Abstract
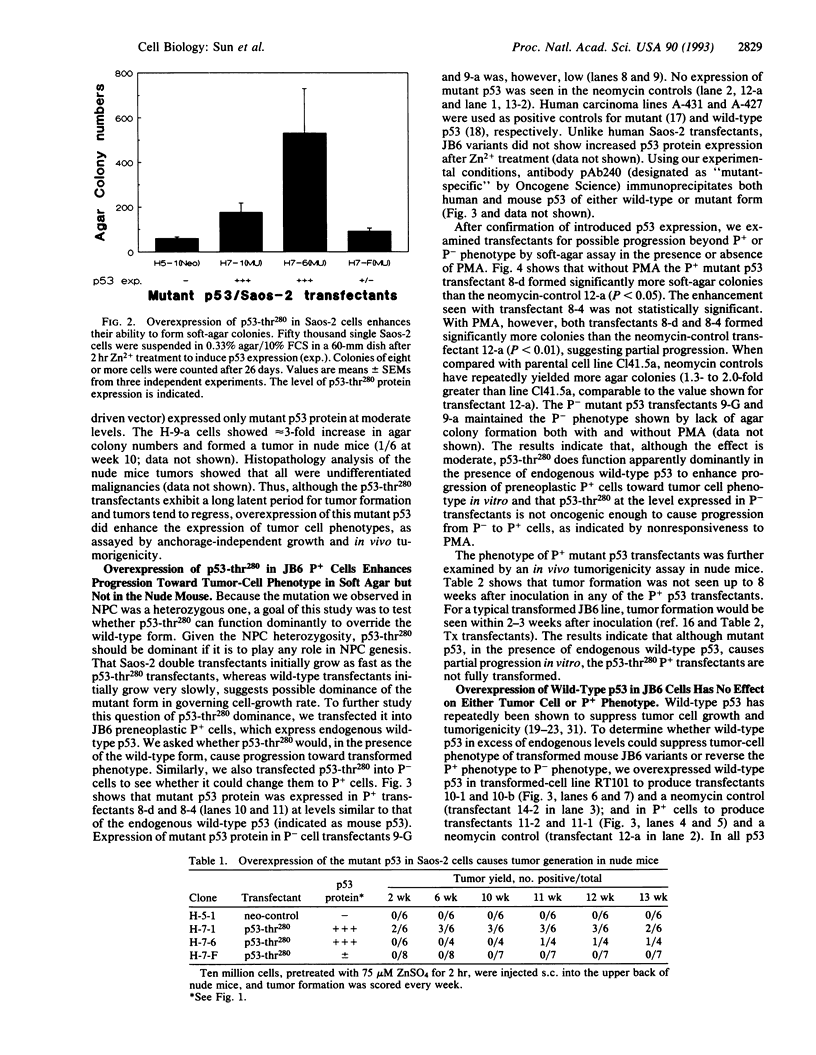
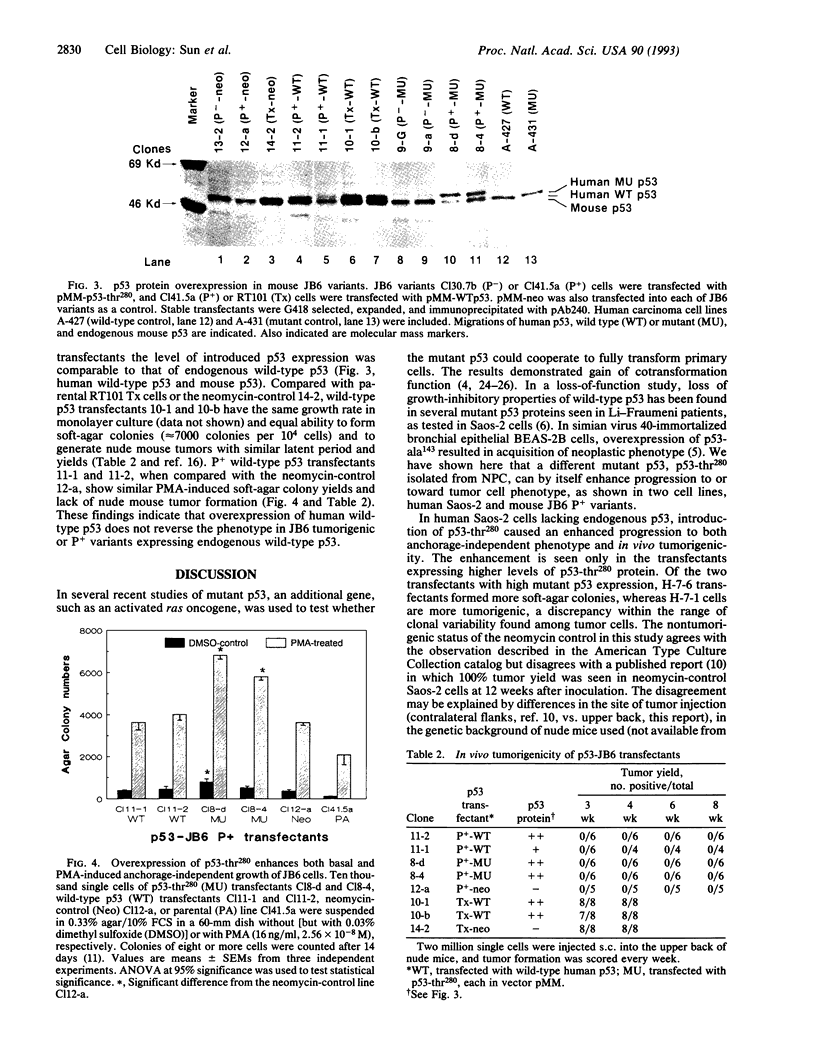
We recently reported the detection of a heterozygous G-->C point mutation at codon 280 of p53 in nasopharyngeal carcinoma, which causes an Arg-->Thr substitution. To test whether this mutant p53 has gained function as an oncogene, we overexpressed the mutant p53 in nontumorigenic cells of two model systems: (i) human Saos-2 cells lacking endogenous p53 and (ii) mouse JB6 variants that bear endogenous wild-type p53. Although they have no growth advantage over the neomycin controls in monolayer culture, human Saos-2 transfectants overexpressing mutant p53 do show enhanced progression to tumor cell phenotype, as assayed by anchorage-independent growth and in vivo tumorigenicity. The enhancement is seen only in transfectants expressing higher levels of p53 protein. In the mouse JB6 system, the mutant p53 functions dominantly in the presence of endogenous wild-type p53 to enhance progression of preneoplastic promotion-sensitive cells toward anchorage-independent phenotype. Mouse JB6 transfectants of mutant p53 are, however, not tumorigenic in nude mice. We conclude from these studies that the G-->C point mutation of p53 at codon 280 is a gain-of-function mutation that appears to operate dominantly and that the mutant p53-thr280 has only moderate oncogenic activity. This mutation may cooperate with other yet-to-be isolated genes in the genesis of nasopharyngeal carcinoma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Cajot J. F., Anderson M. J., Lehman T. A., Shapiro H., Briggs A. A., Stanbridge E. J. Growth suppression mediated by transfection of p53 in Hut292DM human lung cancer cells expressing endogenous wild-type p53 protein. Cancer Res. 1992 Dec 15;52(24):6956–6960. [PubMed] [Google Scholar]
- Cao Y., Sun Y., Poirier S., Winterstein D., Hegamyer G., Seed J., Malin S., Colburn N. H. Isolation and partial characterization of a transformation-associated sequence from human nasopharyngeal carcinoma. Mol Carcinog. 1991;4(4):297–307. doi: 10.1002/mc.2940040408. [DOI] [PubMed] [Google Scholar]
- Casey G., Lo-Hsueh M., Lopez M. E., Vogelstein B., Stanbridge E. J. Growth suppression of human breast cancer cells by the introduction of a wild-type p53 gene. Oncogene. 1991 Oct;6(10):1791–1797. [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Chen P. L., Arnaiz N., Goodrich D., Lee W. H. Expression of wild-type p53 in human A673 cells suppresses tumorigenicity but not growth rate. Oncogene. 1991 Oct;6(10):1799–1805. [PubMed] [Google Scholar]
- Colburn N. H., Former B. F., Nelson K. A., Yuspa S. H. Tumour promoter induces anchorage independence irreversibly. Nature. 1979 Oct 18;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Smith B. M., Wendel E. J., Dowjat W. K., Shimada T. Transfer by pro gene transfection of tumor promoter-sensitive phenotype to promotion-insensitive JB6 cells. Cancer Res. 1988 Mar 1;48(5):1195–1200. [PubMed] [Google Scholar]
- Colburn N. H., Wendel E. J., Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller L., Kassel J., Nelson C. E., Gryka M. A., Litwak G., Gebhardt M., Bressac B., Ozturk M., Baker S. J., Vogelstein B. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. S., Mueller N. E. Viruses and cancer. Causal associations. Ann Epidemiol. 1990 Oct;1(1):71–92. doi: 10.1016/1047-2797(90)90020-s. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Allan G. J., Shanahan F., Vousden K. H., Crook T. p53 is frequently mutated in Burkitt's lymphoma cell lines. EMBO J. 1991 Oct;10(10):2879–2887. doi: 10.1002/j.1460-2075.1991.tb07837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Frebourg T., Kassel J., Lam K. T., Gryka M. A., Barbier N., Andersen T. I., Børresen A. L., Friend S. H. Germ-line mutations of the p53 tumor suppressor gene in patients with high risk for cancer inactivate the p53 protein. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6413–6417. doi: 10.1073/pnas.89.14.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Spillare E., Forrester K., Lehman T. A., Kispert J., Welsh J. A., Pfeifer A. M., Lechner J. F., Baker S. J., Vogelstein B. Mutant p53 can induce tumorigenic conversion of human bronchial epithelial cells and reduce their responsiveness to a negative growth factor, transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2759–2763. doi: 10.1073/pnas.89.7.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Michalovitz D., Oren M. Different tumor-derived p53 mutants exhibit distinct biological activities. Science. 1990 Oct 5;250(4977):113–116. doi: 10.1126/science.2218501. [DOI] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Carter B. S., Ewing C. M. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991 Sep 1;51(17):4716–4720. [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Lehman T. A., Bennett W. P., Metcalf R. A., Welsh J. A., Ecker J., Modali R. V., Ullrich S., Romano J. W., Appella E., Testa J. R. p53 mutations, ras mutations, and p53-heat shock 70 protein complexes in human lung carcinoma cell lines. Cancer Res. 1991 Aug 1;51(15):4090–4096. [PubMed] [Google Scholar]
- Lerman M. I., Hegamyer G. A., Colburn N. H. Cloning and characterization of putative genes that specify sensitivity to neoplastic transformation by tumor promoters. Int J Cancer. 1986 Feb 15;37(2):293–302. doi: 10.1002/ijc.2910370219. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lu X., Park S. H., Thompson T. C., Lane D. P. Ras-induced hyperplasia occurs with mutation of p53, but activated ras and myc together can induce carcinoma without p53 mutation. Cell. 1992 Jul 10;70(1):153–161. doi: 10.1016/0092-8674(92)90541-j. [DOI] [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Hegamyer G., Cheng Y. J., Hildesheim A., Chen J. Y., Chen I. H., Cao Y., Yao K. T., Colburn N. H. An infrequent point mutation of the p53 gene in human nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6516–6520. doi: 10.1073/pnas.89.14.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Pommier Y., Colburn N. H. Acquisition of a growth-inhibitory response to phorbol ester involves DNA damage. Cancer Res. 1992 Apr 1;52(7):1907–1915. [PubMed] [Google Scholar]
- Takahashi T., Carbone D., Takahashi T., Nau M. M., Hida T., Linnoila I., Ueda R., Minna J. D. Wild-type but not mutant p53 suppresses the growth of human lung cancer cells bearing multiple genetic lesions. Cancer Res. 1992 Apr 15;52(8):2340–2343. [PubMed] [Google Scholar]