Abstract
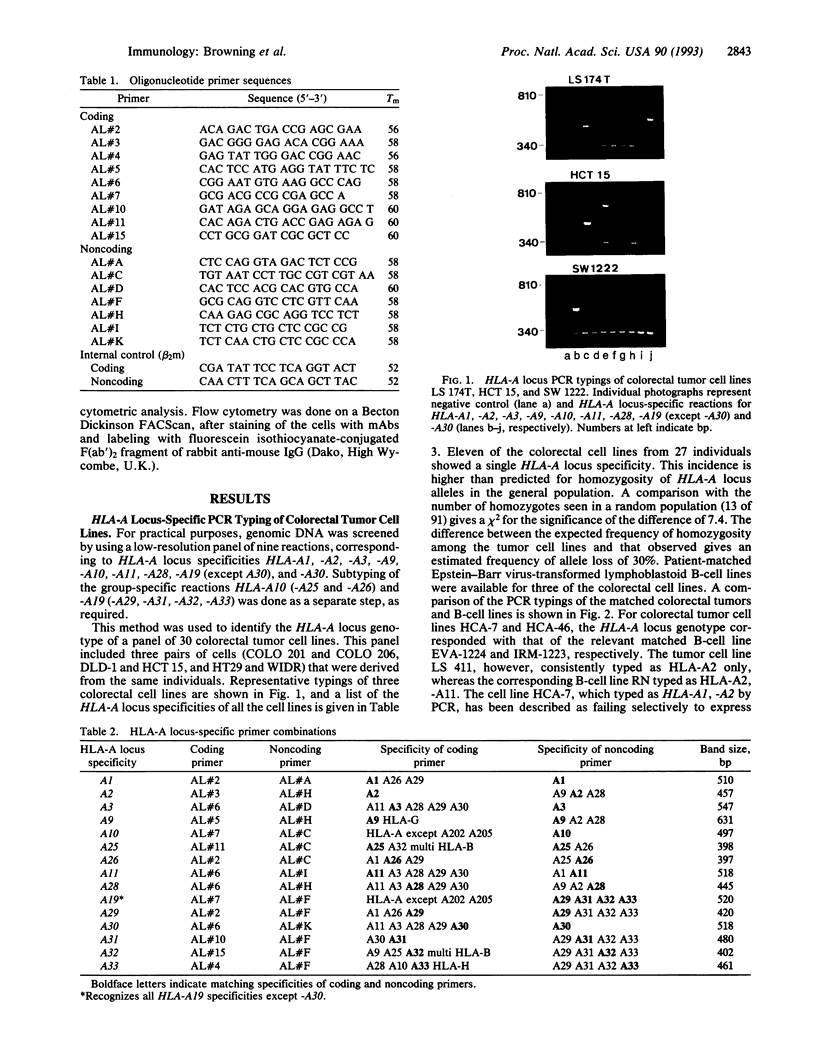
A system devised for tissue typing the HLA-A locus by PCR from genomic DNA has been used to investigate abnormalities of HLA expression in a panel of 30 colorectal tumor cell lines, by comparing the HLA-A locus genotype with surface expression of HLA. Three cell lines showed complete lack of HLA expression associated with failure to express beta 2-microglobulin. In two other cell lines, loss of expression of HLA-A2 was observed, in spite of the presence of the gene in genomic DNA. Eleven cell lines gave a single HLA-A locus specificity on PCR typing. In one of these cell lines we have demonstrated the loss of an HLA-A locus gene in the tumor cell by comparison with DNA from a lymphoblastoid B-cell line derived from the same patient. These data indicate that at least three independent mechanisms were involved in the loss of HLA expression on the colorectal tumor cell lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. The HLA system: structure and function. J Clin Pathol. 1987 Sep;40(9):948–958. doi: 10.1136/jcp.40.9.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J. A., Mason D. W., Morris P. J. Evidence that rat renal allografts are rejected by cytotoxic T cells and not by nonspecific effectors. Transplantation. 1985 Feb;39(2):169–175. doi: 10.1097/00007890-198502000-00012. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Browning M. J., Bodmer W. F. MHC antigens and cancer: implications for T-cell surveillance. Curr Opin Immunol. 1992 Oct;4(5):613–618. doi: 10.1016/0952-7915(92)90036-e. [DOI] [PubMed] [Google Scholar]
- Dexter D. L., Barbosa J. A., Calabresi P. N,N-dimethylformamide-induced alteration of cell culture characteristics and loss of tumorigenicity in cultured human colon carcinoma cells. Cancer Res. 1979 Mar;39(3):1020–1025. [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin H., Bodmer W. F. A sensitive micro-immunoassay using beta-galactosidase/anti-beta-galactosidase complexes. J Immunol Methods. 1987 Feb 26;97(1):19–27. doi: 10.1016/0022-1759(87)90100-1. [DOI] [PubMed] [Google Scholar]
- Fleming K. A., McMichael A., Morton J. A., Woods J., McGee J. O. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981 Jul;34(7):779–784. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni-Celli S., Kirsch K., Timpane R., Isselbacher K. J. Beta 2-microglobulin gene is mutated in a human colon cancer cell line (HCT) deficient in the expression of HLA class I antigens on the cell surface. Cancer Res. 1992 Mar 1;52(5):1201–1204. [PubMed] [Google Scholar]
- Greenhalgh D. A., Kinsella A. R. c-Ha-ras not c-Ki-ras activation in three colon tumour cell lines. Carcinogenesis. 1985 Oct;6(10):1533–1535. doi: 10.1093/carcin/6.10.1533. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Bevan M. J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992 Jun 1;175(6):1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. H., Schacter B. Z., Delaney N. L., Miller T. B., McKusick V. A., Kennett R. H., Bodmer J. G., Young D., Bodmer W. F. Genetic characteristics of the HeLa cell. Science. 1976 Jan 30;191(4225):392–394. doi: 10.1126/science.1246620. [DOI] [PubMed] [Google Scholar]
- Hérin M., Lemoine C., Weynants P., Vessière F., Van Pel A., Knuth A., Devos R., Boon T. Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer. 1987 Mar 15;39(3):390–396. doi: 10.1002/ijc.2910390320. [DOI] [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Jr, Welch W. R., Knapp R. C., Bhan A. K. Expression of major histocompatibility antigens and nature of inflammatory cellular infiltrate in ovarian neoplasms. Int J Cancer. 1983 Nov 15;32(5):547–554. doi: 10.1002/ijc.2910320505. [DOI] [PubMed] [Google Scholar]
- Kirkland S. C., Bailey I. G. Establishment and characterisation of six human colorectal adenocarcinoma cell lines. Br J Cancer. 1986 Jun;53(6):779–785. doi: 10.1038/bjc.1986.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausa P., Moses J., Bodmer W., Bodmer J., Browning M. HLA-A locus alleles identified by sequence specific PCR. Lancet. 1993 Jan 9;341(8837):121–122. doi: 10.1016/0140-6736(93)92605-s. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Leibovitz A., Stinson J. C., McCombs W. B., 3rd, McCoy C. E., Mazur K. C., Mabry N. D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976 Dec;36(12):4562–4569. [PubMed] [Google Scholar]
- McBain J. A., Weese J. L., Meisner L. F., Wolberg W. H., Willson J. K. Establishment and characterization of human colorectal cancer cell lines. Cancer Res. 1984 Dec;44(12 Pt 1):5813–5821. [PubMed] [Google Scholar]
- Murakami H., Masui H. Hormonal control of human colon carcinoma cell growth in serum-free medium. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3464–3468. doi: 10.1073/pnas.77.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi P., Wallace R., Johnson J., Earley E. M., O'Brien S., Ferrone S., Pellegrino M. A., Milstien J., Needy C., Browne W. Characterization of the WIDR: a human colon carcinoma cell line. In Vitro. 1979 Jun;15(6):401–408. doi: 10.1007/BF02618407. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Buckle B. G., Sheer D., Wigley C. B. The isolation and characterization of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patients. Int J Cancer. 1984 Jul 15;34(1):49–56. doi: 10.1002/ijc.2910340109. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Semple T. U., Quinn L. A., Woods L. K., Moore G. E. Tumor and lymphoid cell lines from a patient with carcinoma of the colon for a cytotoxicity model. Cancer Res. 1978 May;38(5):1345–1355. [PubMed] [Google Scholar]
- Smith M. E., Bodmer J. G., Kelly A. P., Trowsdale J., Kirkland S. C., Bodmer W. F. Variation in HLA expression on tumors: an escape from immune response. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):581–586. doi: 10.1101/sqb.1989.054.01.069. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Marsh S. G., Bodmer J. G., Gelsthorpe K., Bodmer W. F. Loss of HLA-A,B,C allele products and lymphocyte function-associated antigen 3 in colorectal neoplasia. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5557–5561. doi: 10.1073/pnas.86.14.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Suardet L., Gaide A. C., Calmès J. M., Sordat B., Givel J. C., Eliason J. F., Odartchenko N. Responsiveness of three newly established human colorectal cancer cell lines to transforming growth factors beta 1 and beta 2. Cancer Res. 1992 Jul 1;52(13):3705–3712. [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986 Jul;58(3):417–420. [PMC free article] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Van Pel A., Vessière F., Boon T. Protection against two spontaneous mouse leukemias conferred by immunogenic variants obtained by mutagenesis. J Exp Med. 1983 Jun 1;157(6):1992–2001. doi: 10.1084/jem.157.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Vánky F., Li S. L., Végh Z., Persson U., Klein E. Expression of MHC-class-I antigens in human carcinomas and sarcomas analyzed by isoelectric focusing. Int J Cancer Suppl. 1991;6:106–116. doi: 10.1002/ijc.2910470721. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Jones J. K., Gabriel A., Lukies R. E. A new colon carcinoma cell line (LIM1863) that grows as organoids with spontaneous differentiation into crypt-like structures in vitro. Cancer Res. 1987 May 15;47(10):2683–2689. [PubMed] [Google Scholar]
- Zemmour J., Parham P. HLA class I nucleotide sequences, 1991. Tissue Antigens. 1991 Apr;37(4):174–180. doi: 10.1111/j.1399-0039.1991.tb01869.x. [DOI] [PubMed] [Google Scholar]