Abstract
Natural Killer T (NKT) cells have gained widespread attention among immunologists because of their distinct ability to regulate anti-tumor responses and to influence the outcome of infections or autoimmunity. Type I (also called invariant) NKT cells (iNKT) are best characterized mainly because of the availability of lipid antigen-loaded CD1d-tetramer detection reagents. Human iNKT cells present important phenotypic differences relative to their murine counterpart, restricting the extrapolation of findings from experimental murine models to human health and disease states. Particularly, the ontogeny and early life phenotype of iNKT cells largely differ between human and mice, indicating divergent functional properties between species. The high therapeutic potential offered by manipulation of iNKT cells in disease warrants a better understanding of human iNKT cell biology. Here, we discuss characteristics of human iNKT cells and present an efficient and rapid method for their ex vivo purification and characterization.
Keywords: Invariant Natural Killer T cells, IL-2 receptor alpha chain, Cord blood, Fluorescent-activated Cell Sorting, Human, Magnetic Bead Positive Selection
1. Introduction
Natural killer T (NKT) cells are a subset of immune cells that have distinct regulatory properties. They were originally observed in mice (Koseki et al., 1990), and similarly in humans (Porcelli et al., 1993), following identification of a T cell subset expressing an invariant TCR alpha VJ receptor combination [reviewed in (Godfrey et al., 2004)]. NKT cells expressing both the T cell receptor (TCR) and the natural killer cell marker NK1.1 (CD161) were later shown to characteristically produce large amounts of IL-4 upon antigenic stimulation (Bendelac et al., 1994; Bendelac et al., 1995). Murine iNKT cells express a canonical T cell receptor (TCR) composed of a unique variable (V) α14 and junction (J) α18 segment alpha chain paired with a limited β-chain repertoire (Vβ8.2, 7 or 2) (Lantz and Bendelac, 1994; Taniguchi et al., 1996). By homology, iNKT cells expressing a structurally similar variable and junction elements of the TCR alpha chain (Vα24/Jα18) were subsequently identified in humans (Davodeau et al., 1997; Exley et al., 1997; Prussin and Foster, 1997). Two types of NKT cells have been recognized, sharing specificity for the non-polymorphic MHC class I-like CD1d molecule. Type I NKT cells (also called invariant NKT, or iNKT cells) are more studied, largely due to their facilitated identification using the lipid antigen α-galactosylceramide loaded on CD1d tetramers (Berzins et al., 2005).
Experiments in mice indicate a strong influence of iNKT cell activity on the outcome of tumors, infections and autoimmunity (Vincent et al., 2003). Both human and murine iNKT cells appear to share a common, highly conserved, specificity for CD1d-loaded lipid antigens (Borg et al., 2007). However, functional differences between human and mice warrant a better understanding of the biology and therapeutic potential of iNKT cells in humans. Here, we discuss the characteristics of human iNKT cells, more specifically emphasizing the phenotype of neonatal iNKT cells, and present a rapid, efficient method for their ex vivo purification and characterization.
2. Characteristics of human iNKT cells
iNKT cells represent a small proportion of CD3-positive cells in human adults peripheral blood (between 0.005 to 0.2 % in most cases), but their proportion has been reported as high as 3% in some individuals (Sakamoto et al., 1999). This proportion appears relatively stable over short periods of time and seems to be genetically determined (Jordan et al., 2004). iNKT cells are also present in the liver (Doherty et al., 1999; Norris et al., 1999), although for obvious practical reasons their investigation in humans has primarily been restricted to blood. In mice, iNKT cells are most abundant among hepatic lymphocytes (20–40 % of T cells) whereas they are present at lower frequencies (~0.5 to 1 %) among splenic and peripheral blood T cells (Godfrey et al., 2010; Berzins et al., 2011).
Given their low numbers in peripheral blood, functional analysis of human iNKT cells has primarily focused on cultured cells following expansion in vitro using antigen-loaded APCs (e.g. CD3-depleted blood mononuclear cells or CD1d-transfected cells lines). Two main subsets of human iNKT cells have been described: CD4positive and double negative (CD8negativeCD4negative) iNKT cells that differ in their effector functions (Gumperz et al., 2002; Lee et al., 2002). CD4positive iNKT cells dominate in fetal and neonatal blood (>90% the iNKT cell population) and secrete diverse cytokines such as IL-4, GM-CSF, IFN-γ, IL-13, TNF-α and MIP-1α/β (Gumperz et al., 2002; Kim et al., 2002; Lee et al., 2002). CD4negative iNKT cells are mainly cytotoxic to tumors, but also to autologous CD1d-expressing antigen-presenting cell targets under some circumstances, indicating a distinct potential for autoreactivity (Gapin, 2010). Evidence suggests that CD4positive iNKT cells directly expand from the thymus, whereas expansion of CD4negative iNKT cells mainly occurs in periphery and through homeostatic mechanisms (Baev et al., 2004). In addition, a small subset of α-galactosylceramide/CD1d-restricted Vα24-expressing CD8positive iNKT cell that mainly produces IFN-γ has been described (Takahashi et al., 2002).
With aging, iNKT cell proportions increase in mice (Faunce et al., 2005) whereas studies have shown a decrease in humans, significantly more in males (DelaRosa et al., 2002; Crough et al., 2004; Jing et al., 2007; Ladd et al., 2010). This latter characteristic limits the investigation of iNKT cells in older men and makes it essential to control for age and gender in associations with diseases.
3. Phenotype of human neonatal iNKT cells
Human neonatal (cord blood) iNKT cells display an intriguing phenotype that differs from adults, but also from mice. A higher, more consistent proportion of iNKT cells are found in human cord blood (~0.1% of CD3-expressing cells), whereas in mice iNKT cells are largely absent before the second post-natal week (Fowlkes et al., 1987; Hammond et al., 1998; Sandberg et al., 2004; Faunce et al., 2005; de Lalla et al., 2008). Both neonatal and adult iNKT cells predominantly express the CD45RO isoform memory T cell marker (D’Andrea et al., 2000; van Der Vliet et al., 2000; Eger et al., 2006). Human cord blood iNKT cells ex vivo also constitutively express the IL-2Rα chain (CD25) molecule (D’Andrea et al., 2000; van Der Vliet et al., 2000). In contrast, expression of the IL-2Rα chain was not detected, in resting murine iNKT cells (Ding J, Sharma AA and Lavoie PM, unpublished data).
CD25 is necessary for high affinity binding of IL-2 to its receptor complex and important for lymphocyte proliferation in humans (Roifman, 2000; Caudy et al., 2007). It is normally only expressed by resting or activated Tregs, or transiently following activation of conventional T cells (Malek, 2008). Expression of CD25 on neonatal iNKT cells is associated with a substantially reduced threshold to proliferation compared to adult iNKT, conventional neonatal or adult T cells and with an apparent initial lack of requirement for IL-2 to drive cells into cycle (Ladd et al., 2010). The reasons underlying this unique phenotype are unclear. However, given the importance of the CD25 receptor for T cell expansion in humans, the relatively limited iNKT cell proportion observed in cord blood suggests a potentially unique role for the CD25-expressing phenotype in regulating early life iNKT cell homeostasis.
4. Ex vivo purification of human iNKT cells
4.1 Identification of iNKT cells
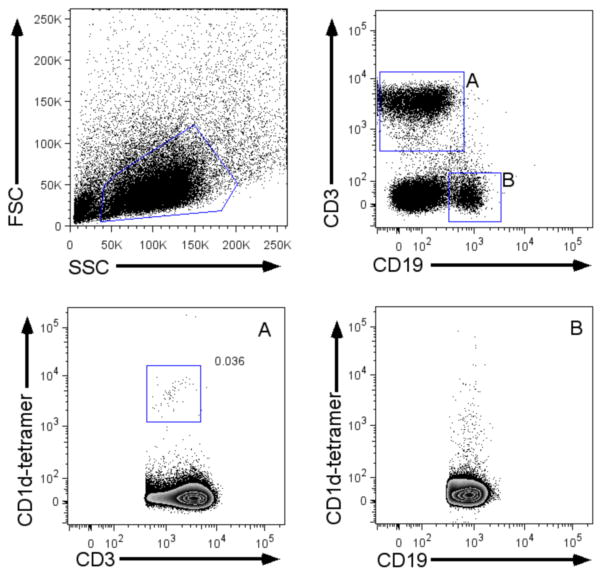
Human iNKT cells are best identified in flow cytometry using conjugated antigen-loaded tetramerized CD1d molecules (Benlagha et al., 2000) combined with an anti-CD3 antibody. The prototypic high affinity iNKT antigen α-galactosylceramide (α-GC, also referred to as KRN7000) displays poor aqueous solubility (Morita et al., 1995). For increased stability, tetramer loaded with structural analogs (e.g. PBS57) are used for labeling and purifying cells (Liu et al., 2006).1 Alternatively, an iNKT TCR-specific monoclonal antibody (denoted 6B11; BD Bioscience) of lower avidity, but greater specificity for the Vα24/Jα18 TCR can be used (Exley et al., 2008). Anti-Vα24 and anti-Vβ11 TCR antibodies may be used in combination with an anti-CD3 antibody for cell identification, although neither TCR chains are sufficiently exclusive to iNKT cells for purification (Benlagha et al., 2000). The flow cytometry gating strategy used to identify iNKT cells in peripheral blood mononuclear cells is shown in figure 1. The use of an anti-CD19 antibody is preferable in flow cytometry analyses in order to exclude non-specifically stained B cells, which share forward and side scatter characteristics similar to iNKT cells.
Figure 1. Gating strategy for flow cytometry identification of iNKT cells among blood mononuclear cells.
Lower panels: PBS57/CD1d-tetramer staining on (A) CD3positive cells or (B) CD19positive cells. Note the significant background PBS57/CD1d-tetramer staining on (CD19-expressing) B cells warranting use of an anti-CD19 antibody as a dump channel.
4.2 Purification of iNKT cells
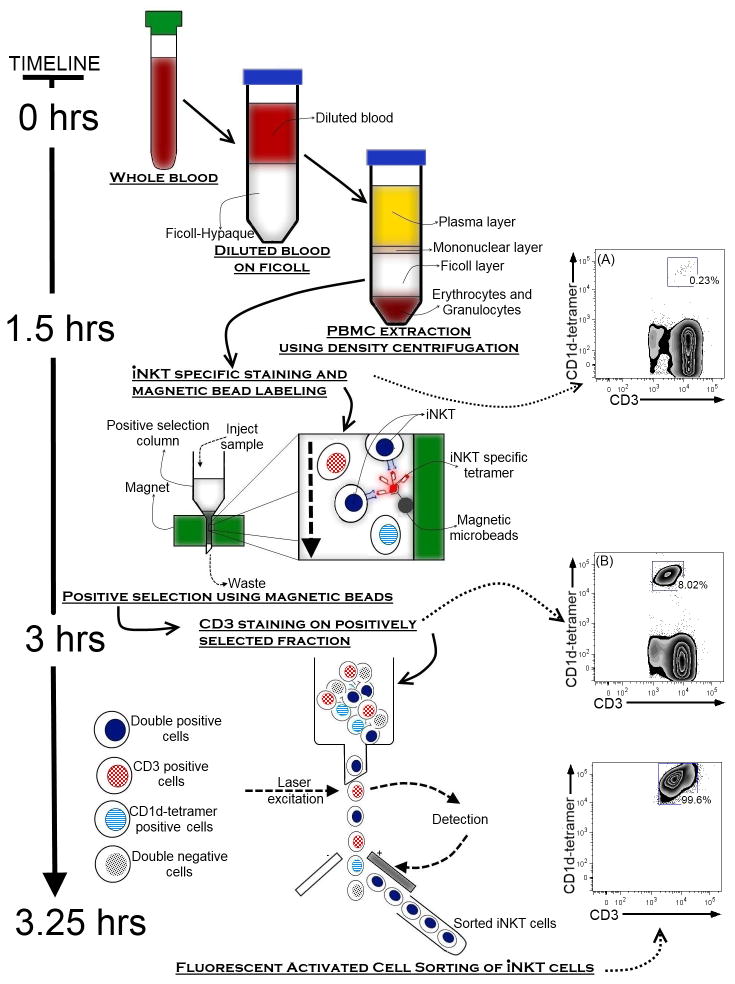
Due to the low occurrence of iNKT cells in human blood, efficient purification of a relatively large amount of cells ex vivo is best achieved using a multi-step procedure: Mononuclear cells are initially extracted using Ficoll-hypaque gradient centrifugation, followed by two positive selection steps using first, magnetic beads and second, fluorescent-activated cell sorting (FACS; figure 2). The initial magnetic bead enrichment of iNKT cells considerably reduces the time required for high purity FACS sorting of these cells (≫98%). However, the magnetic bead separation step may induce iNKT cell pre-activation, which can be minimized by performing the purification at 4°C and with the preferential use of the 6B11 antibody (data not shown). A positive selection is necessary to avoid contamination with conventional T or NK cell types. Due to the lack of validated lineage-specific iNKT cell surface markers in humans, negative selection methods may inevitably result in exclusion of important iNKT subsets.
Figure 2. Multi-step protocol for purification of human iNKT cells.
The low proportion of iNKTs present in human mononuclear cells (A) can be enriched using an initial magnetic bead column purification step followed by (B) Fluorescent Activated Cell Sorting (FACS).
The starting material can be cord blood (to study neonatal iNKT cells) or the “buffy coat” fraction of an adult blood sample (to study sufficient number of adult iNKT cells). Blood (>50–100 mL) is collected in heparinized tubes. Ficoll-hypaque purified mononuclear cells are resuspended (20–50x106 cells/mL) in Dulbecco’s Phosphate Buffered Saline (dPBS) containing 2% Fetal Calf Serum (FCS) and labeled with a fluorescent-conjugated antigen-loaded human CD1d-tetramers (using the lowest saturating amount as determined by titration on a standard mononuclear cell preparation) (Karadimitris et al., 2001; Gumperz et al., 2002; Kita et al., 2002; Li et al., 2008) or a biotinylated 6B11 antibody (Miltenyi Biotech), for 30 minutes at 4°C. Stained cells are then washed twice in dPBS/2%FCS, followed by resuspension in cold (4°C) MACS buffer (Miltenyi Biotech) and incubation for 15 minutes at 4°C with MACS magnetic beads (4 μL of beads/107 cells2, Miltenyi Biotech) conjugated to an antibody directed against the same fluorescent marker used for labeling of cells (when using CD1d-tetramers) or streptavidin (when using the biotin-6B11 antibody).3 Labeled iNKT cells are then passed onto a positive selection MACS MS column (Miltenyi Biotech) and the column is washed again twice with 500 μL of cold MACS buffer. After washing, cells are eluted from the column using 1 mL of cold MACS buffer. This first positive selection step typically yields a purity of about 4–20% (100 to 1000-fold enrichment). Following magnetic bead purification, cells are re-suspended in dPBS/2%FCS at a concentration of 10–20 x 106 cells/mL and stained with a conjugated anti-CD3 antibody at 4°C for 30 minutes. Cells are then washed twice, resuspended in dPBS/2%FCS and sorted at 4°C on double CD3positive/CD1d-tetramerpositive cells by fluorescence-activated cell sorting (FACS). This second positive selection step typically yields >98% iNKT cell purity (figure 2).
5. Characterization of ex vivo purified human iNKT cells
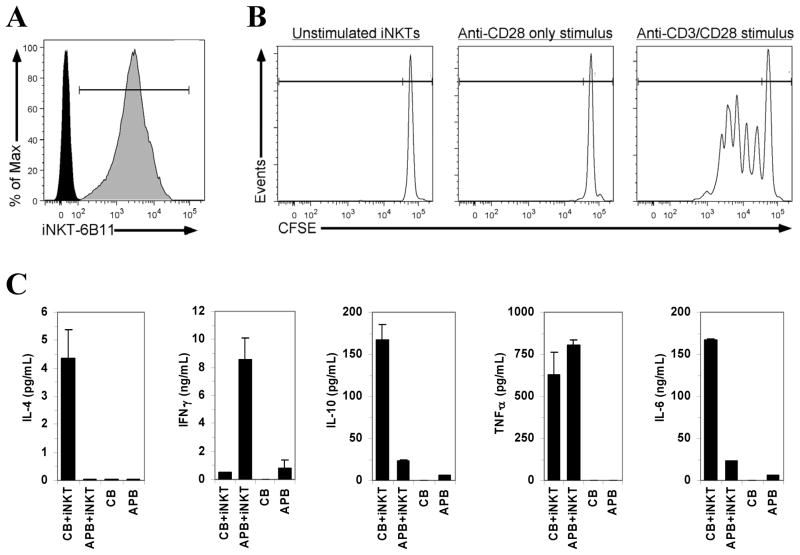
Purification of iNKT cells using this approach yields up to 250,000 cells from a single cord blood or adult blood donor (table 1). Identity of CD1d-tetramer-purified iNKT cells is confirmed by a positive staining of CD1d-tetramer-purified iNKT cells with the 6B11 antibody (figure 3A). Cells display a viability ≫95% when using fresh blood (<3 hours of collection) as assessed immediately after sorting (using 7-AAD and Annexin V, supplementary figure). Purified iNKT cells obtained with this method proliferate following TCR and CD28 co-stimulation (figure 3B). An example of the cytokine response obtained with cord or adult peripheral blood iNKT cells stimulated with α-galactosylceramide in presence of autologous CDd1-depleted mononuclear cells is shown in figure 3C. For polyclonal expansion, purified iNKT cells can be activated with α-galactosylceramide (10–100 ng/mL) in the presence of approximate 1.4 to 1 ratio of irradiated CD3-depleted blood mononuclear cells and 200U/mL of recombinant IL-2 for culture in RPMI medium containing 10% FCS at 37°C under a 5% CO2 atmosphere. (not shown). Purified iNKT cells can also be used in proliferation assays (e.g. using 3H-thymiding incorporation or the proliferation dye carboxyfluorescein succinimidyl ester, CFSE), cytokine analysis (by ELISA or intracellular staining), etc.
Table 1.
Typical number of iNKT cells extracted from adult or neonatal (cord) blood.
| Adult Blood (n = 5) | Adult Buffy Coat (n = 3) | Cord Blood (n = 15) | |
|---|---|---|---|
| Starting Volume (mL) | 150–200 | 50 | 50–150 |
| Mononuclear cells/mL of blood | 1–2 million/mL | 20–25 million/mL | 4–6 million/mL |
| iNKT cells/CD3+ cells (%) | 0.001–0.1% | 0.001–0.1% | 0.05–0.3% |
| Percentage iNKT cells after purification | >95% | >95% | >95% |
| Number of iNKT cells extracted | 3,000–50,000 | 7,000–100,000 | 80,000–250,000 |
Figure 3. Characterization of purified human iNKT cells.
(A) Positive staining of CD1d tetramer-purified iNKT-cell with the 6B11 antibody. (B) iNKT cell proliferation when stimulated with plate-bound anti-CD3 (OKT3) and soluble anti-CD28 antibodies (as described in Ladd et al., 2010). (C) Cytokine profile of purified iNKT cells reconstituted (1 iNKT:1000 mononuclear cells) with CD1d-tetramer-depleted (FACS) autologous cord blood (CB) or adult peripheral blood (APB) mononuclear cells following stimulation with α-galactosylceramide (100 ng/mL; 96 hours). Cytokine levels are presented (mean, error bars: standard deviation of duplicate ELISA analyses) after subtraction of unstimulated cells.
6. Concluding remarks
A large number of human studies have reported associations between iNKT cell proportions and phenotype in disease states such as cancer, infections or autoimmune diseases (Novak et al., 2007; Molling et al., 2008; Gao et al., 2009). The unique immunomodulatory characteristics of iNKT cells have generated great interest in developing functionally altered iNKT agonists to enhance vaccine responses or in cancer therapies. Efficient methods for identification and purification of human iNKT cells, such as that presented in this article should greatly facilitate ex vivo investigations of these cells.
Supplementary Material
Viability of purified iNKT cells following magnetic bead and FACS purification, as assessed using 7-AAD and Annexin V staining. Staining of (positive) control, heat shock-treated peripheral blood mononuclear cells.
Acknowledgments
This research was funded (in part) by the Child & Family Research Institute (CFRI) and a SickKids Foundation New Investigator Grant (XG09-015R). PML acknowledges support from a Child & Family Research Institute Investigators Award. We are thankful to our colleagues at CFRI (John Priatel, Brian Chung, Peter van den Elzen) for revising this manuscript.
Abbreviations
- α-GC
α-galactosylceramide
Footnotes
Antigen-loaded CD1d tetramers may be obtained from the NIH Tetramer Facility at http://tetramer.yerkes.emory.edu.
The amount of magnetic beads necessary can usually be titrated down compared to the amount specific by the supplier.
We have used either PE- or APC-conjugated CD1d-tetramers in combination with anti-PE or anti-APC capture beads with similar efficiency.
References
- Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, Metelitsa LS. Distinct homeostatic requirements of CD4+ and CD4− subsets of Valpha24-invariant natural killer T cells in humans. Blood. 2004;104:4150–6. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–8. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–42. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Smyth MJ, Godfrey DI. Working with NKT cells--pitfalls and practicalities. Curr Opin Immunol. 2005;17:448–54. doi: 10.1016/j.coi.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–9. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–7. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Valpha24(+)Vbeta11(+) NKT cells by age, malignancy and conventional anticancer therapies. Br J Cancer. 2004;91:1880–6. doi: 10.1038/sj.bjc.6602218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea A, Goux D, De Lalla C, Koezuka Y, Montagna D, Moretta A, Dellabona P, Casorati G, Abrignani S. Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur J Immunol. 2000;30:1544–50. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Davodeau F, Peyrat MA, Necker A, Dominici R, Blanchard F, Leget C, Gaschet J, Costa P, Jacques Y, Godard A, Vie H, Poggi A, Romagne F, Bonneville M. Close phenotypic and functional similarities between human and murine alphabeta T cells expressing invariant TCR alpha-chains. J Immunol. 1997;158:5603–11. [PubMed] [Google Scholar]
- de Lalla C, Festuccia N, Albrecht I, Chang HD, Andolfi G, Benninghoff U, Bombelli F, Borsellino G, Aiuti A, Radbruch A, Dellabona P, Casorati G. Innate-like effector differentiation of human invariant NKT cells driven by IL-7. J Immunol. 2008;180:4415–24. doi: 10.4049/jimmunol.180.7.4415. [DOI] [PubMed] [Google Scholar]
- DelaRosa O, Tarazona R, Casado JG, Alonso C, Ostos B, Pena J, Solana R. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213–7. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- Eger KA, Sundrud MS, Motsinger AA, Tseng M, Kaer LV, Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PLoS ONE. 2006;1:e50. doi: 10.1371/journal.pone.0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8− T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley MA, Hou R, Shaulov A, Tonti E, Dellabona P, Casorati G, Akbari O, Akman HO, Greenfield EA, Gumperz JE, Boyson JE, Balk SP, Wilson SB. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur J Immunol. 2008;38:1756–66. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol. 2005;175:3102–9. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, Pardoll DM. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987;329:251–4. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–28. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L. iNKT cell autoreactivity: what is ‘self’ and how is it recognized? Nat Rev Immunol. 2010;10:272–7. doi: 10.1038/nri2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998;10:1491–9. doi: 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S, Deng Y. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719–32. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Fletcher J, Baxter AG. Genetic control of NKT cell numbers. Immunol Cell Biol. 2004;82:276–84. doi: 10.1111/j.0818-9641.2004.01264.x. [DOI] [PubMed] [Google Scholar]
- Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, Chen JL, Koezuka Y, Roberts IA, Price DA, Dusheiko G, Milstein C, Fersht A, Luzzatto L, Cerundolo V. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98:3294–8. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–6. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL, Kaplan M, Gershwin ME. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–43. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M. Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc Natl Acad Sci U S A. 1990;87:5248–52. doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd M, Sharma A, Huang Q, Wang AY, Genowati I, Levings MK, Lavoie PM. Natural Killer T cells constitutively expressing the IL-2 receptor alpha chain early in life are primed to respond to lower antigenic stimulation. Immunology. 2010;131:289–99. doi: 10.1111/j.1365-2567.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen N, McMichael AJ, Screaton GR, Xu XN. Generation and characterisation of CD1d tetramer produced by a lentiviral expression system. J Immunol Methods. 2008;330:57–63. doi: 10.1016/j.jim.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–9. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Molling JW, Moreno M, van der Vliet HJ, van den Eertwegh AJ, Scheper RJ, von Blomberg BM, Bontkes HJ. Invariant natural killer T cells and immunotherapy of cancer. Clin Immunol. 2008;129:182–94. doi: 10.1016/j.clim.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- Novak J, Griseri T, Beaudoin L, Lehuen A. Regulation of type 1 diabetes by NKT cells. Int Rev Immunol. 2007;26:49–72. doi: 10.1080/08830180601070229. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8− alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C, Foster B. TCR V alpha 24 and V beta 11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862–70. [PubMed] [Google Scholar]
- Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr Res. 2000;48:6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Oishi Y, Kurasawa K, Kita Y, Saito Y, Iwamoto I. Characteristics of T-cell receptor Valpha24JalphaQ T cells, a human counterpart of murine NK1 T cells, from normal subjects. J Allergy Clin Immunol. 1999;103:S445–51. doi: 10.1016/s0091-6749(99)70160-0. [DOI] [PubMed] [Google Scholar]
- Sandberg JK, Stoddart CA, Brilot F, Jordan KA, Nixon DF. Development of innate CD4+ alpha-chain variable gene segment 24 (Valpha24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc Natl Acad Sci U S A. 2004;101:7058–63. doi: 10.1073/pnas.0305986101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, Juji T, Hirai H. Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2002;168:3140–4. doi: 10.4049/jimmunol.168.7.3140. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Essential requirement of an invariant V alpha 14 T cell antigen receptor expression in the development of natural killer T cells. Proc Natl Acad Sci U S A. 1996;93:11025–8. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Vliet HJ, Nishi N, de Gruijl TD, von Blomberg BM, van den Eertwegh AJ, Pinedo HM, Giaccone G, Scheper RJ. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95:2440–2. [PubMed] [Google Scholar]
- Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nat Immunol. 2003;4:517–23. doi: 10.1038/ni0603-517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Viability of purified iNKT cells following magnetic bead and FACS purification, as assessed using 7-AAD and Annexin V staining. Staining of (positive) control, heat shock-treated peripheral blood mononuclear cells.