Abstract
Background
Children and adolescents, who either acquire HIV infection perinatally, from contaminated blood products or via sexual transmission early in life, have the greatest cumulative exposure to the negative direct and indirect effects of HIV infection and ART on bone, which may lead to increased lifetime risk for osteoporosis and fracture. We conducted a systematic review to evaluate the literature on bone health in children and adolescents with HIV.
Methods
We performed a comprehensive search of the Medline, Scopus, and Cochrane Library databases (up to April 1, 2014) for studies that reported on bone imaging or bone fractures in HIV-infected children, adolescents, or young adults.
Results
A total of 32 publications met our inclusion criteria. Seventeen studies were cross-sectional and 15 were longitudinal. The majority of studies were conducted in high-income countries, three in middle-income countries and none in low-income countries. Overall, the studies we reviewed indicate that measures of bone mass are reduced, with increased prevalence of low BMD in children and adolescents with HIV. However, the studies are highly variable with respect to comparison sources, measurement methods, adjustment techniques for body size or growth retardation, and highlighted risk factors, including aspects related to medication exposures as well as the effects of HIV infection per se.
Conclusion
HIV infection appears to be associated with decreased bone accrual throughout childhood and adolescence. Initial studies indicate that sub-optimal bone accrual may be persistent and result in reduced peak bone mass, an important determinant of future risk of osteoporosis and fracture. Important areas for future research include evaluation of bone mass, bone quality and fracture risk across the life course among those with early-life infection with HIV, particularly in resource-limited settings where the majority of children with HIV live.
INTRODUCTION
Low bone mineral density (BMD) and increased fracture rates have been reported in HIV-infected individuals, particularly in older men and postmenopausal women [1–5]. Decreased bone mass has also been reported in children, adolescents and young adults who acquire HIV early in life from perinatal or sexual transmission [6, 7]. Multiple factors appear to be involved, including effects of HIV-1 viral proteins, inflammatory cytokines and antiretroviral therapy (ART) on bone cells and bone turnover,[8–14]. Children and adolescents infected with HIV have the largest cumulative exposure to the negative direct and indirect effects of HIV infection and ART on bone metabolism, which may lead to an increase in the lifetime risk for osteoporosis and fracture.
As approximately 85–90% of final adult bone mass is attained during childhood and adolescence, impaired bone accrual during these critical periods of skeletal maturation may compromise peak bone mass (PBM), the maximum amount of bony tissue at the end of skeletal maturation, which is an important determinant of adult osteoporosis and fracture risk [15–17]. Potent ART has transformed HIV from a fatal illness to a manageable chronic infection with near normal life expectancy [18, 19]. With approximately 6 million people age 24 and under living with HIV and possibly more than 1.5 million on ART [20], the long-term outcome of bone development across the life course and causes of suboptimal bone development have emerged as an important areas of investigation. Thus, the aim of this study was to systematically review the literature, summarize the publications concerning bone health in children, adolescents, and young adults with HIV, and identify areas where further research is needed.
METHODS
Search process
We conducted a comprehensive systematic search for original publications reporting bone imaging or bone fractures in HIV-infected children, adolescents, or young adults. Journal articles were retrieved using Medline, Scopus and The Cochrane Library databases up to April 1, 2014. Studies that were electronically published ahead of print publication during this time period were eligible for inclusion. Specific search criteria are reported in Table 1.
Table 1.
Database search terms
| Database | Time Period | Search Terms | # |
|---|---|---|---|
| Medline | Up to April 1, 2014 | (“bone and bones”[MeSH Terms] OR (“bone”[All Fields] AND “bones”[All Fields]) OR “bone and bones”[All Fields] OR “bone”[All Fields]) AND (“hiv”[MeSH Terms] OR “hiv”[All Fields]) AND ((“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields]) OR (“adolescent”[MeSH Terms] OR “adolescent”[All Fields] OR “adolescents”[All Fields]) OR (“young adult”[MeSH Terms] OR (“young”[All Fields] AND “adult”[All Fields]) OR “young adult”[All Fields] OR (“young”[All Fields] AND “adults”[All Fields]) OR “young adults”[All Fields])) | 676 |
| Scopus | Up to April 1, 2014 | HIV AND bone AND (children OR adolescents OR young adults) in “Article Title, Abstract and Keywords” | 668 |
| Cochrane Library | Up to April 1, 2014 | HIV and bone | 6 |
Study selection and data collection
Two investigators (SS, CMA) independently reviewed all publications for inclusion. A study was eligible for inclusion if it reported on bone imaging or bone fractures in HIV-infected children, adolescents, or young adults (up to age 24) and had a full-text article in English. Studies with and without a control group were included, however, studies were excluded if they included less than five HIV-infected individuals.
The following data was abstracted, when available, from each publication that met the inclusion criteria: study name, authors, year of publication, study location and design, total number of participants, number of HIV-infected participants and controls or reference source used for comparison (if applicable). Detailed information on HIV-infected participants, including age, sex, stage of biologic maturation, race/ethnicity, and transmission status were obtained. Where available, we recorded information on bone fractures as well as imaging methods used including dual-energy x-ray absorptiometry (DXA), quantitative computed tomography (QCT), or quantitative ultrasound (QUS). We also recorded specific measurements, including bone mineral content (BMC), bone mineral density (BMD), bone area (BA), cortical and trabecular thickness, speed of sound (SoS), broadband ultrasound attenuation (BUA), bone transmission time (BTT), and skeletal sites, including whole body (WB), lumbar spine (LS), femoral neck (FN), total hip (TH), 1/3 distal radius (R13), tibia, phalanges, and calcaneus. In addition, we recorded techniques for adjustments of bone measures, risk factors, and key findings.
RESULTS
Selection of studies and study characteristics
Results from the literature search and study selection process are shown in Figure 1. A total of 32 articles met inclusion criteria. The characteristics of these studies published between 2001 and 2014 are summarized in Table 2. Most studies were conducted in high income countries, including the United States [6, 7, 21–32], Italy [33–44], Canada [45], and the Netherlands [46]. Three studies were reported from middle-income countries, including one from Thailand [47] and two from Brazil [48, 49]. One study was conducted in three countries: the United States, Brazil, and Panama [50]. No studies were conducted in low-income countries. While the ethnic and racial breakdown varied across studies, several included only white/Caucasian participants [33, 35–40, 43]. The source of comparison group varied across studies. Many recruited a healthy control group [31, 35, 37–40] and several included age, sex, ethnicity, or pubertal stage as criteria for selection [6, 7, 25, 36, 44, 51]. Two studies enrolled children known to be HIV-uninfected but exposed to HIV in utero as a control group [29, 30] and one study recruited HIV-uninfected siblings [24]. Other studies drew comparisons with a number of existing cross-sectional or longitudinal normative databases comprised of healthy children from single or multiple study sites [21–23, 26–28, 34, 45, 47] or provided by the densitometer manufacturer [24, 41, 43, 46, 48, 49]. Bone densitometry by DXA was the predominant method for evaluation. A sole manufacturer densitometer (Hologic, Bedford, MA or GE Lunar, Madison, WI) was used in all but 7 studies [6, 7, 24, 30, 47, 50] which used both [52].
Figure 1.
Flow diagram of literature search and study selection
Table 2.
Results of the systematic review on bone health in HIV-infected children, adolescents, and young adults
| Ref (Year) | Country | Study design |
Comparison source |
N HIV+/Control/Total |
HIV+ sex (%M) |
HIV+ Age (years) |
HIV+ race/ ethnicity |
Measurements | Key Findings |
|---|---|---|---|---|---|---|---|---|---|
| Mora 2001 [33] | Italy | Cross sectional | Recruited healthy control group (N=314) | 40/314/354 | 45 | Range: 6–17 | 100% W | DXA (Lunar): WB BMD, LS BMD |
|
| O’Brien 2001 [21] | USA | Cross sectional | Single site database of healthy children (Ellis 1996, Children’s Nutrition Research Center database) (N=483) | 19/483/502 | 0 | Mean (SD): 9.2 (2.6) Range: 5.9–15.2 |
89% B 11% W |
DXA (Hologic): WB BMC, WB BMD |
|
| Arpadi 2002 [22] | USA | Cross sectional | HIV-uninfected children enrolled in body composition study at the same site (N=262) | 51/262/313 | 51 | Range: 4.2–14.7 | 51% H 41.5% B 7.5% W |
DXA (Lunar): WB BMC |
|
| Gaughan 2002 [32] | USA | Longitudinal | Exposed but uninfected children (N=849) | 2014/849/2863 | 50 | Median: 5.3 10th, 90th: 1, 12 |
16% W 52% B 31% H 1% O |
Legg-Calve-Perthes disease (LCPD) |
|
| Zamboni 2003 [34] | Italy | Cross sectional | Normal prepubertal population (N=198) | 13/198/211 | 31 | Range: 4–12 | Not specified | DXA (Lunar): LS BMD, calculated vBMD |
|
| Mora 2004 [35] | Italy | Longitudinal | Recruited control group of healthy volunteers of comparable ages (N=381) | 32/381/413 | 53 | Mean (SD): 12.4 (0.5) Range: 6.3–17.7 |
100% W | DXA (Lunar): WB BMD, LS BMD |
|
| Stagi 2004 [36] | Italy | Cross-sectional | Recruited control group matched by age, pubertal stage, and sex (N=55) and also used normative database (CUBA for age 5–15 years, Falcini 2003 for under age 5 years) | 44/55/99 | 48 | Median: 8.4 Range: 4.6–12.4 |
100% W | QUS (McCue Ultrasonics): heel BUA |
|
| Giacomet 2005 [37] | Italy | Longitudinal | Recruited control group of healthy white volunteers (N=166) | 16/166/182 | Not reported | Mean 13.3 Range 6.4–17.9 |
100% W | DXA (Lunar): LS BMC, LS BMD, WB BMC, WB BMD |
|
| Hazra 2005 [23] | USA | Longitudinal | Databases Bachrach 1999, Faulkner 1996 | 18/NS/18 | 61 | Mean (SD): 12 (2.5) Range: 8.3–16.2 |
33% W 55% B 6% H 6% O |
DXA (Hologic): LS BMD |
|
| Jacobson 2005 [24] | USA | Longitudinal | Siblings (N=9) and single site multiethnic database (Ellis 2001, Children’s Nutrition Research Center database) | 37/9/46 | 49 | Median: 11.6 Range: 9.6–13.8 |
40% B 27% H 24% W |
DXA (Lunar or Hologic): WB BMC, WB BMD |
|
| Mora 2005 [38] | Italy | Cross-sectional | Recruited control group of healthy children (N=119) | 16/119/135 | 38 | Mean (SD): 9.3 (3.9) Range: 4.4–16.0 |
100% W | DXA (Lunar): LS BMC & WB BMC |
|
| Pitukcheewanont 2005 [25] | USA | Cross sectional | Recruited control group matched for age, gender, ethnicity (N=58) | 58/58/116 | 45 | Mean (SD): 12.03 (3.88) Range: 5–19.39 |
“of multiple ethnicities” | DXA (Hologic): LS bone area, LS BMC, LS BMD, WB bone area, WB BMC, WB BMD QCT (General Electric Hilite Advantage): vertebral BD, vertebral height, vertebral CSA |
|
| Rosso 2005 [39] | Italy | Cross sectional | Recruited control group from schools (N=1227) | 44/1227/1271 | 48 | Median: 10.7 Mean (SD): 10.4 (4.0) Range: 3–17 |
100% W | QUS: phalangeal SOS, BTT |
|
| Gafni 2006 [26] | USA | Longitudinal | For children >9 years: single site multi-ethnic longitudinal database (N=423) (Bachrach 1999) For children <8 years old: single site longitudinal database (Faulkner 1996) |
15/NS/15 | 67 | Range: 4–18 | NS | DXA (Hologic): LS BMD, FN BMD, TH BMD, LS BMAD |
|
| Mora 2007 [40] | Italy | Longitudinal | Control group of healthy children (N=336) | 27/336/363 | 48 | Range: 4.9–17.3 | 100% W | DXA (Lunar): LS BMD, WB BMD |
|
| Purdy 2008 [27] | USA | Longitudinal | None | 6/NA/6 | 67 | Median: 12.8 Range: 11.3–17.5 |
N/A | DXA (Hologic): LS BMD |
|
| Mora 2009 [41] | Italy | Cross sectional | Manufacturer’s software (DXA: DPX-L version, version 1.5; QUS: BeamMed) | 88/NS/88 | 49 | Range: 4.8–22.1 | 78/88 W 10/88 B |
DXA (Lunar): LS BMC & BMD, WB BMC & BMD QUS (BeamMed): tibia, radius SOS |
|
| Jacobson 2010 [6] | USA | Cross sectional | Recruited control group of uninfected children into 3 Tanner strata with similar overall distribution for sex and race/ethnicity as the HIV+ | 236/143/379 | 53 | Median: 12.6 Range: 7–24 |
13.1% W 54.7% B 32.2% H |
DXA (Lunar or Hologic): LS BMC & BMD, WB BMC & BMD |
|
| Rosso 2010 [42] | Italy | Longitudinal | Manufacturer software (not specified) | 8/NS/8 | 75 | Median: 11.2 Range: 3.8–18.2 |
NS | QUS (not specified): SOS and BTT |
|
| Vigano 2010 [43] | Italy | Longitudinal | Manufacturer software (enCORE software, version 13, GE medical systems) | 21/NS/21 | 48 |
At baseline: Median: 12.1 Range: 4.9–17.9 |
100% W | DXA (Lunar): WB BMD and LS BMD |
|
| Zuccotti 2010 [44] | Italy | Cross sectional | Recruited healthy controls of comparable age (N=194) | 86/194/280 | 45 | Range: 4.8–22.1 | NS | DXA (Lunar): LS BMC & BMD, WB BMC & BMD |
|
| Arpadi 2012 [28] | USA | Longitudinal | Multicenter study of healthy children (Bone Mineral Density of Childhood Study Kalkwarf 2007) | 59/NS/59 | NS | Range: 6–16 | 63% B 37% H |
DXA (Hologic): WB BMC and WB BMD |
|
| Della Negra 2012 [50] | USA, Brazil, Panama | Longitudinal | Manufacturer software (not specified but used age-matched, sex-matched, and race-matched healthy controls) | 90/NS/90 | 44 | Mean (SD): 14 (1.5) | 100% H | DXA (Lunar or Hologic): LS BMD, WB BMD |
|
| Mulligan 2012 [7] | USA | Cross sectional | Recruited control group of seronegative men from the same age range at same sites as HIV+ (N=53) | 199/53/252 | 100 | Median: 21 Range 14–25 |
59.8% B 28.1% H 12.1% O |
DXA (Lunar or Hologic): TH BMD, LS BMD, WB BMD, TH BMC, LS BMC, WB BMC |
|
| Puthanakit 2012 [47] | Thailand | Cross sectional | Data from cohort of 199 HIV-uninfected children aged from 12–18 years (N=199) | 101/199/300 | 51 | Median: 14.3 IQR: 13.0–15.7 |
100% Thai | DXA (Lunar or Hologic): LS BMD |
|
| Siberry 2012 [29] | USA | Longitudinal | Exposed but uninfected children | 1326/649/1975 | 49 | Mean: 7.1 Range: 5.0, 10.0 |
62% B 11% W 24% H 2% O |
Fractures |
|
| Schtscherbyna 2012 [49] | Brazil | Cross sectional | Manufacturer software (Prodigy software version 11.4) | 74/NS/74 | 45 | Mean (SD): 17.3 (1.8) | 36.5% W 63.5% not W |
DXA (Lunar) WB, LS BMD |
|
| Bunders 2013 [46] | Netherlands | Longitudinal | Manufacturer software (unspecified) | 66/NS/66 | 45 | Median: 6.7 IQR: 4.4–10.3 |
62% B | DXA (Hologic): LS BMD, left FN BMD |
|
| DiMeglio 2013 [30] | USA and Puerto Rico | Cross sectional | Exposed uninfected children (N=160) | 350/160/510 | 46 | Median: 12.6 IQR: 10.2, 14.4 |
26% H 66% B 8% O/W |
DXA (Lunar or Hologic): WB BMD, LS BMD |
|
| Lima 2013 [48] | Brazil | Cross sectional | Database from NHANES (Kelly 2009) | 48/NS/48 | 50 | Mean (SD): 12.7 (2.7) Range: 7–17 |
53.3% W 43.8% B |
DXA (Hologic): WB BMD, LS BMD |
|
| MacDonald 2013 [45] | Canada | Longitudinal | Healthy controls who were participants in the University of British Columbia Healthy Bones III follow-up study (N=883) |
31/883/914 | 61 | Median: 13.6 IQR: 11.6, 16.0 |
32.2% Mixed 25.8% B 22.6% Aboriginal 12.9% W 6.5% A |
DXA (Hologic): WB BMC, LS BMC, femur BMC Peripheral QCT (Norland/Stratec XCT): muscle CSA, total and cortical bone area, cortical BMD, thickness and strength strain index at tibial shaft |
|
| Yin 2014 [31] | USA | Cross-sectional | Recruited control group of HIV-uninfected controls (N=15) | 30/15/45 | 100 | Mean (SD): 22.5 (0.3) | 60% B 40% H |
DXA (Hologic): LS BMC, LS BMD, FN BMC, FN BMD, TH BMC, TH BMD, R13 BMC, R13 BMD, UD BMC, UD BMD HR peripheral QCT (XtremeCT Scanco): radius CSA, radius vBMD, radius microarchitecture, tibia CSA, tibia vBMD, tibia microarchitecture |
|
Abbreviations: A, asian; ART, antiretroviral therapy; B, black; BD, bone density; BTT, bone transmission time; CSA, cross sectional area; BMC, bone mineral content; BMD, bone mineral density; BUA, broadband ultrasound attenuation; DXA, dual X-ray absorptiometry; FN, femoral neck; H, hispanic/latino; HR, high-resolution; IQR, interquartile range; LS, lumbar spine; NA, not applicable; NHANES, national health and nutrition examination survey; NNRTI, non-nucleoside reverse transcriptase inhibitor; NS, not specified; O, other; QCT, quantitative computed tomography; QUS, quantitative ultrasound; PI, protease inhibitor; PTH, parathyroid hormone; R13, 1/3 distal radius; SD, standard deviation; SOS, speed of sound; TH, total hip; UDR, ultradistal radius; WB, whole body; W, white
A total of 17 cross sectional and 15 longitudinal studies were identified (Table 2). Among cross-sectional studies, 16 reported significant decreases in one or more bone measures in those with HIV including lower BMC and BMD, both WB as well as LS. Some, but not all, reported bone outcomes adjusted for variables such as age, sex, race/ethnicity, weight, height, body composition, and pubertal status. A recent study by DiMeglio et al. observed that Z-score differences between those with HIV and the comparison group were attenuated after adjusting for sex, race/ethnicity, weight, height, and puberty stage [30], which was similar to an earlier study which adjusted for sex, weight, and bone area [34]. Jacobson et al. found that reductions in bone mass were most marked among boys who achieved biological maturation [6]. Sex differences, however, were not confirmed in a recent study conducted in Brazil [48].
A longitudinal study of 32 HIV-infected children aged 6.3 to 17.7 years on long-term ART observed that although WB BMD increased over time, the annual increment was less in those with HIV compared to healthy controls [35]. A small prospective study of 18 perinatally HIV-infected children (mean age 11.3 years) primarily on ART (>80%) found that while all healthy control subjects had increased or stable WB BMD over a 1–3 year period, this was true for only 44% of the HIV-infected group (p=0.09) [24]. A larger study with 66 HIV-infected subjects, median age 6.7 years, with DXA assessments at 2–3 year intervals reported improved LS and FN BMD Z-scores during follow up [46]. A study of older perinatally-infected children ages 11–16 years (mean age 13.6 years) at baseline with 1 and 2 year DXA assessments found a similar result with BMD [45]. Declines in BMD have also been reported in several studies and appear to be primarily associated with changes in ART [23, 26, 50].
Although osteoporosis, i.e. low bone mass and fragility fractures [53], is not reported in children and adolescents with HIV, increased prevalence of low BMD (Z-score ≤−2.0) alone was reported in 6 studies from high and middle-income countries including Italy [30], the Netherlands [46], the United States [25], Brazil [48, 49], and Thailand [47]. In a study of 101 HIV-infected Thai adolescents ages 12–20 years, 24% met criteria for low WB BMD [47]. Studies conducted in Brazil reported low WB and or LS BMD in 32% of 74 perinatally HIV-infected children (mean age 17.3 years) [49] and low WB BMD in 17% of 48 children (mean age 12.7 years) [48]. Much lower prevalence was observed by DiMeglio et al. who found in a study of 350 Italian children (mean age 12.6 years) that 7% had low WB BMD and 4% had low LS BMD compared to 1% for both WB and LS among HIV-uninfected children [30]. Similarly, in a smaller study of 66 perinatally HIV-infected children in the Netherlands (mean age 6.7 years) who were mainly receiving a nelfinavir-containing regimen, 8% had low LS BMD [46]. In these studies we did not observe patterns with respect to sex, race, age group, or anatomic site and prevalence of low BMD.
Reflecting the epidemiology, there are few studies of BMD in children and adolescents with HIV infection acquired by means other than mother-to-child transmission [7, 31, 38]. In a study of 16 ART-naïve children and adolescents (mean age 9.3±3.9 years) with recently acquired HIV (mean duration of infection 2.8 years) due to contaminated blood products, no differences in LS or WB BMC were observed compared to controls, adjusted for sex, weight, and bone area [38]. HIV acquired via sexual transmission during adolescence or young adulthood may have severe impact on bone integrity. DXA Z-scores were lower and bone microarchitecture by high resolution peripheral QCT of the radius and tibia was abnormal in young men ages 20–25 who were infected during adolescence than uninfected controls [31]. BMD and microarchitectural indices in these adolescence-infected men were similar to those of perinatally-infected men despite marked differences in the duration of HIV infection and ART exposure [31]. In addition, Mulligan et al. reported on 199 young men (ages 14–25 years) soon after acquiring HIV infection by sexual transmission, finding lower WB BMC Z-scores among those on ART [7].
Bone mass decrements among those with perinatally acquired HIV may become more pronounced with increasing age and biologic maturation [6, 22, 24] although this was not a consistent finding [8]. Initial studies suggest that peak bone mass, the maximum amount of bony tissue at the end of skeletal maturation is lower among those who acquire HIV perinatally or during adolescence [31].
Calculated vBMD from DXA (BMAD)
Three of the published studies estimated the LS vBMD from DXA output, with each applying a different method for calculating vertebral body volume [6, 26, 34]. This reflects the lack of consensus on assumptions regarding underlying bone geometry, making it difficult to directly compare results. Calculated vBMD derived by dividing the DXA-measured LS BMC obtained by the lateral scan by the body vertebral volume, was found by Zamboni et al. to be reduced in 6 out of 13 patients (mean age 7.8 years); however, the source of comparison was not specified [34]. When compared to longitudinal data obtained in healthy Canadian children, BMAD calculated as described by Carter et al. [LS BMC/(LS area)1.5] among 15 HIV-infected children of unspecified race was reduced (median −0.9, range −3.2–12) [26, 54]. Jacobson et al. used the formula by Katzman et al. [55] to estimate LS BMAD, and the findings and conclusions were the same as those with LS BMD (Table 2) [6].
Measured vBMD
Studies that directly measured vBMD (g/cm3) had variable findings. Pitukcheewanont et al. used QCT in addition to DXA in a study of 58 HIV-infected Thai children and adolescents (age 5–19 years) and age-matched healthy controls [25]. Areal BMD measured by DXA correlated only moderately well with vBMD by QCT (r2=0.302). Although HIV-infected children and adolescents had lower aBMD and aBMC by DXA compared to uninfected controls, no difference in vBMD by CT was observed between groups, underscoring the importance of bone size [25].
Other measurement methods
Two recent studies used peripheral QCT to assess cortical and trabecular thicknesses and vBMD as well as indices of bone strength [31, 45]. In a study by Macdonald et al., no differences in total and cortical bone area Z-scores at the tibia were observed between 31 HIV-infected subjects ages 9–18 years and a database of healthy subjects; however, ethnic-specific norms were not available [45]. Interestingly, cortical vBMD appeared to be higher in those with HIV and was positively associated with non-nucleoside reverse transcriptase inhibitor (NNRTI) use. In addition, cortical thickness was negatively associated with PI use. In contrast, Yin et al. found that total and trabecular vBMD and cortical and trabecular thickness at both the radius and tibia were between 6 and 19% lower in young men ages 20–25 years with HIV acquired perinatally or during adolescence than healthy age-matched controls using high resolution peripheral QCT. The authors point out that these differences are similar to the magnitude observed among post-menopausal women with fractures compared to those without fractures [56]. This study also used high resolution peripheral QCT to evaluate the orientation and characteristics of plate and rod trabecular elements and revealed that HIV-infected men had significant deficiencies in plate-related parameters and 14–17% lower bone stiffness, an accepted measure of bone strength [57, 58].
QUS, which estimates bone density based on the characteristics of ultrasound wave transmission through bone, has also been used in several published studies of HIV-infected children, adolescents, and young adults [36, 39, 41]. QUS devices are portable, simple to operate and do not involve exposure to radiation and may prove to be an attractive alternative for research in low-resource countries where DXA may not be available. QUS devices can evaluate the cortex of long bones at the tibia, radius or phalanges, which are largely comprised of cortical or compact bone or the calcaneus which is mainly trabecular or cancellous in nature. In a study that included children 3–17 years of age the mean phalangeal SOS (m/s), the travel time of sound waves through the region of interest, adjusted for age, skeletal age, height, and BMI was significantly lower in those with HIV infection compared to healthy controls [39]. BUA (dB/MHz), the differential attenuation of sound waves transmitted through bone, has also been reported to be lower in HIV-infected children with severe disease, but not those with mild-moderate disease compared to healthy controls [36]. Although the physical properties measured by QUS and DXA are not identical, they appear to be well correlated in children with HIV [41]. Mora et al. observed that radial SOS was significantly correlated with LS and WB BMC and BMD (R=0.57–0.60) after corrections for sex, weight, and height, and that tibial SOS related to all DXA BMC and BMD measurements (R=0.58–0.66). Z-scores by QUS (tibial and radial) and DXA did not differ for WB BMC and WB BMD but LS BMC and BMD Z-scores were significantly lower than those obtained by radial and tibial QUS as might be expected given the greater proportion of cortical bone in WB compared to the spine [41]. In addition, DXA and QUS yielded comparable results for identification of those with <−2.0 Z-score.
Risk Factors
Several factors traditionally associated with low bone mass were identified in published studies involving HIV-infected children and adolescents, including low weight and height for age, and delayed biologic maturation [24, 30, 47]. We identified two studies that evaluated the relationships between lean body mass and bone [24, 45]. MacDonald et al. reported that lean mass Z-score was lower in subjects with HIV compared to the reference group and was significantly associated with WB BMC as well as FN BMC and peripheral femur BMC but not LS BMC [45]. Of interest, despite lower muscle cross-sectional area, a proxy of muscle force, total and cortical bone area Z-scores were not significantly different from healthy controls, suggesting an intact relationship between muscle force and bone mass. Jacobson et al. also reported a positive correlation between arm muscle circumference and WB BMD; however this finding was not sustained in multivariable analysis [24]. In addition, there is evidence that lipodystrophy, which refers to a number of abnormalities in regional fat distribution that occurs in children as well as adults with HIV, is associated with lower BMC and BMD [33].
In a study conducted in the United States in which calcium intake was 20–50% below the recommended intake, O’Brien et al. reported evidence that inadequate dietary intake of calcium contributed to reductions in bone mass [21]. This is in contrast to a recent Brazilian study in which calcium intake did not significantly influence bone mass [48]. Inadequate intake of vitamin D and vitamin D deficiency (i.e. low plasma concentrations of 25-hydroxyvitamin D) are also reported to be highly prevalent but neither has been established as a significant risk factor for low bone mass [24, 47]. Further, a 2-year randomized placebo controlled trial of vitamin D and calcium supplementation failed to detect improvements in bone mass despite achieving adequacy in plasma concentrations of 25-hydroxyvitamin D among those receiving supplementation [28, 59].
Assessments of physical activity, an important determinant of muscle mass on bone accrual, have not been extensively conducted. Though DiMeglio et al. reported greater (self-reported on questionnaire) physical activity to be associated with higher LS BMD, Lima et al. did not find bone indices to be significantly associated with accelerometer-assessed levels of physical activity [30, 48]. Mulligan et al. also found no statistically significant association between low bone mass and regular exercise measured by questionnaire [7]. A longitudinal study by Mora et al. found that in HIV-infected children, annual incremental changes in leg BMD were comparable to healthy children whereas arm BMD, a component of WB BMD, was reduced, pointing towards a potential for weight-bearing physical activity to be important for bone development in children with HIV [35].
The question of whether HIV per se negatively affects bone mass during childhood was addressed by 4 studies. Three relatively small studies that evaluated bone mass accrual among treatment-naïve perinatally HIV-infected children and adolescents report no deficits in WB or LS BMD compared to healthy controls [25, 33, 38]. Similarly, no short-term differences in bone measures were detected among treatment-naïve and demographically similar seronegative subjects and199 relatively recently horizontally infected males ages 14–25 years (mean time since diagnosis 1.3 years) [7].
Among those living with HIV, associations between bone measures and plasma HIV RNA concentration (negative), CD4 count (positive), and stage of HIV (lower with advanced disease) are reported across many studies [24, 30, 47, 51]. However, study results vary on some of these factors; several studies found no association between advanced stage of HIV and bone measures [21, 35, 48] while others found a negative association [24, 36]. In a study of HIV-infected children treated at single hospital in the Netherlands with repeated DXA scans, those with higher CD4% at the time of the scan had higher LS BMD Z-scores and those with a higher plasma HIV RNA concentration had lower LS BMD Z-scores [46]. BMD was also positively correlated with CD4 count in a recent study conducted by MacDonald et al. [45].
Exposure to antiretroviral medications was evaluated in included studies. Two identified in our search [7, 26] found decreases in BMD by 2–6% following initiation of treatment-naïve patients on ART regimens [7] as well as treatment-experienced patients switching to new drug regimens due to poorly controlled HIV [26]. In a study of recently horizontally infected young men on treatment for <6 months, Mulligan et al. reported both lower WB BMD and Z-score WB BMC. Those on both NNRTI and protease inhibitor-containing combination ART regimens had lower WB BMC Z-scores than HIV-uninfected controls [7]. Only those on protease inhibitor-containing ART had lower BMD (hip, FN, trochanter, and Ward’s) than the ART naïve group (e.g. those on NNRTI therapy did not differ from naïve) [7]. Protease inhibitors, especially older formulations, are the most extensively evaluated. in published studies. Some, but not all studies in which categorical analyses of exposure to any drug in the protease inhibitor class, reported associations with decreased bone acquisition [7, 21, 24, 30, 35, 44]. A report from Bunders et al., however, indicates that different protease inhibitor agents may affect bone development differently; for example, nelfinavir was positively associated with bone mass indices while ritonavir-boosted lopinavir was negatively associated [46]. An association between use of ritonavir or ritonavir-boosted protease inhibitors and lower measures of bone mass has also been reported in other publications [6, 30, 44, 48]. Studies involving subjects on established ART, reported lower BMC in children related to exposure to drugs in the nucleoside reverse transcriptase inhibitor class including stavudine [44, 46] and zidovudine [6] as well as higher BMD associated with NNRTI drug class such as nevirapine [6, 24].
Some, but not all studies indicate that initiation of tenofovir adversely affects bone acquisition in HIV-infected youth, although there may be less impact among those who are virologically controlled at the time of switch compared to those who are switched under less optimal conditions. Schtscherbyna et al. reported lower LS and WB BMD Z-scores among those on TDF, and the length of time of TDF was indirectly correlated with LS and WB BMD Z-score [49]. A non-comparative study reported a decline in BMD of >6% at the FN, TH, and LS, during the initial 24–48 weeks in 6 of 15 treatment experienced children ages 12–16 years who were switched to tenofovir [26]. However, in a 48 week randomized trial involving 90 adolescents ages 12–18 years on failing ART regimens who were switched to optimized background regimen with and without tenofovir demonstrated a decline in LS and WB BMD in both groups during the first 24 weeks after randomization but no group difference in mean spine or WB BMD Z-score at 48 weeks. In addition, 18% of children switched to a tenofovir-containing regimen experienced a significant decline in LS BMD (>4%) as compared to only 3% switched to regimens without tenofovir; however, these differences were not statistically significant [50]. Recent data also suggest that there are adverse effects on bone mass and early growth in HIV-uninfected infants with fetal exposure to tenofovir used as either part of maternal combination ART or as prophylaxis against mother to child transmission, providing additional evidence of a possible toxic effect of tenofovir to bone development [60, 61].
In addition, the duration of antiretroviral exposure is also significantly associated with bone mass measures in children and adolescents but not all studies agree on the direction. In a longitudinal study in the Netherlands, duration of ART was associated with an increase in LS BMD Z-score (coefficient, 0.08; P <0.001); this association remained after adjusting for BMI, CD4% and plasma HIV RNA concentration [46]. DiMeglio et al. found that LS BMD Z-scores was associated with more years on HAART (−0.03 per year, p=0.14) [30].
No histomorphometric studies have been conducted in HIV-infected children or adolescents; therefore, evaluations of pathogenic mechanisms in the studies in this review have relied on correlations with biochemical markers. Several studies found elevated concentrations of bone turnover markers (e.g. bone formation and resorption) among HIV-infected children and adolescents [33, 34, 43, 62], and were interpreted to be indicative of increased bone remodeling. Mora et al. found that HIV-infected children on ART had higher concentrations of bone alkaline phosphatase (BALP), a bone formation marker, and N-telopeptide (NTX), a bone resorption marker, than untreated HIV-infected children and HIV-uninfected children [33]. In contrast, Tan et al. reported higher bone formation, as measured by osteocalcin, in HIV-infected children than controls and lower resorption measured by carboxyterminal telopeptide of type I collagen (ICTP) in HIV-infected children on protease inhibitors (PIs) [62].
Stagi et al. reported that reduced bone mass and quality in HIV-infected children were related to reduced IGF-1, a potent modulator of osteoblast-osteoclast interactions [63], longitudinal bone growth and bone mass acquisition during childhood [64, 65], and possibly related to overproduction of IL-6 [36], similar to findings from an earlier, smaller study [34]. Finally, an increased incidence of osteonecrosis of the hip (i.e. Legg-Calvé-Perthes disease) is reported in one study [32]. A single study evaluating the risk of fracture in HIV-infected children did not detect an increase in fracture rate [29]. This study compared rates of fracture in a cohort of 1326 HIV-infected and 649 HIV-uninfected children with a mean age of 5.8–7.1 with a median of 2.26–4.97 years and found similar rates of fracture in the HIV-infected and uninfected groups (1.2 vs. 1.1 per 1000 person-years) [29].
DISCUSSION
We systematically reviewed the literature to identify studies that reported on bone imaging or bone fractures in HIV-infected children, adolescents, or young adult. Although the studies vary greatly with respect to comparison sources, adjustment techniques for body size or growth retardation, and highlighted risk factors, most studies we reviewed found that measures of bone mass are reduced in children and adolescents with HIV. Reductions in bone mass are reported in association with antiretroviral medications, with specific antiretroviral classes and agents such as protease inhibitors and tenofovir appearing more prominently in the literature than other classes or agents, but study results are inconsistent.
The heterogeneity with respect to the sources used for comparison in the published studies poses a problem for assessing the severity of deficits in bone mass acquisition and the extent to which the observed reductions in bone mass may be attributable to factors other than HIV known to be important to bone accrual [66], such as small size for age and delayed biologic maturation which frequently accompany HIV infection in children and adolescents. It also limits comparison of findings across studies. There does not appear to be a single optimal comparison group and the best choice may vary with the questions being asked. For example, while inclusion of siblings of HIV-infected children as controls may be optimal for race/ethnicity, genetics, and other factors, this approach may not provide the best estimation of the status of bone mass as compared to the overall population. For example, Jacobson et al. found that children with HIV had lower WB BMD than expected compared to a normative database but no difference when compared to siblings [24]. Another potential problem with this approach is that some uninfected siblings may have had intrauterine exposure to antiretrovirals and the inflammatory environment of maternal HIV infection. Use of large longitudinal normative databases that have recently become available [67–69] as well as inclusion of additional healthy control subjects recruited for study purposes can overcome these limitations. The studies included in this review also differed in the approaches used for adjusting for bone size, height, lean body mass, skeletal age, and degree of biologic maturation. DXA does not measure the anteroposterior diameter of bone and provides only a two-dimensional areal estimate of BMD (aBMD, g/cm2), which may underestimate true volumetric BMD (vBMD, g/cm3) particularly in children with impaired growth or maturational delays [70]. The International Society for Clinical Densitometry advises that in children with short stature, whole body BMC and aBMD results should be adjusted using height-for-age Z-score (HAZ), and that either BMAD or HAZ should be used for LS results [71]. The literature we reviewed used a number of different approaches to account for skeletal size and maturational delays. Age, sex, height, weight, BMI, race, and pubertal stage were variably included and several studies adjusted for bone age [25, 38]. Only Jacobson et al. included HAZ which in healthy children is the strategy least likely to result in biased estimates of BMC and BMD in older, shorter children (i.e. HAZ <−1.0) [66].
Although in vitro studies suggest that HIV-specific proteins alter osteoblast function by means of disrupting the differentiation of precursor stem cells into osteoblasts, stimulating osteoblast apoptosis and altering osteoblast activity, evidence from clinical studies to support a direct role for HIV is limited, and distinguishing direct effects of HIV from those due to inflammation poses a challenge. HIV infection is characterized by chronic immune activation with polyclonal B cell activation, production of cytokines and increased T cell activation and turnover [72, 73].
Chronic immune activation is among the putative causes of bone loss in adults with HIV but has not been carefully studied in children and adolescents. Despite effective ART and viral suppression, the proportion of activated (CD38+) T cells and serum pro-inflammatory cytokines remains elevated [74–78]. Chronic immune activation and increased levels of pro-resorptive cytokines like TNFα, IL-6 and RANKL are thought to increase osteoclastic resorption. In addition, some antiretroviral agents may also reduce osteoblast activity via induction of inflammatory activity [9]. If osteoblast-mediated bone formation is unable to match or exceed resorption during periods of growth, then inadequate bone acquisition could result. The role of persistent residual immune activation despite viral suppression on bone formation and resorption during growth is an additional important line of investigation.
While reductions in bone mass linked to antiretroviral agents are reported, there are inconsistencies in the studies under review. Some of the inconsistencies regarding ART exposures and bone measures in the studies under review may be attributed to variability in time elapsed between ART initiation or change in ART regimen and the ascertainment of bone measurement. Evidence from studies conducted in adults [79–83], as well as studies identified in our search [7, 26], indicate that BMD declines during initial phases of treatment in relation to a number of different ART regimens. In addition, as antiretroviral drugs are ordinarily used in combination and often sequentially, attributing a specific bone outcome finding to a single drug is difficult in an observational study. Further, many of these observational studies have low power to detect differences in bone accrual by treatment regimen due to the inclusion of small numbers of subjects on a multiplicity of drug combinations [33, 44]. In some studies, specific drug agents were assessed separately while in others analyses were conducted using all members of a pharmacologic class, which may contribute to inconsistent results in the literature. Several antiretroviral agents may be of particular clinical and public health importance due to their potential impact on bone metabolism and their widespread use and warrant further study. Ritonavir-boosted protease inhibitors, among the more widely used medications for children and adolescents, is associated with declines in BMD. In addition, a meta-analysis of adult studies that revealed an association between protease inhibitor use and low BMD and in vitro studies suggesting that ritonavir alters osteoclast as well as osteoblast gene expression resulting in dysregulation of cell differentiation, expression of growth factors, and enzymes that favor bone resorption [8, 9, 84] provides additional rationale to investigate further. Questions also remain regarding short- and long-term effects of tenofovir, another important drug agent increasingly used in children and adolescents, on bone. There are several potential mechanisms for adverse bone effects for tenofovir. In vitro studies suggest that tenofovir may have a direct effect on bone formation by affecting osteoblast proliferation and increasing apoptosis [85], and clinical studies suggest that tenofovir induces functional vitamin D deficiency [86].
Although disturbances in body composition in pediatric HIV are well described, the relationships between fat or lean tissue compartments and bone mass have not been evaluated extensively [87, 88]. In light of the potential importance of increased muscle forces and mechanical loading on bone development [89], assessments of the role of physical activity warrant further examination as this may have therapeutic implications.
As presented in Figure 2, possible increases in osteoporosis and fracture later in life is of far greater concern associated with impaired bone acquisition early in life. The accrual of bone mass takes place from fetal life all the way through early adulthood, and the majority (95%) of PBM is attained by young adulthood. One recent study suggests that those who acquire HIV early in life do not achieve optimal PBM [31]. PBM is a key predictor of osteoporosis and fracture risk [15]. Mathematical models indicate that relatively small increases (10%) in PBM acquisition in healthy females could delay onset of osteoporosis by as many as 13 years [90]. The reductions in PBM and microarchitectural abnormalities observed by Yin et al. may have important implications for increasing future risk of fracture and requires further study.
Figure 2. Hypothetical evolution of bone acquisition with HIV infection early in life.
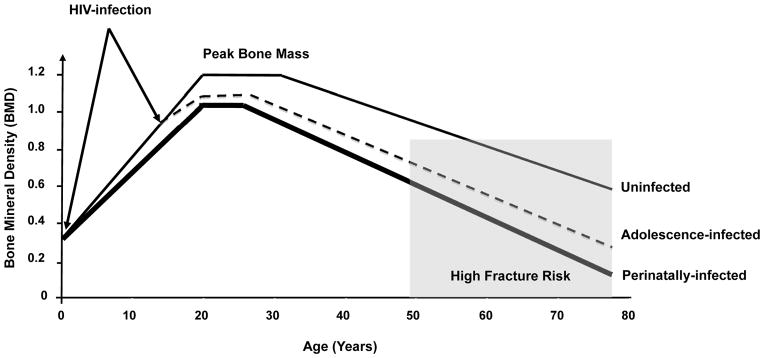
Adapted from Heaney, Osteoporosis Int., 2000
There is a substantial gap in studies from low- and middle-income countries, where the majority of children with HIV live, but where access to bone imaging by DXA is limited. Alternative methods such as QUS could facilitate expansion of bone-related research in these settings. There is also a paucity of evaluation of interventions for optimizing bone accrual among HIV-infected children and adolescents either by selection of “bone sparing” ART, maximizing weight bearing physical activity. Although results of one study of vitamin D and calcium supplementation in children and adolescents did not find an effect [59], vitamin D supplementation may prove useful as a specific means to prevent bone loss with tenofovir exposure [86].
There are a number of limitations to this review. The search was limited to three databases and therefore may not have been comprehensive. In addition, the search criteria may have inadvertently excluded relevant publications. As discussed, heterogeneity across studies made comparisons challenging.
There is a strong need for further, high-quality studies, particularly in resource-constrained settings. Studies to determine fracture risk as well as to mitigate the adverse effects of HIV infection, co-morbid conditions and associated therapeutics and to optimize bone accrual during childhood and adolescence are timely and important for the over 8 million children and youth currently living with HIV worldwide.
References
- 1.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 4.Young B, Dao CN, Buchacz K, Baker R, Brooks JT Investigators HIVOS. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52:1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 5.Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS. 2013;27:1949–1957. doi: 10.1097/QAD.0b013e328361d241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson DL, Lindsey JC, Gordon CM, Moye J, Hardin DS, Mulligan K, et al. Total body and spinal bone mineral density across Tanner stage in perinatally HIV-infected and uninfected children and youth in PACTG 1045. AIDS. 2010;24:687–696. doi: 10.1097/QAD.0b013e328336095d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulligan K, Harris DR, Emmanuel P, Fielding RA, Worrell C, Kapogiannis BG, et al. Low bone mass in behaviorally HIV-infected young men on antiretroviral therapy: Adolescent Trials Network Study 021B. Clin Infect Dis. 2012;55:461–468. doi: 10.1093/cid/cis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150:67–78. doi: 10.1007/s00705-004-0395-7. [DOI] [PubMed] [Google Scholar]
- 9.Malizia AP, Cotter E, Chew N, Powderly WG, Doran PP. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts. AIDS Res Hum Retroviruses. 2007;23:243–250. doi: 10.1089/aid.2006.0084. [DOI] [PubMed] [Google Scholar]
- 10.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 11.Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, et al. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest. 2004;114:206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 13.Cotter EJ, Chew N, Powderly WG, Doran PP. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res Hum Retroviruses. 2011;27:187–199. doi: 10.1089/aid.2010.0114. [DOI] [PubMed] [Google Scholar]
- 14.Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. The Journal of infectious diseases. 2012;205(Suppl 3):S391–398. doi: 10.1093/infdis/jis199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 16.Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15:2245–2250. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 17.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 18.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. Jama. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 19.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 20.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. 2013 [Google Scholar]
- 21.O’Brien KO, Razavi M, Henderson RA, Caballero B, Ellis KJ. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr. 2001;73:821–826. doi: 10.1093/ajcn/73.4.821. [DOI] [PubMed] [Google Scholar]
- 22.Arpadi SM, Horlick M, Thornton J, Cuff PA, Wang J, Kotler DP. Bone mineral content is lower in prepubertal HIV-infected children. J Acquir Immune Defic Syndr. 2002;29:450–454. doi: 10.1097/00126334-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hazra R, Gafni RI, Maldarelli F, Balis FM, Tullio AN, DeCarlo E, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics. 2005;116:e846–854. doi: 10.1542/peds.2005-0975. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson DL, Spiegelman D, Duggan C, Weinberg GA, Bechard L, Furuta L, et al. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr. 2005;41:339–346. doi: 10.1097/01.mpg.0000174468.75219.30. [DOI] [PubMed] [Google Scholar]
- 25.Pitukcheewanont P, Safani D, Church J, Gilsanz V. Bone measures in HIV-1 infected children and adolescents: disparity between quantitative computed tomography and dual-energy X-ray absorptiometry measurements. Osteoporos Int. 2005;16:1393–1396. doi: 10.1007/s00198-005-1849-9. [DOI] [PubMed] [Google Scholar]
- 26.Gafni RI, Hazra R, Reynolds JC, Maldarelli F, Tullio AN, DeCarlo E, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–718. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 27.Purdy JB, Gafni RI, Reynolds JC, Zeichner S, Hazra R. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J Pediatr. 2008;152:582–584. doi: 10.1016/j.jpeds.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arpadi SM, McMahon DJ, Abrams EJ, Bamji M, Purswani M, Engelson ES, et al. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. Am J Clin Nutr. 2012;95:678–685. doi: 10.3945/ajcn.111.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siberry GK, Li H, Jacobson D. Fracture risk by HIV infection status in perinatally HIV-exposed children. AIDS Res Hum Retroviruses. 2012;28:247–250. doi: 10.1089/aid.2011.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiMeglio LA, Wang J, Siberry GK, Miller TL, Geffner ME, Hazra R, et al. Bone mineral density in children and adolescents with perinatal HIV infection. AIDS. 2013;27:211–220. doi: 10.1097/QAD.0b013e32835a9b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin MT, Lund E, Shah J, Zhang CA, Foca M, Neu N, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. Aids. 2014;28:345–353. doi: 10.1097/QAD.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaughan DM, Mofenson LM, Hughes MD, Seage GR, 3rd, Ciupak GL, Oleske JM. Osteonecrosis of the hip (Legg-Calve-Perthes disease) in human immunodeficiency virus-infected children. Pediatrics. 2002;109:E74–74. doi: 10.1542/peds.109.5.e74. [DOI] [PubMed] [Google Scholar]
- 33.Mora S, Sala N, Bricalli D, Zuin G, Chiumello G, Vigano A. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2001;15:1823–1829. doi: 10.1097/00002030-200109280-00011. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni G, Antoniazzi F, Bertoldo F, Lauriola S, Antozzi L, Tato L. Altered bone metabolism in children infected with human immunodeficiency virus. Acta Paediatr. 2003;92:12–16. doi: 10.1111/j.1651-2227.2003.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 35.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 36.Stagi S, Bindi G, Galluzzi F, Galli L, Salti R, de Martino M. Changed bone status in human immunodeficiency virus type 1 (HIV-1) perinatally infected children is related to low serum free IGF-I. Clin Endocrinol (Oxf) 2004;61:692–699. doi: 10.1111/j.1365-2265.2004.02150.x. [DOI] [PubMed] [Google Scholar]
- 37.Giacomet V, Mora S, Martelli L, Merlo M, Sciannamblo M, Vigano A. A 12-month treatment with tenofovir does not impair bone mineral accrual in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:448–450. doi: 10.1097/01.qai.0000184860.62189.c8. [DOI] [PubMed] [Google Scholar]
- 38.Mora S, Zamproni I, Giacomet V, Cafarelli L, Figini C, Vigano A. Analysis of bone mineral content in horizontally HIV-infected children naive to antiretroviral treatment. Calcif Tissue Int. 2005;76:336–340. doi: 10.1007/pl00020973. [DOI] [PubMed] [Google Scholar]
- 39.Rosso R, Vignolo M, Parodi A, Di Biagio A, Sormani MP, Bassetti M, et al. Bone quality in perinatally HIV-infected children: role of age, sex, growth, HIV infection, and antiretroviral therapy. AIDS Res Hum Retroviruses. 2005;21:927–932. doi: 10.1089/aid.2005.21.927. [DOI] [PubMed] [Google Scholar]
- 40.Mora S, Zamproni I, Cafarelli L, Giacomet V, Erba P, Zuccotti G, et al. Alterations in circulating osteoimmune factors may be responsible for high bone resorption rate in HIV-infected children and adolescents. AIDS. 2007;21:1129–1135. doi: 10.1097/QAD.0b013e32810c8ccf. [DOI] [PubMed] [Google Scholar]
- 41.Mora S, Vigano A, Cafarelli L, Pattarino G, Giacomet V, Gabiano C, et al. Applicability of quantitative ultrasonography of the radius and tibia in HIV-infected children and adolescents. J Acquir Immune Defic Syndr. 2009;51:588–592. doi: 10.1097/QAI.0b013e3181adc838. [DOI] [PubMed] [Google Scholar]
- 42.Rosso R, Parodi A, Torrisi C, De Terlizzi F, Viscoli C, Vignolo M. A tailored dose of tenofovir could reduce its impact on bone mass in HIV Type 1-infected children and adolescents: a report from 5 years of clinical experience. AIDS Res Hum Retroviruses. 2010;26:1265–1266. doi: 10.1089/aid.2010.0122. [DOI] [PubMed] [Google Scholar]
- 43.Vigano A, Zuccotti GV, Puzzovio M, Pivetti V, Zamproni I, Cerini C, et al. Tenofovir disoproxil fumarate and bone mineral density: a 60-month longitudinal study in a cohort of HIV-infected youths. Antivir Ther. 2010;15:1053–1058. doi: 10.3851/IMP1650. [DOI] [PubMed] [Google Scholar]
- 44.Zuccotti G, Vigano A, Gabiano C, Giacomet V, Mignone F, Stucchi S, et al. Antiretroviral therapy and bone mineral measurements in HIV-infected youths. Bone. 2010;46:1633–1638. doi: 10.1016/j.bone.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Macdonald HM, Chu J, Nettlefold L, Maan EJ, Forbes JC, Cote H, et al. Bone geometry and strength are adapted to muscle force in children and adolescents perinatally infected with HIV. J Musculoskelet Neuronal Interact. 2013;13:53–65. [PubMed] [Google Scholar]
- 46.Bunders MJ, Frinking O, Scherpbier HJ, van Arnhem LA, van Eck-Smit BL, Kuijpers TW, et al. Bone mineral density increases in HIV-infected children treated with long-term combination antiretroviral therapy. Clin Infect Dis. 2013;56:583–586. doi: 10.1093/cid/cis917. [DOI] [PubMed] [Google Scholar]
- 47.Puthanakit T, Saksawad R, Bunupuradah T, Wittawatmongkol O, Chuanjaroen T, Ubolyam S, et al. Prevalence and risk factors of low bone mineral density among perinatally HIV-infected Thai adolescents receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;61:477–483. doi: 10.1097/QAI.0b013e31826ea89b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima LR, Silva RC, de Giuliano IC, Sakuno T, Brincas SM, Carvalho AP. Bone mass in children and adolescents infected with human immunodeficiency virus. J Pediatr (Rio J) 2013;89:91–99. doi: 10.1016/j.jped.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Schtscherbyna A, Pinheiro MF, Mendonca LM, Gouveia C, Luiz RR, Machado ES, et al. Factors associated with low bone mineral density in a Brazilian cohort of vertically HIV-infected adolescents. Int J Infect Dis. 2012;16:e872–878. doi: 10.1016/j.ijid.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 50.Della Negra M, de Carvalho AP, de Aquino MZ, da Silva MT, Pinto J, White K, et al. A randomized study of tenofovir disoproxil fumarate in treatment-experienced HIV-1 infected adolescents. Pediatr Infect Dis J. 2012;31:469–473. doi: 10.1097/INF.0b013e31824bf239. [DOI] [PubMed] [Google Scholar]
- 51.Negredo E, Domingo P, Ferrer E, Estrada V, Curran A, Navarro A, et al. Peak bone mass in young HIV-infected patients compared with healthy controls. J Acquir Immune Defic Syndr. 2014;65:207–212. doi: 10.1097/01.qai.0000435598.20104.d6. [DOI] [PubMed] [Google Scholar]
- 52.Faulkner KG, Roberts LA, McClung MR. Discrepancies in normative data between Lunar and Hologic DXA systems. Osteoporos Int. 1996;6:432–436. doi: 10.1007/BF01629574. [DOI] [PubMed] [Google Scholar]
- 53.Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7:17–26. doi: 10.1385/jcd:7:1:17. [DOI] [PubMed] [Google Scholar]
- 54.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 55.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 56.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 57.Liu XS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006;21:1608–1617. doi: 10.1359/jbmr.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu XS, Shane E, McMahon DJ, Guo XE. Individual trabecula segmentation (ITS)-based morphological analysis of microscale images of human tibial trabecular bone at limited spatial resolution. J Bone Miner Res. 2011;26:2184–2193. doi: 10.1002/jbmr.420. [DOI] [PubMed] [Google Scholar]
- 59.Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nurutdinova D, Onen NF, Hayes E, Mondy K, Overton ET. Adverse effects of tenofovir use in HIV-infected pregnant women and their infants. Ann Pharmacother. 2008;42:1581–1585. doi: 10.1345/aph.1L083. [DOI] [PubMed] [Google Scholar]
- 61.Siberry GK, Jacobson DL, Kalkwarf HJ. Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate. Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MA. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan BM, Nelson RP, Jr, James-Yarish M, Emmanuel PJ, Schurman SJ. Bone metabolism in children with human immunodeficiency virus infection receiving highly active anti-retroviral therapy including a protease inhibitor. J Pediatr. 2001;139:447–451. doi: 10.1067/mpd.2001.117005. [DOI] [PubMed] [Google Scholar]
- 63.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 64.Yanovski JA, Sovik KN, Nguyen TT, Sebring NG. Insulin-like growth factors and bone mineral density in African American and White girls. J Pediatr. 2000;137:826–832. doi: 10.1067/mpd.2000.109151. [DOI] [PubMed] [Google Scholar]
- 65.Zofkova I. Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol Res. 2003;52:657–679. [PubMed] [Google Scholar]
- 66.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Hangartner TN, et al. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95:1690–1698. doi: 10.1210/jc.2009-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 69.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majaliwa ES, Mohn A, Chiarelli F. Growth and puberty in children with HIV infection. J Endocrinol Invest. 2009;32:85–90. doi: 10.1007/BF03345686. [DOI] [PubMed] [Google Scholar]
- 71.Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 73.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 74.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 76.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 77.Lichtfuss GF, Hoy J, Rajasuriar R, Kramski M, Crowe SM, Lewin SR. Biomarkers of immune dysfunction following combination antiretroviral therapy for HIV infection. Biomark Med. 2011;5:171–186. doi: 10.2217/bmm.11.15. [DOI] [PubMed] [Google Scholar]
- 78.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 80.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 81.van Vonderen MG, Lips P, van Agtmael MA, Hassink EA, Brinkman K, Geerlings SE, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 2009;23:1367–1376. doi: 10.1097/QAD.0b013e32832c4947. [DOI] [PubMed] [Google Scholar]
- 82.Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 83.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem. 2002;277:19247–19250. doi: 10.1074/jbc.C200069200. [DOI] [PubMed] [Google Scholar]
- 85.Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Carlson AE, Mansky KC. Tenofovir treatment of primary osteoblasts alters gene expression profiles: implications for bone mineral density loss. Biochem Biophys Res Commun. 2010;394:48–53. doi: 10.1016/j.bbrc.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Havens PL, Stephensen CB, Hazra R, Flynn PM, Wilson CM, Rutledge B, et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis. 2012;54:1013–1025. doi: 10.1093/cid/cir968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arpadi SM, Horlick MN, Wang J, Cuff P, Bamji M, Kotler DP. Body composition in prepubertal children with human immunodeficiency virus type 1 infection. Arch Pediatr Adolesc Med. 1998;152:688–693. doi: 10.1001/archpedi.152.7.688. [DOI] [PubMed] [Google Scholar]
- 88.Arpadi S, Shiau S, Strehlau R, Martens L, Patel F, Coovadia A, et al. Metabolic abnormalities and body composition of HIV-infected children on Lopinavir or Nevirapine-based antiretroviral therapy. Arch Dis Child. 2013;98:258–264. doi: 10.1136/archdischild-2012-302633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frost HM. Muscle, bone, and the Utah paradigm: a 1999 overview. Med Sci Sports Exerc. 2000;32:911–917. doi: 10.1097/00005768-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]