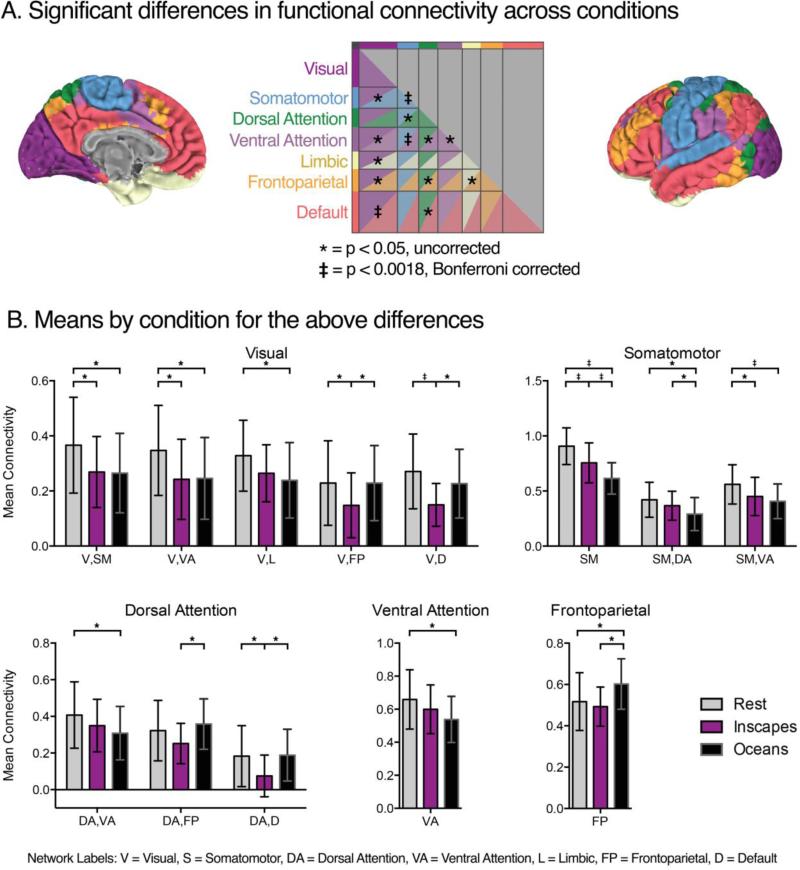
Starting with the 200×200 functional parcellation connectivity matrices (Row A, Figure 6), we performed a series of 28 repeated-measures ANOVAs that tested the effect of condition (Inscapes, Oceans, Rest) on the subject-level mean Fischer z-transformed r-values quantifying intra- and inter-network FC. Results of the ANOVAs are summarized in Panel A. Post hoc t-tests were performed where a significant main effect of condition was obtained, the results of which are depicted in Panel B (p < 0.05), along with the group-level means for each condition. Although there were multiple differences among conditions at an uncorrected threshold, only three main effects of condition survived Bonferroni correction (p < 0.0018): intra-network FC of the somatomotor network; inter-network FC of somatomotor-ventral attention networks; and inter-network FC of default-visual networks.
Qualitatively, the 200×200 matrices of ROI-based FC are similar across conditions. To quantitatively assess similarity, we assigned each ROI to a functional network (using the Yeo et al. 7-network scheme) and computed the average within-subject correlation between Rest and each of the movie conditions for mean intra-network and inter-network FC (21 unique pairs). A high degree of correlation was evident for both movies. For Inscapes, 20 out of 28 correlations (7 intra-network and 21 inter-network) with Rest had an r > 0.5. In Oceans, this fell to 13 of 28 (X(1)2 = 3.62, p = 0.057). When we directly compared subject-level correlations with Rest between movie conditions, we found that the correlation was significantly higher for Inscapes in 22 of 28 comparisons at p < 0.05, 11 of which exceeded Bonferroni correction for 28 comparisons. Of note, all 7 of the intra- and inter-network relationships involving the default network were significantly more correlated with Rest during Inscapes than during Oceans, 6 of which exceeded Bonferroni threshold.
We also quantitatively assessed for dissimilarity across conditions using a series of repeated-measures ANOVAs. There were three main effects of condition that were significant at a Bonferroni-corrected threshold (p < 0.0018): FC within the somatomotor network; internetwork FC of the somatomotor and ventral attention networks; and inter-network FC of the default and visual networks. For all three of these differences, mean FC was strongest during Rest. For completeness, Figure 7 shows the condition means and results of t-test comparisons for main effects of condition that were significant at an uncorrected threshold (p < 0.05). In most cases, FC is stronger during Rest than during the movie conditions.
Network-based Mean Functional Connectivity (Figure 8)
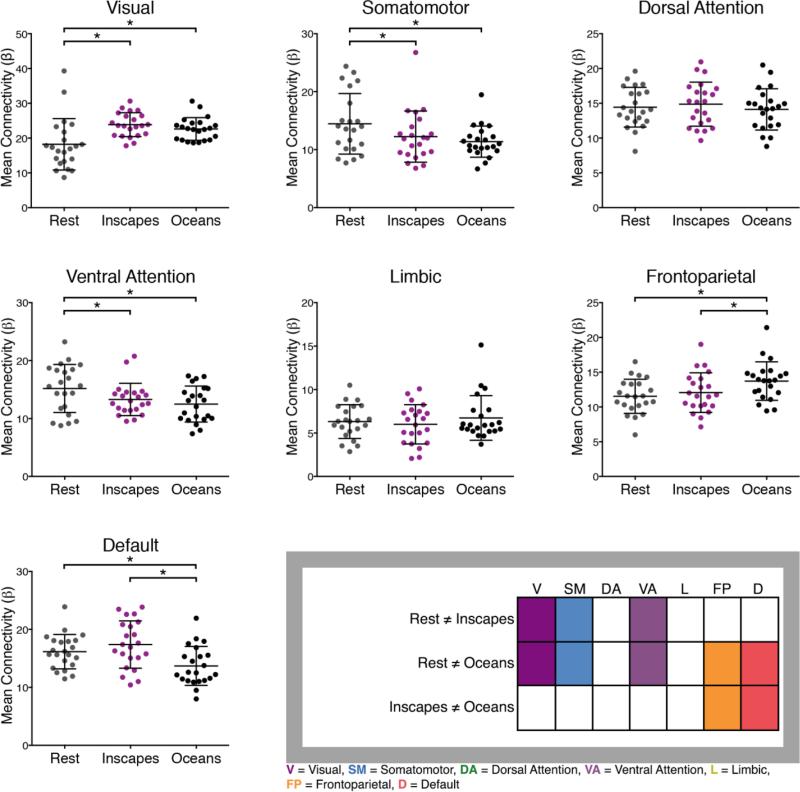
Figure 8. Mean network functional connectivity (FC) in healthy adults across different acquisition conditions, n = 22.
A dual regression analysis was used to obtain mean voxel-wise FC scores for each network for each subject (Filippini et al., 2009). Network masks were based on the Yeo 7-network scheme (Yeo et al., 2011). To evaluate differences in mean FC, we performed a series of repeated-measures ANOVAs to test for effect of condition (Inscapes, Oceans, Rest). A significant main effect of condition on mean FC was demonstrated for 5 out of 7 networks, with no significant difference observed for the dorsal attention and limbic networks. The table in the bottom right summarizes the results of the post hoc t-tests, with colored boxes indicating those networks that exhibited significant differences across conditions. The visual, somatomotor and ventral attention networks differed significantly for both movies when compared to Rest, while no significant difference was found across the movies. Additionally, the frontoparietal and default networks differed significantly between Oceans and Rest and Oceans and Inscapes, while no significant difference was found across Inscapes and Rest.
A series of repeated-measures ANOVAs demonstrated a significant main effect of condition on mean within-network voxel-wise FC for 5 of 7 networks (F (2,21) = 8.89, p < 0.005), with no significant differences observed for the dorsal attention and limbic networks. For the visual, somatomotor and ventral attention networks, post hoc t-tests showed that both movies differed significantly from Rest, while the movies did not differ significantly from one another. For the frontoparietal and default networks, Oceans differed from both Rest (p < 0.001) and Inscapes (p < 0.05). Intra-network mean voxel-wise FC was therefore significantly different from Rest in 5 networks for Oceans, and in 3 networks for Inscapes.
4. Discussion
4.1 Novelty of the paradigm
Here we introduce Inscapes as a novel movie paradigm designed to improve participant compliance during the collection of functional MRI data while minimizing cognitive demand. Inscapes features a fixed camera angle and has no scene cuts or sudden transitions in the audio or visuals. The absence of a plot or social narrative and the focus on abstract imagery are intended to sustain engagement and mental alertness in a unique, low-demand way.
4.2 Pragmatic advantages
Our first goal was to improve compliance during fMRI scanning. Our data show that movies decreased head movement in both children and adults compared to Rest, and that Inscapes was as effective at improving compliance as fast-paced narrative-based movies. As hypothesized, the degree of improvement in head motion was greater for children than for adults. Inscapes also reduced the number of adult participants self-reporting falling asleep during scanning by half. Oceans performed the best in this regard with zero reports of sleep. The overall conclusion regarding participant compliance is that all movies tested performed significantly better than Rest in both children and adults. The motion improvements were most advantageous for children, and the sleep improvements were most advantageous for adults.
To our knowledge, these are the first data quantifying a significant improvement in framewise displacement using movies in children in this age range (4-7 years old). Notably, the children in this study were in the MRI simulator for at least 25 minutes. The minimization of head movement in this population throughout a substantial period of time is an important step in enabling widespread scanning of awake children younger than seven years of age. The amount and amplitude of head movement in the pediatric group, though vastly improved, remains problematic for studies of functional connectivity (FC). Ideally, efforts to prevent head movement using movies would comprise one part of a multi-faceted approach that would include the use of optimized MRI acquisition sequences to decrease the effects of head movement on signal (Bright and Murphy, 2013, Kundu et al., 2013, Kundu et al., 2015) and advanced postprocessing techniques for motion correction (Murphy et al., 2013, Yan et al., 2013, Pruim et al., 2015).
New data continue to emerge regarding both the ideal and minimal acquisition times needed for functional connectivity analyses. For example, the Human Connectome Project has allotted 15 minutes per scan for healthy adults, and in general, longer acquisition times are recommended for studies of temporal dynamics. When considering minimal possible scan times, recent data indicate that shorter scans can produce useful results. For example, Bright and Murphy showed that networks observed using 5.5 minutes of resting state data were also observable in shortened datasets, even when as few as the first 10% of volumes were analyzed (Bright and Murphy 2015). In school-age children (ages 6-8, mean age 7.1 ± 0.6) with low head movement, eight networks were found to exhibit stable FC at a scan length of 5.5 minutes (White et al., 2014). For investigators electing to pursue longer acquisition times, Inscapes has two features that could be used to lengthen scan duration beyond 7 minutes. First, the visual forms in the movie were designed so that it can be shown both forward and in reverse. The reverse version provides another 7 minutes that replicates the amount and type of visual and auditory stimulation while providing some degree of novelty. Both the forward and reverse versions of the visuals are designed to fit well with the score in its original form, so the auditory components of the stimulus are the same in both versions. Second, because the film begins and ends with five seconds of fixation, it can also be seamlessly looped for repetitive runs. Given the lack of a narrative arc, it would also be relatively simple to add new scenes in the future.
4.3 BOLD-signal correlations during Inscapes
To explore the extent to which evoked responses were shared across subjects, we compared intersubject correlations (ISCs) during Inscapes, Oceans and Rest. We found that Inscapes evoked an intermediate level of ISCs, with more widespread and stronger ISCs than Rest and spatially more limited and weaker ISCs than Oceans. Given the nature of Inscapes, we infer that the intermediate level of ISCs during Inscapes reflects an intermediate level of stimulus-evoked information processing. However, it is also possible that Inscapes evokes the same level of cognitive processing as Oceans, but due to the abstract nature of the stimulus, participants engage in cognitive processes that are not temporally synchronized across subjects, resulting in weaker ISCs.
To further compare conditions, we calculated whole-brain matrices of FC based on a previously defined functional parcellation (Craddock et al., 2012). The resulting FC matrices show that intra- and inter-network FC relationships were highly similar across all conditions. This finding is in line with studies that have shown only slight modulation of FC patterns even during highly complex viewing conditions such as movies (Moeller et al., 2009, Betti et al., 2013). Statistical comparison of these between-condition correlations (i.e., comparing the correlation between Rest and Inscapes with the correlation between Rest and Oceans) showed significantly stronger correlations between Rest and Inscapes than between Rest and Oceans for 11 out of 28 intra- and internetwork comparisons. When testing for differences, significant differences in mean FC were sparse, and mainly involved the somatomotor and visual networks. For these differences, mean FC was strongest during Rest.
The second FC analysis used a dual regression approach to calculate mean voxel-wise FC within each of seven networks (Yeo et al., 2011). Similar to the results of the parcellation analysis (Figure 7), the dual-regression approach also revealed significant differences in FC within the somatomotor, visual and ventral attention networks. In this analysis, the means for these three networks differed significantly between each movie and rest, but not between the two movies. Our conclusions based on these data are that movies modulated mean FC within the visual, somatomotor and ventral attention networks. Additionally, a complex, conventional movie like Oceans modulated FC within frontoparietal and default networks.
In both the parcellation and dual regression analyses, the patterns of default network FC stand out as being more similar between Inscapes and Rest than between Inscapes and Oceans. In the Crad-200 matrices (see Figure 6), this was true for both intra- and inter-network connectivity. The dual regression analysis suggested that mean network connectivity of the default network did not differ significantly between Inscapes and Rest (Figure 8). Taken together, these data suggest that Oceans modulates within and between-network FC of the default network more than Inscapes. The difference analyses shown in Figure 7 indicate that an exception to this observation may be the inter-network mean FC between the default and visual networks, which was significantly lower during Inscapes than either Rest or Oceans.
The use of different movies and tasks by various research groups to investigate functional connectivity modulation makes cross-study comparisons difficult. For example, Betti et al. report that FC within the visual network was modulated by their movie condition, with lower FC during the movie than during rest (Betti et al., 2013). Our data support the observation that visual network connectivity differs during movies compared to Rest, but we found higher mean connectivity during both movies compared to Rest, not lower (Figure 8). The same paper also reported that watching a conventional, complex movie resulted in decreased functional connectivity within the default network compared to rest, which aligns with our finding of lower default network connectivity during Oceans. However, because of the use of different movies (i.e., The Good, the Bad and the Ugly and Ocean's Eleven), it is difficult to know how much weight to give either these conflicting or converging results. As was the case with task-based fMRI, establishing standardized movie paradigms that can be used by many groups may lead to more reliable and convergent findings more quickly. We are hopeful that movies may be better suited than conventional tasks to sharing across research groups because a single movie can facilitate numerous model-based and model-free analytic approaches, because multiple brain regions can be interrogated, and because multiple task-based and functional connectivity metrics can be obtained from a single data set.
4.4 Limitations
The analyses used in this study were not selected to capture or isolate specific aspects of network modulation known to occur during naturalistic viewing conditions, but rather to assess the sum of these evoked effects on relationships within and between large-scale functional networks. Both of our FC analyses used predetermined functional parcellations of the cortex. Such schemas are coarse-grained, and because they impose a structural organization onto the data, they are not designed to delineate spatial shifts in network topography. Additionally, our analyses collapsed data across time and consequently could not capture possible differences in FC temporal dynamics during the three 7-minute conditions. For example, our analyses do not tell us if the temporal dynamics of default network FC observed during Inscapes are similar to those observed during Rest. These questions will be addressed in future studies.
Another limitation in our design is that self-reported sleep likely underestimates the number of adult participants who actually fell asleep and would be a stronger metric if accompanied by physiological measures of sleep and arousal such as simultaneous electroencephalography. The decision to incorporate the scanner sound into the soundtrack of the movie is necessarily scanner- and sequence-specific, and it is unclear how this will affect the musicality of the movie in other situations. Finally, though we selected “conventional” movies for comparison, it is possible that they are unique in ways that limit generalization of the compliance or FC findings in terms of classifying these as responses to “high-demand” or “low-demand” conditions.
Revisiting the assertion that “rest” is an arbitrary task state (Buckner et al., 2013), we would argue that movie-watching data (as passive viewing tasks) should be discussed in the same way: movies result in a pattern of functional connectivity that arises from both intrinsic and evoked connectivity relationships, and are, in a sense, also arbitrary. We are not suggesting that movies are a proxy to Rest, but rather that movie-watching provides a valid state during which FC can be assessed. Inscapes was designed to provide a high-compliance, low-demand condition for fMRI data collection, and the initial characterization of ISC and FC metrics presented here suggests that we accomplished this goal. Furthermore, if movie-watching is conceptualized as a task state with potential performance differences, further efforts to quantify and control for task performance during movie-watching by way of eye-tracking and physiological measures of arousal that could be time-locked to the stimulus could provide levels of experimental control that are not possible during unconstrained rest.
4.5 Conclusions
The movies we tested significantly improved head movement in scanning environments in both young children and adults. Movies also significantly reduced the number of healthy adults self-reporting sleep when compared to resting state conditions during 7-minute runs. Finally, our novel abstract, nonsocial movie, Inscapes, appears to be associated with patterns of FC that more closely resemble Rest than those of conventional movies while still significantly improving compliance.
4.6 Future Directions
We are currently studying the effect of Inscapes on head movement in school age children and adolescents, as well as testing if improved compliance can be attained across sites and across multiple scanning sessions. Ongoing analyses will examine the temporal dynamics of functional connectivity (particularly in the default network) during Inscapes. Inscapes is currently being used by several research groups, and is available for public download in an uncompressed format at headspacestudios.org/inscapes, or by emailing the corresponding author.
Supplementary Material
Highlights.
- Functional connectivity studies in some populations are limited by head movement and sleep.
- We created a new movie to maximize compliance while minimizing cognitive load for data collection.
- The abstract movie, Inscapes, is compared to resting state conditions and to a conventional movie clip.
- The new movie decreased head movement and sleep during scanning.
- Compared to a typical movie, functional connectivity for Inscapes more closely resembled rest.
Acknowledgements
The authors thank Pierre Bellec and Uri Hasson for helpful comments and discussions during manuscript preparation. Dr. Vanderwal acknowledges the Allison Family Foundation and the American Academy of Child and Adolescent Psychiatry for funding support. Dr. Kelly acknowledges funding received from the Leon Levy Foundation and the National Institute of Mental Health (R03MH104334). Dr. Kelly is now affiliated with Trinity College Institute of Neuroscience, the School of Psychology and School of Medicine at Trinity College Dublin. We also thank Randy Smith for sound recording and Gregg Leonard for sound design with Inscapes. Lastly, we thank Krishna Somandepalli for data processing, and Karen Martin for her expertise during scanning. Inscapes is copyright of Yale University, 2013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Reproducibility of single-subject functional connectivity measurements. AJNR American journal of neuroradiology. 2011;32:548–555. doi: 10.3174/ajnr.A2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain--natural viewing conditions reveal a time-based anatomy of the brain. NeuroImage. 2004;22:419–433. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions--a new guide for mapping connectivity in vivo. NeuroImage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S, Logothetis NK. Natural vision reveals regional specialization to local motion and to contrast-invariant, global flow in the human brain. Cerebral cortex. 2008;18:705–717. doi: 10.1093/cercor/bhm107. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, Mantini D, Marzetti L, Romani GL, Corbetta M. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron. 2013;79:782–797. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, Pelegrini-Issac M, Maquet P, Benali H. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5856–5861. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MG, Murphy K. Removing motion and physiological artifacts from intrinsic BOLD fluctuations using short echo data. NeuroImage. 2013;64:526–537. doi: 10.1016/j.neuroimage.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MG, Murphy K. Is fMRI “noise” really noise? Resting state nuisance regressors remove variance with network structure. NeuroImage. 2015;114:158–169. doi: 10.1016/j.neuroimage.2015.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human brain mapping. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Li R. Neural activity during natural viewing of Sesame Street statistically predicts test scores in early childhood. PLoS biology. 2013;11:e1001462. doi: 10.1371/journal.pbio.1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy BR, Singer BD, Guntupalli JS, Ramadge PJ, Haxby JV. Inter-subject alignment of human cortical anatomy using functional connectivity. NeuroImage. 2013;81:400–411. doi: 10.1016/j.neuroimage.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human brain mapping. 2012;33:1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. Reprint of: Mapping connectivity in the developing brain. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2014;32:41–57. doi: 10.1016/j.ijdevneu.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr., Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cerebral cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Haider B, Krause MR, Duque A, Yu Y, Touryan J, Mazer JA, McCormick DA. Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation. Neuron. 2010;65:107–121. doi: 10.1016/j.neuron.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Gelbard H, Vallines I, Harel M, Minshew N, Behrmann M. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2009;2:220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Furman O, Clark D, Dudai Y, Davachi L. Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron. 2008;57:452–462. doi: 10.1016/j.neuron.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Malach R, Heeger DJ. Reliability of cortical activity during natural stimulation. Trends in cognitive sciences. 2010;14:40–48. doi: 10.1016/j.tics.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain's default mode network during deep sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Human brain mapping. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kauppi JP, Jaaskelainen IP, Sams M, Tohka J. Inter-subject correlation of brain hemodynamic responses during watching a movie: localization in space and frequency. Frontiers in neuroinformatics. 2010;4:5. doi: 10.3389/fninf.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JJDL, Koch BL, Curtwright LA, Dickerson JM, Hardin JL, Hutchinsaon S, Wright J, Gessner KE. A program to decrease the need for pediatric sedation for CT and MRI. Applied Radiology. 2007;36:4. [Google Scholar]
- Kundu P, Benson BE, Baldwin KL, Rosen D, Luh WM, Bandettini PA, Pine DS, Ernst M. Robust resting state fMRI processing for studies on typical brain development based on multi-echo EPI acquisition. Brain imaging and behavior. 2015;9:56–73. doi: 10.1007/s11682-014-9346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vertes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16187–16192. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski JM, Salmi J, Jaaskelainen IP, Lampinen J, Glerean E, Tikka P, Sams M. Stimulus-related independent component and voxel-wise analysis of human brain activity during free viewing of a feature film. PloS one. 2012;7:e35215. doi: 10.1371/journal.pone.0035215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Martin S, Tricklebank MD, Schwarz AJ, Gilmour G. Task-induced modulation of intrinsic functional connectivity networks in the behaving rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015a;35:658–665. doi: 10.1523/JNEUROSCI.3488-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kehoe EG, McGinnity TM, Coyle D, Bokde AL. Modulation of effective connectivity in the default mode network at rest and during a memory task. Brain connectivity. 2015b;5:60–67. doi: 10.1089/brain.2014.0249. [DOI] [PubMed] [Google Scholar]
- Lv Y, Margulies DS, Villringer A, Zang YF. Effects of finger tapping frequency on regional homogeneity of sensorimotor cortex. PloS one. 2013;8:e64115. doi: 10.1371/journal.pone.0064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Data-driven analysis of analogous brain networks in monkeys and humans during natural vision. NeuroImage. 2012a;63:1107–1118. doi: 10.1016/j.neuroimage.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Hasson U, Betti V, Perrucci MG, Romani GL, Corbetta M, Orban GA, Vanduffel W. Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nat Methods. 2012b;9:277–282. doi: 10.1038/nmeth.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cerebral cortex. 2013;23:223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Nallasamy N, Tsao DY, Freiwald WA. Functional connectivity of the macaque brain across stimulus and arousal states. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5897–5909. doi: 10.1523/JNEUROSCI.0220-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. NeuroImage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajula J, Kauppi JP, Tohka J. Inter-subject correlation in fMRI: method validation against stimulus-model based analysis. PloS one. 2012;7:e41196. doi: 10.1371/journal.pone.0041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Lee M, Buechler R, Christodoulou JA, Chang M, Vakil M, Stering PL, Gaab N. Making MR imaging child's play - pediatric neuroimaging protocol, guidelines and procedure. Journal of visualized experiments : JoVE. 2009 doi: 10.3791/1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabuncu MR, Singer BD, Conroy B, Bryan RE, Ramadge PJ, Haxby JV. Function-based intersubject alignment of human cortical anatomy. Cerebral cortex. 2010;20:130–140. doi: 10.1093/cercor/bhp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. Watching You Watch Movies: Using Eye Tracking to Inform Cognitive Film Theory. In: Shimamura A, editor. Psychocinematics: Exploring Cognition at the Movies. Oxford University Press; New York: 2013. [Google Scholar]
- Spiers HJ, Maguire EA. Decoding human brain activity during real-world experiences. Trends in cognitive sciences. 2007;11:356–365. doi: 10.1016/j.tics.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Gleiser PM, Czisch M. Frontoparietal Connectivity and Hierarchical Structure of the Brain's Functional Network during Sleep. Frontiers in neurology. 2012;3:80. doi: 10.3389/fneur.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL. Development of the brain's functional network architecture. Neuropsychology review. 2010;20:362–375. doi: 10.1007/s11065-010-9145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Muetzel R, Schmidt M, Langeslag SJ, Jaddoe V, Hofman A, Calhoun VD, Verhulst FC, Tiemeier H. Time of acquisition and network stability in pediatric resting-state functional magnetic resonance imaging. Brain Connect. 2014;4:417–427. doi: 10.1089/brain.2013.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Molnar-Szakacs I, Iacoboni M. Beyond superior temporal cortex: intersubject correlations in narrative speech comprehension. Cerebral cortex. 2008;18:230–242. doi: 10.1093/cercor/bhm049. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.