Abstract
Next generation sequencing and proteomics have helped to comprehensively characterize gene expression in tick salivary glands at both the transcriptome and the proteome level. Functional data are, however, lacking. Given that tick salivary secretions are critical to the success of the tick transmission lifecycle and, as a consequence, for host colonization by the pathogens they spread, we thoroughly review here the literature on the known interactions between tick saliva (or tick salivary gland extracts) and the innate and adaptive vertebrate immune system. The information is intended to serve as a reference for functional characterization of the numerous genes and proteins expressed in tick salivary glands with an ultimate goal to develop novel vector and pathogen control strategies.
Keywords: adaptive immunity, innate immunity, saliva, salivary glands, tick
Graphical abstract
Introduction
Ticks are obligatory blood-feeding arthropods that belong to the subclass Acari, order Ixodida, and three families: Ixodidae (hard ticks), Argasidae (soft ticks), and Nuttallielidae. Soft ticks feed repeatedly for minutes to hours, while hard ticks usually stay attached to their hosts and feed for several days or even weeks, but only once in each life stage [1, 2]. The amount of blood ingested is species and life-stage specific, with females of some tick species increasing their volume up to 200 times by the end of blood feeding [3].
Ticks are important vectors that transmit a wide range of pathogens. The most common tick-borne pathogens are viruses and bacteria, but fungi, protozoa, and helminths can also be transmitted [4]. Clinically and epidemiologically, the most important tick-borne diseases are: tick-borne encephalitis (TBE), caused by the TBE virus; Lyme disease, caused by spirochetes belonging to the Borrelia burgdorferi sensu lato complex in Europe and B. burgdorferi sensu stricto in the USA; tick-borne spotted fever, caused by Rickettsia spp.; anaplasmosis, caused by Anaplasma spp.; and babesiosis, caused by Babesia spp. protozoa [5, 6]. Pathogens have different life cycles, but the transmission usually begins with a tick biting an infected vertebrate host and pathogen uptake by the tick in the blood meal. Pathogens, e.g. Borrelia spp. spirochetes then stay in the midgut and wait until next feeding, which triggers their proliferation and migration through the midgut wall to haemocoel and, ultimately, to the salivary glands. Moreover, spirochetes interact with some midgut and salivary components that induce Borrelia proliferation or increase their infectious potential [7]. When the tick bites its next vertebrate host, pathogens are transmitted via tick saliva. In some tick species the pathogens are transmitted transovarially from the female to laid eggs, thus keeping the level of prevalence in the tick population [8]. Tick saliva has been shown to facilitate pathogen transfer to the vertebrate host by virtue of its pharmacological properties, including modulation of the vertebrate immune system [9–11]. Moreover, tick saliva contains toxins belonging to families also found in venomous animals, such as spiders or snakes, and that can induce paralysis and other toxicoses [12].
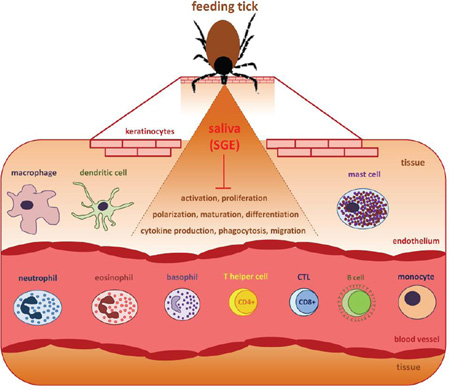
To secure uninterrupted blood uptake, ticks suppress and evade the complex physiological host immune and homeostatic responses that are raised against them. Hemostasis, which includes coagulation, vasoconstriction, and platelet aggregation, is the first innate host defense mechanism against the mechanical injury caused by intrusion of tick mouthparts into the host skin. This early vertebrate host response further includes complement activation and inflammation, with the host inflammatory response including, among other factors, rapid leukocyte infiltration after skin injury [13]. Keratinocytes, endothelial cells, and resident leukocytes such as mast cells, dendritic cells, and macrophages make immediate contact with tick saliva or the tick hypostome and are activated. Pro-inflammatory chemokines and cytokines including interleukin-8 (IL-8), tumor necrosis factor (TNF), and IL-1β are released to recruit neutrophils and other inflammatory cells to the area of tick infestation [14]. Following tick feeding, there is activation of both the cellular and humoral branches of vertebrate adaptive immunity [15]. Activated memory T and B cells (in the case of secondary infestation) amplify the host inflammatory response to ticks by releasing specific cytokines and producing antibodies that target tick salivary or mouthpart-derived antigens to activate complement or sensitize mast cells and basophils [9, 14, 15]. The strength and specificity of the host immune response and its effect on tick physiology depend on the host and tick species, the host’s health, and its genotype [16]. The same is true for tick defense mechanisms, since both tick salivary components and host immune mechanisms have been co-evolving. As a result, the tick-host interaction can be considered an “arms race” between the new defense mechanisms developed by the host and the evasion strategies developed by ticks [17]. As an adaptation to blood feeding, ticks secrete a complex mixture of immunomodulatory substances in their saliva that suppress both innate and adaptive host immune responses that can cause pain, itch, blood flow disruption in the tick feeding cavity, or even direct damage to the tick, thereby subverting tick rejection and death [18–20]. Despite the specificity of tick salivary component targets, there is also redundancy at the molecular, cellular, and functional level [9, 13]. The richness and diversity of tick salivary compounds have been established in several transcriptomic studies over the last fifteen years and, more recently, by next generation sequencing (NGS) studies.
The rapid developments in NGS and proteomics are reflected in the recent progress made in tick research, in which several transcriptomic and proteomic studies have been published over the last few years. These studies represent a rich data source that provides the basis for functional studies and investigation of gene expression dynamics during tick feeding and different physiological states. For instance, significant differences in the salivary proteome of partially and fully engorged female Rhipicephalus (Boophilus) microplus ticks have been described [21]. More recently, a transcriptomic study described over 800 immuno-proteins in Amblyomma americanum saliva during 24–48 hours of feeding [22]. A transcriptomic analysis of Dermacentor andersoni salivary glands resulted in over 500 singletons and 200 clusters in which a number of sequences with similarity to mammalian genes associated with immune response regulation, tumor suppression, and wound healing were identified [23]. By combining transcriptomic and proteomic approaches, nearly 700 proteins were identified in D. andersoni saliva after two and five days of feeding, from which 157 were postulated to be involved in immunomodulation and blood feeding [24]. Schwarz and colleagues performed a comprehensive study of Ixodes ricinus salivary and midgut transcriptomes and proteomes and found that the transcriptomic and proteomic dynamics did not 100% overlap in different tick tissues [25]. A recent study by Kotsyfakis and colleagues characterized transcriptional dynamics in the I. ricinus female and nymph salivary glands and midguts at various feeding time points [26], and established that some gene families show stage- and time-specific expression, possibly via epigenetic control. In addition, the genes encoding secreted proteins exhibited a high mutation rate, possibly representing a mechanism of antigenic variation, and analysis of the midgut transcriptome revealed several novel enzymes, transporters, and antimicrobial peptides [26]. A transcriptomic analysis of Amblyomma maculatum salivary glands revealed almost 3500 contigs with a secretory function [27]. Another sialome (salivary gland transcriptome) of Amblyomma ticks was published by Garcia and colleagues [28]: the authors analyzed samples from A. triste nymphs and females, A. cajennese females, and A. parvum females and focused on putative transcripts encoding anticoagulants, immunosuppressants, and anti-inflammatory molecules. A further study characterized A. americanum nymph and adult proteomes and compared the data with other Amblyomma species [29]. A Rhipicephalus pulchellus tick sialome study revealed differences between males and females [30], with the sequences identified used for a preliminary proteomic study to identify 460 male and over 2000 female proteins. A sialomic study was also performed in Haemaphysalis flava that revealed tens of thousands of genes, some of which were putative secreted salivary proteins thought to be involved with blood feeding and ingestion [31]. A Rhipicephalus sanguineus salivary proteome showed recycling of host proteins and their secretion back into the host [32]. Lewis and colleagues used a transcriptomic approach to characterize immunogenic I. scapularis salivary proteins present after 24 hours of feeding [33]; these appeared to be involved in tick feeding even before the majority of pathogens could be transmitted.
In addition to analysis of secreted tick salivary proteins, tick-feeding lesions on the host have been analyzed by high-throughput and histological methods. Recently, the feeding lesion of D. andersoni was described in detail together with microarray analysis of host gene expression dynamics, thereby characterizing the inflammatory infiltrate at the feeding site and the changes occuring in the epidermal and dermal compartments near the tick [34, 35]. The skin lesions examined from rats infested by Ornithodoros brasiliensis showed edema, muscle degeneration, and hemorrhage [36], with the rats themselves presenting with a bleeding tendency and signs of toxicosis. O. brasiliensis salivary gland homogenates delayed wound healing and had anti-proliferative or even cytotoxic activity on cultured epithelial cells [37]. An analysis of skin-draining lymph nodes in goats repeatedly infested with A. cajennese nymphs revealed an increased number of antigen presenting cells (APCs) such as B lymphocytes, macrophages, and dendritic cells [38]. A skin lesion from a human infested with female Amblyomma testudinarium was characterized by an inflammatory infiltrate and an eosinophilic cement in the center of the lesion [39]. Feeding lesions from rabbits injected with salivary gland extract (SGE) collected from R. sanguineus ticks after 2, 4, and 6 days of feeding showed signs of inflammation, especially at day four [40], suggesting that molecules present in R. sanguineus SGE have high immunogenicity and that immune reaction raised against SGE is stronger than the immunomodulatory action of R. sanguineus salivary effectors.
Such high-throughput studies in both ticks and hosts and complemented with histological information and detailed characterization of salivary components have made a valuable contribution to our knowledge of the dynamic processes occurring at the tick-host interface. However, experiments with saliva or SGE highlight the complexity of host modulation by the tick in vivo. Characterizing individual salivary components can help link specific pathophysiological events to particular molecules to provide a complete picture of tick-host interactions. In this review, we focus on the immunomodulatory actions of whole tick saliva or salivary gland extracts (SGE) rather than the effects of the individual salivary components, since these are reviewed elsewhere [13, 41, 42].
The role of tick saliva in modulating host hemostasis and complement
Ticks have developed various mechanisms to counteract the hemostatic responses of the host so that they can successfully feed on blood for many days [13, 19]. Serine proteases are key players in host hemostasis and, therefore, are specifically targeted by the wide range of serine protease inhibitors present in tick saliva. The net result is that the physiological balance between host proteases and endogenous anti-proteases is impaired. Tick salivary secretions also contain vasodilators, platelet activation inhibitors, and coagulation modulators, as reviewed elsewhere [14, 43, 44].
Complement is a cascade of proteolytically-activated components that eventually leads to the creation of pores in the walls of microbes, leading to their destruction. There are three main complement activation pathways: classical, alternative, and lectin; the central reaction in all pathways is the conversion of complement component C3 to C3a and C3b [45, 46]. The inhibition of the host alternative complement pathway is crucial for tick feeding and, indeed, the saliva of several Ixodes species inhibits this pathway [47, 48]. In an in vitro study, the ability of tick saliva to counteract complement activity varied according to the animal species source of serum, with specificity shown toward the most common hosts for each Ixodes species [49]. Several anti-complement molecules have been identified to date; however, a detailed description is beyond the scope of this review. Further information about the role of complement in tick-host interactions can be found in the reviews by Schroeder and colleagues [50] or Wikel [14].
Innate immunity and tick saliva
Innate immune responses against tick feeding involve the activation of resident immune cells that initiate and promote the local inflammatory response as a reaction to skin damage. The resident leukocytes are macrophages, Langerhans cells (LCs), mast cells, or innate lymphoid cells, and pro-inflammatory mediators are also released by endothelial cells and keratinocytes [51]. These mediators and complement components are chemotactic for circulating inflammatory cells including neutrophils and monocytes.
Interaction of macrophages and monocytes with tick saliva
Macrophages are APCs as well as cytokine and chemokine producers [52]. They can be further divided into two different subpopulations: (i) bone marrow-derived hematopoietic macrophages, which circulate as monocytes and, after extravasation at the site of inflammation, differentiate into pro-inflammatory [53] or alternatively-activated macrophages [54] and (ii) tissue-resident macrophages of yolk sac origin that are found in many organs including the skin; the latter tend to be more immune-modulatory [55]. These macrophage subpopulations differ with respect to cytokine production, receptor expression, and their overall effect on any subsequent immune response [54, 56, 57].
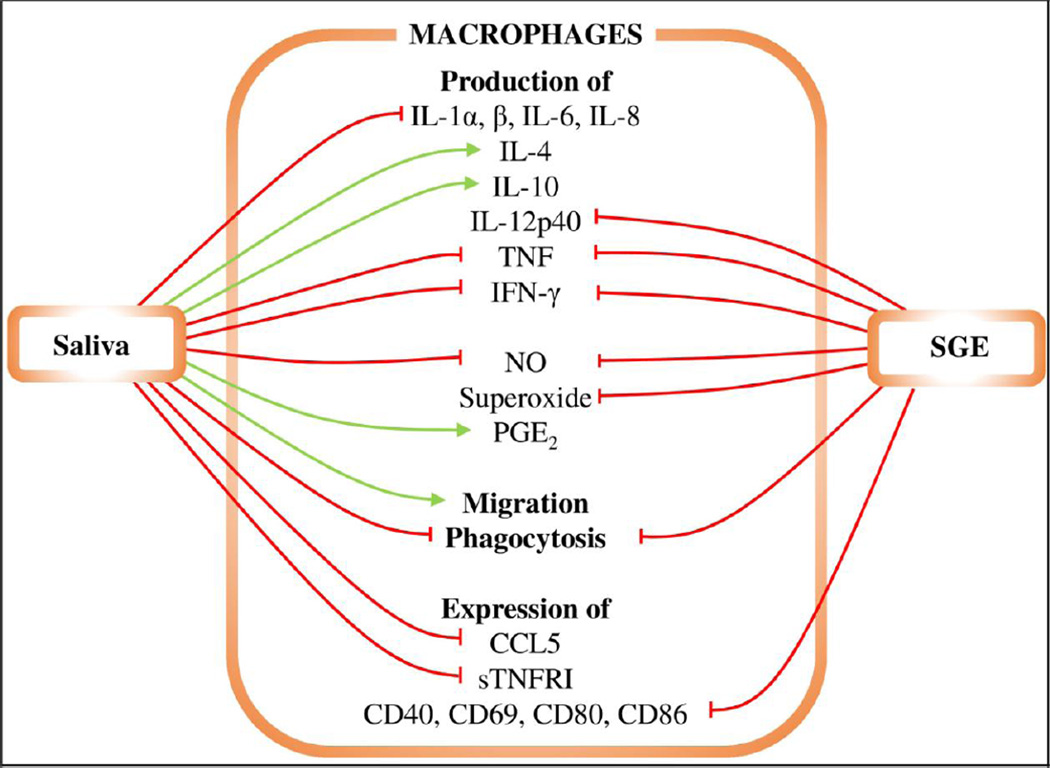
Numerous interactions have been identified between macrophages, tick saliva or SGE, and pathogens, suggesting that they play a major role in host defenses against ticks and tick-borne infectious agents. The effects of saliva or SGE on macrophages are summarized in Figure 1. I. ricinus SGE inhibited superoxide and nitric oxide (NO) production by Borrelia afzelii-activated macrophages, which led to the inhibition of Borrelia killing in a murine host [58]. I. ricinus SGE also reduced phagocytosis of B. afzelii spirochetes by murine macrophages and inhibited IFN-γ- and B. afzelii-stimulated TNF production by macrophages [59]. It was recently shown that I. ricinus saliva could induce the production of monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein 2 (MIP-2) in splenocytes [60]. MCP-1 attracts monocytes, and MIP-2 is a chemokine secreted by monocytes and macrophages that is chemotactic for neutrophils.
Figure 1. The effects of saliva and SGE on macrophages.
Red lines represent inhibition, green lines enhancement. Tick saliva inhibits production of IL-1α, IL-1β, IL-6, IL-8, TNF, IFN-γ, NO, superoxide, and CCL5, as well as expression of sTNFRI and phagocytosis. Tick saliva increases production of IL-4, IL-10, and PGE2 and macrophage migration. Tick SGE inhibits production of IL-12p40, TNF, IFN-γ, and NO, expression of CD40, CD69, CD80, and CD86, and phagocytosis.
Similarly, I. scapularis saliva inhibited TNF, IL-1β, IL-6, and IL-12p40 production by murine bone marrow-derived macrophages (BMDMs) after stimulation with lipopolysaccharide (LPS) or A. phagocytophilum. It was further reported to inhibit IL-8 secretion by human peripheral blood mononuclear cells (PBMCs) after TNF stimulation [61] and NO synthesis upon LPS stimulation [62].
Incubation with SGE isolated from R. microplus, a tick of veterinary importance, resulted in diminished expression of the co-stimulatory molecules CD80, CD86, CD40, and CD69 on the surface of bovine macrophages after 24 hours of LPS stimulation, which was accompanied by a decrease in TNF, IFN-γ, and IL-12 production [63]. Conversely, CD86 expression was increased in the murine macrophage cell line RAW 264.7 in response to R. microplus SGE and LPS but not SGE alone. Furthermore, SGE had no effect on CD40 and CD80 expression [64]. However, both bovine primary macrophages and murine macrophage cell line displayed an increase of CD86 expression after 6 hours incubation with LPS and SGE. [64]. These partially contradictory observations may be attributed to the host specific response. The difference may origin also from altered signaling in immortalized cell line, as CD86 upregulation was shown to be at least partially dependent on the ERK1/2 pathway and may, therefore, promote polarization of the immune response towards a less pro-inflammatory Th2 profile (see below) [64]. In another study, R. sanguineus saliva diminished NO production by IFN-γ-activated macrophages and thus impaired Trypanosoma cruzi killing. The authors suggested that decreased NO production was due to a saliva-induced cytokine imbalance, leading to decreased NO synthase activity [65]. Similar to the results with primary macrophages, SGE from Rhipicephalus appendiculatus affected cytokine production by the murine macrophage cell line JA-4. SGE from R. appendiculatus inhibited the transcription of IL-1α, IL-10, and TNF after macrophage stimulation with LPS. NO production was also lower, in accordance with the similar effect observed with I. ricinus saliva [58, 66].
Dermatocentor variabilis saliva has been shown to impair phagocytosis and alter gene expression in the murine macrophage cell line IC-21, as well as increase basal and platelet-derived growth factor (PDGF)-stimulated macrophage migration and the expression of the Th2-specific cytokines IL-4 and IL-10 [67].
The tick salivary component prostaglandin E2 (PGE2) subverted macrophage secretion of proinflammatory mediators and was able to recruit fibroblasts to heal tick-bite wound [68]. In addition to PGE2 from tick saliva, the saliva of D. variabilis upregulated PGE2 secretion in IC-21 murine peritoneal macrophages and reduced secretion of the pro-inflammatory mediators CCL5, TNF, and soluble TNF receptor I (sTNFRI) via a PGE2-dependent mechanism mediated by cAMP [68].
In summary, the tick saliva of various tick species inhibits the pro-inflammatory activities of macrophages, supporting a major role for macrophages in anti-tick defenses.
Dendritic cells and tick saliva
Dendritic cells (DCs) are APCs and are part of the innate immune system. After immature (unstimulated) DCs recognize and phagocytose pathogens, they mature and migrate to draining lymph nodes where they present antigens derived from the processed pathogen to CD4+ T cells, which subsequently launch an adaptive immune response. Thus, DCs initiate host adaptive immunity via presentation of pathogenic antigens. Two DC states exist: an immature form present in skin or mucosae and a mature form in lymphoid tissues. Langerhans cells (LCs) are a specialized resident cell type found in the vertebrate skin. Similar to macrophages, LCs have two origins and share many properties with macrophages [69]; therefore, they are sometimes considered to be a subtype of tissue macrophage [57]. Immature DCs primarily have an antigen uptake and presenting function, while mature DCs effectively stimulate T cells but have limited phagocytic activity. Several studies suggest that there are interactions between tick saliva and DCs [70–72]. For a review of the interactions between DCs, tick saliva, and Borrelia, see [73].
Oliveira and colleagues studied the effect of R. saguineus saliva on DC migration and function, and found that tick saliva reduced immature DC migration toward macrophage inflammatory proteins MIP-1α and MIP-1β but not MIP-3β [74]. Tick saliva also inhibited the chemokine RANTES by reducing expression of its surface receptor CCR5 [74]. DC maturation was impaired via toll-like receptor (TLR) signaling [75]. However, the inhibition of migration was limited to immature DCs. DC maturation and differentiation was inhibited in the presence of A. cajennese saliva [76]; in this study, the DCs showed reduced expression of CCR5 and CCR7 and, therefore, diminished migration toward the corresponding chemokines. Furthermore, tick saliva polarized cytokine production toward a Th2 phenotype. The authors suggested that most of the observed effects were due to the presence of PGE2 in tick saliva [76]. I. scapularis saliva has displayed various effects on bone marrow-derived DCs: it inhibited TNF and IL-12 production upon stimulation of different TLRs, in particular TLR-2, TLR-4, or TLR-9 [77], and the DC’s ability to stimulate antigen-specific CD4+ proliferation and IL-2 production was also suppressed [77]. LC-deficient mice induced Th1 responses after I. scapularis infestation, demonstrating the requirement for LCs in attenuating tick-mediated Th1 responses in regional lymph nodes [78].
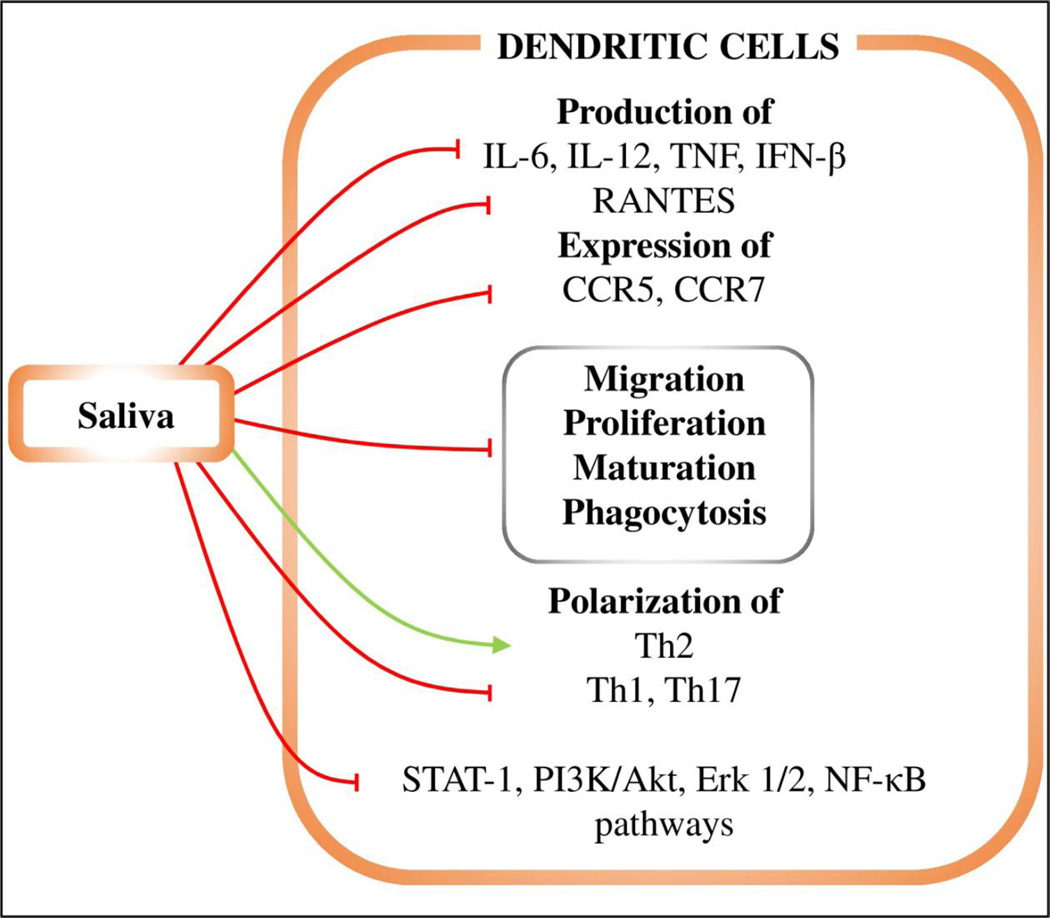
CD40 or TLR3, 7, and 9 ligation impaired DC maturation, and I. ricinus saliva inhibited DC migration in vivo and antigen presentation [79]. I. ricinus saliva has also been shown to impair Th1 and Th17 polarization in DCs [79] and activation of specific CD4+ T lymphocyte subsets by Borrelia-exposed DCs [80]. In the latter study, I. ricinus saliva decreased DC phagocytosis of B. afzelii. Interestingly, I. ricinus saliva inhibited DC production of both Th1 cytokines (TNF and IL-6) and the Th2 cytokine IL-10 after 48 hours (but not 24 hours) of incubation with B. afzelii [80]. I. ricinus saliva also impaired DC maturation and production of TNF and IL-6 in response to infection with TBE virus [81]. Lieskovská and Kopecký studied the signaling pathways activated in DCs via TLR-2 ligand and B. afzelii in the presence of tick saliva [82]; upon both types of activation, the NF-κB and phosphatidylinositol-3-kinase (PI3K)/Akt pathways were inhibited by I. ricinus saliva. When activated by Borrelia spirochetes, TNF levels decreased in DCs due to selective suppression of ERK1/2, Akt, and NF-κB as a result of tick saliva mimicking the native inhibitors. Tick saliva also attenuated IFN-β production, and IFN-β triggered signal transducer and activator of transcription-1 (STAT-1) activation [83]. A summary of the known interactions between DCs and tick saliva is shown in Figure 2.
Figure 2. The effects of saliva on dendritic cells.
Red lines represent inhibition, green lines enhancement. Tick saliva inhibits production of IL-6, IL-12, TNF, IFN-β, and RANTES cytokines. It also inhibits expression of CCR5 and CCR7, DC migration, proliferation, maturation, and phagocytosis, and STAT-1, PI3K/Akt, Erk1/2, and NF-κB signaling pathways. The saliva induces Th2 polarization while suppressing Th1 and Th17 differentiation.
Mast cells and tick saliva
Mast cells serve as sentinel cells and reside in many tissues. They are divided into two main types based on the presence of mast cell-specific proteases: connective tissue mast cells, which produce both tryptase and chymase (MCTC), and mucosal mast cells, which produce only tryptase (MCT) [84]; skin mast cells are of the first type. Upon exposure to pathogens or other stimuli, activated mast cells degranulate and release a variety of pre-stored mediators including vasoactive compounds, serine proteases, histamine, and cytokines. Activated mast cells also secrete newly synthesized mediators to recruit more inflammatory cells [85].
The immunological importance of mast cells in tick-host interactions remains unclear. Mast cell numbers increase after secondary or subsequent tick infestations, but remain unchanged during primary tick infestations [86–88]. The number of degranulated mast cells is also significantly higher after repeated tick infestations. Mast cell-deficient mice have been shown to develop some resistance to D. variabillis after repeated exposure, similar to wild type mice [89]. On the other hand, mast cell-deficient mice were not resistant to Haemaphysalis longicornis, with tick resistance re-established after mast cell injection [90, 91]. Such differences might be due to species-specific host responses or other unknown factors. Highly tick-resistant zebuine cattle breeds have more dermal mast cells than taurine breeds [92]. F2 crossbreeds of these two cattle were resistant to R. microplus infestation, with feeding with R. microplus larvae inducing significant increases in dermal mast cell numbers. Mast cells are major producers of the inflammatory mediator histamine, and ticks can affect histamine actions by either binding histamine via histamine-binding lipocalins [93, 94] or by promoting its secretion via histamine release factor [95], further evidence of the ambiguous role for mast cells in tick feeding responses. One explanation for histamine binding followed by its release can be explained by the need to suppress inflammatory responses at the early stage of feeding, followed by an increased need for vascular permeability during the rapid engorgement phase of tick feeding.
Granulocytes and tick saliva
Granulocytes are bone marrow-derived myeloid leukocytes that contain granules in their cytoplasm. The granulocyte group consists of three major cell types: basophils, eosinophils, and neutrophils [96].
Basophils and tick saliva
Basophils are IgE-activated granulocytes that, unlike tissue-resident mast cells, circulate in the blood. They play a critical role in the IgE-mediated development of chronic allergic reactions and inflammation [97, 98], and they can also promote polarization towards Th2 responses by IgE-independent antigen presentation in mice [99, 100]. Basophils are recruited to a tick-feeding site and accumulate in the host skin during second and consequent (but not primary) tick infestation, where they act as important tick rejection factors [101, 102]. After migration to the site of injury, basophils degranulate and release mediators such as histamine to reject ticks in a host reaction known as cutaneous basophil hypersensitivity [103]. Similar to mast cells, histamine release from basophils can be mediated by tick histamine release factor binding [95]. Several studies have confirmed the role of basophils in acquired immunity against ticks in mice [102, 104, 105]. Basophils expressing the immunoglobulin Fc receptor were found to be responsible for antibody-mediated acquisition of H. longicornis resistance [102], with selective basophil ablation by diphtheria toxin leading to loss of resistance to H. longicornis feeding in subsequent tick infestations [102]. Basophils appear to play a non-redundant role in antibody-mediated acquired immunity against ticks [102].
As noted above, I. ricinus saliva increased MCP-1 production by stimulated splenocytes [60]. MCP-1 is a potent basophil activator that triggers their degranulation and histamine release [106].
Basophils can cause cutaneous basophilia, a mechanism of tick resistance [104, 105]. The susceptibility or resistance of cattle to ticks (R. microplus) was associated with the number of basophils at the feeding site, with skin biopsies from tick-resistant breeds contain significantly more basophils than biopsies from susceptible breeds [107–109].
Eosinophils and tick saliva
Eosinophils are mainly present in mucosal areas in contact with the external environment such as the gut or lung mucosae. Their circulating levels are relatively low in healthy organisms, but increase during allergic reactions or parasitic infections [46]. Eosinophils produce cytokines, chemokines, and other mediators, some of which (e.g., indoleamine 2,3 deoxygenase; IDO) induce apoptosis and inhibit T cell proliferation [110, 111]. Eosinophils are also rich in granules that contain cytotoxic effectors such as eosinophil peroxidase, eosinophil cationic protein, eosinophil-derived neurotoxin, or major basic protein, which can cause mast cell (and probably also basophil) degranulation [112]. Finally, eosinophils are an important source of inflammatory and tissue repair-related molecules such as the transforming growth factors TGF-α and TGF-β1 and the extracellular matrix glycoprotein tenascin [113, 114].
Repeated exposure to both soft and hard tick species raised eosinophil levels at the feeding site in many host species including cattle [115, 116], dogs [117], guinea pigs [118, 119], rabbits [86], mice [88], woolless lambs [120], rats [36], capybaras [121], and even anteaters and armadillos [122]. The relationship between eosinophil number and tick resistance is not clear. Similar to mast cells, the susceptibility or resistance to ticks in cattle was associated with the number of eosinophils (and basophils) at the feeding site. Cattle breeds with more eosinophils (Bos taurus indicus, Nelores breed) appeared to be more resistant to R. microplus feeding than the Bos taurus taurus Holstein breed with fewer eosinophils [107]. In contrast, the tick count on Nguni and Bonsmara cattle was positively correlated with the eosinophil count in skin biopsies from tick feeding sites, while the correlation was negative in the case of mast cells and basophils [109].
Ticks inhibit the chemokine-mediated attraction of eosinophils to tick feeding sites. SGE from many tick species blocked CCL3, CCL5, or CCL11 (eotaxin) eosinophil chemoattractant activity [123–126].
Neutrophils and tick saliva
Neutrophils are granulocytes with both phagocytosis and degranulation roles. They are highly motile cells and they have a relatively short lifespan. Neutrophils play an important role in the early stages of vertebrate immune homeostasis, such as during acute inflammation, but they also play a role in some chronic inflammatory diseases. Neutrophils are generally activated by pathogens and secrete effectors and mediators that promote inflammation by recruiting other leukocytes, and they also directly kill pathogens by releasing their granules [46, 127]. They can also phagocytose and kill pathogens intracellularly [127].
Tick saliva modulates a local cutaneous immune response at the tick feeding site almost immediately after tick attachment, as shown by gene expression analysis of skin biopsies taken at several time points after the initiation of I. scapularis nymph feeding [128]. The expression of neutrophil-specific chemokines (CXCL1 and 5) was induced as early as 12 hours after tick attachment to the host [128]. Neutrophil abundance in the skin was high during the first tick infestation compared to other cell types but decreased during subsequent tick infestations of the same host [120, 129]. It is unknown whether the absence of neutrophils affects resistance of the host to ticks.
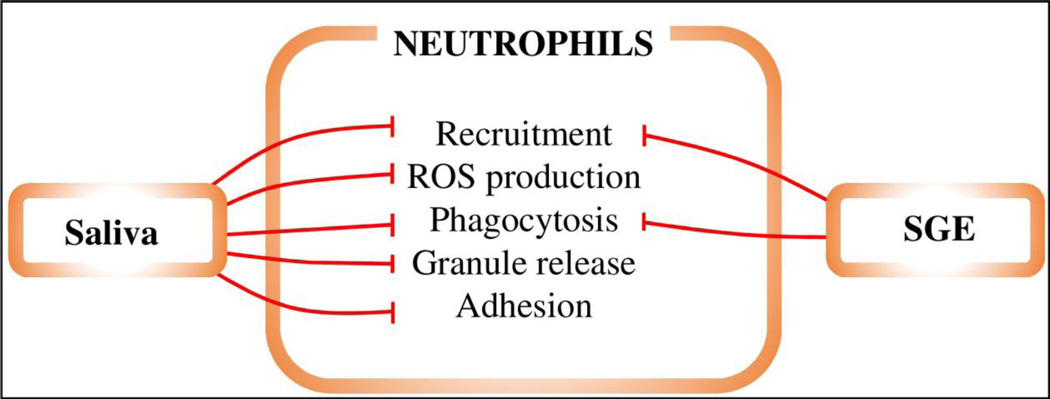
Saliva or SGE from soft and hard ticks have been shown to attenuate neutrophil functions such as recruitment by interfering with the neutrophil chemoattractants CXCL8 (IL-8) or CCL3 [123, 124, 126, 130, 131]. In one study, I. ricinus saliva significantly decreased neutrophil reactive oxygen species (ROS) production [132]. In contrast, the formation of neutrophil extracellular traps (NETs), which are formed by extrusion of neutrophil DNA and can retain and kill bacteria, was not affected by saliva [132]. I. scapularis (published as Ixodes dammini) saliva inhibited granule release and neutrophil infiltration and had an inhibitory effect on neutrophil phagocytosis of B. burgdorferi [133]. I. scapularis saliva also reduced polymorphonuclear leukocyte (PMN) adhesion by downregulating β2-integrin expression and signaling, which decreased pro-inflammatory functions of PMNs [134]. Finally, SGE from R. microplus inhibited neutrophil phagocytic activity in cattle [135]. These data show that tick saliva inhibits several pro-inflammatory neutrophil properties that are deleterious to tick feeding but does not affect anti-bacterial NET formation, suggesting that tick salivary activity is specific. The effects of tick saliva and SGE on neutrophils are illustrated in Figure 3.
Figure 3. The effects of saliva and SGE on neutrophils.
Red lines represent inhibition, green lines enhancement. Tick saliva inhibits neutrophil recruitment, phagocytosis, adhesion, granule release, and production of ROS.
T and B lymphocytes and tick saliva
Adaptive immunity relies on a wide range of antigen receptors (with varying antigen recognition specificities), which are clonally distributed in two types of lymphocytes: T cells and B cells. The induction of a specific immune response is only possible when a foreign antigen is recognized by the corresponding receptor. This first recognition signal is consolidated by the interaction of co-stimulatory molecules on T or B cells with those on APCs - such as dendritic cells or macrophages – that belong to the innate immune system. In this way, links are made between the cell populations that play dominant roles in the two branches of vertebrate immunity [136].
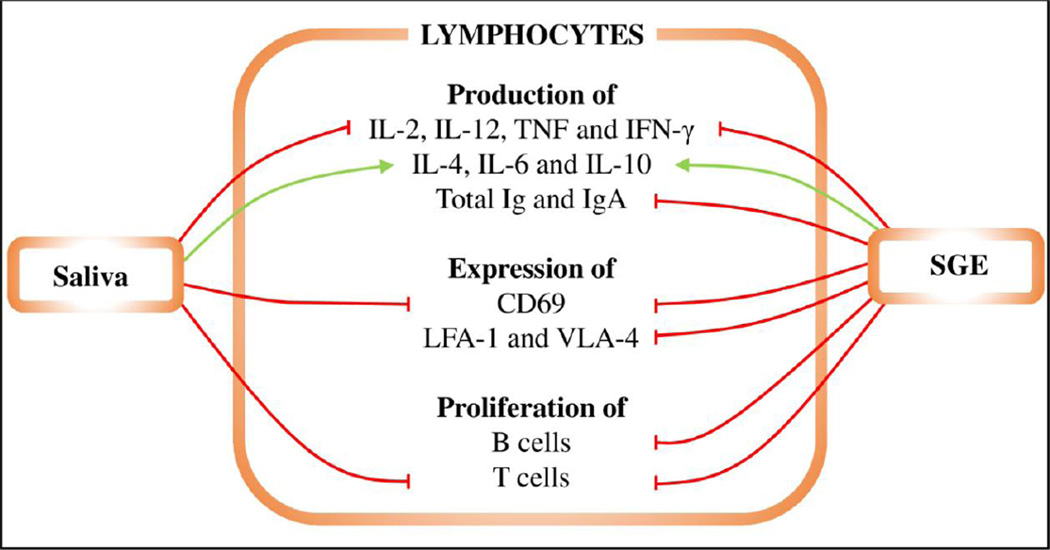
T cells are produced in the bone marrow from lymphoid progenitors and differentiate in the thymus. Mature T cells then migrate to the peripheral lymphoid tissues; they also circulate throughout the body [46]. Two major T cell subpopulations are recognized based on the co-receptor molecule expressed at the cell surface: CD4+ (T helper cells) and CD8+ T cells (which develop into cytotoxic T lymphocytes, CTLs). According to the secreted cytokine profile, CD4+ T helper cells can be further divided into several subpopulations that have different roles in immune responses [137], with Th1 and Th2 populations the most thoroughly studied in tick-host interactions thus far. Th1 populations are associated with host cellular and inflammatory responses, and Th2 populations with host humoral responses against ticks [138, 139]. Figure 4 illustrates how tick saliva and SGE influence T and B cell functions.
Figure 4. The effects of saliva and SGE on B and T lymphocytes.
Red lines represent inhibition, green lines enhancement. Tick saliva inhibits T cell proliferation, CD69 expression, and production of IL-2, IL-12, TNF, and IFN-γ by lymphocytes. In contrast, it increases production of IL-4, IL-6, and IL-10. Tick SGE has the same effects as tick saliva and, furthermore, suppresses LFA-1 and VLA-4 expression, proliferation of B cells, and total Ig and IgA production.
In 1985, I. scapularis (dammini) tick saliva was shown to inhibit IL-2 production by T lymphocytes, with PGE2 proposed to be responsible for this effect [140]. Urioste and colleagues confirmed diminished IL-2 levels in the presence of I. scapularis saliva, and showed profoundly inhibited splenic T cell proliferation in response to stimulation with concanavalin A (ConA) or phytohemagglutinin in the presence of saliva [62]; however, they disproved the PGE2 hypothesis, providing evidence that IL-2 is in fact inhibited by a proteinaceous salivary component. Later, in 2001, an unknown salivary component from I. scapularis was reported to bind IL-2 and inhibit T lymphocyte proliferation [141].
The inhibition of lymphocyte proliferation by SGE has also been reported in other tick species such as I. ricinus, Amblyomma variegatum, and R. appendiculatus, with species- and sex-specific differences shown for the effects of tick salivary gland antigens on human lymphocyte proliferation [142]. I. ricinus SGE suppressed isolated B cell proliferation and IL-10 production in response to LPS. CD69 activation marker expression on both activated T and B cells was also reduced [143]. I. ricinus saliva inhibited splenic T cell proliferation in response to ConA, and both SGE and saliva reduced the responsiveness of T cells draining to lymph nodes and sensitized splenic T cells [144]. The same observation was made with naïve splenic T cells [145]. T lymphocytes from mice infested nine days previously with I. ricinus nymphs displayed suppressed responses to ConA stimulation compared to cells from naïve mice [145]. In contrast, the lymph node cell response to LPS was increased in infested mice compared to naïve mice [145]. The authors attributed the observed effect to increased B lymphocyte numbers or activity [145]. On the other hand, soluble salivary gland antigens derived from female I. ricinus ticks stimulated lymph node T cells from mice infested with I. ricinus larvae or nymphs, but not those infested with Amblyomma hebraeum nymphs [146]. A 65 kDa protein fraction (IrSG65) isolated from the salivary glands of partially fed I. ricinus females induced specific T cell proliferation in lymph node cells obtained from mice infested with I. ricinus nymphs [146]. Feeding of I. ricinus nymphs on BALB/c mice revealed that CD4+ T cells were more abundant than CD8+ cells [147], which changed from 2:1 upon primary tick infestation to 7:1 in tertiary tick infestation. The ratio of CD3+ and CD4+ T lymphocytes was identical in I. ricinus infested and control mice [148].
D. andersoni SGE reduced ConA-induced proliferation of T cells [149, 150]. R. microplus feeding on cattle decreased the T lymphocyte percentage in peripheral blood lymphocytes (PBLs) [151], with the B lymphocyte percentage only lowering after repeated heavy infestations [151]. R. microplus saliva also suppressed PBL responsiveness to phytohemagglutinin [151]. R. sanguineus feeding on dogs suppressed ConA, phytohemagglutinin, and pokeweed mitogen-induced lymphocyte responses [152]. In the same study, SGE also suppressed all mitogen-stimulated blastogenic responses of lymphocytes from healthy dogs in vitro. Feeding of the Haemaphysalis bispinosa and Hyalomma anatolicum anatolicum ticks on sheep resulted in reduced circulating T lymphocyte counts as tick feeding progressed [153]. The authors showed that depletion of CD8+ populations and increased CD4+ T cell levels accounted for the observed effects [153]. Feeding of these two ticks also suppressed in vitro proliferation of T cells isolated from the tick-infested animals [153]. The CD4+/CD8+ and B/T lymphocyte ratios were increased in all sheep during infestation with either H. bispinosa and H. anatolicum anatolicum [153]. Interestingly, reduced CD4/CD8 T cell ratios were observed in skin biopsies taken at primary and secondary infestation with H. anatolicum anatolicum ticks on sheep compared to healthy skin biopsies [154].
B cells also originate from lymphoid progenitors in the bone marrow [46]. Their further differentiation involves migration from the bloodstream to the spleen, where they develop into mature B cells. Mature B cells circulate between the spleen and lymph nodes. The role of B cells lies in the surface expression and secretion of immunoglobulins upon activation [155]. In immunity against ticks, B cells produce specific antibodies against tick salivary and gut antigens.
Both primary and secondary infestations of sheep with H. anatolicum anatolicum ticks caused a significant increase in circulating B lymphocytes over several days [153]. In dogs, R. sanguineus SGE was shown to suppress total immunoglobulin and IgA (but not IgM) production by PBLs in vitro upon activation with LPS or pokeweed mitogen [156]. It has also been observed that anti-BSA IgG and IgM levels decreased in mice immunized with BSA during I. ricinus feeding [148]. However, anti-BSA IgG and IgM production was not decreased when BSA was injected prior to tick infestation. Interestingly, this study did not demonstrate a shift towards the Th2-type immune response when anti-BSA IgG1 and IgG2a antibody levels were compared between mice groups [148]. It was later shown that total IgG and IgM antibody levels were not reduced in animal sera by tick infestation, anti-BSA antibody production was not delayed, and memory cell formation did not appear to be inhibited by tick saliva [157]. Tick saliva did not affect memory B cell production of either anti-BSA IgG or IgM [157].
Experiments with tick saliva or SGE have shown polarization of the immune response from Th1 to Th2 branches by suppression of Th1 and upregulation of Th2 cytokines in both mice and humans. This polarization leads to an attenuated inflammatory response, which is beneficial for tick survival and feeding [15, 158]. Briefly, saliva or SGE inhibited secretion of IL-2, IL-12, TNF, and IFN-γ. In contrast, IL-4, IL-6, and IL-10 secretion was stimulated [66, 139, 159]. IL-10-specific neutralizing antibodies abrogated the suppressive effect of I. ricinus SGE on IFN-γ production [160]. IL-1α secretion was inhibited in JA-4 macrophage cells exposed to R. appendiculatus SGE [66]. In contrast, and in spite of their pro-inflammatory properties, IL-1α and IL-1β production was increased by Th1 lymphocytes and splenocytes after treatment with I. ricinus SGE [161, 162]. This can be explained by the fact that IL-1 can also act as a co-stimulator for Th2 lymphocyte proliferation. One of the mechanisms described for the action of I. ricinus saliva involves a negative effect on DCs, which then prime naive CD4+ T cells to induce Th2 cell differentiation in vitro and in vivo [71].
Feeding of D. andersoni decreased expression of two integrins, leukocyte function-associated antigen-1 (LFA-1) and very late activation-4 (VLA-4), by lymphocytes [163]. The same effect was achieved by exposing tick-naïve mouse lymphocytes to both D. andersoni saliva and SGE [163]. Infestation with D. andersoni nymphs or intradermal administration of female or male tick SGE increased IL-4 and IL-10 transcript levels in the draining lymph nodes and skin of the host [164]. Intracellular IL-4 levels were significantly increased in CD4+ T cells [164], and increased IL-4 levels were also observed during I. scapularis nymph feeding or by intradermal application of SGE [165]. Primary I. scapularis infestation on mice was characterized by late induction of an innate immune response and by inhibition of proinflammatory Th17 immunity. During secondary tick infestation, a mixed Th1/Th2 response was elicited [35].
Ticks have evolved various ways to circumvent adaptive immunity. Their saliva inhibits lymphocyte proliferation to reduce immune responses. Furthermore, ticks actively direct the immune response towards the Th2 arm that favors their feeding. The immunosuppressive properties of tick saliva also include inhibition of antibody production by B cells that could damage tick mouthparts and activate other cells or complement. The effects of tick saliva and SGE on lymphocytes are illustrated in Figure 4.
Natural killer cells and tick saliva
Despite their lymphoid origin, natural killer (NK) cells are part of the innate immune system [46]. Their main function is microbial or tumor cell killing and the regulation of endothelial cell, dendritic cell, and macrophage interactions with T lymphocytes [166]. SGE from female Dermatocentor reticulatus ticks that fed for 3–6 days on mice decreased human NK cell activity, while SGE from unfed or one day-fed ticks had no effect. Weaker activity was reported for SGE from A. variegatum and Haemaphysalis inermis ticks [167, 168], and NK cell cytotoxicity was suppressed after treatment with I. ricinus SGE [169].
Conclusions
Tick saliva clearly contains numerous different pharmacologically-active molecules that affect various immune cell populations and facilitate tick feeding. In this “systems biology” era, the effects of tick saliva described in this review can help in the design of experiments to discover specific salivary molecules that account for those effects. Although molecular biology and biochemical methods such as transcriptome and proteome analyses have provided excellent information about the genes expressed in the salivary glands of different tick species, the number of identified and functionally characterized salivary molecules remains limited. Ultimately, the goal is to fully uncover the complexity of how ticks modulate the host immune system so that this information can be used to pioneer the development of novel control strategies for ticks and tick-borne diseases and aid drug discovery.
Table 1.
The effects of tick saliva, SGE, or feeding on immune cell populations.
| Macrophages | |||
|---|---|---|---|
| Tick | Saliva/SGE/feeding | Effect | Reference |
|
Dermatocentor variabilis |
saliva | impaired phagocytosis and altered gene expression, stimulation of migration |
[67] |
| stimulation of PGE2 production, inhibition of cytokine production |
[68] | ||
| Ixodes ricinus | SGE | inhibition of superoxide and NO production | [58] |
| inhibition of phagocytosis and TNF production |
[59] | ||
| Ixodes scapularis | saliva | inhibition of cytokine production | [61] |
| inhibition of NO production | [62] | ||
|
Rhipicephalus appendiculatus |
SGE | inhibition of cytokine and NO production | [66] |
|
Rhipicephalus microplus |
SGE | altered surface molecule expression, inhibition of cytokine production |
[63, 64] |
|
Rhipicephalus sanguineus |
saliva | inhibition of NO production | [65] |
| Dendritic cells | |||
| Tick | Saliva/SGE/feeding | Effect | Reference |
| Amblyomma cajennese | saliva | inhibited maturation and differentiation; reduced migration due to decreased expression of receptors; polarization towards Th2 cytokines |
[76] |
| Ixodes ricinus | saliva | inhibited maturation, migration and antigen presentation; blocked Th1 and Th17 polarization |
[79] |
| inhibited proliferation, phagocytosis and cytokine production |
[80] | ||
| impaired maturation and cytokine production |
[81] | ||
| inhibition of signaling pathways | [82, 83] | ||
| Ixodes scapularis | saliva | inhibition of proliferation and cytokine production |
[77] |
|
Rhipicephalus sanguineus |
saliva | reduced migration, maturation and cytokine production |
[74, 75] |
| Basophils | |||
| Tick | Saliva/SGE/feeding | Effect | Reference |
| Amblyomma cajennense | feeding | increased amount of basophils in feeding cavity |
[121] |
| Amblyomma dubitatu | feeding | increased amount of basophils in feeding cavity |
[121] |
| Eosinophils | |||
| Tick | Saliva/SGE/feeding | Effect | Reference |
| soft and hard ticks | feeding | increased amount of eosinophils in feeding cavity |
[36, 88, 120–122] |
| hard ticks | SGE | inhibition of attraction to the feeding site | [123, 124] |
| Ixodes ricinus | saliva | basophil activation via MCP-1 released from splenocytes |
[60] |
| Neutrophils | |||
| Tick | Saliva/SGE/feeding | Effect | Reference |
| soft and hard ticks | SGE | anti-IL-8 activity | [123, 130] |
|
Amblyomma americanum |
SGE | altered dynamics of chemokine activity | [125] |
| Ixodes ricinus | saliva | decrease in ROS production | [132] |
| Ixodes scapularis | saliva | inhibition of granule release, infiltration, phagocytosis |
[133] |
| reduced adhesion of polymorphonuclear leukocytes |
[134] | ||
|
Rhipicephalus appendiculatus |
SGE | altered cytokines mRNA production by peripheral blood leukocytes |
[170] |
|
Rhipicephalus microplus |
SGE | inhibition of phagocytosis | [135] |
| Lymphocytes | |||
| Tick | Saliva/SGE/feeding | Effect | Reference |
| soft and hard ticks | saliva, SGE | polarization of the immune response towards Th2 via cytokines |
[66, 71, 139, 159, 161, 162, 171, 172] |
| Amblyomma variegatum | SGE | inhibition of lymphocyte proliferation | [142] |
| Dermacentor andersoni | SGE | reduced T cells proliferation | [149, 150] |
| reduced Th1 cytokine production | [173, 174] | ||
| saliva, SGE, feeding | inhibition of integrin expression | [163] | |
| SGE, feeding | increased IL-4 and IL-10 levels | [164] | |
|
Haemaphysalis bispinosa |
feeding | reduction in T lymphocyte count and proliferation, increased CD4+/CD8+ ratio |
[153] |
|
Hyalomma anatolicum anatolicum |
feeding | reduction in T lymphocyte count and proliferation, increased CD4+/CD8+ ratio, increase in circulating B lymphocyte count |
[153] |
| Ixodes ricinus | SGE | inhibition of lymphocyte proliferation | [142] |
| suppression of B cell proliferation, inhibition of IL-10 production, reduction of markers on the surface of T and B cells |
[143] | ||
| saliva | inhibition of T cell proliferation | [144] | |
| induction of Th2 differentiation of CD4+ T cells via dendritic cells |
[71] | ||
| feeding | increased CD4+/CD8+ ratio | [147] | |
| inhibited proliferation and responsiveness | [145] | ||
| reduced amount of specific Ig against antigen, no change in total Ig amount |
[148, 157] | ||
| Ixodes scapularis | saliva | inhibition of IL-2 production by T cells, inhibition of splenic T cell proliferation |
[62, 140, 141] |
| feeding | inhibition of Th17 immunity, priming of a mixed Th1/Th2 response during secondary infestation |
[35] | |
| SGE, feeding | increased IL-4 levels | [165] | |
|
Rhipicephalus appendiculatus |
SGE | inhibition of lymphocyte proliferation | [142] |
|
Rhipicephalus microplus |
feeding | decreased T and B lymphocyte percentage among PBLs |
[151] |
| saliva | decreased PBL responsiveness to phytohemagglutinin |
[151] | |
| inhibition of the blastogenic response of mononuclear cells |
[175] | ||
|
Rhipicephalus sanguineus |
feeding | suppressed response to mitogens | [152] |
| saliva | suppressed response to mitogens | [152] | |
| SGE | suppressed Ig production by PBL | [156] | |
| NK cells | |||
| Tick | Saliva/SGE/feeding | Effect | Reference |
| Amblyomma variegatum | SGE | decreased NK cell activity | [168] |
|
Dermatocentor reticulatus |
SGE | decreased NK cell activity | [167] |
| Haemaphysalis inermis | SGE | decreased NK cell activity | [168] |
| Ixodes ricinus | SGE | suppression of NK cell cytotoxicity | [169] |
Significance.
We overview all the known interactions of tick saliva with the vertebrate immune system. The provided information is important, given the recent developments in high-throughput transcriptomic and proteomic analysis of gene expression in tick salivary glands, since it may serve as a guideline for the functional characterization of the numerous newly-discovered genes expressed in tick salivary glands.
Highlights.
Tick salivary secretion is important in the interaction of ticks with the vertebrate hosts and the pathogens they transmit.
Recent Systems Biology approaches have characterized massively gene expression in tick salivary glands.
An important function of tick salivary secretion is to modulate vertebrate innate and adaptive immunity.
We overview the available literature about the immunomodulatory properties of tick saliva.
Our review may serve as a guideline for the discovery of the genes that mediate the specific function of tick saliva.
Acknowledgements
We thank Nextgenediting (www.nextgenediting.com) for providing editorial assistance and the anonymous reviewers for their constructive comments. This work was supported by the Grant Agency of the Czech Republic (GACR grant P502/12/2409 to MK), the Academy of Sciences of the Czech Republic (grant no. RVO60077344 to the Biology Center-Institute of Parasitology), and the 7th Framework Program of the European Union (EU FP7; Marie Curie Reintegration grant PIRG07-GA-2010-268177 to MK). This publication reflects only the authors’ views and the European Union is not liable for any use that may be made of the information contained herein. MK and JHFP were supported by the National Institutes ofHealth (R01 AI093653) and JHFP was supported by start-up funds provided by the University of Maryland, Baltimore School of Medicine. JFA and IMBF were supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; USA). MK received support from the Academy of Sciences of the Czech Republic (Jan Evangelista Purkyne Fellowship).
Because JFA and IMBF are government employees and this is government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. Rights can be established outside of the United States subject to a government use license.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
List of abbreviations
- Akt
protein kinase B
- BMDMs
bone marrow-derived macrophages
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- CCL
chemokine (C-C motif) ligand
- CCR
C-C motif receptor
- CD
cluster of differentiation
- ConA
concanavalin A
- CTL
cytotoxic T lymphocytes
- CXCL
chemokine (C-X-C motif) ligand
- DC
dendritic cell
- ERK
extracellular signal-regulated kinase
- IDO
indoleamine 2,3 deoxygenase
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- IRAK
interleukin-1 receptor-associated kinase
- LC
Langerhans cell
- LFA-1
leukocyte function-associated antigen-1
- LPS
lipopolysaccharide
- MC
mast cell
- MCP
monocyte chemotactic protein
- MIP
macrophage inflammatory protein
- NET
neutrophil extracellular trap
- NF-κB
nuclear factor kappa light chain-enhancer of activated B cells
- NK
natural killer
- NO
nitric oxide
- PBL
peripheral blood leukocytes
- PGE2
prostaglandin E2
- PI3k
phosphatidylinositol-3 kinase
- PMNs
polymorphonuclear lymphocytes
- RANTES
regulated upon activation, normal T cell expressed and secreted
- ROS
reactive oxygen species
- SGE
salivary gland extract
- STAT
signal transducer and activator of transcription
- sTNFRI
soluble TNF receptor I
- TGF
transforming growth factor
- Th
helper T cell
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- VLA-4
very late activation-4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that no conflicts of interest exist.
References
- 1.Brusca RC, Brusca GJ. Invertebrates. 2nd ed. Sunderland, Mass: Sinauer Associates; 2003. [Google Scholar]
- 2.Sonenshine DE, Roe RM. Biology of ticks. 2nd ed. New York: Oxford University Press; 2013. [Google Scholar]
- 3.Capinera JL. Encyclopedia of entomology. 2nd ed. New York: Springer; 2008. [Google Scholar]
- 4.Mehlhorn H. Encyclopedia of parasitology. 3rd. ed. New York: Springer; 2008. [Google Scholar]
- 5.Sonenshine DE, Roe RM. Biology of ticks. 2nd ed. New York: Oxford University Press; 2014. [Google Scholar]
- 6.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 7.Tilly K, Rosa PA, Stewart PE. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22:217–234. doi: 10.1016/j.idc.2007.12.013. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell JM, Ueti MW, Palmer GH, Scoles GA, Knowles DP. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J Clin Microbiol. 2007;45:426–431. doi: 10.1128/JCM.01757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman AS, Nuttall PA. Ticks : biology, disease and control. Cambridge, UK; New York: Cambridge University Press; 2008. [Google Scholar]
- 10.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu XY, Bonnet SI. Hard tick factors implicated in pathogen transmission. PLoS Negl Trop Dis. 2014;8:e2566. doi: 10.1371/journal.pntd.0002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabezas-Cruz A, Valdes JJ. Are ticks venomous animals? Front Zool. 2014;11:47. doi: 10.1186/1742-9994-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci (Landmark Ed) 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikel S. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol. 2013;4:337. doi: 10.3389/fmicb.2013.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brossard M, Wikel SK. Tick immunobiology. Parasitology. 2004;129(Suppl):S161–S176. doi: 10.1017/s0031182004004834. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira BR, Szabo MJ, Cavassani KA, Bechara GH, Silva JS. Antigens from Rhipicephalus sanguineus ticks elicit potent cell-mediated immune responses in resistant but not in susceptible animals. Vet Parasitol. 2003;115:35–48. doi: 10.1016/s0304-4017(03)00190-0. [DOI] [PubMed] [Google Scholar]
- 17.Andrade BB, Teixeira CR, Barral A, Barral-Netto M. Haematophagous arthropod saliva and host defense system: a tale of tear and blood. An Acad Bras Cienc. 2005;77:665–693. doi: 10.1590/s0001-37652005000400008. [DOI] [PubMed] [Google Scholar]
- 18.Hovius JW. Spitting image: tick saliva assists the causative agent of Lyme disease in evading host skin's innate immune response. J Invest Dermatol. 2009;129:2337–2339. doi: 10.1038/jid.2009.202. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 20.Chmelar J, Oliveira CJ, Rezacova P, Francischetti IM, Kovarova Z, Pejler G, et al. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood. 2011;117:736–744. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirloni L, Reck J, Terra RM, Martins JR, Mulenga A, Sherman NE, et al. Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS One. 2014;9:e94831. doi: 10.1371/journal.pone.0094831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radulovic ZM, Kim TK, Porter LM, Sze SH, Lewis L, Mulenga A. A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics. 2014;15:518. doi: 10.1186/1471-2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon-Chaidez FJ, Sun J, Wikel SK. Transcriptome analysis of the salivary glands of Dermacentor andersoni Stiles (Acari: Ixodidae) Insect Biochem Mol Biol. 2007;37:48–71. doi: 10.1016/j.ibmb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Mudenda L, Pierle SA, Turse JE, Scoles GA, Purvine SO, Nicora CD, et al. Proteomics informed by transcriptomics identifies novel secreted proteins in Dermacentor andersoni saliva. Int J Parasitol. 2014;44:1029–1037. doi: 10.1016/j.ijpara.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz A, Tenzer S, Hackenberg M, Erhart J, Gerhold-Ay A, Mazur J, et al. A systems level analysis reveals transcriptomic and proteomic complexity in ixodes ricinus midgut and salivary glands during early attachment and feeding. Molecular & cellular proteomics : MCP. 2014;13:2725–2735. doi: 10.1074/mcp.M114.039289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotsyfakis M, Schwarz A, Erhart J, Ribeiro JM. Tissue- and time-dependent transcription in Ixodes ricinus salivary glands and midguts when blood feeding on the vertebrate host. Scientific reports. 2015;5:9103. doi: 10.1038/srep09103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PloS One. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia GR, Gardinassi LG, Ribeiro JM, Anatriello E, Ferreira BR, Moreira HN, et al. The sialotranscriptome of Amblyomma triste, Amblyomma parvum and Amblyomma cajennense ticks, uncovered by 454-based RNA-seq. Parasit Vectors. 2014;7:430. doi: 10.1186/1756-3305-7-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villar M, Popara M, Mangold AJ, de la Fuente J. Comparative proteomics for the characterization of the most relevant Amblyomma tick species as vectors of zoonotic pathogens worldwide. J Proteomics. 2014;105:204–216. doi: 10.1016/j.jprot.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Tan AW, Francischetti IM, Slovak M, Kini RM, Ribeiro JM. Sexual differences in the sialomes of the zebra tick, Rhipicephalus pulchellus. J Proteomics. 2015;117:120–144. doi: 10.1016/j.jprot.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu XL, Cheng TY, Yang H, Yan F, Yang Y. De novo sequencing, assembly and analysis of salivary gland transcriptome of Haemaphysalis flava and identification of sialoprotein genes. Infect Genet Evol. 2015;32:135–142. doi: 10.1016/j.meegid.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira CJ, Anatriello E, de Miranda-Santos IK, Francischetti IM, Sa-Nunes A, Ferreira BR, et al. Proteome of Rhipicephalus sanguineus tick saliva induced by the secretagogues pilocarpine and dopamine. Ticks Tick Borne Dis. 2013;4:469–477. doi: 10.1016/j.ttbdis.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis LA, Radulovic ZM, Kim TK, Porter LM, Mulenga A. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks Tick Borne Dis. 2015 doi: 10.1016/j.ttbdis.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinze DM, Carmical JR, Aronson JF, Alarcon-Chaidez F, Wikel S, Thangamani S. Murine cutaneous responses to the rocky mountain spotted fever vector, Dermacentor andersoni, feeding. Front Microbiol. 2014;5:198. doi: 10.3389/fmicb.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinze DM, Wikel SK, Thangamani S, Alarcon-Chaidez FJ. Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit Vectors. 2012;5:26. doi: 10.1186/1756-3305-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reck J, Bandarra P, Pavarini S, Termignoni C, Driemeier D, Martins JR, et al. Experimentally induced tick toxicosis in rats bitten by Ornithodoros brasiliensis (Chelicerata: Argasidae): a clinico-pathological characterization. Toxicon. 2014;88:99–106. doi: 10.1016/j.toxicon.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Reck J, Marks FS, Termignoni C, Guimaraes JA, Martins JR. Ornithodoros brasiliensis (mouro tick) salivary gland homogenates inhibit in vivo wound healing and in vitro endothelial cell proliferation. Parasitol Res. 2013;112:1749–1753. doi: 10.1007/s00436-013-3333-3. [DOI] [PubMed] [Google Scholar]
- 38.Monteiro GE, Bechara GH, Franzin AM, de Miranda Santos IK. Antigen-presenting cells in draining lymph nodes of goats repeatedly infested by the Cayenne tick Amblyomma cajennense nymphs. Exp Appl Acarol. 2011;53:63–69. doi: 10.1007/s10493-010-9380-x. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Kang HA, Kim SS, Joo HS, Chong WS. Perianal tick-bite lesion caused by a fully engorged female Amblyomma testudinarium. Korean J Parasitol. 2014;52:685–690. doi: 10.3347/kjp.2014.52.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebling LM, Furquim KC, Bechara GH, Camargo-Mathias MI. Inoculation of salivary gland extracts obtained from female of Rhipicephalus sanguineus (Latreille, 1806) (Acari, Ixodidae) with 2, 4, and 6 days of feeding in rabbit: I--histopathology of the feeding lesion. Parasitol Res. 2013;112:577–584. doi: 10.1007/s00436-012-3169-2. [DOI] [PubMed] [Google Scholar]
- 41.Kazimirova M, Stibraniova I. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol. 2013;3:43. doi: 10.3389/fcimb.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stibraniova I, Lahova M, Bartikova P. Immunomodulators in tick saliva and their benefits. Acta Virol. 2013;57:200–216. doi: 10.4149/av_2013_02_200. [DOI] [PubMed] [Google Scholar]
- 43.Francischetti IM. Platelet aggregation inhibitors from hematophagous animals. Toxicon. 2010;56:1130–1144. doi: 10.1016/j.toxicon.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chmelar J, Calvo E, Pedra JH, Francischetti IM, Kotsyfakis M. Tick salivary secretion as a source of antihemostatics. J Proteomics. 2012;75:3842–3854. doi: 10.1016/j.jprot.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rother K, Till GO, Hansch GM. The Complement system. 2nd rev. ed. Berlin; New York: Springer; 1998. [Google Scholar]
- 46.Murphy K, Janeway CA, Mowat A. Janeway's immunobiology. 8th ed. New York: Garland Science; 2012. [Google Scholar]
- 47.Ribeiro JM. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 48.Lawrie CH, Sim RB, Nuttall PA. Investigation of the mechanisms of anti-complement activity in Ixodes ricinus ticks. Mol Immunol. 2005;42:31–38. doi: 10.1016/j.molimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Lawrie CH, Randolph SE, Nuttall PA. Ixodes ticks: serum species sensitivity of anticomplement activity. Exp Parasitol. 1999;93:207–214. doi: 10.1006/expr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder H, Skelly PJ, Zipfel PF, Losson B, Vanderplasschen A. Subversion of complement by hematophagous parasites. Dev Comp Immunol. 2009;33:5–13. doi: 10.1016/j.dci.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 52.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2014;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123:e110–e122. doi: 10.1182/blood-2013-08-520619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 57.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuthejlova M, Kopecky J, Stepanova G, Macela A. Tick salivary gland extract inhibits killing of Borrelia afzelii spirochetes by mouse macrophages. Infect Immun. 2001;69:575–578. doi: 10.1128/IAI.69.1.575-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyckova K, Kopecky J. Effect of tick saliva on mechanisms of innate immune response against Borrelia afzelii. J Med Entomol. 2006;43:1208–1214. [PubMed] [Google Scholar]
- 60.Langhansova H, Bopp T, Schmitt E, Kopecky J. Tick saliva increases production of three chemokines including monocyte chemoattractant protein-1, a histamine-releasing cytokine. Parasite Immunol. 2015;37:92–96. doi: 10.1111/pim.12168. [DOI] [PubMed] [Google Scholar]
- 61.Chen G, Severo MS, Sohail M, Sakhon OS, Wikel SK, Kotsyfakis M, et al. Ixodes scapularis saliva mitigates inflammatory cytokine secretion during Anaplasma phagocytophilum stimulation of immune cells. Parasit Vectors. 2012;5:229. doi: 10.1186/1756-3305-5-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urioste S, Hall LR, Telford SR, 3rd, Titus RG. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brake DK, Perez de Leon AA. Immunoregulation of bovine macrophages by factors in the salivary glands of Rhipicephalus microplus. Parasit Vectors. 2012;5:38. doi: 10.1186/1756-3305-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brake DK, Wikel SK, Tidwell JP, Perez de Leon AA. Rhipicephalus microplus salivary gland molecules induce differential CD86 expression in murine macrophages. Parasit Vectors. 2010;3:103. doi: 10.1186/1756-3305-3-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira BR, Silva JS. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-gamma-induced macrophage microbicidal activity. Vet Immunol Immunopathol. 1998;64:279–293. doi: 10.1016/s0165-2427(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 66.Gwakisa P, Yoshihara K, Long To T, Gotoh H, Amano F, Momotani E. Salivary gland extract of Rhipicephalus appendiculatus ticks inhibits in vitro transcription and secretion of cytokines and production of nitric oxide by LPS-stimulated JA-4 cells. Vet Parasitol. 2001;99:53–61. doi: 10.1016/s0304-4017(01)00445-9. [DOI] [PubMed] [Google Scholar]
- 67.Kramer CD, Poole NM, Coons LB, Cole JA. Tick saliva regulates migration, phagocytosis, and gene expression in the macrophage-like cell line, IC-21. Exp Parasitol. 2011;127:665–671. doi: 10.1016/j.exppara.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Poole NM, Mamidanna G, Smith RA, Coons LB, Cole JA. Prostaglandin E(2) in tick saliva regulates macrophage cell migration and cytokine profile. Parasit Vectors. 2013;6:261. doi: 10.1186/1756-3305-6-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavassani KA, Aliberti JC, Dias AR, Silva JS, Ferreira BR. Tick saliva inhibits differentiation, maturation and function of murine bone-marrow-derived dendritic cells. Immunology. 2005;114:235–245. doi: 10.1111/j.1365-2567.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mejri N, Brossard M. Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naive CD4+T to induce Th2 cell differentiation in vitro and in vivo. Int Immunol. 2007;19:535–543. doi: 10.1093/intimm/dxm019. [DOI] [PubMed] [Google Scholar]
- 72.Nithiuthai S, Allen JR. Langerhans cells present tick antigens to lymph node cells from tick-sensitized guinea-pigs. Immunology. 1985;55:157–163. [PMC free article] [PubMed] [Google Scholar]
- 73.Mason LM, Veerman CC, Geijtenbeek TB, Hovius JW. Menage a trois: Borrelia, dendritic cells, and tick saliva interactions. Trends Parasitol. 2014;30:95–103. doi: 10.1016/j.pt.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Oliveira CJ, Cavassani KA, More DD, Garlet GP, Aliberti JC, Silva JS, et al. Tick saliva inhibits the chemotactic function of MIP-1alpha and selectively impairs chemotaxis of immature dendritic cells by down-regulating cell-surface CCR5. Int J Parasitol. 2008;38:705–716. doi: 10.1016/j.ijpara.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Oliveira CJ, Carvalho WA, Garcia GR, Gutierrez FR, de Miranda Santos IK, Silva JS, et al. Tick saliva induces regulatory dendritic cells: MAP-kinases and Toll-like receptor-2 expression as potential targets. Vet Parasitol. 2010;167:288–297. doi: 10.1016/j.vetpar.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 76.Carvalho-Costa T, Mendes M, da Silva M, da Costa T, Tiburcio M, Anhe A, et al. Immunosuppressive effects of Amblyomma cajennense tick saliva on murine bone marrow-derived dendritic cells. Parasit Vectors. 2015;8:22. doi: 10.1186/s13071-015-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sa-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, et al. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- 78.Vesely DL, Fish D, Shlomchik MJ, Kaplan DH, Bockenstedt LK. Langerhans cell deficiency impairs Ixodes scapularis suppression of Th1 responses in mice. Infect Immun. 2009;77:1881–1887. doi: 10.1128/IAI.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skallova A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol. 2008;180:6186–6192. doi: 10.4049/jimmunol.180.9.6186. [DOI] [PubMed] [Google Scholar]
- 80.Slamova M, Skallova A, Palenikova J, Kopecky J. Effect of tick saliva on immune interactions between Borrelia afzelii and murine dendritic cells. Parasite Immunol. 2011;33:654–660. doi: 10.1111/j.1365-3024.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 81.Fialova A, Cimburek Z, Iezzi G, Kopecky J. Ixodes ricinus tick saliva modulates tick-borne encephalitis virus infection of dendritic cells. Microbes Infect. 2010;12:580–585. doi: 10.1016/j.micinf.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Lieskovska J, Kopecky J. Effect of tick saliva on signalling pathways activated by TLR-2 ligand and Borrelia afzelii in dendritic cells. Parasite Immunol. 2012;34:421–429. doi: 10.1111/j.1365-3024.2012.01375.x. [DOI] [PubMed] [Google Scholar]
- 83.Lieskovska J, Kopecky J. Tick saliva suppresses IFN signalling in dendritic cells upon Borrelia afzelii infection. Parasite Immunol. 2012;34:32–39. doi: 10.1111/j.1365-3024.2011.01345.x. [DOI] [PubMed] [Google Scholar]
- 84.Pejler G, Ronnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 85.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 86.Brossard M, Fivaz V. Ixodes ricinus L.: mast cells, basophils and eosinophils in the sequence of cellular events in the skin of infested or re-infested rabbits. Parasitology. 1982;85(Pt 3):583–592. doi: 10.1017/s0031182000056365. [DOI] [PubMed] [Google Scholar]
- 87.Gill HS. Kinetics of mast cell, basophil and eosinophil populations at Hyalomma anatolicum anatolicum feeding sites on cattle and the acquisition of resistance. Parasitology. 1986;93(Pt 2):305–315. doi: 10.1017/s0031182000051477. [DOI] [PubMed] [Google Scholar]
- 88.Ushio H, Watanabe N, Kiso Y, Higuchi S, Matsuda H. Protective immunity and mast cell and eosinophil responses in mice infested with larval Haemaphysalis longicornis ticks. Parasite Immunol. 1993;15:209–214. doi: 10.1111/j.1365-3024.1993.tb00602.x. [DOI] [PubMed] [Google Scholar]
- 89.Steeves EB, Allen JR. Tick resistance in mast cell-deficient mice: histological studies. Int J Parasitol. 1991;21:265–268. doi: 10.1016/0020-7519(91)90020-8. [DOI] [PubMed] [Google Scholar]
- 90.Matsuda H, Fukui K, Kiso Y, Kitamura Y. Inability of genetically mast cell-deficient W/Wv mice to acquire resistance against larval Haemaphysalis longicornis ticks. J Parasitol. 1985;71:443–448. [PubMed] [Google Scholar]
- 91.Matsuda H, Nakano T, Kiso Y, Kitamura Y. Normalization of anti-tick response of mast cell-deficient W/Wv mice by intracutaneous injection of cultured mast cells. J Parasitol. 1987;73:155–160. [PubMed] [Google Scholar]
- 92.Engracia Filho JR, Bechara GH, Teodoro RL. Dermal mast cell counts in F2 Holstein × Gir crossbred cattle artificially infested with the tick Boophilus microplus (Acari: Ixodidae) Ann N Y Acad Sci. 2006;1081:476–478. doi: 10.1196/annals.1373.070. [DOI] [PubMed] [Google Scholar]
- 93.Paesen GC, Adams PL, Nuttall PA, Stuart DL. Tick histamine-binding proteins: lipocalins with a second binding cavity. Biochim Biophys Acta. 2000;1482:92–101. doi: 10.1016/s0167-4838(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 94.Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI. Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol Cell. 1999;3:661–671. doi: 10.1016/s1097-2765(00)80359-7. [DOI] [PubMed] [Google Scholar]
- 95.Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog. 2010;6:e1001205. doi: 10.1371/journal.ppat.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delves PJ, Roitt IM. 2nd ed. San Diego: Academic Press; 1998. Encyclopedia of immunology. [Google Scholar]
- 97.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 98.Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 99.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 101.Oltean BM, Ernst M, Renneker S, Bakheit MA, Seitzer U, Ahmed J. Whole antigenic lysates of Ixodes ricinus, but not Der-p2 allergen-like protein, are potent inducers of basophil activation in previously tick-exposed human hosts. Transbound Emerg Dis. 2013;60(Suppl 2):162–171. doi: 10.1111/tbed.12151. [DOI] [PubMed] [Google Scholar]
- 102.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown SJ, Askenase PW. Rejection of ticks from guinea pigs by anti-hapten-antibody-mediated degranulation of basophils at cutaneous basophil hypersensitivity sites: role of mediators other than histamine. J Immunol. 1985;134:1160–1165. [PubMed] [Google Scholar]
- 104.Monteiro GE, Bechara GH. Cutaneous basophilia in the resistance of goats to Amblyomma cajennense nymphs after repeated infestations. Ann N Y Acad Sci. 2008;1149:221–225. doi: 10.1196/annals.1428.026. [DOI] [PubMed] [Google Scholar]
- 105.Otavio FS, Bechara GH. Guinea pigs develop cutaneous basophilia after repeated infestations by nymphs of the tick Amblyomma triste. Ann N Y Acad Sci. 2008;1149:226–229. doi: 10.1196/annals.1428.027. [DOI] [PubMed] [Google Scholar]
- 106.Bischoff SC, Krieger M, Brunner T, Dahinden CA. Monocyte chemotactic protein 1 is a potent activator of human basophils. J Exp Med. 1992;175:1271–1275. doi: 10.1084/jem.175.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carvalho WA, Franzin AM, Abatepaulo AR, de Oliveira CJ, More DD, da Silva JS, et al. Modulation of cutaneous inflammation induced by ticks in contrasting phenotypes of infestation in bovines. Vet Parasitol. 2010;167:260–273. doi: 10.1016/j.vetpar.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 108.Carvalho WA, Maruyama SR, Franzin AM, Abatepaulo AR, Anderson JM, Ferreira BR, et al. Rhipicephalus (Boophilus) microplus: clotting time in tick-infested skin varies according to local inflammation and gene expression patterns in tick salivary glands. Exp Parasitol. 2010;124:428–435. doi: 10.1016/j.exppara.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marufu MC, Dzama K, Chimonyo M. Cellular responses to Rhipicephalus microplus infestations in pre-sensitised cattle with differing phenotypes of infestation. Exp Appl Acarol. 2014;62:241–252. doi: 10.1007/s10493-013-9723-5. [DOI] [PubMed] [Google Scholar]
- 110.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 111.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 113.Elovic AE, Ohyama H, Sauty A, McBride J, Tsuji T, Nagai M, et al. IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J Immunol. 1998;160:6121–6127. [PubMed] [Google Scholar]
- 114.Phipps S, Flood-Page P, Menzies-Gow A, Ong YE, Kay AB. Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol. 2004;122:1406–1412. doi: 10.1111/j.0022-202X.2004.22619.x. [DOI] [PubMed] [Google Scholar]
- 115.Allen JR, Doube BM, Kemp DH. Histology of bovine skin reactions to Ixodes holocyclus Neumann. Can J Comp Med. 1977;41:26–35. [PMC free article] [PubMed] [Google Scholar]
- 116.Schleger AV, Lincoln DT, McKenna RV, Kemp DH, Roberts JA. Boophilus microplus: cellular responses to larval attachment and their relationship to host resistance. Aust J Biol Sci. 1976;29:499–512. doi: 10.1071/bi9760499. [DOI] [PubMed] [Google Scholar]
- 117.Szabo MP, Bechara GH. Sequential histopathology at the Rhipicephalus sanguineus tick feeding site on dogs and guinea pigs. Exp Appl Acarol. 1999;23:915–928. doi: 10.1023/a:1006347200373. [DOI] [PubMed] [Google Scholar]
- 118.Brown SJ, Askenase PW. Blood eosinophil and basophil responses in guinea pigs parasitized by Amblyomma americanum ticks. Am J Trop Med Hyg. 1982;31:593–598. doi: 10.4269/ajtmh.1982.31.593. [DOI] [PubMed] [Google Scholar]
- 119.Brown SJ, Worms MJ, Askenase PW. Rhipicephalus appendiculatus: larval feeding sites in guinea pigs actively sensitized and receiving immune serum. Exp Parasitol. 1983;55:111–120. doi: 10.1016/0014-4894(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 120.Prosdocimi CC, Bechara GH, Luduverio DJ, Otavio FM, Del Vecchio RE. Innate immunity in wooless lamb to larvae of Amblyomma cajennense tick (Fabricius, 1787) (Acari: Ixodidae) Transbound Emerg Dis. 2010;57:75–76. doi: 10.1111/j.1865-1682.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 121.van der Heijden KM, Szabo MP, Egami MI, Pereira MC, Matushima ER. Histopathology of tick-bite lesions in naturally infested capybaras (Hydrochoerus hydrochaeris) in Brazil. Exp Appl Acarol. 2005;37:245–255. doi: 10.1007/s10493-005-4155-5. [DOI] [PubMed] [Google Scholar]
- 122.Lima e Silva MF, Szabo MP, Bechara GH. Microscopic features of tick-bite lesions in anteaters and armadillos: Emas National Park and the Pantanal region of Brazil. Ann N Y Acad Sci. 2004;1026:235–241. doi: 10.1196/annals.1307.036. [DOI] [PubMed] [Google Scholar]
- 123.Hajnicka V, Vancova I, Kocakova P, Slovak M, Gasperik J, Slavikova M, et al. Manipulation of host cytokine network by ticks: a potential gateway for pathogen transmission. Parasitology. 2005;130:333–342. doi: 10.1017/s0031182004006535. [DOI] [PubMed] [Google Scholar]
- 124.Vancova I, Hajnicka V, Slovak M, Kocakova P, Paesen GC, Nuttall PA. Evasin-3-like anti-chemokine activity in salivary gland extracts of ixodid ticks during blood-feeding: a new target for tick control. Parasite Immunol. 2010;32:460–463. doi: 10.1111/j.1365-3024.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- 125.Vancova I, Slovak M, Hajnicka V, Labuda M, Simo L, Peterkova K, et al. Differential anti-chemokine activity of Amblyomma variegatum adult ticks during blood-feeding. Parasite Immunol. 2007;29:169–177. doi: 10.1111/j.1365-3024.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 126.Deruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, et al. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med. 2008;205:2019–2031. doi: 10.1084/jem.20072689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–1203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 128.Heinze DM, Carmical JR, Aronson JF, Thangamani S. Early immunologic events at the tick-host interface. PLoS One. 2012;7:e47301. doi: 10.1371/journal.pone.0047301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gill HS, Walker AR. Differential cellular responses at Hyalomma anatolicum anatolicum feeding sites on susceptible and tick-resistant rabbits. Parasitology. 1985;91(Pt 3):591–607. doi: 10.1017/s0031182000062831. [DOI] [PubMed] [Google Scholar]
- 130.Hajnicka V, Kocakova P, Slavikova M, Slovak M, Gasperik J, Fuchsberger N, et al. Anti-interleukin-8 activity of tick salivary gland extracts. Parasite Immunol. 2001;23:483–489. doi: 10.1046/j.1365-3024.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- 131.Peterkova K, Vancova I, Hajnicka V, Slovak M, Simo L, Nuttall PA. Immunomodulatory arsenal of nymphal ticks. Med Vet Entomol. 2008;22:167–171. doi: 10.1111/j.1365-2915.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 132.Menten-Dedoyart C, Faccinetto C, Golovchenko M, Dupiereux I, Van Lerberghe PB, Dubois S, et al. Neutrophil extracellular traps entrap and kill Borrelia burgdorferi sensu stricto spirochetes and are not affected by Ixodes ricinus tick saliva. J Immunol. 2012;189:5393–5401. doi: 10.4049/jimmunol.1103771. [DOI] [PubMed] [Google Scholar]
- 133.Ribeiro JM, Weis JJ, Telford SR., 3rd Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990;70:382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- 134.Montgomery RR, Lusitani D, De Boisfleury Chevance A, Malawista SE. Tick saliva reduces adherence and area of human neutrophils. Infect Immun. 2004;72:2989–2994. doi: 10.1128/IAI.72.5.2989-2994.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turni C, Lee RP, Jackson LA. Effect of salivary gland extracts from the tick, Boophilus microplus, on leucocytes from Brahman and Hereford cattle. Parasite Immunol. 2002;24:355–361. doi: 10.1046/j.1365-3024.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 136.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 137.Kara EE, Comerford I, Fenix KA, Bastow CR, Gregor CE, McKenzie DR, et al. Tailored immune responses: novel effector helper T cell subsets in protective immunity. PLoS Pathog. 2014;10:e1003905. doi: 10.1371/journal.ppat.1003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 139.Ferreira BR, Silva JS. Successive tick infestations selectively promote a T-helper 2 cytokine profile in mice. Immunology. 1999;96:434–439. doi: 10.1046/j.1365-2567.1999.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med. 1985;161:332–344. doi: 10.1084/jem.161.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gillespie RD, Dolan MC, Piesman J, Titus RG. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J Immunol. 2001;166:4319–4326. doi: 10.4049/jimmunol.166.7.4319. [DOI] [PubMed] [Google Scholar]
- 142.Rolnikova T, Kazimirova M, Buc M. Modulation of human lymphocyte proliferation by salivary gland extracts of ixodid ticks (Acari: Ixodidae): effect of feeding stage and sex. Folia Parasitol (Praha) 2003;50:305–312. [PubMed] [Google Scholar]
- 143.Hannier S, Liversidge J, Sternberg JM, Bowman AS. Ixodes ricinus tick salivary gland extract inhibits IL-10 secretion and CD69 expression by mitogen-stimulated murine splenocytes and induces hyporesponsiveness in B lymphocytes. Parasite Immunol. 2003;25:27–37. doi: 10.1046/j.1365-3024.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- 144.Mejri N, Rutti B, Brossard M. Immunosuppressive effects of ixodes ricinus tick saliva or salivary gland extracts on innate and acquired immune response of BALB/c mice. Parasitol Res. 2002;88:192–197. doi: 10.1007/s00436-001-0515-1. [DOI] [PubMed] [Google Scholar]
- 145.Ganapamo F, Rutti B, Brossard M. Immunosuppression and cytokine production in mice infested with Ixodes ricinus ticks: a possible role of laminin and interleukin-10 on the in vitro responsiveness of lymphocytes to mitogens. Immunology. 1996;87:259–263. doi: 10.1046/j.1365-2567.1996.450512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ganapamo F, Rutti B, Brossard M. Identification of an Ixodes ricinus salivary gland fraction through its ability to stimulate CD4 T cells present in BALB/c mice lymph nodes draining the tick fixation site. Parasitology. 1997;115(Pt 1):91–96. doi: 10.1017/s0031182097008913. [DOI] [PubMed] [Google Scholar]
- 147.Mbow ML, Rutti B, Brossard M. Infiltration of CD4+ CD8+ T cells, and expression of ICAM-1, Ia antigens, IL-1 alpha and TNF-alpha in the skin lesion of BALB/c mice undergoing repeated infestations with nymphal Ixodes ricinus ticks. Immunology. 1994;82:596–602. [PMC free article] [PubMed] [Google Scholar]
- 148.Menten-Dedoyart C, Couvreur B, Thellin O, Drion PV, Herry M, Jolois O, et al. Influence of the Ixodes ricinus tick blood-feeding on the antigen-specific antibody response in vivo. Vaccine. 2008;26:6956–6964. doi: 10.1016/j.vaccine.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 149.Ramachandra RN, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J Med Entomol. 1992;29:818–826. doi: 10.1093/jmedent/29.5.818. [DOI] [PubMed] [Google Scholar]
- 150.Wikel SK. Influence of Dermacentor andersoni infestation on lymphocyte responsiveness to mitogens. Ann Trop Med Parasitol. 1982;76:627–632. doi: 10.1080/00034983.1982.11687593. [DOI] [PubMed] [Google Scholar]
- 151.Inokuma H, Kerlin RL, Kemp DH, Willadsen P. Effects of cattle tick (Boophilus microplus) infestation on the bovine immune system. Vet Parasitol. 1993;47:107–118. doi: 10.1016/0304-4017(93)90181-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Inokuma H, Aita T, Ohno K, Onishi T. Effects of infestation by Rhipicephalus sanguineus on lymphocyte blastogenic responses to mitogens in dogs. J Vet Med Sci. 1998;60:1013–1016. doi: 10.1292/jvms.60.1013. [DOI] [PubMed] [Google Scholar]
- 153.Boppana DK, Dhinakar Raj G, John L, Wikel SK, Latha BR, Gomathinayagam S. In vivo immunomodulatory effects of ixodid ticks on ovine circulating T- and B-lymphocytes. Parasite Immunol. 2004;26:83–93. doi: 10.1111/j.0141-9838.2004.00687.x. [DOI] [PubMed] [Google Scholar]
- 154.Boppana DK, Wikel SK, Raj DG, Manohar MB, Lalitha J. Cellular infiltration at skin lesions and draining lymph nodes of sheep infested with adult Hyalomma anatolicum anatolicum ticks. Parasitology. 2005;131:657–667. doi: 10.1017/S0031182005008243. [DOI] [PubMed] [Google Scholar]
- 155.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 156.Matsumoto K, Inokuma H, Okuda M, Onishi T. Effects of salivary gland extract from Rhipicephalus sanguineus on IgG subclass production and cytokine mRNA expression in mononuclear cells of canine peripheral blood. J Vet Med Sci. 2003;65:137–140. doi: 10.1292/jvms.65.137. [DOI] [PubMed] [Google Scholar]
- 157.Menten-Dedoyart C, Couvreur B, Jolois O, Van Lerberghe PB, Duwez L, Drion P, et al. Kinetic study of the antibody response during the blood meal of Ixodes ricinus: implication on plasma cell maturation in vivo and for anti-Ixodes vaccination. Vaccine. 2011;29:2044–2050. doi: 10.1016/j.vaccine.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 158.Wikel SK. Tick modulation of host cytokines. Exp Parasitol. 1996;84:304–309. doi: 10.1006/expr.1996.0118. [DOI] [PubMed] [Google Scholar]
- 159.Mejri N, Franscini N, Rutti B, Brossard M. Th2 polarization of the immune response of BALB/c mice to Ixodes ricinus instars, importance of several antigens in activation of specific Th2 subpopulations. Parasite Immunol. 2001;23:61–69. doi: 10.1046/j.1365-3024.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 160.Kopecky J, Kuthejlova M, Pechova J. Salivary gland extract from Ixodes ricinus ticks inhibits production of interferon-gamma by the upregulation of interleukin-10. Parasite Immunol. 1999;21:351–356. doi: 10.1046/j.1365-3024.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 161.Kovar L, Kopecky J, Rihova B. Salivary gland extract from Ixodes ricinus tick polarizes the cytokine profile toward Th2 and suppresses proliferation of T lymphocytes in human PBMC culture. J Parasitol. 2001;87:1342–1348. doi: 10.1645/0022-3395(2001)087[1342:SGEFIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 162.Kovar L, Kopecky J, Rihova B. Salivary gland extract from Ixodes ricinus tick modulates the host immune response towards the Th2 cytokine profile. Parasitol Res. 2002;88:1066–1072. doi: 10.1007/s00436-002-0714-4. [DOI] [PubMed] [Google Scholar]
- 163.Macaluso KR, Wikel SK. Dermacentor andersoni: effects of repeated infestations on lymphocyte proliferation, cytokine production, and adhesion-molecule expression by BALB/c mice. Ann Trop Med Parasitol. 2001;95:413–427. doi: 10.1080/00034980120059081. [DOI] [PubMed] [Google Scholar]
- 164.Boppana VD, Thangamani S, Alarcon-Chaidez FJ, Adler AJ, Wikel SK. Blood feeding by the Rocky Mountain spotted fever vector, Dermacentor andersoni, induces interleukin-4 expression by cognate antigen responding CD4+ T cells. Parasit Vectors. 2009;2:47. doi: 10.1186/1756-3305-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Muller-Doblies UU, Maxwell SS, Boppana VD, Mihalyo MA, McSorley SJ, Vella AT, et al. Feeding by the tick, Ixodes scapularis, causes CD4(+) T cells responding to cognate antigen to develop the capacity to express IL-4. Parasite Immunol. 2007;29:485–499. doi: 10.1111/j.1365-3024.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- 166.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 167.Kubes M, Fuchsberger N, Labuda M, Zuffova E, Nuttall PA. Salivary gland extracts of partially fed Dermacentor reticulatus ticks decrease natural killer cell activity in vitro. Immunology. 1994;82:113–116. [PMC free article] [PubMed] [Google Scholar]
- 168.Kubes M, Kocakova P, Slovak M, Slavikova M, Fuchsberger N, Nuttall PA. Heterogeneity in the effect of different ixodid tick species on human natural killer cell activity. Parasite Immunol. 2002;24:23–28. doi: 10.1046/j.0141-9838.2001.00434.x. [DOI] [PubMed] [Google Scholar]
- 169.Kopecky J, Kuthejlova M. Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 1998;20:169–174. [PubMed] [Google Scholar]
- 170.Fuchsberger N, Kita M, Hajnicka V, Imanishi J, Labuda M, Nuttall PA. Ixodid tick salivary gland extracts inhibit production of lipopolysaccharide-induced mRNA of several different human cytokines. Exp Appl Acarol. 1995;19:671–676. doi: 10.1007/BF00145255. [DOI] [PubMed] [Google Scholar]
- 171.Ganapamo F, Rutti B, Brossard M. In vitro production of interleukin-4 and interferon-gamma by lymph node cells from BALB/c mice infested with nymphal Ixodes ricinus ticks. Immunology. 1995;85:120–124. [PMC free article] [PubMed] [Google Scholar]
- 172.Wu J, Wang Y, Liu H, Yang H, Ma D, Li J, et al. Two immunoregulatory peptides with antioxidant activity from tick salivary glands. J Biol Chem. 2010;285:16606–16613. doi: 10.1074/jbc.M109.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bergman DK, Ramachandra RN, Wikel SK. Dermacentor andersoni: salivary gland proteins suppressing T-lymphocyte responses to concanavalin A in vitro. Exp Parasitol. 1995;81:262–271. doi: 10.1006/expr.1995.1117. [DOI] [PubMed] [Google Scholar]
- 174.Bergman DK, Ramachandra RN, Wikel SK. Characterization of an immunosuppressant protein from Dermacentor andersoni (Acari: Ixodidae) salivary glands. J Med Entomol. 1998;35:505–509. doi: 10.1093/jmedent/35.4.505. [DOI] [PubMed] [Google Scholar]
- 175.Inokuma H, Kemp DH, Willadsen P. Prostaglandin E2 production by the cattle tick (Boophilus microplus) into feeding sites and its effect on the response of bovine mononuclear cells to mitogen. Vet Parasitol. 1994;53:293–299. doi: 10.1016/0304-4017(94)90193-7. [DOI] [PubMed] [Google Scholar]