Abstract
Thermal adaptations of soil microorganisms could mitigate or facilitate global warming effects on soil organic matter (SOM) decomposition and soil CO2 efflux. We incubated soil from warmed and control subplots of a forest soil warming experiment to assess whether 9 years of soil warming affected the rates and the temperature sensitivity of the soil CO2 efflux, extracellular enzyme activities, microbial efficiency, and gross N mineralization. Mineral soil (0–10 cm depth) was incubated at temperatures ranging from 3 to 23 °C. No adaptations to long‐term warming were observed regarding the heterotrophic soil CO2 efflux (R 10 warmed: 2.31 ± 0.15 μmol m−2 s−1, control: 2.34 ± 0.29 μmol m−2 s−1; Q 10 warmed: 2.45 ± 0.06, control: 2.45 ± 0.04). Potential enzyme activities increased with incubation temperature, but the temperature sensitivity of the enzymes did not differ between the warmed and the control soils. The ratio of C : N acquiring enzyme activities was significantly higher in the warmed soil. Microbial biomass‐specific respiration rates increased with incubation temperature, but the rates and the temperature sensitivity (Q 10 warmed: 2.54 ± 0.23, control 2.75 ± 0.17) did not differ between warmed and control soils. Microbial substrate use efficiency (SUE) declined with increasing incubation temperature in both, warmed and control, soils. SUE and its temperature sensitivity (Q 10 warmed: 0.84 ± 0.03, control: 0.88 ± 0.01) did not differ between warmed and control soils either. Gross N mineralization was invariant to incubation temperature and was not affected by long‐term soil warming. Our results indicate that thermal adaptations of the microbial decomposer community are unlikely to occur in C‐rich calcareous temperate forest soils.
Keywords: enzyme activities, gross N mineralization, soil CO2 efflux, soil warming, substrate use efficiency, thermal adaptation
Introduction
Temperature is the primary environmental driver of soil organic matter (SOM) decomposition in temperate ecosystems. Accordingly, there is concern that global warming accelerates the heterotrophic soil CO2 efflux (Rh), feeding back to atmospheric CO2 concentrations and climate (Cox et al., 2000; Friedlingstein et al., 2006). The long‐term effect of warming on the soil CO2 efflux, however, is unclear as changing SOM availability over time and/or microbial adaptations to warmer conditions affect the temperature sensitivity of Rh and its overall rates. If and to which extent such microbial adaptation mechanisms affect Rh is still a matter of debate (Hartley et al., 2007, 2008, 2009; Bradford et al., 2008; Allison et al., 2010; Vanhala et al., 2011; Frey et al., 2013; Karhu et al., 2014).
Soil warming experiments in the field have proven to be meaningful tools to study the behavior of SOM decomposition and can particularly lead to a better understanding of long‐term warming effects on soil C dynamics. Recent meta‐analyses of in situ warming effects showed strong enhancement of soil respiration in global terrestrial ecosystems (Lu et al., 2013; Wang et al., 2014). However, in some of the longer running manipulation experiments, the initial response to warming declined and diminished over time, suggesting that the warming response of SOM decomposition and soil respiration is transient (Strömgren, 2001; Melillo et al., 2002; Lamb et al., 2011). Two explanations have been put forward for the observed decreases in soil respiration in continuously warmed field soils: (i) depletion of labile C causes heterotrophic respiration to decline (Melillo et al., 2002; Kirschbaum, 2004; Eliasson et al., 2005; Hartley et al., 2007) and/or (ii) thermal adaptation of microbial processes along with changes in microbial community structure (Luo et al., 2001; Bradford et al., 2008; Balser & Wixon, 2009; Allison et al., 2010; Conant et al., 2011; Bradford, 2013). Although the decrease in the warming response could be fully explained by simple soil C pool models (e.g., Kirschbaum, 2004), a simultaneous occurrence of physiological adaptation of the microbial community is concurrent as documented by an array of isolated mycorrhizal fungi (Malcolm et al., 2008) and heterotrophic soil microorganisms (Crowther & Bradford, 2013). The significance of such physiological adaptations with regard to the observed response of Rh to warming remains, however, unclear (Rousk et al., 2012).
Rh represents a complex process, based on activities of several extracellular enzymes mediating the breakdown of soil organic materials, microbial uptake of labile C compounds, and partitioning of these C sources between microbial growth and respiration. The first step in substrate utilization is the breakdown of complex organic macromolecules by extracellular enzymes which generally responds positively to soil temperature (e.g., Trasar‐Cepeda et al., 2007; Wallenstein et al., 2009) as long as soil moisture is not limiting (Brzostek et al., 2012; Steinweg et al., 2012; Suseela et al., 2014). The temperature optimum, as well as the temperature‐specific enzyme conformations, can vary between cold‐ and warm‐adapted microorganisms/enzymes (Zavodszky et al., 1998; Bradford, 2013). Extracellular enzyme activities can adapt to seasonal fluctuations in soil temperature (Fenner et al., 2005; Wallenstein et al., 2009; Jing et al., 2014) and were found to adapt to local conditions along latitudinal temperature gradients (German et al., 2012). Together, this makes potential adaptations of extracellular enzyme kinetics to long‐term soil warming plausible. Once the C‐substrate is taken up by the microbial cell, it is invested in maintenance, growth, or storage. The microbial carbon‐use‐efficiency (CUE; the proportion of C uptake allocated to growth vs. the microbial CO2 production by respiration) has been shown to decrease with increasing soil temperature (Steinweg et al., 2008; Frey et al., 2013; Tucker et al., 2013) or to be invariant with temperature (Hopkins et al., 2014). Decreased CUE in warmer soils could at least partly explain the declining warming response of Rh over time due to decreasing microbial biomass (Tucker et al., 2013). It has also been shown that the temperature sensitivity of CUE depends on the substrate quality and that long‐term warming can lead to the adaptation of microbial physiology toward higher CUE for compounds that are indicators of more stable SOM components (Frey et al., 2013).
Adaptations to warming per se (‘direct’ thermal adaptations) are often difficult to disentangle from concurrent ‘indirect’ warming effects such as induced declines in SOM availability or quality (Bradford, 2013). In this study, we took advantage of a soil warming experiment in Achenkirch/Austria where soil has been warmed by 4 °C during the snow‐free seasons from 2005 until the current study in 2013. In contrast to other long‐term soil warming experiments, the positive response of soil respiration to warming (~40% increase) remained stable throughout the 9 years of warming (Schindlbacher et al., 2012). The sustained enhancement of soil respiration suggests that substrate depletion did not play a major role in this C‐rich soil so far. As the respiration response to warming was sustained over time, we hypothesize (I) that no thermal adaptation of the soil microbial community occurred or that (II) thermal adaptations of microbial processes were not reflected in the soil CO2 efflux. In this study, we therefore analyzed the effect of long‐term soil warming on the temperature sensitivity of Rh, extracellular enzyme activities, biomass‐specific respiration rates, microbial substrate use efficiency, and gross N mineralization. Changed temperature sensitivity of these parameters will be considered as thermal adaptation.
Materials and methods
Site description and soil sampling
The study site is located at 910 m a.s.l. on a north–north‐east slope of a mountain in the Northern Limestone Alps, Achenkirch, Austria (47°34′50″N; 11°38′21″E). The site is characterized by a cool humid climate. The snow‐free period lasts from April/May to November/December. Local mean annual air temperature and precipitation were 6.9 °C and 1506 mm (1992–2012), respectively [Achenkirch village; ~7 km away at similar altitude; data from Zentralanstalt für Meteorologie und Geodynamik (ZAMG)]. The ~130‐year old forest is dominated by Norway spruce (Picea abies), with interspersed European beech (Fagus sylvatica) and silver fir (Abies alba). The soils are a mosaic of shallow Chromic Cambisols and Rendzic Leptosols. The bedrock is formed of dolomite. Soils are characterized by high carbonate content and near neutral pH. Mull is the dominant humus form and the thickness of the litter + O‐layer reach from 0 to 3 cm. A‐horizons show a strong, small‐scale variability in thickness reaching from 10 to 40 cm. Root density is highest in the O‐ and A‐horizons, and few roots were found to reach down to a depth of 60 cm. Organic C stocks were estimated to be ~10 t ha−1 in the organic layer and ~120 t ha−1 in the mineral soil (Schindlbacher et al., 2010).
In 2004, three experimental plots were randomly selected on the site. Each of the three plots consisted of a warmed and a disturbed‐control subplot (Schindlbacher et al., 2009). The subplots had a size of 2 × 2 m each. Warmed subplots were equipped with resistance heating cables (0.4 cm diameter, TECUTE – 0.18 Ohm m−1 per UV, Etherma, Austria). The cables were buried in 3‐cm‐deep slots and had a spacing of 7–8 cm. The soil temperature of each warmed subplot was kept 4 °C above that of the adjacent control subplot during the snow‐free seasons, starting in spring 2005. On disturbed‐control subplots, we inserted cables that were not heated, but had inflicted the same soil disturbance as on the warmed subplots.
For the current study, we sampled soil from the warmed (n = 3) and the disturbed‐control (n = 3, hereinafter ‘control’) subplots. Soil sampling was accomplished during the ninth year of soil warming in early September 2013. We sampled four randomly distributed intact soil cores (diameter 7 cm, depth 10 cm, stainless steel cylinders) from each subplot for the subsequent determination of soil CO2 efflux and its temperature sensitivity. We further sampled similar aliquots of mineral soil (0–10 cm) from four randomly distributed spots within each subplot for basic soil parameters and microbial analyses. These mineral soil samples were pooled, mixed, and sieved (2 mm) to obtain a homogenized bulk sample of each subplot for further analytical treatment. Soil was stored in cooling boxes and transported to the laboratory on the day of sampling. Soil temperature at 5 cm depth and field soil CO2 efflux were measured on the day of soil sampling from permanently installed chambers, as described in Schindlbacher et al. (2012).
Soil CO2 efflux and temperature sensitivity
To assess whether long‐term warming affected the temperature sensitivity of the soil CO2 efflux, we simultaneously incubated soil from warmed and control plots under controlled conditions in the laboratory. Undisturbed soil cores, excised roots, and sieved soil were incubated in this order. The incubation was performed with a temperature controlled automatic system as described in Schindlbacher et al. (2010, 2004). In brief, an incubator hosted 13 glass chambers (750 cm3 each). Twelve chambers were equipped with soil cores, while the 13th chamber was equipped with a stainless steel block of the same size and served as a reference chamber. The system operated as an open‐flow‐through method. Ambient air from inside the incubator was sucked through the chamber headspace to the CO2 analyzer (WMA‐4, PP‐Systems, www.ppsystems.com). The CO2 efflux from each soil core was calculated from the airflow (1 L min−1) and the difference in the headspace CO2 concentration of the soil chamber and of the subsequently measured reference chamber (Schindlbacher et al., 2008). Chambers were measured consecutively (6 min soil chamber + 2 min reference chamber). A single CO2 measuring cycle for all 12 cores lasted ~1.5 h.
Two cores from each plot were immediately placed in the incubator and adjusted to a temperature of 3 °C overnight. The remaining cores were stored in a fridge at 3 °C until incubation. We incubated the undisturbed soil cores at incrementing temperatures from 3 to 23 °C (2.5 °C increments). Incubation at each temperature level lasted for 6 h and allowed for four CO2 efflux measurement cycles. Only the last CO2 measurement cycle (h 4.5–6) was used for the CO2 efflux calculation. Time prior (h 0–4.5) was considered to be for soil temperature increase and for a short equilibration period to the higher temperature. Soil temperature was measured in an additional soil core with a PT100 temperature sensor. The whole incubation procedure lasted for 56 h. The second set of undisturbed soil cores was incubated immediately after the first set was finished (same procedure).
The intact soil cores were disaggregated after the incubation was finished and all the roots were picked out. Fine roots (<2 mm diameter) were washed with water and then placed on wet inert glass fiber mats which were wrapped into the cylinders. To obtain enough root material to establish a measureable CO2 efflux, we pooled the roots from the four cores of each subplot. To keep incubation time short, we incubated the excised roots at only five temperatures (3, 8, 13, 18, and 23 °C). As root temperature was considered to equilibrate faster with the incubator temperature, only two CO2 measurement cycles were performed at each temperature (3 h) and only the second cycle was used for flux calculation. This allowed finishing the whole root incubation within 15 h.
The root‐free mineral soil of each subplot was homogenized and sieved through a 2‐mm sieve. The homogenized mineral soil from each subplot was repacked into two cylinders at the mean bulk density of the undisturbed soil cores. The sieved soil cores were stored at 3 °C for an equilibration period of ~24 h and were incubated under the same procedure as described above for the undisturbed soil cores.
Basic soil parameters and microbial biomass
All analyzes described below started 1 day after soil sampling in the field. To determine water content, the sieved mineral soil samples were dried at 80 °C for 48 h. Sieved soils were extracted with 1 m KCl (1 : 7.5 w : v) for 60 min and filtered through ash‐free cellulose filters. Ammonium and nitrate concentrations were determined photometrically following Kandeler & Gerber (1988) and Miranda et al. (2001) as modified by Hood‐Nowotny et al. (2010), respectively. Total free amino acids (TFAA) were determined fluorometrically following the method described by Jones et al. (2002) with modifications by Prommer et al. (2014). Microbial C and N were estimated using chloroform fumigation–extraction (Brookes et al., 1985) modified after Kaiser et al. (2010): Soil samples were fumigated over chloroform for 48 h and were afterward, together with nonfumigated samples, extracted with 1 m KCl solution. Dissolved organic C (DOC) and total dissolved N (TN) were determined in both sets of extracts using a DOC/TN analyzer (Shimadzu TOC‐VCPH/CPN/TNM‐1, Vienna, Austria). Microbial C and N were calculated as the difference between fumigated and nonfumigated samples, without correction for extraction efficiency. DOC, TN, ammonium, nitrate, TFAA, and microbial C and N concentrations were determined in three analytical replicates for each subplot.
Potential extracellular enzyme activities
Potential enzyme activities were determined fluorometrically and photometrically using a microtiter plate assay (modified after Kaiser et al. (2010)). In short, for each subplot three times 1 g of soil was suspended in 100 mL sodium acetate buffer each (100 mm, pH 5.5) and homogenized by ultrasonication. For each sample and each enzyme, 5 wells of a black microtiter plate were filled with 200 μL of the soil slurry. The respective wells were amended with MUF (4‐methylumbelliferyl) labeled substrates: β‐D‐glucopyranoside (0.25 mm) for β‐glucosidase (BG), β‐D‐cellobioside (0.5 mm) for cellobiohydrolase (CBH), and N‐acetyl‐β‐D‐glucosaminide (1 mm) for N‐acetyl‐glucosaminidase (NAG). L‐leucine‐7‐amido‐4‐methyl coumarin (1 mm) was used as substrate for leucine aminopeptidase (LAP). Analytical replicated plates for the assays of BG, CBH, NAG, and LAP were incubated simultaneously at five temperatures (3, 8, 13, 18, and 23 °C) for 140 min. Afterward, activity was measured fluorometrically (excitation 365 nm and emission 450 nm). The ratio of C : N acquisition by enzymes was calculated as the sum of the log‐transformed BG and CBH activities divided by the sum of the log‐transformed activities of LAP and NAG. Phenoloxidase (POX) and peroxidase (PEX) activities were measured using L‐3,4‐dihydroxyphenylalanine (DOPA) as substrate in a photometric assay. Three times 1 mL of the original soil slurry was mixed with 1 mL of a 20 mm DOPA solution. After shaking and centrifugation, two wells of each transparent microtiter plates were filled with 250 μL of the supernatant. One of these wells additionally received 10 μL H2O2 (0.3%) for determination of peroxidase activity. Plates for oxidative enzyme activities were measured photometrically (absorbance 450 nm) at the beginning and after incubation for 20 h at the five temperatures. POX activities were then calculated as the increase in color during the incubation time. PEX activities were calculated as the increase in color during the incubation time from the results of the wells that received H2O2 minus the results of the wells without H2O2 addition. Because of condensation on the transparent microtiter plates, which had been incubated at 3 and 8 °C, and which has biased the photometric measurements, only the POX and PEX measurements from the three higher temperatures (13, 18, and 23 °C) were used for further calculations.
Substrate use efficiency and mass‐specific respiration
To gain an estimate for microbial CUE, we incubated sieved soil samples with a mixture of 13C labeled substrates for 24 h and traced 13C into CO2 and microbial biomass. Such an approach tends to overestimate actual CUE as it does not fully capture microbial growth and maintenance respiration (Sinsabaugh et al., 2013). We therefore report our results as substrate use efficiency (SUE).
Samples were incubated with a mixture of uniformly 13C‐labeled sugars, amino sugar, organic acids, and amino acids, enriched at 10.4% 13C. The overall C : N ratio of the mixture was 20, and the overall degree of reduction, a measure of the chemical energy per unit mole of C, was 4.0. This mixture was chosen to contain low molecular weight compounds available in soils for microbial consumption (Van Hees et al., 2005; Manzoni et al., 2012). From each subplot and for each temperature (6 subplots × 5 temperatures), soil samples (aliquots of 2 g) were placed in glass bottles (250 mL). The dissolved substrate mixture equivalent to 40 μg C was added to soil samples. The bottles were sealed with butyl rubber plugs (Glasgerätebau Ochs Laborfachhandel e.K., Bovenden, Germany). Using a syringe, 20 mL headspace samples were taken from the bottles and injected into evacuated Exetainers® (Labco Ltd., Ceredigion, UK), directly after adding the 13C labeled mixture. The syringe was purged with ambient air between samples. The air removed from the bottles was replaced from a gas bag with known CO2 concentration and carbon isotope composition. Samples were incubated at the indicated temperature for 24 h, after which a second set of gas samples was taken. At the end of the incubation period, soil samples were split into equal portions and microbial biomass C (Cmic) was estimated by chloroform fumigation–extraction. Aliquots of fumigated and nonfumigated K2SO4 extracts were used to determine δ 13C of DOC, by direct injection (without column, direct mode) on an HPLC (Dionex Corporation, Sunnyvale, CA, USA) connected through a Finnigan LC‐IsoLink Interface (Thermo Fisher Scientific, Waltham, MA, USA) to a Finnigan Delta V Advantage Mass Spectrometer (Thermo Fisher, Bremen, Germany). δ 13C signatures of CO2 of air samples were analyzed by headspace gas sampler (GasBench II, Thermo Fisher) coupled to an isotope ratio mass spectrometer (Delta V Advantage, Thermo Fisher). CO2 reference gas was calibrated using ISO‐TOP gas standards (Air Liquide) with certified 13C concentrations. SUE was calculated as follows: SUE = 13Cmic/(13Cmic + 13CO2) where 13Cmic is the substrate 13C incorporated into biomass and 13CO2 is the cumulative substrate 13C respired during incubation. Biomass incorporation was calculated as the difference between 13C in DOC of chloroform‐fumigated and nonfumigated samples. Cumulative respiration was corrected for the air replaced at the start of the incubation.
Mass‐specific respiration (Rmass) rates were calculated as respiration rates per unit microbial biomass. Rmass was calculated from the SUE incubation data (above). Rh (corrected for the contribution of 13C respiration by subtracting the amount of 13CO2 from total CO2) at each incubation temperature (3, 8, 13, 18, and 23 °C) was divided by the microbial biomass content of each sample at the corresponding temperature.
Gross N mineralization
Gross rates of N mineralization were simultaneously determined for all incubation temperatures (3, 8, 13, 18, and 23 °C) in three replicates per subplot, as described by Kaiser et al. (2011). In short, samples were amended with 500 μL of 15N labeled (NH4)2SO4 (0.125 mm, 10 at%, Sigma‐Aldrich) in duplicates of 2 g soil, for each temperature. Replicated samples were incubated simultaneously for 4 and 24 h at the respective temperatures and were then extracted with 15 mL of 2 m KCl. NH4 + was diffused into acid traps which were measured with an elemental analyzer–isotope ratio mass spectrometer (EA‐IRMS) system consisting of a CE Instrument EA 1110 elemental analyzer coupled to a Finnigan MAT DeltaPlus IRMS with a Finnigan MAT ConFlo II Interface. Gross rates were calculated as described by Wanek et al. (2010).
Data analysis
The temperature response of the soil core CO2 efflux, of enzyme activities, of gross N mineralization, and of SUE was determined by means of an exponential Q 10 function (Janssens & Pilegaard, 2003):
| (1) |
in which R is the measured process rate (soil CO2 efflux, gross N mineralization, Rmass, SUE, 13C incorporation, and 13C respiration), R 10 is the simulated process rate at 10 °C, Q 10 is the temperature sensitivity of the process rate, and T, the independent variable, is the soil temperature. The R 10 and Q 10 were fitted to the measured R and temperature data by means of a nonlinear least square fitter (SigmaPlot for Windows, version 12.0; SysStat Software, Inc., San Jose, CA, USA).
Q 10 and R 10 values were calculated for each individual subplot; that is, the Q 10 function was fitted to the mean CO2 efflux of the replicated soil cores from each subplot (intact cores: 4 replicates per subplot; sieved soil: 2 replicates; roots: 1 replicate). Q 10 and R 10 values were calculated for each subplot of the control and warming treatment for statistical analysis. Mean Q 10 and R 10 of CO2 efflux from undisturbed cores, roots, and sieved soil cores (n = 3) were statistically analyzed using a t‐test (SigmaPlot for Windows, version 12.5, SysStat Software, Inc.).
Q 10 and R 10 of enzyme activities, gross N mineralization, SUE, 13C incorporation as well as 13C respiration were statistically analyzed using t‐tests as well (SigmaPlot for Windows, version 12.5, SysStat Software, Inc.). In addition, the effects of incubation temperature during the experiment and of soil warming in situ (treatment effect), as well as their interaction, were tested by two‐way anova, after checking for homogeneity of variance using the Levene test. anovas were calculated using Statgraphics Centurion XVI software (StatPoint Technologies, Inc., Warrenton, VA, USA).
Results
2012 and 2013 soil temperatures at warmed and control subplots are presented in Fig. 1. Mean soil temperatures at 5 cm depth during soil sampling (03 Sept 2013 12:00) were 15.6 ± 0.2 °C at the warmed and 11.6 ± 0.4 °C at the control subplots. Corresponding soil temperatures at 15 cm depth were 10.2 ± 0.3 °C at the warmed and 13.4 ± 0.3 °C at the control subplots. Mean field soil CO2 efflux at 03 September 2013 was 6.2 ± 1.1 μmol m−2 s−1 at the warmed subplots and 4.1 ± 0.6 μmol m−2 s−1 at the control subplots (not shown). Soils at both, warmed and control, subplots showed high soil moisture contents (Table 1). All basic soil parameters showed a trend (though nonsignificant) toward lower values in the warmed subplots (Table 1).
Figure 1.
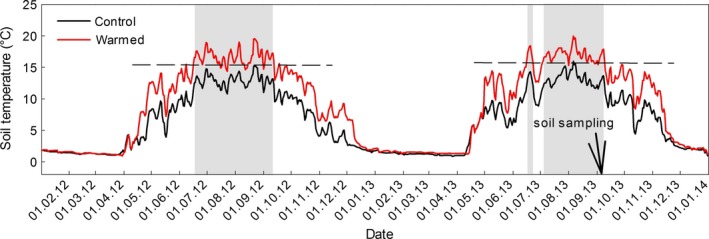
Daily mean temperatures at 5 cm soil depth at control and warmed subplots during 2012 and 2013. Soil was not warmed during snow cover. Gray bars indicate periods during which the warmed subplot temperatures exceeded the maximum control subplot temperature of the corresponding year (dashed line). The arrow indicates the date of soil sampling for incubation.
Table 1.
Basic mineral soil (0–10 cm soil depth, means ± SE, n = 3) parameters from warmed and control subplots. Samples were obtained during the ninth year of soil warming. n.s. not significant
| Control | Warmed | t‐Test | |
|---|---|---|---|
| Water content (% fresh soil) | 48.3 ± 3.8 | 44.1 ± 2.5 | n.s. |
| Extractable organic C (μg C g−1 dw) | 123 ± 6.5 | 104 ± 6.8 | n.s. |
| Extractable N (μg N g−1 dw) | 29.8 ± 1.5 | 24.7 ± 1.86 | n.s. |
| Ammonium (μg N g−1 dw) | 2.17 ± 0.17 | 2.44 ± 0.37 | n.s. |
| Nitrate (μg N g−1 dw) | 4.44 ± 0.32 | 3.07 ± 0.51 | n.s. |
| Total free amino acids (μg N g−1 dw) | 2.31 ± 0.10 | 2.15 ± 0.13 | n.s. |
| Microbial C (μg C g−1 dw) | 847 ± 143 | 696 ± 25 | n.s. |
| Microbial N (μg N g−1 dw) | 182 ± 31 | 154 ± 5 | n.s. |
CO2 efflux from all incubated soil cores increased exponentially with increasing incubation temperature (Fig. 2). The Q 10 function showed an excellent fit for CO2 effluxes of all undisturbed cores (R 2 0.995–0.999) and the sieved soil cores (R 2 0.993–0.999). The fit of the root CO2 effluxes was weaker but still highly significant (R 2 0.913–0.997). The mean CO2 efflux of the intact soil cores from the warmed subplots was slightly higher as the CO2 efflux of the control subplot cores across all incubation temperatures (Fig. 2), but the difference in R 10 was not statistically significant (Table 2). The temperature sensitivity of soil CO2 efflux (Q 10) from intact cores did not differ significantly between warmed and control subplots (Table 2). Mean mass of fine roots was similar in warmed subplot root cores (5.5 ± 1.5 g d w core−1) and control subplot root cores (5.7 ± 0.7 g d w core−1) as was the root CO2 efflux (Fig. 2) and its temperature sensitivity (Table 2). Sieved soil core CO2 efflux rates and their temperature sensitivity were similar for both, warmed and control, subplots at all incubation temperatures (Fig. 2) producing correspondingly similar R 10 and Q 10 values (Table 2).
Figure 2.
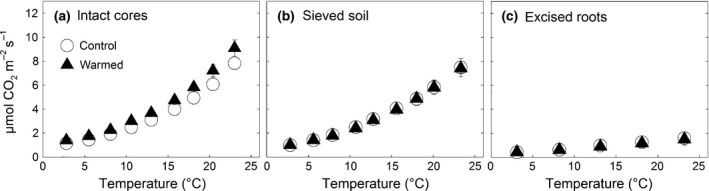
Effect of increasing soil temperature on the CO2 efflux from (a) intact soil cores, (b) cores packed with sieved mineral soil, and (c) the roots which were excised during the disaggregation of the intact soil cores. Soil cores (0–10 cm soil depth) were sampled from subplots which had been exposed to soil warming throughout 9 seasons (full triangles, means ± SE, n = 3) and from subplots which had experienced the corresponding natural temperature conditions (open circles, means ± SE, n = 3).
Table 2.
Mean Q 10 and R 10 values (± SE, n = 3) of soil respiration rates, extracellular enzyme activities [β‐glucosidase (BG), cellobiohydrolase (CBH), N‐acetyl‐glucosaminidase (NAG), leucine aminopeptidase (LAP), phenoloxidase (POX), and peroxidase (PEX)], substrate use efficiency and its components, mass‐specific respiration (Rmass), and gross N mineralization. Soil was incubated at temperatures ranging from 3 to 23 °C. (n.s. not significant; n.a. not analyzed)
| Process | Q 10 | R 10 | ||||
|---|---|---|---|---|---|---|
| Control | Warmed | t‐Test | Control | Warmed | t‐Test | |
| Soil respiration | ||||||
| Intact soil cores (μmol CO2 m−2 s−1) | 2.56 ± 0.04 | 2.52 ± 0.06 | n.s. | 2.30 ± 0.11 | 2.77 ± 0.23 | n.s. |
| Sieved soil cores (μmol CO2 m−2 s−1) | 2.45 ± 0.04 | 2.45 ± 0.06 | n.s. | 2.34 ± 0.29 | 2.31 ± 0.15 | n.s. |
| Roots (μmol CO2 m−2 s−1) | 1.85 ± 0.02 | 1.81 ± 0.05 | n.s. | 0.73 ± 0.02 | 0.73 ± 0.24 | n.s. |
| Extracellular enzyme activities | ||||||
| BG (pmol g−1 dry soil s−1) | 2.06 ± 0.06 | 1.95 ± 0.18 | n.s. | 12.6 ± 1.5 | 17.3 ± 0.8 | n.s. |
| CBH (pmol g−1 dry soil s−1) | 1.46 ± 0.04 | 1.49 ± 0.08 | n.s. | 66.0 ± 5.5 | 71.4 ± 4.7 | n.s. |
| NAG (pmol g−1 dry soil s−1) | 1.48 ± 0.07 | 1.56 ± 0.13 | n.s. | 172 ± 18.3 | 142 ± 8.9 | n.s. |
| LAP (pmol g−1 dry soil s−1) | 1.61 ± 0.03 | 1.66 ± 0.05 | n.s. | 33.7 ± 4.0 | 27.2 ± 1.0 | n.s. |
| POX (pmol g−1 dry soil s−1) | 4.77 ± 0.24 | 4.21 ± 0.32 | n.s. | 20.1 ± 7.0 | 16.6 ± 1.5 | n.s. |
| PEX (pmol g−1 dry soil s−1) | 2.82 ± 0.19 | 2.85 ± 0.10 | n.s. | 101 ± 29.4 | 78.5 ± 5.3 | n.s. |
| Ratio of C : N acquisition | n.s. | n.s. | n.a. | n.s. | n.s. | n.a. |
| Substrate use efficiency | ||||||
| 13C respiration (pmol C g−1 dry soil s−1) | 1.47 ± 0.04 | 1.47 ± 0.02 | n.s. | 11.2 ± 0.7 | 10.2 ± 0.8 | n.s. |
| 13C incorp. (pmol C g−1 dry soil s−1) | n.s. | n.s. | n.a. | n.s. | n.s. | n.a. |
| Substrate use efficiency | 0.88 ± 0.01 | 0.84 ± 0.03 | n.s. | 0.72 ± 0.02 | 0.74 ± 0.01 | n.s. |
| Microbial C (μmol g−1 dry soil) | n.s. | n.s. | n.a. | n.s. | n.s. | n.a. |
| SOM respiration (pmol C g−1 dry soil s−1) | 2.75 ± 0.17 | 2.54 ± 0.23 | n.s. | 45.3 ± 7.83 | 42.4 ± 10.1 | n.s. |
| Rmass (nmol C g−1 Mic C s−1) | 2.62 ± 0.25 | 2.45 ± 0.19 | n.s. | 37.1 ± 5.55 | 42.0 ± 11.4 | n.s. |
| Gross nitrogen processes | ||||||
| N mineralization | n.s. | n.s. | n.a. | n.s. | n.s. | n.a. |
Potential extracellular enzyme activities all showed a significant positive relationship with incubation temperature (Fig. 3, Tables 2 and 3). The model fit was weaker than for soil respiration (R 2 0.686–0.999) but significant in all cases (P < 0.05). Q 10 and R 10 values of potential enzyme activities did not differ significantly for any enzyme between the treatments (Table 2). The temperature sensitivity of the different individual enzymes varied substantially (Q 10 1.5–4.8), the oxidative enzymes being the most sensitive to soil temperature. According to two‐way anova, BG activity was significantly higher in warmed subplot soils, whereas NAG and LAP activities were significantly lower in warmed subplot soils (Table 3). The ratio of C : N acquisition, calculated as the sum of BG and CBH over the sum of NAG and LAP, showed no increase with incubation temperature, but was significantly higher in soils from warmed subplots (Table 3, Fig. 4).
Figure 3.
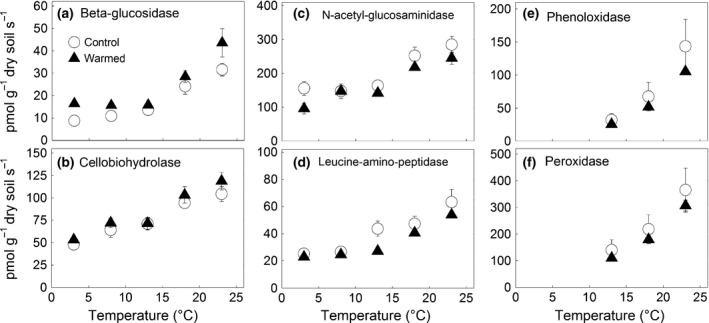
Temperature effects on the extracellular enzyme activities of the mineral top‐soil ((a) β‐glucosidase, (b) cellobiohydrolase, (c) N‐acetyl‐glucosaminidase, (d) leucine aminopeptidase, (e) phenoloxidase, and (f) peroxidase) from subplots which had been exposed to soil warming throughout 9 seasons (full triangles, means ± SE, n = 3) and from subplots which had experienced the corresponding natural temperature conditions (open circles, means ± SE, n = 3). Mineral soil from each subplot was incubated simultaneously at the indicated temperatures.
Table 3.
Results of two‐way anova analysis of the factors soil warming treatment and incubation temperature and their interaction on soil respiratory processes, extracellular enzyme activities, microbial substrate use efficiency and its components, mass‐specific respiration (Rmass), and gross N mineralization. Variance homogeneity was checked by Levene's test and was given for all data. Significant effects are highlighted in bold
| Process | Warming treatment | Incubation temperature | Interaction | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Soil respiratory processes | ||||||
| Intact soil cores | 6.3 | <0.001 | 35.9 | <0.001 | 0.2 | 0.886 |
| Sieved soil cores | 0.1 | 0.873 | 49.6 | <0.001 | 0.0 | 1.000 |
| Root respiration | 0.1 | 0.838 | 4.9 | 0.007 | 0.0 | 0.999 |
| Soil dry mass‐based/enzyme activities | ||||||
| β‐Glucosidase | 8.5 | 0.009 | 20.6 | <0.001 | 0.6 | 0.650 |
| Cellobiohydrolase | 2.0 | 0.177 | 17.2 | <0.001 | 0.2 | 0.944 |
| Chitinase | 5.2 | 0.034 | 16.1 | <0.001 | 0.5 | 0.711 |
| Leucine aminopeptidase | 5.3 | 0.033 | 15.9 | <0.001 | 0.7 | 0.609 |
| Phenoloxidase | 1.1 | 0.318 | 8.5 | 0.005 | 0.2 | 0.792 |
| Peroxidase | 0.9 | 0.360 | 7.7 | 0.007 | 0.0 | 0.965 |
| Ratio of C : N acquisition | 29.8 | <0.001 | 1.2 | 0.360 | 1.2 | 0.327 |
| Substrate use efficiency | ||||||
| Microbial C | 3.2 | 0.088 | 0.15 | 0.962 | 0.1 | 0.977 |
| 13C‐respiration | 2.5 | 0.131 | 21.2 | <0.001 | 0.1 | 0.974 |
| 13C‐incorporation | 3.0 | 0.099 | 0.5 | 0.763 | 0.3 | 0.862 |
| Substrate use efficiency | 0.1 | 0.806 | 9.6 | <0.001 | 0.3 | 0.857 |
| SOM respiration | 1.2 | 0.297 | 20.6 | <0.001 | 0.4 | 0.805 |
| Rmass | 0.1 | 0.829 | 18.7 | <0.001 | 0.1 | 0.965 |
| Gross N processes | ||||||
| N mineralization | 0.5 | 0.492 | 0.3 | 0.851 | 0.1 | 0.973 |
Figure 4.
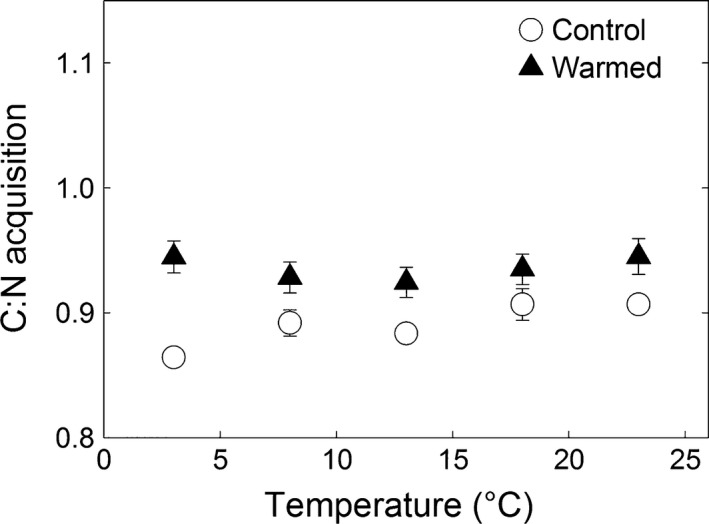
Ratio of enzymatic C : N acquisition, calculated as the sum of the log‐transformed activities of beta‐glucosidase and cellobiohydrolase activity over the sum the log‐ transformed activities of N‐acetyl‐glucosaminidase and leucine aminopeptidase activity at different incubation temperatures. Full triangles represent the values for soil which was exposed to 9 years of warming (means ± SE, n = 3). Open circles represent the values for soil which had experienced the corresponding natural temperature conditions (means ± SE, n = 3).
Microbial SUE did not differ between soils from warmed and control subplots (Table 3). For both treatments, 13C respiration increased exponentially with increasing incubation temperature, whereas 13C incorporation into the microbial biomass showed no response to incubation temperature (Fig. 5). Accordingly, microbial SUE decreased with increasing incubation temperature (Fig. 5). There was no significant field warming effect on the rates of 13C incorporation or 13C respiration (Table 3). The temperature sensitivity of microbial SUE did not differ between warmed and untreated soils either (Tables 2 and 3).
Figure 5.
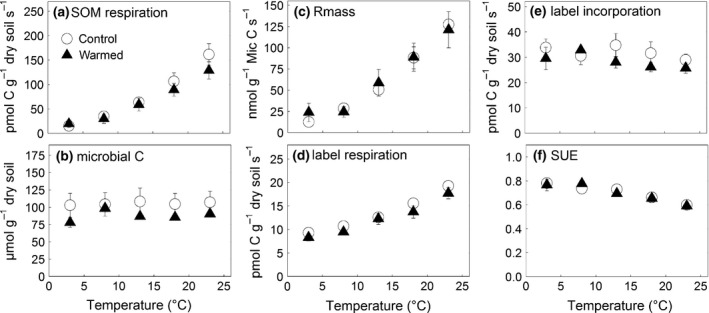
Effects of soil temperature on decomposer efficiency. Heterotrophic respiration of SOM, corrected for 13C respiration (a); microbial biomass C (b); mass‐specific respiration (c); respired 13C label (d); 13C label incorporated into microbial biomass (e); and substrate use efficiency (SUE, f). Full triangles represent the values from long‐term warmed soil (means ± SE, n = 3). Open circles represent the values of soil which had experienced the corresponding natural temperature conditions (means ± SE, n = 3). Mineral soil from each subplot was incubated simultaneously at the indicated soil temperatures for 24 h.
Rmass increased exponentially with incubation temperature (Fig. 5, Table 3) as microbial biomass C was unaffected by incubation temperature and the respiration corrected for the 13C label (SOM respiration) increased in a similar manner as observed for the undisturbed cores incubation (Tables 2 and 3). Long‐term field soil warming did not affect the temperature sensitivity of Rmass (Table 2).
Gross N mineralization showed, in contrast to respiration and enzyme activities, no increase with incubation temperature (Table 3, Fig. 6) and was not affected by long‐term warming (Table 3).
Figure 6.
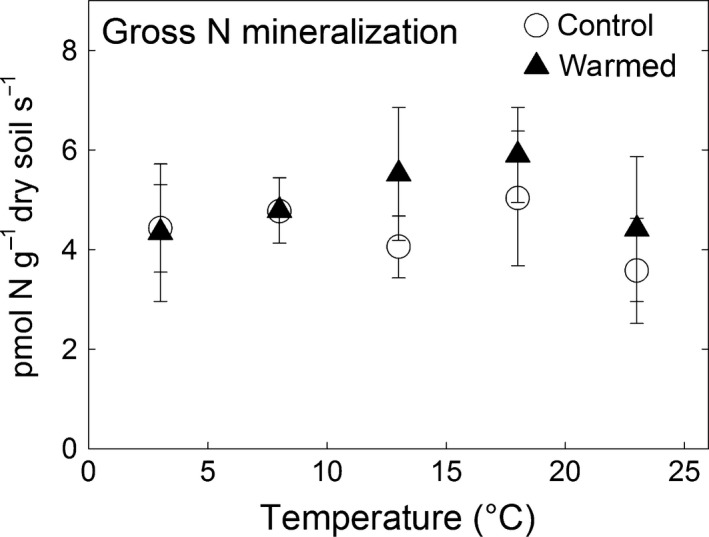
Effect of soil temperature on gross N mineralization. Full triangles represent the values from long‐term warmed soil (means ± SE, n = 3). Open circles represent the values of soil which had experienced the corresponding natural temperature conditions (means ± SE, n = 3). Mineral soil from each subplot was incubated simultaneously at the indicated soil temperatures.
Discussion
Thermal adaptation of microbial processes could strongly impact future soil C stocks and atmospheric CO2 concentrations by accelerating or mitigating the response of Rh to global warming (Wieder et al., 2013; Hagerty et al., 2014; Karhu et al., 2014), but our results indicate that such thermal adaptations are, at least for the studied soil type, unlikely to occur.
Rh as well as the temperature sensitivity of Rh did not differ between the soil which was warmed during 9 consecutive growing seasons and the soil which had experienced natural temperature conditions when soil was incubated under controlled conditions. This suggests that no thermal adaptations of the decomposer community had occurred as of yet. The sustained strong response of the soil CO2 efflux to field warming (~50% higher CO2 efflux at warmed subplots during the day of soil sampling), and the almost equal CO2 efflux from the incubated sieved soil indicate that ‘indirect’ effects such as substrate limitation had not affected the decomposer community in the studied soil until now. With the consecutive incubation of intact soil cores, roots, and sieved soil, we aimed to account for potential effects of soil disruption on the temperature sensitivity of Rh during the incubation. As the temperature sensitivity of Rh is influenced by SOM quality/recalcitrance (Conant et al., 2008; Karhu et al., 2010), disruption of soil aggregates and a release of easily decomposable SOM during soil sieving could alter Rh rates and their temperature sensitivity. Such a disruption effect can, however, be neglected in our study as the Q 10 values from intact soil cores and sieved soil were similar and showed the same pattern when compared to long‐term soil warming. Fine root amount, root respiration rates, and their temperature sensitivity were similar in cores from warmed and control subplots. Therefore, with regard to Rh, the comparability of the intact soil core CO2 efflux from warmed and control subplots was given as well. The slightly higher soil CO2 efflux of the intact cores from the warmed plots indicates a higher availability of labile C at the warmed plots, which might, at least partly, be explained by increased fine root turnover due to long‐term soil warming (A. Schindlbacher, unpublished data). Neither the low contribution of root derived CO2 to the soil core efflux, nor the temperature sensitivity of the CO2 efflux of cutoff roots should be considered representative of field conditions because roots were cut off several days prior to the incubation.
Although the uniform response of Rh to increasing soil temperature indicates the absence of thermal adaptations, it is possible that individual microbial processes have adapted to the long‐term warming, but that individual adaptations outbalanced each other. We therefore separately assessed a number of underlying C cycling processes. The first step in microbial substrate and nutrient acquisition is the production and release of extracellular enzymes which break down complex organic macromolecules and provide soluble low molecular weight products for microbial uptake and assimilation. The effect of long‐term soil warming on extracellular enzyme activities has, however, rarely been studied in the field. In accordance with Allison & Treseder (2008), Jing et al. (2014), Steinweg et al. (2013), and Weedon et al. (2014), we could not find a clear response of enzyme activities to the long‐term soil warming. Like us, Allison & Treseder (2008) observed slightly lower NAP activity in an artificially warmed boreal forest soil. Other enzyme activities were not affected by warming in their study. We observed higher BG activity in warmed soil together with a constantly higher ratio of enzymatic C : N acquisition. This indicates that the microbial communities in the warmed soil had shifted their energy investments toward C acquisition. The enhanced investment in C‐acquiring enzymes could be a strategy to cope with the increased temperature and the concomitant higher demand for C for respiration (discussed below). In accordance with Jing et al. (2014), we did not observe any thermal adaptation in the temperature sensitivity of the enzyme activities. The study of Jing et al. (2014) is based on seasonal changes in enzyme activities, whereas we conducted a point in time assessment. As the extracellular enzyme activity can adapt seasonally (Wallenstein et al., 2009), it cannot be excluded that thermal adaptations in extracellular enzyme kinetics had occurred during other seasons than during soil sampling in our study. The likelihood of such adaptations, however, is low as the microbial community composition at our site experiences only minor seasonal variations and was not affected by soil warming (Schindlbacher et al., 2011; Kuffner et al., 2012).
Extracellular enzymes provide the compounds that can be assimilated by soil microorganisms. The CO2 of aerobic respiration is produced within the microbial cells and the heterotrophic soil CO2 efflux therefore results from intracellular reactions. Therefore, trade‐offs between microbial energy investments in different growth and maintenance strategies affect the decomposition (and formation) of SOM and correspondingly determine Rh rates (Bradford, 2013). Rmass gives a first clue about potential adaptations in microbial efficiency. Rmass increased with incubation temperature but was not different in long‐term warmed soil and control soil. Accordingly, thermal adaptations regarding Rmass did not occur in our long‐term warming experiment. Our Rmass values may hold some uncertainty as the 24‐h incubation period might not have been sufficiently long enough to allow for full equilibration of microbial biomass to the different temperature regimes. However, we periodically assessed microbial biomass in the field from 2008 until 2010 (4th–6th year of warming) and found that warming had not affected microbial biomass C, while the soil CO2 efflux was substantially increased (Schindlbacher et al., 2011). Therefore, warming had persistently enhanced Rmass in the field, confirming our incubation results.
In contrast to Rmass, microbial SUE decreased with increasing incubation temperature, indicating that some energy demanding processes outbalanced the microbial C gain at increasing temperatures. The increase in 13C respiration (Fig. 3) suggests that primarily maintenance energy costs increased with temperature (Manzoni et al., 2012). The decrease in microbial SUE of ~0.009 °C−1 is consistent with the estimate of 0.009 °C−1 derived by Steinweg et al. (2008) using a similar approach and with the decrease of microbial CUE by 0.011‐ 0.017 °C−1 in the study of Tucker et al. (2013). Frey et al. (2013) did not observe a negative response of microbial CUE to soil temperature for glucose but a strong negative response of more recalcitrant compounds (glutamic acid – 0.9 °C−1, phenol – 1.1 °C−1), whereas Hagerty et al. (2014) did not observe any temperature response. The frequently observed negative relationship between microbial CUE and soil temperature raised the question whether this relationship could be a cause of thermal acclimation of soil respiration (Tucker et al., 2013). Our results do not support this hypothesis. The temperature sensitivity of microbial SUE was, however, similar. Hagerty et al. (2014) proposed that the apparent decline in microbial CUE at higher soil temperatures is actually caused by increased microbial turnover rates. This would, however, not have affected our estimates of microbial SUE as this was assessed only over a 24‐h period. Applying their higher microbial turnover scenario, the model predicts a strong decrease in the microbial biomass pool in the longer term (Hagerty et al., 2014). Such a decrease in microbial biomass did not occur in our field experiment so far.
The increase in enzymatic C : N acquisition ratios in warmed soils points to possible impacts on the soil N cycle. However, we found no thermal adaptation of gross N mineralization in the long‐term warming treatment, similar as reported in a recent meta‐analysis of the few explicit studies available on this subject (Bai et al., 2013). Surprisingly, gross N mineralization showed no temperature sensitivity in our incubation study which concurs with results found for managed temperate soils (Lang et al., 2010) but contrasts with findings from others where gross N mineralization increased with incubation temperature (Binkley et al., 1994; Cookson et al., 2002, 2007; Huber et al., 2007; Schütt et al., 2014). Gross N mineralization is driven by the rate of organic N release in soils (and microbial organic N uptake) and the microbial nitrogen‐use efficiency (NUE) (Mooshammer et al., 2014). Similar to CUE, microbial NUE depicts how much of the organic N taken up is incorporated into microbial biomass, the excess being excreted as ammonium thereby fueling gross N mineralization (Mooshammer et al., 2014). The results observed here indicate that organic N release through extracellular enzymes increased with temperature (see NAG and LAP). The absence of short‐term stimulation of gross N mineralization by temperature can therefore be reconciled by increasing microbial NUE with temperature which lowers the fraction of organic N taken up by microbes that is mineralized. The proposed increase in microbial NUE could possibly be triggered by the increase in the release of organic C relative to organic N with temperature, causing an increased microbial N demand (or N limitation).
As thermal adaptations were documented for a variety of decomposer microorganisms, the question remains why microbial traits did not adapt to elevated soil temperature in our study. The answer may lie in the heterogeneity of the microbial community (Kuffner et al., 2012) but probably also in the strong seasonal and diurnal temperature fluctuations of this ecosystem. Field soil temperatures fluctuate daily, seasonally, and interannually (Fig. 1). This implies that soil in unwarmed subplots experienced, with some time lag, similar temperatures as the warmed subplots. Only during summer, warmed subplot temperatures actually exceed the temperatures which are experienced by the control soils (Fig. 1). Therefore, it should be questioned, if, and to which extent physiological adaptations to higher or lower temperatures, which were typically observed under rather static temperature conditions, apply in ecosystems which are characterized by recurrent and strong temperature fluctuations (Wallenstein & Hall, 2012).
We did not warm the soil during the dormant season because it would eliminate the isolating snow cover. In subalpine and alpine climate zones, the consequences of warmer air temperatures for soil temperatures during winter rely on the existence and condition of the snow cover. In the study region, climate warming will reduce the duration of snow cover with a general trend toward warmer soils during wintertime (Kreyling & Henry, 2011). However, a missing snow cover can also cause severe soil frost during cold winters with respective implications for soil C cycling (Groffman et al., 2001; Muhr et al., 2009). At our study site, soil temperature might be not or less affected at future elevated winter air temperatures as long as snow covers the soil surface.
Our data show that even 9 years of intensive soil warming do not necessarily lead to a thermal adaptation of microbial processes. Reasons for this might be that the investigated soil, with its high SOM content, provided the C to fulfill the higher microbial energy demand at the higher temperatures, but also the nutrients to produce the enzymes that were necessary to access additional C sources. This may change when warming reduces the substrate pool to a level at which microbes become limited in C supply. Limited substrate availability can alter the microbial community structure (Frey et al., 2008; DeAngelis et al., 2015) and function (Bradford et al., 2008). If and how such ‘microbial community response’ (Karhu et al., 2014) affects future soil C cycling, however, also depends on the response of the above‐ and belowground biomass to global change (Bronson et al., 2008). Increased plant productivity and C input into the soil could retain the availability of SOM. Our results indicate that under such circumstances, a thermal adaptation of microbial physiology to higher soil temperatures seems unlikely. Therefore, global warming could cause substantial C losses from C‐rich temperate forest soils to the atmosphere and thereby offset the predicted increase of C inputs to soils from vegetation. Microbial physiology can adapt when soils experience temperatures above the optimum conditions for microbial growth and C mineralization (Bárcenas‐Moreno et al., 2009; Birgander et al., 2013). At our site, however, soil temperatures in the warming plots were far below the optimum temperatures for SOM decomposition.
Acknowledgments
The study was funded by the Austrian Science Fund (FWF; project P‐23222‐B17). We thank three reviewers for their constructive input. We further thank our technician Christian Holtermann for keeping the soil warm during all the years.
References
- Allison SD, Treseder KK (2008) Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Global Change Biology, 14, 2898–2909. [Google Scholar]
- Allison SD, Wallenstein MD, Bradford MA (2010) Soil‐carbon response to warming dependent on microbial physiology. Nature Geoscience, 3, 336–340. [Google Scholar]
- Bai E, Li S, Xu W, Li W, Dai W, Jiang P (2013) A meta‐analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytologist, 199, 441–451. [DOI] [PubMed] [Google Scholar]
- Balser TC, Wixon DL (2009) Investigating biological control over soil carbon temperature sensitivity. Global Change Biology, 15, 2935–2949. [Google Scholar]
- Bárcenas‐Moreno G, Gómez‐Brandón M, Rousk J, Bååth E (2009) Adaptation of soil microbial communities to temperature: comparison of fungi and bacteria in a laboratory experiment. Global Change Biology, 15, 2950–2957. [Google Scholar]
- Binkley D, Stottlemyer R, Suarez F, Cortina J (1994) Soil nitrogen availability in some arctic ecosystems in northwest Alaska: responses to temperature and moisture. Ecoscience, 1, 64–70. [Google Scholar]
- Birgander J, Reischke S, Jones DL, Rousk J (2013) Temperature adaptation of bacterial growth and 14C‐glucose mineralisation in a laboratory study. Soil Biology & Biochemistry, 65, 294–303. [Google Scholar]
- Bradford MA (2013) Thermal adaptation of decomposer communities in warming soils. Frontiers in Microbiology, 4, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MA, Davies CA, Frey SD et al (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecology Letters, 11, 1316–1327. [DOI] [PubMed] [Google Scholar]
- Bronson DR, Gower ST, Tanner M, Linder S, Vanherk I (2008) Response of soil CO2 flux in a boreal forest to ecosystem warming. Global Change Biology, 14, 856–867. [Google Scholar]
- Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology & Biochemistry, 17, 837–842. [Google Scholar]
- Brzostek ER, Blair JM, Dukes JS et al (2012) The effect of experimental warming and precipitation change on proteolytic enzyme activity: positive feedbacks to nitrogen availability are not universal. Global Change Biology, 18, 2617–2625. [Google Scholar]
- Conant RT, Steinweg JM, Haddix ML, Paul EA, Plante AF, Six J (2008) Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology, 89, 2384–2391. [DOI] [PubMed] [Google Scholar]
- Conant RT, Ryan MG, Ågren GI et al (2011) Temperature and soil organic matter decomposition rates – synthesis of current knowledge and a way forward. Global Change Biology, 17, 3392–3404. [Google Scholar]
- Cookson WR, Cornforth IS, Rowarth JS (2002) Winter soil temperature (2‐15 degrees C) effects on nitrogen transformations in clover green manure amended or unamended soils; a laboratory and field study. Soil Biology & Biochemistry, 34, 1401–1415. [Google Scholar]
- Cookson WR, Osman M, Marschner P et al (2007) Controls on soil nitrogen cycling and microbial community composition across land use and incubation temperature. Soil Biology & Biochemistry, 39, 744–756. [Google Scholar]
- Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon‐cycle feedbacks in a coupled climate model. Nature, 408, 184–187. [DOI] [PubMed] [Google Scholar]
- Crowther TW, Bradford MA (2013) Thermal acclimation in widespread heterotrophic soil microbes. Ecology Letters, 16, 469–477. [DOI] [PubMed] [Google Scholar]
- DeAngelis KM, Pold G, Topcuoglu BD et al (2015) Long‐term forest soil warming alters microbial communities in temperate forest soils. Frontiers in Microbiology, 6, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson PE, Mcmurtie RE, Pepper DA, Strömgren M, Linder S, Ågren GI (2005) The response of heterotrophic CO2 flux to soil warming. Global Change Biology, 11, 167–181. [Google Scholar]
- Fenner N, Freeman C, Reynolds B (2005) Observations of a seasonally shifting thermal optimum in peatland carbon‐cycling processes; implications for the global carbon cycle and soil enzyme methodologies. Soil Biology & Biochemistry, 37, 1814–1821. [Google Scholar]
- Frey SD, Drijber R, Smith H, Melillo JM (2008) Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biology & Biochemistry, 40, 2904–2907. [Google Scholar]
- Frey SD, Lee J, Melillo JM, Six J (2013) The temperature response of soil microbial efficiency and its feedback to climate. Nature Climate Change, 3, 395–398. [Google Scholar]
- Friedlingstein P, Cox P, Betts R et al (2006) Climate‐carbon cycle feedback analysis: results from the C4MIP model intercomparison. Journal of Climate, 19, 3337–3353. [Google Scholar]
- German DP, Marcelo KRB, Stone MM, Allison SD (2012) The Michaelis‐Menten kinetics of soil extracellular enzymes in response to temperature: a cross‐latitudinal study. Global Change Biology, 18, 1468–1479. [Google Scholar]
- Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL (2001) Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry, 56, 135–150. [Google Scholar]
- Hagerty SB, Van Groenigen KJ, Allison SD et al (2014) Accelerated microbial turnover but constant growth efficiency with warming in soil. Nature Climate Change, 4, 903–906. [Google Scholar]
- Hartley IP, Heinemeyer A, Ineson P (2007) Effects of three years of soil warming and shading on the rate of soil respiration: substrate availability and not thermal acclimation mediates observed response. Global Change Biology, 13, 1761–1770. [Google Scholar]
- Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA (2008) Soil microbial respiration in arctic soil does not acclimate to temperature. Ecology Letters, 11, 1092–1100. [DOI] [PubMed] [Google Scholar]
- Hartley IP, Hopkins DW, Garnett MH, Sommerkorn M, Wookey PA (2009) No evidence for compensatory thermal adaptation of soil microbial respiration in the study of Bradford et al. (2008). Ecology Letters, 12, E12–E14. [DOI] [PubMed] [Google Scholar]
- Hood‐Nowotny R, Hinko‐Najera Umana N, Inselbacher E, Oswald‐Lachouani P, Wanek W (2010) Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Science Society of America Journal, 74, 1018–1027. [Google Scholar]
- Hopkins FM, Filley TR, Gleixner G, Lange M, Top SM, Trumbore SE (2014) Increased belowground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biology & Biochemistry, 76, 57–69. [Google Scholar]
- Huber E, Wanek W, Gottfried M et al (2007) Shift in soil‐plant nitrogen dynamics of an alpine‐nival ecotone. Plant and Soil, 301, 65–76. [Google Scholar]
- Janssens IA, Pilegaard K (2003) Large seasonal changes in Q10 of soil respiration in a beech forest. Global Change Biology, 9, 911–918. [Google Scholar]
- Jing X, Wang YH, Chung HG, Mi ZR, Wang SP, Zeng H, He JS (2014) No temperature acclimation of soil extracellular enzymes to experimental warming in an alpine grassland ecosystem on the Tibetan Plateau. Biogeochemistry, 117, 39–54. [Google Scholar]
- Jones DL, Owen AG, Farrar JF (2002) Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biology & Biochemistry, 34, 1893–1902. [Google Scholar]
- Kaiser C, Koranda M, Kitzler B et al (2010) Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist, 187, 843–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C, Fuchslueger L, Koranda M et al (2011) Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology, 92, 1036–1051. [DOI] [PubMed] [Google Scholar]
- Kandeler E, Gerber H (1988) Short‐term assay of soil urease activity using colorimetric determination of ammonium. Biology and Fertility of Soils, 6, 68–72. [Google Scholar]
- Karhu K, Fritze H, Hämäläinen K et al (2010) Temperature sensitivity of soil carbon fractions in boreal forest soil. Ecology, 91, 370–376. [DOI] [PubMed] [Google Scholar]
- Karhu K, Auffret MD, Dungait JAJ et al (2014) Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature, 513, 81–84. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MUF (2004) Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Global Change Biology, 10, 1870–1877. [Google Scholar]
- Kreyling J, Henry HAL (2011) Vanishing winters in Germany: soil frost dynamics and snow cover trends, and ecological implications. Climate Research, 46, 269–276. [Google Scholar]
- Kuffner M, Hai B, Rattei T et al (2012) Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiology Ecology, 82, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb EG, Han S, Lanoil BD, Henry GHR, Brummell ME, Banerjee S, Siciliano SD (2011) A high arctic soil ecosystem resists long‐term environmental manipulations. Global Change Biology, 17, 3187–3194. [Google Scholar]
- Lang M, Cai ZC, Mary B, Hao XY, Chang SX (2010) Land‐use type and temperature affect gross nitrogen transformation rates in Chinese and Canadian soils. Plant and Soil, 334, 377–389. [Google Scholar]
- Lu M, Zhou XH, Yang Q et al (2013) Responses of ecosystem carbon cycle to experimental warming: a meta‐analysis. Ecology, 94, 726–738. [DOI] [PubMed] [Google Scholar]
- Luo Y, Wan S, Hui D, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature, 413, 622–624. [DOI] [PubMed] [Google Scholar]
- Malcolm GM, López‐Gutiérrez JC, Koide RT, Eissenstat DM (2008) Acclimation to temperature and temperature sensitivity of metabolism by ectomycorrhizal fungi. Global Change Biology, 14, 1–12. [Google Scholar]
- Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI (2012) Environmental and stoichiometric controls on microbial carbon‐use efficiency in soils. New Phytologist, 196, 79–91. [DOI] [PubMed] [Google Scholar]
- Melillo JM, Steudler PA, Aber JD et al (2002) Soil warming and carbon‐cycle feedbacks to the climate system. Science, 298, 2173–2176. [DOI] [PubMed] [Google Scholar]
- Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide, 5, 62–71. [DOI] [PubMed] [Google Scholar]
- Mooshammer M, Wanek W, Hämmerle I et al (2014) Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil N cycling. Nature Communications, 5, 3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J, Borken W, Matzner E (2009) Effects of soil frost on soil respiration and its radiocarbon signature in a Norway spruce forest soil. Global Change Biology, 15, 782–793. [Google Scholar]
- Prommer J, Wanek W, Hofhansl F et al (2014) Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS ONE, 9, e86388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk J, Frey SD, Baath E (2012) Temperature adaptation of bacterial communities in experimentally warmed forest soils. Global Change Biology, 18, 3252–3258. [DOI] [PubMed] [Google Scholar]
- Schindlbacher A, Zechmeister‐Boltenstern S, Butterbach‐Bahl K (2004) Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. Journal of Geophysical Research, 109, D17302. [Google Scholar]
- Schindlbacher A, Zechmeister‐Boltenstern S, Kitzler B, Jandl R (2008) Experimental forest soil warming: response of autotrophic and heterotrophic soil respiration to a short‐term 10 C temperature rise. Plant and Soil, 303, 323–330. [Google Scholar]
- Schindlbacher A, Zechmeister‐Boltenstern S, Jandl R (2009) Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? Global Change Biology, 15, 901–913. [Google Scholar]
- Schindlbacher A, De Gonzalo C, Díaz‐Pinés E et al (2010) Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. Journal of Geophysical Research, 115, G03018. [Google Scholar]
- Schindlbacher A, Rodler A, Kuffner M, Kitzler B, Sessitsch A, Zechmeister‐Boltenstern S (2011) Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biology & Biochemistry, 43, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindlbacher A, Wunderlich S, Borken W, Kitzler B, Zechmeister‐Boltenstern S, Jandl R (2012) Soil respiration under climate change: prolonged summer drought offsets soil warming effects. Global Change Biology, 18, 2270–2279. [Google Scholar]
- Schütt M, Borken W, Spott O, Stange CF, Matzner E (2014) Temperature sensitivity of C and N mineralization in temperate forest soils at low temperatures. Soil Biology & Biochemistry, 69, 320–327. [Google Scholar]
- Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecology Letters, 16, 930–939. [DOI] [PubMed] [Google Scholar]
- Steinweg JM, Plante AF, Conant RT, Paul EA, Tanaka DL (2008) Patterns of substrate utilization during long‐term incubations at different temperatures. Soil Biology & Biochemistry, 40, 2722–2728. [Google Scholar]
- Steinweg JM, Dukes JS, Wallenstein MD (2012) Modeling the effects of temperature and moisture on soil enzyme activity: linking laboratory assays to continuous field data. Soil Biology & Biochemistry, 55, 85–92. [Google Scholar]
- Steinweg JM, Dukes JS, Paul EA, Wallenstein MD (2013) Microbial responses to multi‐factor climate change: effects on soil enzymes. Frontiers in Microbiology, 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömgren M (2001) Soil‐Surface CO2 Flux and Growth in a Boreal Norway Spruce Stand: Effects of Soil Warming and Nutrition. Acta Universitatis Agriculturae Sueciae, Uppsala. [Google Scholar]
- Suseela V, Tharayil N, Xing BS, Dukes JS (2014) Warming alters potential enzyme activity but precipitation regulates chemical transformations in grass litter exposed to simulated climatic changes. Soil Biology & Biochemistry, 75, 102–112. [Google Scholar]
- Trasar‐Cepeda C, Gil‐Sotres F, Leiros MC (2007) Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biology & Biochemistry, 39, 311–319. [Google Scholar]
- Tucker CL, Bell J, Pendall E, Ogle K (2013) Does declining carbon‐use efficiency explain thermal acclimation of soil respiration with warming? Global Change Biology, 19, 252–263. [DOI] [PubMed] [Google Scholar]
- Van Hees PAW, Jones DL, Finlay R, Godbold DL, Lundström US (2005) The carbon we do not see ‐ the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biology & Biochemistry, 37, 1–13. [Google Scholar]
- Vanhala P, Karhu K, Tuomi M, Björklöf K, Fritze H, Hyvärinen H, Liski J (2011) Transplantation of organic surface horizons of boreal soils into warmer regions alters microbiology but not the temperature sensitivity of decomposition. Global Change Biology, 17, 538–550. [Google Scholar]
- Wallenstein MD, Hall EK (2012) A trait‐based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry, 109, 35–47. [Google Scholar]
- Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Global Change Biology, 15, 1631–1639. [Google Scholar]
- Wanek W, Mooshammer M, Blöchl A, Hanreich A, Richter A (2010) Determination of gross rates of amino acid production and immobilization in decomposing leaf litter by a novel 15N isotope pool dilution technique. Soil Biology & Biochemistry, 42, 1293–1302. [Google Scholar]
- Wang X, Liu LL, Piao S et al (2014) Soil respiration under climate warming: differential response of heterotrophic and autotrophic respiration. Global Change Biology, 10, 3229–3237. [DOI] [PubMed] [Google Scholar]
- Weedon J, Aerts R, Kowalchuk G, Van Bodegom P (2014) No effects of experimental warming but contrasting seasonal patterns for soil peptidase and glycosidase enzymes in a sub‐arctic peat bog. Biogeochemistry, 117, 55–66. [Google Scholar]
- Wieder WR, Bonan GB, Allison SD (2013) Global soil carbon projections are improved by modelling microbial processes. Nature Climate Change, 3, 909–912. [Google Scholar]
- Zavodszky P, Kardos J, Svingor A, Petsko GA (1998) Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proceedings of the National Academy of Sciences of the United States of America, 95, 7406–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]


