Abstract
Introduction
Studies of cerebral hemodynamics during motor learning have mostly focused on neurorehabilitation interventions and their effectiveness. However, only a few imaging studies of motor learning and the underlying complex cognitive processes have been performed.
Methods
We measured cerebral hemodynamics using near-infrared spectroscopy (NIRS) in relation to acquisition patterns of motor skills in healthy subjects using character entry into a touch-screen terminal. Twenty healthy, right-handed subjects who had no previous experience with character entry using a touch-screen terminal participated in this study. They were asked to enter the characters of a randomly formed Japanese syllabary into the touch-screen terminal. All subjects performed the task with their right thumb for 15 s alternating with 25 s of rest for 30 repetitions. Performance was calculated by subtracting the number of incorrect answers from the number of correct answers, and gains in motor skills were evaluated according to the changes in performance across cycles. Behavioral and oxygenated hemoglobin concentration changes across task cycles were analyzed using Spearman’s rank correlations.
Results
Performance correlated positively with task cycle, thus confirming motor learning. Hemodynamic activation over the left sensorimotor cortex (SMC) showed a positive correlation with task cycle, whereas activations over the right prefrontal cortex (PFC) and supplementary motor area (SMA) showed negative correlations.
Conclusions
We suggest that increases in finger momentum with motor learning are reflected in the activity of the left SMC. We further speculate that the right PFC and SMA were activated during the early phases of motor learning, and that this activity was attenuated with learning progress.
Introduction
Humans accomplish a wide variety of motor and cognitive tasks in everyday life. Furthermore, we continue acquiring new complex motor skills to overcome various challenges. Therefore, it is important to study the underlying changes in cerebral hemodynamics during motor-cognitive adaptation. In recent years, various functional brain imaging techniques that enable noninvasive visualization of brain activity have advanced greatly [1–8]. Cerebral dynamics during motor-cognitive task adaptations have been investigated by employing various neuroimaging techniques, such as positron emission tomography (PET) or functional magnetic resonance imaging (fMRI), while the subject is in a supine position [9–14]. However, in daily life, motor-cognitive adaptation tasks are usually performed in upright positions such as sitting or standing. To address the issue of limited ecological validity and enable task performance in a more natural setting, we used near-infrared spectroscopy (NIRS) as a less-limiting imaging method that enables measurement of cortical activation during activities of daily life.
Although NIRS is characterized by marked limitations in temporal and spatial resolution, its safety, non-restrictiveness, and portability enable broader and more flexible use than other brain imaging methods [15]. A number of studies have examined cerebral hemodynamics using NIRS during a wide variety of motor activities such as a walking or running [16–20], cycling [21–23], apple peeling [24], and finger tapping [25–28]. Additionally, researchers have examined cerebral hemodynamics during cognitive tasks such as trail making [29–32], the rock-paper-scissors game [33,34], maze navigation [35], and sequential finger touching [36,37].
To our knowledge, NIRS studies that examine motor skill learning with ongoing adaptation processes are rare. Two NIRS studies have reported changes of hemodynamics over time related to motor skill learning using eye-hand coordination in pursuit rotor [38] or target reaching tasks [39]. These NIRS studies evaluated specific sensorimotor tasks. However, these studies did not address changes in hemodynamic activity during motor learning of complex skills with concurrent cognitive processing such as working memory and executive function. Gentili et al. reported changes in cerebral hemodynamics as measured by NIRS during performance of a motor-cognitive adaptation task and demonstrated activation only in the prefrontal cortex (PFC) [40]. However, the NIRS probe in their study only covered the forehead. Therefore, no studies are available that examine the hemodynamic responses of other cortical regions during performance of motor-cognitive adaptation tasks using a NIRS system that also includes the posterior half-head.
Information technology has substantially affected modern society, and researchers concerned with neurorehabilitation should examine interventions relevant to the lifestyle of modern people. In recent years, the number of touch-screen terminal users has increased remarkably and smartphones have spread all over the world. eMarketer, a U.S. research company, speculated that the number of smartphone users will total 1.75 billion in 2014 and further increase to 2.5 billion in 2017 [41]. The operation of a smartphone requires flick inputs to create an email sentence in Japanese, and the entry of the required characters is difficult and will need to be considered in the field of neurorehabilitation in the future. However, there are no studies measuring cerebral hemodynamics related to motor skill learning that address the underlying complex cognitive processing in tasks such as character entry into a touch-screen terminal. Therefore, in this study, we examined cerebral hemodynamics using NIRS associated with acquisition patterns of motor-cognitive skills in healthy subjects using character entry into a touch-screen terminal. Based on previous studies, we hypothesized that the behavioral improvements resulting from adaptation would be accompanied by distinct patterns of activation over the various cortical regions.
Methods
Subjects
Twenty healthy subjects (9 men and 11 women; mean age, 27.5±5.5 years) participated in this study. All subjects were self-reported as right-handed (S1 Dataset). Exclusionary criteria included any medical illness affecting central nervous system function, psychiatric or neurological disorders, history of head trauma, or current substance abuse. None of the subjects had previous experience with character entry using a touch-screen terminal. Written informed consent was obtained from each subject. The study was approved by the local ethics committee of Nagasaki University Graduate School of Biomedical and Health Sciences. All of the experimental procedures were conducted in accordance with the Declaration of Helsinki.
Tasks and procedures
The subjects sat on a chair 80 cm away from a PC monitor (Epson LD1957S, Japan, 19-inch, resolution: 1024 × 768 pixels) (Fig 1A). All subjects performed the task with their right thumb for 15 s alternating with 25 s of rest for 30 repetitions (cycles 1 to 30) (Fig 1B). Gains in motor skills were evaluated according to the number of characters entered into the touch-screen terminal (Apple iPod Touch 4, Japan, 3.5-inch, resolution: 960×640 pixels) (Fig 1C). All subjects were asked to enter the characters of a randomly formed Japanese syllabary presented on a PC monitor, starting with the character from the upper left, into the touch-screen terminal as quickly as possible (Fig 1D). The character string in the monitor was constructed in five lines and nine rows, resulting in 45 characters. The characters of the randomly formed Japanese syllabary were changed every cycle (S1 Supporting Information). All subjects were asked to fixate on a single point at the center of the screen during rest and to stay relaxed. In English, the letter combinations “P Q R S” and “W X Y Z” involve a three-way operation in the upper, right, and left direction, whereas the input otherwise involves operation in two ways. In Japanese, the combinations “ya yu yo” and “wa wo n” are two-way operations in the right and left directions, whereas the input otherwise operates in four ways in the upper, lower, right, and left directions. Therefore, operation of these devices using Japanese input is more complicated than that using English input. The task of the present study was not simply a matter of entering as many characters as possible; the only characters that were meant to be entered were those displayed. There was thus a trade-off between the number of characters and the number of incorrect entries. Thus, the task performance of each subject was calculated by subtracting the number of errors from the number of correct answers in each cycle.
Fig 1. Experimental setup of touch-screen terminal task.
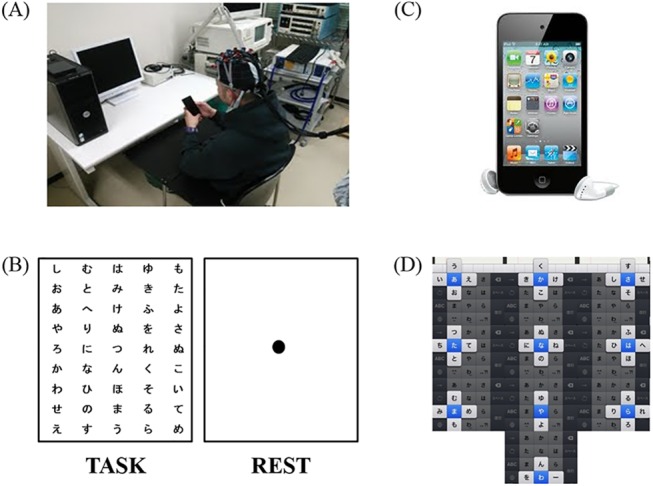
(A) Experimental setup of character entry into touch-screen terminal showing a subject with the custom-made cap with the NIRS device. (B) Display of task and rest conditions. The display showing the task condition changed with each cycle. (C) The iPod Touch 4. The character entry into screen mode was used to measure the number of character entries. All subjects performed the task with their right thumb for 15 s interleaved with rest periods of 25 s for 30 repetitions. (D) Methods of character entry into touch-screen terminal. All subjects were asked to enter the characters of a randomly formed Japanese syllabary into the touch-screen terminal.
NIRS measurements were performed using a continuous wave system (ETG-4000, Hitachi Medical Corp., Tokyo, Japan) equipped with 4 × 4 optode probe sets (8 incident light and 8 detector fibers), resulting in a total of 24 channels with an inter-optode distance of 3.0 cm. The continuous-wave NIRS system utilizes two different wavelengths (~625 and 830 nm). Relative changes in the absorption of near-infrared light were sampled at 10 Hz, and these measures were converted into related concentration changes for oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb), based on the modified Beer-Lambert approach [42]. The moving average method (window: 5 s) was used to exclude short-term motion artifacts in the analyzed data. The obtained data were analyzed in the integral mode, which calculates average waveform. Pre-task baseline was defined as the 5-s period immediately prior to task onset. In this study, we used changes in oxy-Hb concentration as an indicator of changes in regional cerebral blood volume, as an earlier NIRS signal study using a perfused rat brain model proposed that oxy-Hb, rather than deoxy-Hb, is the more sensitive parameter for the study of activation [43]. The NIRS channels were placed according to the international 10–20 system [44]. Regarding the positions of the optodes, we followed previous NIRS studies of motor-related areas [37,45]. The optodes were positioned using a custom-made cap that covered the right and left PFC, presupplementary motor area (preSMA), supplementary motor area (SMA), dorsal premotor cortex (PMC), and sensorimotor cortex (SMC). The areas and optodes were as follows: left SMC, channels 18 and 22; right SMC, channels 21 and 24; motor area, channels 19, 20, and 23; SMA, channels 9, 12, 13, and 16; preSMA, channels 2, 5, and 6; left PMC, channels 8, 11, and 15; right PMC, channels, 10, 14, and 17; left PFC, channels 1 and 4; and right PFC, channels 3 and 7. The Cz position in the international 10–20 system was used as a marker for ensuring replicable placement of the optodes (Fig 2).
Fig 2. Location of the optodes and brain areas.
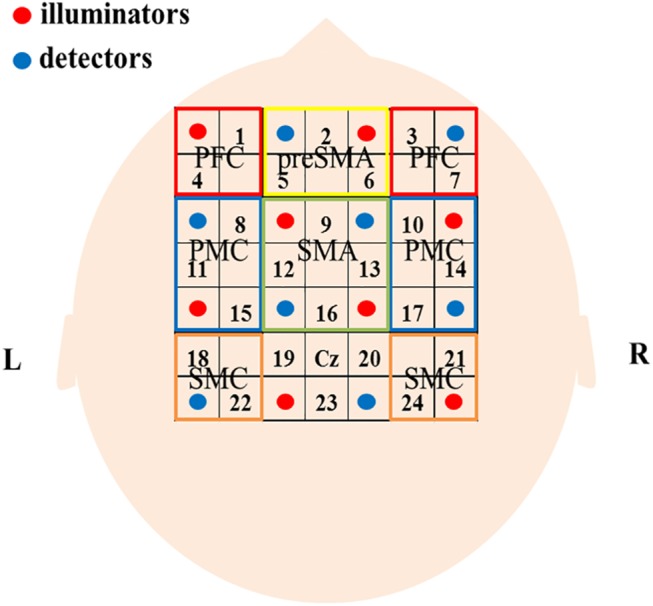
Sixteen optodes, comprising 8 light source fibers (red) and 8 detectors (blue) that enabled 24-channel measurements, were arranged on the scalp. The channels covering SMC, SMA, preSMA, PMC, and PFC are shown. See text for details.
Data analysis
Changes in performance across task cycles were analyzed by calculating Spearman’s rank correlation coefficients; serial changes (1–30 cycles) in the level of oxy-Hb associated with cycle repetition in the various regions were also evaluated using Spearman’s rank correlation coefficients. These correlation coefficients tested for associations between the number of task cycles and changes in oxy-Hb level for each region.
Results
Fig 3 shows the average performance (i.e., number of correct entries minus incorrect entries into the touch-screen terminal) during the touch-screen task over 30 cycles. There was a highly significant positive correlation between task cycle and performance (ρ = 0.924, p < 0.001) (S2 Dataset). Fig 4 shows the mean changes in oxy-Hb concentration for three cortical regions over the 30 cycles of the touch-screen task. A significant positive correlation between task cycle and oxy-Hb concentration for the channels covering the area of the left SMC (ρ = 0.387, p < 0.05) was observed (S3 Dataset). In contrast, significant negative correlations between task cycle and oxy-Hb concentration for the channels covering the SMA (ρ = -0.513, p < 0.01) (S4 Dataset) and right PFC (ρ = -0.364, p < 0.05) (S5 Dataset) were obtained. There were no significant correlations between task cycle and oxy-Hb changes for the channels covering the other areas (left PFC, preSMA, bilateral PMC, and right SMC) (S6 Dataset).
Fig 3. Correlation between performance (number of correctly entered characters minus incorrectly entered characters) and number of task cycles.
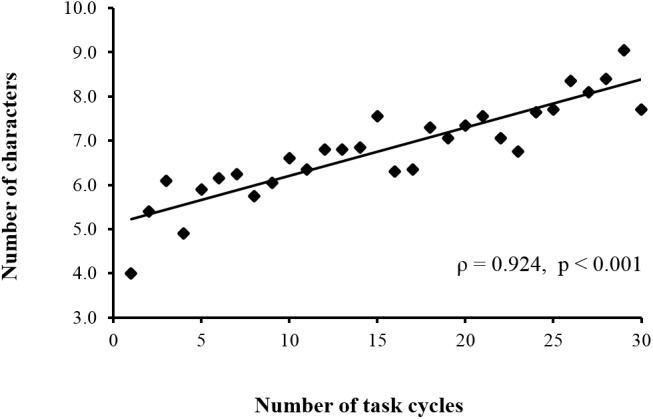
Performance significantly increased with cycle repetitions.
Fig 4. Correlations between oxy-Hb changes over three regions and number of task cycles.
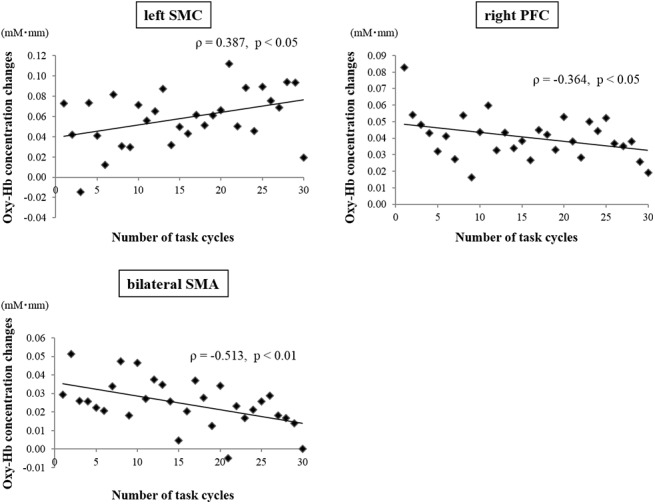
Vertical axis represents mean oxy-Hb concentration changes (in mM*mm). Left SMC activation significantly increased, whereas SMA and right PFC activation significantly decreased with cycle repetition.
Discussion
Flick input is one of the entry methods for Japanese characters in a touch-screen terminal. However, in general, flick input operation is difficult. The user of the touch-screen terminal has to learn to input a letter quickly to effectively convey information. Because the subjects of this study were inexperienced in touch-screen terminal operation, this task was equivalent to motor learning combined with complex cognitive processing. In recent years, activities involving complex cognitive processing during operation of touch-screen terminals have become abundant in everyday life. However, the cerebral blood flow dynamics during motor learning with complex cognitive processing in humans are not well understood. Therefore, we used NIRS to examine the cerebral blood flow dynamics of various cortical regions during motor learning of flick input operation of a touch-screen terminal.
Role of SMC in motor learning
The left SMC was activated with an increasing number of task cycles, whereas the right SMC did not show such an activation pattern. The left SMC is equivalent to a region including primary motor and primary sensory areas primarily controlling operation of the hand [46]. PET and NIRS studies have reported that the cerebral blood flow volume of the contralateral primary motor area of the operating hand increased with the frequency of finger tapping [26,47,48]. In addition, the primary motor and primary sensory areas contralateral to the operating hand became activated during motor learning of the finger. In the present study, the number of character entries significantly increased with the task cycle. During the course of one experiment, motor learning of the finger occurred in all subjects, as confirmed by the improvement of the flick input operation. Therefore, we speculate that increases in the momentum of the finger reflect motor learning.
Role of SMA in motor learning
The SMA extracts motor programs that depend on memory information and the initiation of spontaneous movements. Thus, the SMA plays an important role in situations where a compound movement is controlled [49–53]. The examination of SMA activity during motor learning can be accomplished by various neuroimaging techniques. However, there is little consensus on the activity of the SMA during motor learning [54–60]. In a previous NIRS study, SMA activation gradually increased with motor learning [38]. That study used a pursuit rotor task requiring simple motor learning and therefore differs from the motor learning task of the current study that involved complex cognitive processing. Although the SMA showed greatly increased activity immediately after experiment initiation, its activity was gradually attenuated with increasing task cycles.
We suggest that these changes in cerebral blood flow were caused by motor inhibition. All subjects of this study owned a feature phone and inputted characters using ten keys that operated in a toggle manner. During the experiment, all subjects were required to input letters using a method that differed from the operation they had mastered previously. We speculate that the two divergent character input methods competed, leading to motor inhibition that occurred in all subjects during this task. Preliminary research has suggested that the SMA is activated by motor inhibition [61,62]. We further suggest that the SMA is activated initially by motor inhibition; as learning progresses, SMA activity is attenuated. Although our findings demonstrate activation of SMA by motor inhibition, we could not find similar reports that examined changes of cerebral blood flow showing motor inhibition with cognitive learning over time. Therefore, this study may be the first to report cerebral blood flow dynamics in SMA with cognitive learning involving motor inhibition. However, the task required complex cognitive processes including visuomotor adaptation and working memory. Therefore, the current task was not specifically designed to test motor inhibition. In future experiments, we thus need to examine the dynamics of cerebral blood flow over the SMA during more specific tasks requiring motor inhibition.
This may be the first study to report the activity of the SMA during a motor learning task with complex cognitive processing using character entry into a touch-screen terminal over time. Therefore, further research is needed to examine the role of SMA during motor learning using other complex tasks (e.g., character input on a personal computer, trail making, serial reaction time, or other visuomotor adaptation and motor sequence learning tasks that involve complex cognitive processing).
Role of the PFC in motor learning
The PFC is an important neural substrate for visual working memory [63]. As the task of this study consisted of entering a letter presented on a PC screen into a hand-held touch-screen terminal, working memory was required to successfully accomplish this task. Memory encoding has been associated with lateral PFC activation across a variety of experimental paradigms in functional neuroimaging [64,65]. In the current study, the right PFC activation was gradually attenuated with increasing task cycle. However, activation of the left PFC continued without attenuation as the task cycles progressed. Activity of PFC regions has been reported to gradually decrease with learning [66–71]. Therefore, acquisition of the cognitive skill associated with working memory in our subjects may have attenuated the activity of the right PFC. As all subjects in our study were right-handed, we assume that their left hemisphere was language-dominant. Thus, the left PFC was likely involved in linguistic information manipulation [63,72,73] and continued to be recruited during all task cycles, thereby maintaining the activity of the left PFC. Previous studies have reported PFC asymmetry for memory encoding of verbal and nonverbal code [74,75]. The right PFC is activated during a nonverbal task, whereas the left PFC is activated during a verbal task, supporting the validity of our hypothesis.
Role of other regions
The PMC is involved in choice and control of movements that depend on sensory information [76,77]. During the 1–30 cycles, the PMC continued to be activated bilaterally. As the task of this study consisted of a visuomotor problem, we speculate that the activity of PMC was maintained because the subjects depended on visual information throughout all task cycles. The preSMA controls the aspect of the task that uses the rich entry from the premotor area, including order decision of the complex movements during preparation and movement choice; this also includes motor learning of changes in movement style and timing of the movement initiation. Furthermore, the preSMA is activated by cognitive control in the absence of movement control [78–82]. The activity of the preSMA was previously reported to be attenuated with motor learning [38]. However, in this study, the activity of preSMA continued during all task cycles.
The subjects performed a movement control problem with complex cognitive information processing by translating the letter presented on a monitor to the movement direction of their finger. We suggest that this step represents early stages of cognitive-motor learning. In the current task, sustained attentional engagement from the subjects was required during cognitive processing, and we speculate that this led to the continued activity of preSMA without attenuation.
Limitations of this study
First, as our measurements included only the initial learning period of 30 cycles, it was not possible to determine how cerebral blood flow dynamics might have changed after completion of the 30-cycle learning stage. Therefore, further studies are needed to investigate cerebral blood flow dynamics after task training.
Second, we used the number of characters entered minus the number of incorrect entries as the performance index in this study, and investigated its relationship with Oxy-Hb concentration changes in each region in this study,. However, it is also possible that the Oxy-Hb concentration changes for each region would show a different course if the speed of entering the characters and reaction time (i.e. the time to enter a character appearing on a monitor upon confirmation) were investigated separately as a performance index. Further studies should be performed to address this issue.
Third, activation in deeper structures such as the basal ganglia, which are closely linked with the frontal cortices, cannot be detected because of the technical limitations of NIRS. We thus could not address functional connectivity between brain areas, but it would be interesting to investigate functional connectivity between the right PFC and SMA during complex motor learning tasks. In future research, it will be necessary to consider other neuroimaging techniques that measure cerebral blood flow dynamics in humans during motor learning of flick input operation of a touch-screen terminal.
Conclusions
We examined cerebral blood flow dynamics during motor learning of flick input into a touch-screen terminal using NIRS. The number of character inputs significantly increased with repetition of task cycles. These results show that motor learning occurred in all subjects during the course of one experiment. In the left SMC, SMA, and right PFC, there was a significant change of cerebral blood flow dynamics as the task cycles progressed, indicating motor learning over time. Changes in activity over the left SMC, SMA, and right PFC likely reflect distinct aspects of acquisition of the motor task such as increase in finger momentum, motor inhibition, and visual working memory, respectively.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(PPTX)
Acknowledgments
The authors would like to thank all the participants in this study. Takehito Yonezawa, Kengo Fujiwara, and Akira Nakashima contributed significantly with their flexible support of the participants during data acquisition. We thank Hitachi Medical Corp., Japan for their skilled technical support whenever needed. Moreover, we would particularly like to thank Takumi Inakazu for his continuous support during the data acquisition and processing phase. We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- NIRS
near-infrared spectroscopy
- PFC
prefrontal cortex
- PFC
left dorsolateral prefrontal cortex
- preSMA
presupplementary motor area
- SMA
supplementary motor area
- PMC
dorsal premotor cortex
- SMC
sensorimotor cortex
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25: 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005;6: 77–85. [DOI] [PubMed] [Google Scholar]
- 3. Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Debener S, Ullsperger M, Siegel M, Engel AK. Single-trial EEG-fMRI reveals the dynamics of cognitive function. Trends Cogn Sci. 2006;10: 558–63. [DOI] [PubMed] [Google Scholar]
- 5. Norris DG. Principles of magnetic resonance assessment of brain function. J Magn Reson Imaging. 2006;23: 794–807. [DOI] [PubMed] [Google Scholar]
- 6. Otte A, Halsband U. Brain imaging tools in neurosciences. J Physiol Paris. 2006;99: 281–92. [DOI] [PubMed] [Google Scholar]
- 7. Stern JM. Simultaneous electroencephalography and functional magnetic resonance imaging applied to epilepsy. Epilepsy Behav. 2006;8: 683–92. [DOI] [PubMed] [Google Scholar]
- 8. Van Eimeren T, Siebner HR. An update on functional neuroimaging of parkinsonism and dystonia. Curr Opin Neurol. 2006;19: 412–9. [DOI] [PubMed] [Google Scholar]
- 9. Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophysiol. 2008;99: 1836–45. 10.1152/jn.01187.2007 [DOI] [PubMed] [Google Scholar]
- 10. Anguera JA, Reuter-lorenz PA, Willingham DT, Seidler RD. Contributions of Spatial Working Memory to Visuomotor Learning. J Cogn Neurosci. 2009;22: 1917–1930. [DOI] [PubMed] [Google Scholar]
- 11. King BR, Fogel SM, Albouy G, Doyon J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci. 2013;7: 142 10.3389/fnhum.2013.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bédard P, Sanes JN. Brain representations for acquiring and recalling visual-motor adaptations. Neuroimage. Elsevier B.V. 2014;101: 225–35. 10.1016/j.neuroimage.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deluca C, Golzar A, Santandrea E, Lo Gerfo E, Eštočinová J, Moretto G, et al. The cerebellum and visual perceptual learning: evidence from a motion extrapolation task. Cortex. 2014;58: 52–71. 10.1016/j.cortex.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 14. Stewart JC, Tran X, Cramer SC. Age-related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults. Neuroimage. Elsevier Inc.; 2014;86: 326–34. 10.1016/j.neuroimage.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okamoto M, Dan I. Functional near-infrared spectroscopy for human brain mapping of taste-related cognitive functions. J Biosci Bioeng. 2007;103: 207–15. [DOI] [PubMed] [Google Scholar]
- 16. Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14: 1186–92. [DOI] [PubMed] [Google Scholar]
- 17. Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, et al. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52: 188–94. [DOI] [PubMed] [Google Scholar]
- 18. Hiroyuki H, Hamaoka T, Sako T, Nishio S, Kime R, Murakami M, et al. Oxygenation in vastus lateralis and lateral head of gastrocnemius during treadmill walking and running in humans. Eur J Appl Physiol. 2002;87: 343–9. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage. 2004;23: 1020–6. [DOI] [PubMed] [Google Scholar]
- 20. Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp brain Res. 2009;193: 445–54. 10.1007/s00221-008-1643-y [DOI] [PubMed] [Google Scholar]
- 21. Subudhi AW, Dimmen AC, Roach RC. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J Appl Physiol. 2007;103: 177–83. [DOI] [PubMed] [Google Scholar]
- 22. Subudhi AW, Miramon BR, Granger ME, Roach RC. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol. 2009;106: 1153–8. 10.1152/japplphysiol.91475.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ide K, Horn A, Secher NH, Trangmar SJ, Chiesa ST, Stock CG, et al. Cerebral metabolic response to submaximal exercise maximal exercise in trained humans maximal exercise in trained humans Cerebral metabolic response to submaximal exercise. 2014; 1604–1608.
- 24. Okamoto M, Dan H, Shimizu K, Takeo K, Amita T, Oda I, et al. Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. Neuroimage. 2004;21: 1275–88. [DOI] [PubMed] [Google Scholar]
- 25. Kuboyama N, Nabetani T, Shibuya K-I, Machida K, Ogaki T. The effect of maximal finger tapping on cerebral activation. J Physiol Anthropol Appl Human Sci. 2004;23: 105–10. [DOI] [PubMed] [Google Scholar]
- 26. Kuboyama N, Nabetani T, Shibuya K, Machida K, Ogaki T. Relationship between Cerebral Activity and Movement Frequency of Maximal Finger Tapping. J Physiol Anthropol Appl Human Sci. 2005;24: 201–208 [DOI] [PubMed] [Google Scholar]
- 27. Ito M, Fukuda M, Suto T, Uehara T, Mikuni M. Increased and decreased cortical reactivities in novelty seeking and persistence: a multichannel near-infrared spectroscopy study in healthy subjects. Neuropsychobiology. 2005;52: 45–54. [DOI] [PubMed] [Google Scholar]
- 28. Holper L, Biallas M, Wolf M. Task complexity relates to activation of cortical motor areas during uni- and bimanual performance: a functional NIRS study. Neuroimage. Elsevier Inc.; 2009;46: 1105–13. 10.1016/j.neuroimage.2009.03.027 [DOI] [PubMed] [Google Scholar]
- 29. Shibuya K, Kuboyama N. Human motor cortex oxygenation during exhaustive pinching task. Brain Res. 2007;1156: 120–4. [DOI] [PubMed] [Google Scholar]
- 30. Kubo M, Shoshi C, Kitawaki T, Takemoto R, Kinugasa K, Yoshida H, et al. Increase in prefrontal cortex blood flow during the computer version trail making test. Neuropsychobiology. 2008;58: 200–10. 10.1159/000201717 [DOI] [PubMed] [Google Scholar]
- 31. Nakahachi T, Ishii R, Iwase M, Canuet L, Takahashi H, Kurimoto R, et al. Frontal cortex activation associated with speeded processing of visuospatial working memory revealed by multichannel near-infrared spectroscopy during Advanced Trail Making Test performance. Behav Brain Res. Elsevier B.V.; 2010;215: 21–7. 10.1016/j.bbr.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 32. Ohsugi H, Ohgi S, Shigemori K, Schneider EB. Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neurosci. BMC Neuroscience; 2013;14: 10 10.1186/1471-2202-14-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kikuchi S, Iwata K, Onishi Y, Kubota F, Nisijima K, Tamai H, et al. Prefrontal cerebral activity during a simple “rock, paper, scissors” task measured by the noninvasive near-infrared spectroscopy method. Psychiatry Res. 2007;156: 199–208. [DOI] [PubMed] [Google Scholar]
- 34. Yamauchi Y, Kikuchi S, Miwakeichi F, Matsumoto K, Nishida M, Ishiguro M, et al. Relation between parametric change of the workload and prefrontal cortex activity during a modified version of the “rock, paper, scissors” task. Neuropsychobiology. 2013;68: 24–33. 10.1159/000350948 [DOI] [PubMed] [Google Scholar]
- 35. Miyata H, Watanabe S, Minagawa-Kawai Y. Two successive neurocognitive processes captured by near-infrared spectroscopy: prefrontal activation during a computerized plus-shaped maze task. Brain Res. Elsevier B.V.; 2011;1374: 90–9. 10.1016/j.brainres.2010.12.047 [DOI] [PubMed] [Google Scholar]
- 36. Suto T, Fukuda M, Ito M, Uehara T, Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol Psychiatry. 2004;55: 501–11. [DOI] [PubMed] [Google Scholar]
- 37. Amemiya K, Ishizu T, Ayabe T, Kojima S. Effects of motor imagery on intermanual transfer: a near-infrared spectroscopy and behavioural study. Brain Res. Elsevier B.V.; 2010;1343: 93–103. 10.1016/j.brainres.2010.04.048 [DOI] [PubMed] [Google Scholar]
- 38. Hatakenaka M, Miyai I, Mihara M, Sakoda S, Kubota K. Frontal regions involved in learning of motor skill—A functional NIRS study. Neuroimage. 2007;34: 109–16. [DOI] [PubMed] [Google Scholar]
- 39. Ikegami T, Taga G. Decrease in cortical activation during learning of a multi-joint discrete motor task. Exp brain Res. 2008;191: 221–36. 10.1007/s00221-008-1518-2 [DOI] [PubMed] [Google Scholar]
- 40.Gentili RJ, Shewokis P a, Ayaz H, Contreras-Vidal JL. Functional near-infrared spectroscopy-based correlates of prefrontal cortical dynamics during a cognitive- [DOI] [PMC free article] [PubMed]
- 41.eMarketer. eMarketer. Smartphone Users Worldwide Will Total 1.75 Billion in 2014. January 16, 2014. Available: http://www.emarketer.com/Article/Smartphone-Users-Worldwide-Will-Total-175-Billion-2014/1010536. Accessed 6 December 2014
- 42. Obrig H, Villringer A. Beyond the visible—imaging the human brain with light. J Cereb Blood Flow Metab. 2003;23: 1–18. [DOI] [PubMed] [Google Scholar]
- 43. Hoshi Y, Kobayashi N, Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol. 2001;90: 1657–62. [DOI] [PubMed] [Google Scholar]
- 44. Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21: 99–111 [DOI] [PubMed] [Google Scholar]
- 45. Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34: 2866–70. [DOI] [PubMed] [Google Scholar]
- 46. Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285: 2136–9. [DOI] [PubMed] [Google Scholar]
- 47. Grafton ST, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand. A PET functional imaging study. Exp brain Res. 2002;146: 369–78. [DOI] [PubMed] [Google Scholar]
- 48. Kuboyama N, Nabetani T, Shibuya K-I, Machida K, Ogaki T. The effect of maximal finger tapping on cerebral activation. [Internet]. Journal of physiological anthropology and applied human science. 2004. pp. 105–10. [DOI] [PubMed] [Google Scholar]
- 49. Roland PE, Larsen B, Lassen N a, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43: 118–36. [DOI] [PubMed] [Google Scholar]
- 50. Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol. 1996;6: 782–7. [DOI] [PubMed] [Google Scholar]
- 51. Picard N, Strick P. Motor areas of the medial wall: a review of their location and functional activation. Cereb cortex. 1996;6: 342–53. [DOI] [PubMed] [Google Scholar]
- 52. Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9: 856–69. 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- 53. Mita A, Mushiake H, Shima K, Matsuzaka Y, Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat Neurosci. 2009;12: 502–7. 10.1038/nn.2272 [DOI] [PubMed] [Google Scholar]
- 54. Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowick RS, Phelps ME. Functional Anatomy of Human Procedural Learning Determined with Regional Cerebral Blood Flow and PET. J Neurosci. 1992;12: 2542–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8: 50–61. [DOI] [PubMed] [Google Scholar]
- 56. Hund-Georgiadis M, von Cramon D. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp brain Res. 1999;125: 417–25. [DOI] [PubMed] [Google Scholar]
- 57. Krings T, Töpper R, Foltys H, Erberich S, Sparing R, Willmes K, et al. Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett. 2000;278: 189–193. [DOI] [PubMed] [Google Scholar]
- 58. Hatta A, Nishihira Y, Higashiura T, Kim SR, Kaneda T. Long-term motor practice induces practice-dependent modulation of movement-related cortical potentials (MRCP) preceding a self-paced non-dominant handgrip movement in kendo players. Neurosci Lett. 2009;459: 105–8. 10.1016/j.neulet.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 59. Lefebvre S, Dricot L, Gradkowski W, Laloux P, Vandermeeren Y. Brain activations underlying different patterns of performance improvement during early motor skill learning. Neuroimage. Elsevier Inc.; 2012;62: 290–9. 10.1016/j.neuroimage.2012.04.052 [DOI] [PubMed] [Google Scholar]
- 60. Wright DJ, Holmes PS, Di Russo F, Loporto M, Smith D. Differences in cortical activity related to motor planning between experienced guitarists and non-musicians during guitar playing. Hum Mov Sci. Elsevier B.V.; 2012;31: 567–77. 10.1016/j.humov.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 61. Whitmer AJ, Banich MT. Brain activity related to the ability to inhibit previous task sets: an fMRI study. Cogn Affect Behav Neurosci. 2012;12: 661–70. 10.3758/s13415-012-0118-6 [DOI] [PubMed] [Google Scholar]
- 62. Nakata H, Sakamoto K, Honda Y, Kakigi R. Somato-motor inhibitory processing in humans: evidence from neurophysiology and neuroimaging. J Physiol Sci. 2014;64: 233–52. 10.1007/s12576-014-0320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93: 13473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shallice T. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368: 633–635. [DOI] [PubMed] [Google Scholar]
- 65. Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of episodic memory. Trends Neurosci. 1997;20: 213–8. [DOI] [PubMed] [Google Scholar]
- 66. Jenkins I, Brooks D, Nixon P, Frachowick S, Passingham R. Motor sequence learning: A Study with Positron Emission Tomography. J Neurosci. 1994;14: 3775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol. 1997;78: 977–91. [DOI] [PubMed] [Google Scholar]
- 68. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coodination task. Neuropsychologia. 2004;42: 855–67. [DOI] [PubMed] [Google Scholar]
- 69. Floyer-Lea a, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94: 512–8. [DOI] [PubMed] [Google Scholar]
- 70. Leff DR, Elwell CE, Orihuela-Espina F, Atallah L, Delpy DT, Darzi AW, et al. Changes in prefrontal cortical behaviour depend upon familiarity on a bimanual co-ordination task: an fNIRS study. Neuroimage. 2008;39: 805–13. [DOI] [PubMed] [Google Scholar]
- 71. James DRC, Leff DR, Orihuela-Espina F, Kwok K-W, Mylonas GP, Athanasiou T, et al. Enhanced frontoparietal network architectures following “gaze-contingent” versus “free-hand” motor learning. Neuroimage. Elsevier Inc.; 2013;64: 267–76. 10.1016/j.neuroimage.2012.08.056 [DOI] [PubMed] [Google Scholar]
- 72.Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of Storage and Rehearsal in Verbal Working Memory: Evidence From Positron Emission Tomography. 1996. pp. 25–31.
- 73. Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional Cerebral Blood Flow During Word and Nonword Reading. Hum Brain Mapp. 1997;92: 84–92. [DOI] [PubMed] [Google Scholar]
- 74. Opitz B, Mecklinger A, Friederici A. Functional asymmetry of human prefrontal cortex: encoding and retrieval of verbally and nonverbally coded information. Learn Mem. 2000;7: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Floel A, Poeppel D, Buffalo EA, Braun A, Wu CW-H, Seo H, et al. Prefrontal Cortex Asymmetry for Memory Encoding of Words and Abstract Shapes. Cereb Cortex. 2004;14: 404–9. [DOI] [PubMed] [Google Scholar]
- 76. Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements Neuronal Activity in the Primate Premotor, Supplementary, and Precentral Motor Cortex During Vi. J Neurophysiol. 1991;66: 705–18. [DOI] [PubMed] [Google Scholar]
- 77. Murray E a, Bussey TJ, Wise SP. Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Exp brain Res. 2000;133: 114–29. [DOI] [PubMed] [Google Scholar]
- 78. Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B. Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol. 1996;76: 617–21. [DOI] [PubMed] [Google Scholar]
- 79. Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, et al. The Role of Rostral Brodmann Area 6 in Mental-operation Tasks: an Integrative Neuroimaging Approach. Cereb Cortex. 2002;12: 1157–70. [DOI] [PubMed] [Google Scholar]
- 80. Leek EC, Johnston SJ. Functional specialization in the supplementary motor complex. Nat Rev Neurosci. 2009;10: 78; author reply 78. 10.1038/nrn2478-c1 [DOI] [PubMed] [Google Scholar]
- 81. Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. Elsevier Inc.; 2013;67: 283–97. 10.1016/j.neuroimage.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wilson TW, Kurz MJ, Arpin DJ. Functional specialization within the supplementary motor area: a fNIRS study of bimanual coordination. Neuroimage. Elsevier Inc.; 2014;85 Pt 1: 445–50. 10.1016/j.neuroimage.2013.04.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(PPTX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


