Abstract
Recent studies have shown that the Gram-positive bacterium Lactococcus lactis can be exploited for the expression of heterologous proteins; however, a versatile set of vectors suitable for inducible extracellular protein production and subsequent purification of the expressed proteins by immobilized metal affinity chromatography was so far lacking. Here we describe three novel vectors that, respectively, facilitate the nisin-inducible production of N- or C-terminally hexa-histidine (His6)-tagged proteins in L. lactis. One of these vectors also encodes a tobacco etch virus (TEV) protease cleavage site allowing removal of the N-terminal His6-tag from expressed proteins. Successful application of the developed vectors for protein expression, purification and/or functional studies is exemplified with six different cell wall-bound or secreted proteins from Staphylococcus aureus. The results show that secretory production of S. aureus proteins is affected by the position, N- or C-terminal, of the His6-tag. This seems to be due to an influence of the His6-tag on protein stability. Intriguingly, the S. aureus IsdB protein, which is phosphorylated in S. aureus, was also found to be phosphorylated when heterologously produced in L. lactis, albeit not on the same Tyr residue. This implies that this particular post-translational protein modification is to some extent conserved in S. aureus and L. lactis. Altogether, we are confident that the present vector set combined with the L. lactis expression host has the potential to become a very useful tool in optimization of the expression, purification and functional analysis of extracytoplasmic bacterial proteins.
Electronic supplementary material
The online version of this article (doi:10.1007/s00253-015-6778-8) contains supplementary material, which is available to authorized users.
Keywords: L. lactis, Expression vectors, NICE, Usp45, Histidine tag, Secretion
Introduction
Over the past decades, a wide range of bacterial expression systems for heterologous protein production has been developed (Zerbs et al. 2009). Today, the Gram-negative bacterium Escherichia coli is one of the most commonly used organisms for large-scale heterologous protein production (Terpe 2006). This is due to the ease of handling, the multitude of available expression vectors and the relatively simple fermentation procedures for E. coli (Zerbs et al. 2009; Chen 2012). Despite these advantages, some clear disadvantages of the use of E. coli are evident. In the first place, E. coli is not capable of efficiently secreting heterologous proteins into the growth medium since exported proteins usually remain confined in the periplasm. Secondly, overexpression of heterologous proteins in E. coli often leads to the formation of high-density aggregates of misfolded proteins known as inclusion bodies. Thirdly, the post-translational modification of proteins that are heterologously produced in E. coli is likely to be different from the modification that these proteins undergo in their original host. Lastly, the inherent production of the well-known endotoxin lipopolysaccharide (LPS) is a major drawback for the clinical application of E. coli-derived recombinant proteins (Braun et al. 1999; Petsch and Anspach 2000; Sarvas et al. 2004; Westers et al. 2004; Neef et al. 2014).
While E. coli has become a preferred host for the cytoplasmic production of structurally simple biotherapeutics, other bacterial species, especially Gram-positive bacteria, are preferred hosts for the secretory production of structurally more challenging types of proteins. For example, Bacillus species are highly popular expression platforms for enzymes (Terpe 2006). Importantly, organisms such as Bacillus subtilis are generally regarded as safe (GRAS). Moreover, they can secrete proteins directly into the fermentation broth to high concentrations, thereby simplifying their downstream processing. However, bacilli often secrete endogenous proteases at high levels, which often requires the use of multiple protease-deficient strains (Li et al. 2004; Krishnappa et al. 2013). Alternatively, the Gram-positive bacterium Lactococcus lactis has been successfully applied for the secretory production of protease-sensitive proteins (Morello et al. 2008; Neef et al. 2014). This relates to the fact that this GRAS organism produces only two proteases that can potentially interfere with protein production. These two proteases, the cytoplasmic ClpP protease and the extracytoplasmic HtrA protease, are completely dispensable and their removal strongly reduces product degradation (Morello et al. 2008; Poquet et al. 2000; Miyoshi et al. 2002; Cortes-Perez et al. 2006). Moreover, the unwanted autolysis of L. lactis cells is prevented by the removal of the major autolysin AcmA which, combined with an htrA deletion, leads to the stable and efficient production of secreted proteinaceous antigens of Staphylococcus aureus (Neef et al. 2014).
Several inducible expression systems have been developed for L. lactis (Morello et al. 2008) of which the nisin-inducible (NICE) system is the most efficient and extensively used (Mierau 2005). This system is based on the regulation of the nisA promoter by the food-grade lantibiotic nisin, which activates the NisRK two-component regulatory system (De Ruyter et al. 1996). The NICE system has thus been used for production of a wide range of homologous and heterologous proteins, including vaccines (Zhou et al. 2006).
The purification of overproduced proteins can be facilitated by particular tags that bind with high affinity to a specific matrix. The hexa-histidine (His6)-tag is the most widely used tag and ensures efficient separation by metal affinity chromatography (Jones et al. 1995). However, the exact placement of these tags can influence the solubility and/or stability of overproduced proteins (Woestenenk et al. 2004). To circumvent the latter problems, changing the location of the His6-tag from the N- to the C-terminus or vice versa may prove beneficial. Notably, although the His6-tag has usually limited impact on protein structure or function (Terpe 2003), it is desirable to remove it prior to structure-function studies (Arnau et al. 2011). Therefore, a specific protease cleavage site, e.g. for the tobacco etch virus (TEV) protease, is often placed between the target protein and the affinity tag.
In this study, we describe an expression vector set that facilitates convenient exploration of nisin-inducible protein production in L. lactis. As shown with a representative panel of extracytoplasmic proteinaceous antigens from S. aureus, the overproduced proteins can be purified by metal affinity chromatography using N- or C-terminal His6-tags. The latter is useful since our present results show that, also in L. lactis, the exact position of the His6-tag affects production efficiency and/or protein stability. In one vector configuration, the His6-tag can be removed from the expressed proteins by cleavage with the TEV protease. Of note, we show that L. lactis is capable of phosphorylating the IsdB protein of S. aureus.
Materials and methods
Bacterial strains and growth conditions
Strains and plasmids are listed in Table 1. E. coli was grown at 37 °C in Lysogeny broth (LB; Becton Dickinson, Breda, The Netherlands) with ampicillin (100 μg/ml) for plasmid selection. L. lactis was grown at 30 °C in M17 broth (Oxoid Limited, Hampshire, UK) supplemented with 0.5 % glucose (w/v) (GM17). Chloramphenicol (5 μg/ml) was added when needed. For nisin production, the L. lactis NZ9700 strain was cultured in GM17 and the cell-free supernatant was used for induction of the PnisA promoter in a 1:1000 dilution at OD600 ~ 0.5 (Kuipers et al. 1997).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant phenotype(s) or genotype(s) | Source and reference |
|---|---|---|
| Strains | ||
| L. lactis NZ9700 | Nisin producer | NIZO culture collection, Ede, The Netherlands (Kuipers et al. 1993) |
| L. lactis PA1001 | MG1363 pepN::nisRK, allows nisin-inducible expression, ΔacmA ΔhtrA | Mucosis culture collection, Groningen, The Netherlands (Bosma et al. 2006) |
| S. aureus Newman | NCTC 8178 clinical isolate | PHE culture collection NCTC8178 (Duthie and Lorenz 1952) |
| S. aureus USA300 | Community-acquired MRSA isolate | ATCC strain BAA-1717 (McDougal et al. 2003) |
| S. aureus N315 | Hospital-acquired MRSA | (Kuroda et al. 2001) |
| S. aureus NCTC8325 | Restriction-deficient derivative of NCTC 8325; cured of all known prophages | WDCM culture collection 154 (Kreiswirth et al. 1983) |
| Plasmids | ||
| pET302/NT | ApR, containing PT7, lacO, MCS, his 6, T7 terminator | Life Technologies |
| pEF110 | pET302/NT derivative, ApR, containing PT7, his 6, BamHI/EcoRI-XbaI/NotI cloning sites | Laboratory collection J.A.G. van Strijp |
| pEF111 | pET302/NT derivative, ApR, containing PT7, his 6, TEV cleavage site, BamHI/EcoRI-XbaI/NotI cloning sites | Laboratory collection J.A.G. van Strijp |
| pEF210 | pET302/NT derivative, ApR, containing PT7, BamHI/EcoRI-XbaI/NotI cloning sites, his 6 | Laboratory collection J.A.G. van Strijp |
| pNG400 | CmR, containing PnisA, SSusp45, and a transcription terminator | (Bosma et al. 2006) |
| pNG4110 | pNG400 derivative, containing his 6, BamHI/EcoRI-XbaI/NotI cloning sites | This study |
| pNG4111 | pNG400 derivative, containing his 6, TEV cleavage site, BamHI/EcoRI-XbaI/NotI cloning sites | This study |
| pNG4210 | pNG400 derivative, containing BamHI/EcoRI-XbaI/NotI cloning sites, his 6 followed by a Stop codon | This study |
Ap R ampicillin resistance gene, Cm R chloramphenicol resistance gene, P T7 IPTG inducible T7-promoter, P nisA nisin-inducible promoter, his 6 6 histidine tag, SS usp45 signal sequence of usp45, MCS multiple cloning site
General molecular biology
Enzymes and buffers were from New England Biolabs (Ipswich, UK) and Fermentas (Landsmeer, The Netherlands). PCR was performed using a Bio-Rad C1000 Thermal Cycler (Richmond, CA). Primers were from Eurogentec (Maastricht, The Netherlands) (Table 2 and Table S1). The polymerases PFU (Fermentas, Landsmeer, The Netherlands), Pwo (Roche, Woerden, The Netherlands) and Taq (Life Technologies, Bleiswijk, The Netherlands) were used according to the manufacturer. PCR products were purified using the High Pure PCR Purification Kit from Roche (Woerden, The Netherlands). Plasmid purification was performed using the Plasmid Isolation Kit from Analytik Jena AG (Jena, Germany); L. lactis was lysed by incubation with lysozyme (4 mg/ml; Sigma-Aldrich, Zwijndrecht, The Netherlands) for 10 min at 55 °C in resuspension buffer followed by addition of lysis buffer. L. lactis was transformed by electroporation using a Gene Pulser (Biorad; Leenhouts and Venema 1993). Nucleotide sequence analysis was performed by Eurofins DNA (Ebersberg, Germany).
Table 2.
Primers used for the construction of the expression vectors
| Primer | 5′ → 3′ nucleotide sequencea | Restriction siteb |
|---|---|---|
| NHis_F | TATGCACCATCACCATCACCATGGATCCGAATTCCTCGAGGCGGCCGCATAAA | |
| NHis_R | ACGTGGTAGTGGTAGTGGTACCTAGGCTTAAGGAGCTCCGCCGGCGTATTTCTAG | |
| CHis_F | TATGGGATCCGAATTCCTCGAGGCGGCCGCACACCATCACCATCACCATTAAA | |
| CHis_R | ACCCTAGGCTTAAGGAGCTCCGCCGGCGTGTGGTAGTGGTAGTGGTAATTTCTAG | |
| NHisTEV_F | TATGCACCATCACCATCACCATGAGAACCTGTACTTCCAGGGATCCGAATTCCTCGAGGCGGCCGCATAAA | |
| NHisTEV_R | ACGTGGTAGTGGTAGTGGTACTCTTGGACATGAAGGTCCCTAGGCTTAAGGAGCTCCGCCGGCGTATTTCTAG | |
| pEF110.f | CCCCGTCTC CCATGCACCATCACCATCATCATGGATCCGAA | BsmBI |
| pEF110.r | CCCAAGCTT TTA CCATGGTGCGGCCGCCTCGAGG | HindIII and NotI |
| pEF111.f | CCCCGTCTC CCATGCACCATCACCATCACCATGAG | BsmBI |
| pEF111.r | CCCAAGCTT TTATGCGGCCGCCTCGAGG | HindIII |
| pEF210.f | CCCCCATGGGATCCGAATTCCTCGAGGC | NcoI |
| pEF210.r | CCCAAGCTT TTAATGGTGATGGTGATGGTGTGC | HindIII |
aRestriction site underlined, stop codon in bold
b BsmBI restriction resulting in NcoI overhang in italic
Construction of expression vectors
The E. coli pEF vector set is based on plasmid pET302/NT. A total of three primer pairs were created (NHis_F/NHis_R for pEF110, CHis_F/CHis_R for pEF210, NHisTEV_F/NHisTEV_R for pEF111) (Table 2) containing an NdeI and BglII overhang at their 5′ and 3′ ends. These primers were annealed by incubation at a 1:1 ratio at 94 °C and stepwise-controlled temperature drops to room temperature. The fragments were ligated into the NdeI and BamHI digested pET302/NT vector resulting in deletion of the original vector-derived BamHI site and introduction of a multiple cloning site comprising BamHI, EcoRI, XhoI and NotI restriction sites (Fig. 1). Following transformation in E. coli TOP10F cells, the prepared constructs were sequence-verified.
Fig. 1.
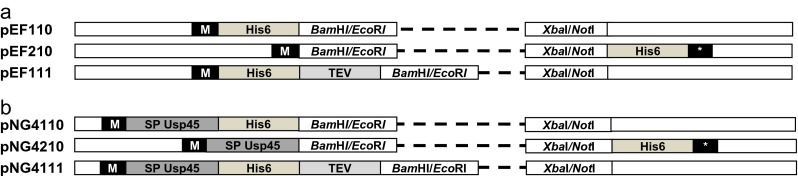
Expression cassettes present in the vectors for heterologous protein production in E. coli or L. lactis. a Expression cassettes of the E. coli pEF vectors, encoding an N-terminal His6-tag (pEF110), a C-terminal His6-tag (pEF210) or a TEV-removable (TEV) N-terminal His6-tag (pEF111). Restriction sites for cloning (BamHI/EcoRI or XbaI/NotI, respectively), start codons (M) and stop codons (*) are indicated. b Expression cassettes of the L. lactis pNG vectors, encoding an N-terminal His6-tag (pNG4110), a C-terminal His6-tag (pNG4210) or a TEV-removable (TEV) N-terminal His6-tag (pNG4111). Restriction sites for cloning (BamHI/EcoRI or XbaI/NotI, respectively), start codons (M), the Usp 45 signal sequence (SP Usp45) and stop codons (*) are indicated
L. lactis vectors were constructed based on the pEF vectors by amplifying the multiple cloning site together with the His6-tag and the TEV cleavage site (encoded by the specific amino acid sequence EQLYFOG (Arnau et al. 2011) using the primers in Table 2. For construction of pNG4110 and pNG4111, the primer combinations pEF110.fw/pEF110.rev and pEF111.fw/pEF111.rev were used with plasmids pEF110 and pEF111 as templates, respectively. PCR products were digested using HindIII and BsmBI resulting in HindIII and NcoI overhangs and ligated into plasmid pNG400 digested with HindIII and NcoI. For the construction of pNG4210, primers pEF210.fw/pEF210.rev were used with plasmid pEF210 as template DNA. The PCR products and receiving plasmid pNG400 were digested using NcoI and HindIII. After ligation, the resulting vectors were used to transform L. lactis PA1001. For expression of extracellular proteins of S. aureus in L. lactis, the respective genes were amplified by PCR with Pwo polymerase from chromosomal DNA of S. aureus strains USA300 or NCTC8325 (see Table 3). Genes amplified with the f1/r2 primer sets were cloned in pNG4110 and pNG4111, and genes amplified with the f1/r1 primer sets in pNG4210 using the BamHI/NotI restriction sites (Table S1). Ligation mixtures were introduced into L. lactis PA1001. The resulting vectors were sequence-verified.
Table 3.
S. aureus proteins used in this study
| Name and function | NCBIa | Aab | pIc | kDad | Constructs |
|---|---|---|---|---|---|
| SA0620, Secretory antigen SsaA homologue (ssaA like) | USA300HOU_0686 | 26-265 | 6.44 | 27.2 | pNG4110-sa0620 |
| 6.26 | 28.0 | pNG4111-sa0620 | |||
| 6.44 | 27.1 | pNG4210-sa0620 | |||
| FtsL, cell division | USA300HOU_1120 | 66-133 | 9.41 | 8.9 | pNG4110-ftsL |
| 9.02 | 9.7 | pNG4111-ftsL | |||
| 9.41 | 9.1 | pNG4210-ftsL | |||
| ClfB, clumping factor B | USA300HOU_2630 | 45-567 | 4.68 | 57.8 | pNG4110-clfB |
| 4.65 | 58.6 | pNG4111-clfB | |||
| 4.68 | 57.9 | pNG4210-clfB | |||
| Hypothetical protein SA2100, similar to autolysin E | USA300HOU_2288 | 27-258 | 8.95 | 27.7 | pNG4110-sa2100 |
| 8.62 | 28.5 | pNG4111-sa2100 | |||
| 8.95 | 27.9 | pNG4210-sa2100 | |||
| Pro-Atl, pro-peptide autolysin Atl | SAOUHSC_00994 | 29-199 | 9.02 | 19.0 | pNG4110-pro-atl |
| 7.87 | 19.8 | pNG4111-pro-atl | |||
| 9.02 | 18.9 | pNG4210-pro-atl | |||
| IsdB, iron-regulated heme-iron binding protein | 41-609 | 9.18 | 64.9 | pNG4110-isdB | |
| 9.10 | 65.7 | pNG4111-isdB | |||
| 9.18 | 65.2 | pNG4210-isdB |
aNCBI genome annotation of S. aureus strain USA300_TCH1516 (SAUSA300) or NCTC8325 (SAOUHSC)
bNumbers of first and last amino acid residues of the proteins expressed in L. lactis
cIsoelectric point
dMolecular weight
Lithium dodecyl sulphate-polyacrylamide gel electrophoresis and Western blotting
For lithium dodecyl sulphate-polyacrylamide gel electrophoresis (LDS-PAGE), cells were resuspended in LDS buffer (Life Technologies) and disrupted by bead beating with 0.1 μm glass beads (Biospec Products, Bartlesville, USA) using a Precellys24 (Bertin Technologies, Montigny-le-Bretonneux, France), while secreted proteins in the culture medium were precipitated with 10 % trichloroacetic acid (TCA). Protein samples were incubated for 10 min at 95 °C, separated by LDS-PAGE using 10 % NuPAGE gels (Invitrogen) and stained with SimplyBlueTM SafeStain (Life Technologies). For Western blotting, proteins were transferred to a nitrocellulose membrane (Protran®, Schleicher & Schuell, Dassel, Germany). Immunodetection was performed using anti-His-tag antibodies (Life Technologies). Bound antibodies were visualized using fluorescently labeled secondary antibodies (IRDye 800 CW from LiCor Biosciences, NE, USA). Membranes were scanned for fluorescence at 800 nm using the Odyssey Infrared Imaging System (LiCor Biosciences).
Protein production and isolation
Overnight cultures of L. lactis were diluted 1:20 in GM17 medium containing chloramphenicol. Induction of PnisA with nisin was performed for 16 h. Cells producing S. aureus protein SA0620 were resuspended in binding buffer (20 mM sodium phosphate, pH 7.4, 0.5 mM NaCl, 50 mM imidazole) and disrupted by bead beating. The S. aureus proteins SA2100 and pro-Atl were precipitated from the growth medium of L. lactis with 10 % TCA. The SA0620 protein in cell-free extracts and the TCA-precipitated SA2100 and pro-Atl proteins were purified by metal affinity chromatography with His Mag SepharoseTM Ni beads (Mag beads; GE Healthcare, Little Chalfont, UK). Incubation with Mag beads was performed for ~1 h at room temperature; unbound proteins were removed by washing with binding buffer, and bound proteins were eluted with elution buffer (20 mM sodium phosphate, pH 7.4, 0.5 M NaCl and 500 mM imidazole).
Protein activity assays
To analyse the possibility of the TEV cleavage, L. lactis culture medium containing His6-TEV-FtsL, was dialysed against phosphate buffered saline (PBS) and incubated 16 h at 4 °C with 10 U TurboTEV protease (Eton Bioscience, Inc., San Diego, CA). Proteins were separated on a 10 % NuPAGE gel and stained with SimplyBlue.
Cell wall hydrolase activity of His6-tagged derivatives of the SA2100 protein was analysed in zymograms using sodium dodecyl sulfate-polyacrylamide (SDS-PAA) gels (12.5 %) containing 0.15 % autoclaved, lyophilized Micrococcus lysodeikticus ATCC 4698 cells (Sigma-Aldrich.), as described (Buist et al. 1997).
Clumping activity of ClfB was analysed by growing induced PA1001 cells expressing clfB in a 12-well microtitre plate. After overnight induction with nisin, the plate was gently stirred. Cell clumping was visualized using a G-Box Chemi XT16 (Syngene, Cambridge, UK).
Analysis of phosphorylation
Phosphorylation of His6-IsdB and His6-TEV-IsdB was visualized by LDS-PAGE and subsequent staining with Pro-Q® Diamond phosphoprotein gel stain (Life Technologies). Cytosolic cell fractions were produced by bead beating of overnight nisin-induced cells. Cell debris was removed by centrifugation. Prior to separation by LDS-PAGE, cytosolic and secreted proteins were delipidated and desalted according to the instructions for use of the Pro-Q stain. Protein staining was visualized with the G-Box.
Gel pieces containing putatively phosphorylated proteins were prepared for mass spectrometric (MS) analysis as described (Bonn et al. 2014). Peptides were eluted and subjected to high-resolution and high mass accuracy MS measurements on an Orbitrap Elite coupled online to a Proxeon EASY-nLC 1000. The Orbitrap Elite was operated in data-dependent MS/MS mode at a resolution of R = 60,000 in the MS1 with the lockmass option enabled. Data-dependent triggering of fragment scans was set on the 20 most intense precursor ions. Multistage activation (MSA) was used for enhanced fragmentation of putative phosphate group containing ions as described (Basell et al. 2014). MS/MS spectra were searched against a target-decoy database including all protein sequences of S. aureus USA300 extracted from the UniProt database in addition to common laboratory contaminants and an appended set of the reversed sequences. Database searching was performed by Sequest (Thermo Fisher Scientific, San Jose, CA, USA; version v.27, rev. 11). Peptide hits were filtered with Scaffold (version Scaffold_4.3.4, Proteome Software Inc., Portland, OR). Mass tolerance for peptide identification on MS and MS/MS peaks were 10 ppm and 1 Da, respectively. Up to two missed tryptic cleavages were allowed. Methionine oxidation and cysteine carbamidomethylation, as well as phosphorylation at serine, threonine or tyrosine, were set as variable modifications. Peptide identifications were accepted if they matched the following criteria: deltaCn scores of greater than 0.10 and XCorr scores of greater than, 2.5, 3.5 and 3.5 for doubly, triply and quadruply charged peptides. Protein identifications were accepted if they contained at least two identified peptides. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Results
Development of a pNG vector set for heterologous protein production in L. lactis
In previous studies, the pEF vector set was successfully used for heterologous protein expression in E. coli (Bardoel et al. 2012; Pel et al. 2014). This vector set offered convenient possibilities for N- or C-terminal His6-tagging of expressed proteins (pEF110 and pEF210, respectively) and for TEV-mediated tag removal (pEF111; Fig. 1a). We therefore implemented the respective cloning sites, His6-tag sequences and TEV cleavage site sequence in the pNG series of L. lactis expression vectors. In addition, the pNG vectors were provided with the nisA promoter and the signal sequence of Usp45 for nisin-inducible secretory protein production. Specifically, this resulted in pNG4110 for expression of N-terminally His6-tagged proteins, pNG4210 for expression of C-terminally His6-tagged proteins and pNG4111 for expression of proteins with a TEV protease-removable N-terminal His6-tag (Fig. 1b).
The versatility of the pNG vectors was tested by expressing six different extracytoplasmic proteins of S. aureus. Notably, these included a secreted protein (SA0620), a membrane-associated protein (FtsL), two covalently cell wall-bound proteins (ClfB and IsdB), a non-covalently cell wall-bound protein (SA2100) and the pro-peptide of the major autolysin Atl (Table 2). To express these proteins, the L. lactis strain PA1001 was used, which has an improved stability due to a deletion of the acmA gene and a reduced proteolytic activity due to deletion of the htrA gene (Neef et al. 2014).
Position of the His6-tagging affects SA0620 production in L. lactis
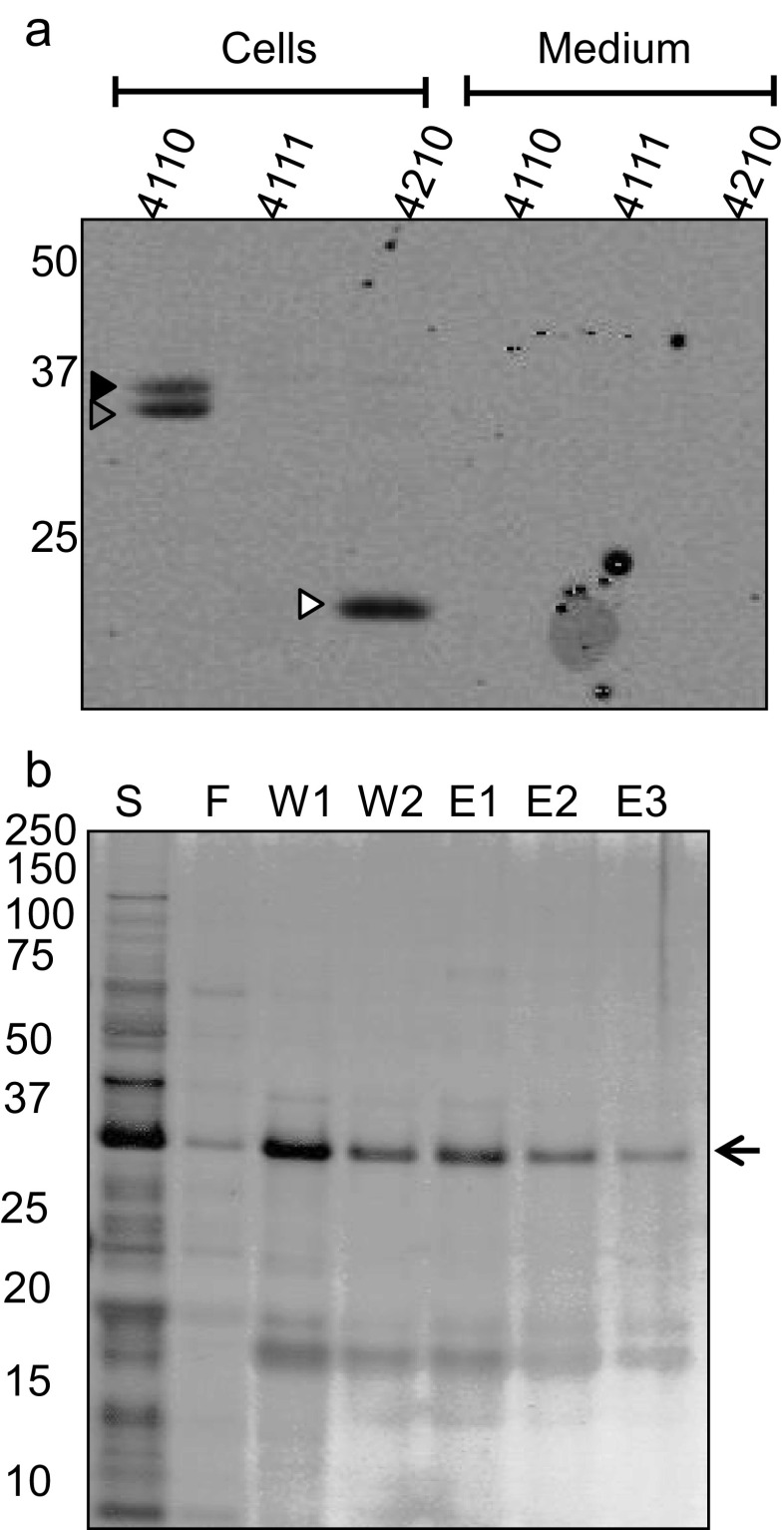
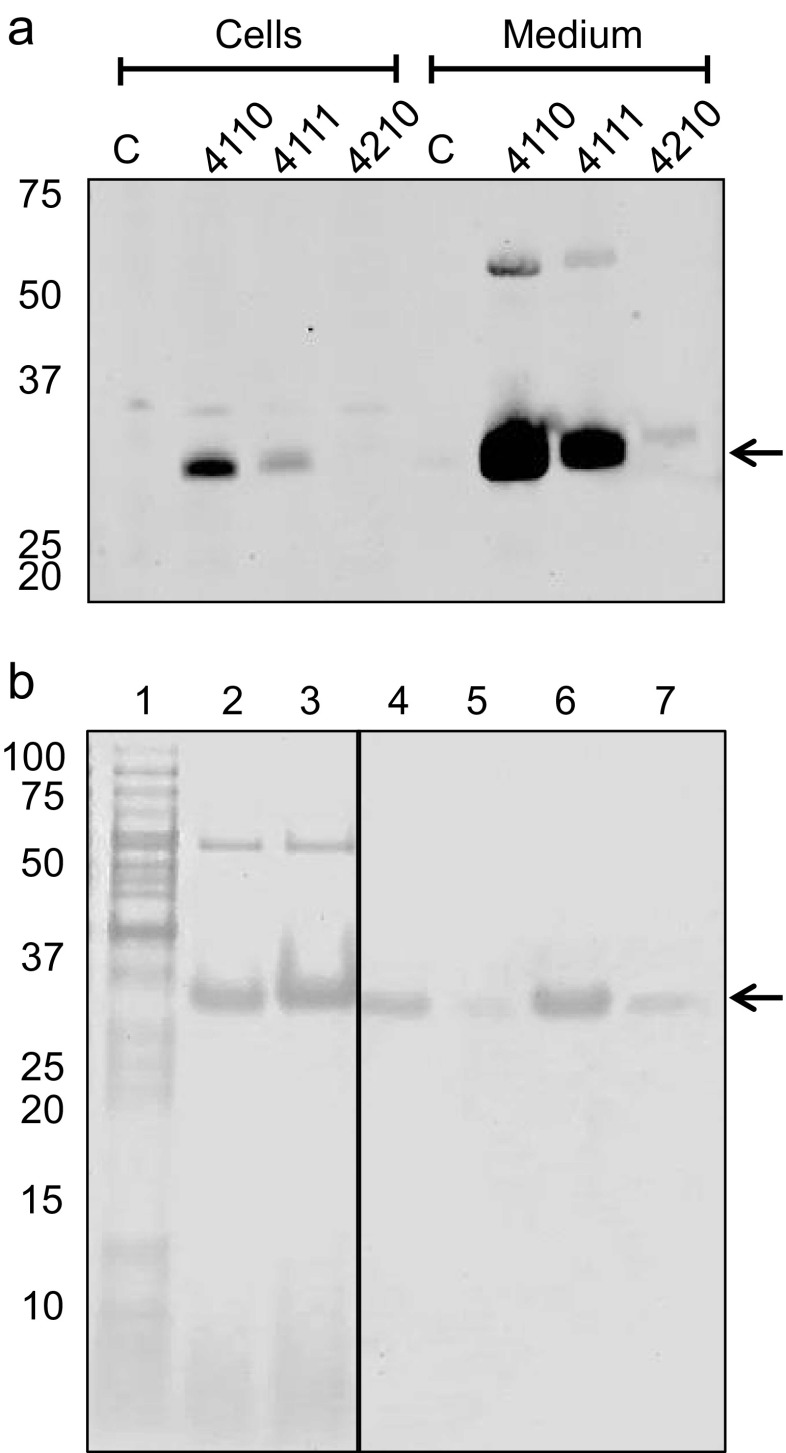
In a previous study, we observed that the secretory antigen SsaA homologue SA0620 was not produced in L. lactis PA1001 when expressed with a C-terminal His6-tag from plasmid pNG400 (Neef et al. 2014). To test whether this might be due to the location of the His6-tag, the gene for SA0620 was cloned in plasmids pNG4110, pNG4111 and pNG4210. After overnight induction with nisin, the expression of the resulting His6-tagged proteins was analysed by LDS-PAGE and Western blotting. This showed that effective expression of the full-length SA0620 precursor and mature proteins (30.4 and 27.7 kDa, respectively) was only achieved when this protein was synthesized with an N-terminal His6-tag as provided by pNG4110 (Fig. 2a). Precursor forms of SA0620 were observed in low amounts when expressed from pNG4111 or pNG4210, and in the latter case, also a degradation product of approximately 17 kDa was detectable (Fig. 2a). For the His6-TEV-SA0620 variant produced from pNG4111, not even a degradation product was detectable. Notably, despite the fusion of SA0620 to the signal peptide of Usp45, no SA0620 or fragments thereof were detectable in the growth medium (Fig. 2a). Nevertheless, the expression in pNG4110 did allow purification of the mature SA0620 protein and a degradation product from cells disrupted by bead beating and subsequent treatment with 6 M urea, as illustrated in Fig. 2b. Together, these data show that the N-terminal location of the His6-tag is critical for successful production and subsequent isolation of the full-length S. aureus antigen SA0620.
Fig. 2.
Production and purification of the S. aureus SA0620 protein. a Cell lysates (Cells) and TCA-precipitated growth medium (Medium) of L. lactis PA1001 expressing His6-SA0620 (4110), His6-TEV-SA0620 (4111) or SA0620-His6 (4210) were analysed by Western blotting using anti-His6 antibodies. The black arrowhead indicates a potential precursor form of SA0620, the grey arrowhead indicates the mature-sized SA0620 protein and the white arrowhead indicates a degradation product. b The His6-SA0620 (4110) was purified form the disrupted cells in the presence of 6 M urea. The start material (S), flow-through fraction (F), wash fractions (W) and elution fractions (E) were analysed by LDS-PAGE and separated proteins were detected by silver staining. The position of His6-SA0620 is indicated by an arrow and a co-purified degradation product of this protein is marked (*). The positions of Mw marker proteins are indicated
TEV protease-mediated His6-tag removal from the secreted His6-TEV-FtsL protein
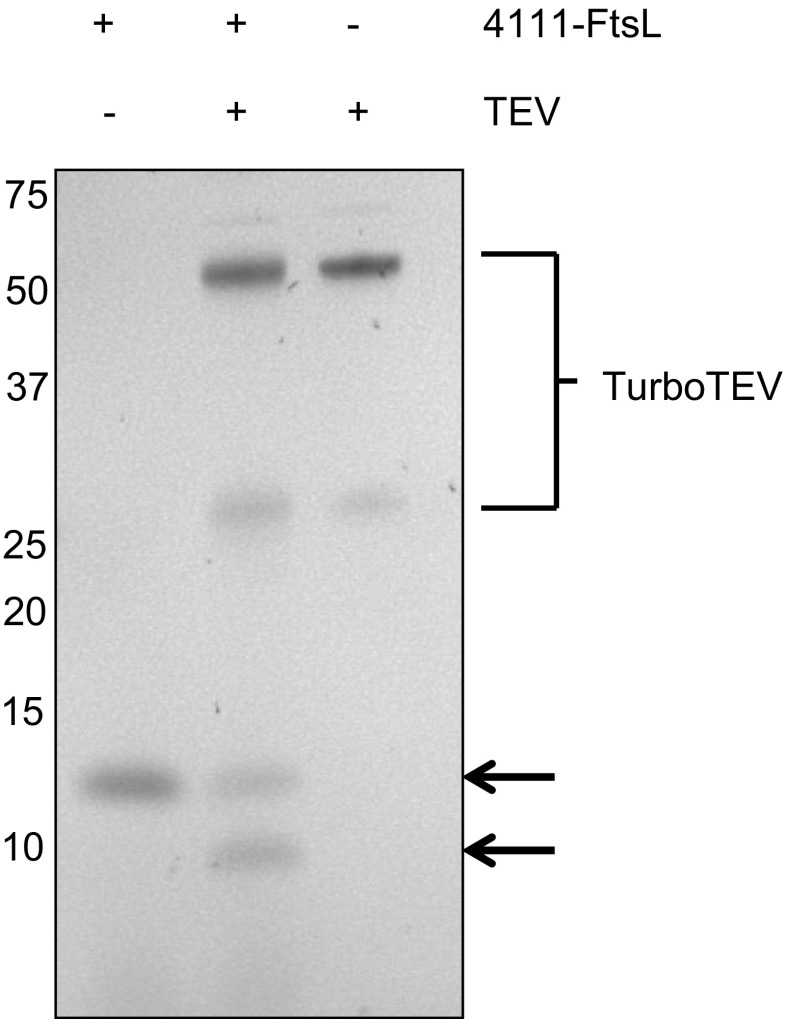
To determine whether the His6-tag can proteolytically be removed from proteins expressed from the pNG4111 vector, by means of the encoded TEV cleavage site, the S. aureus cell division membrane protein FtsL was used. Specifically, the ftsL gene was expressed lacking the transmembrane helix. After induction with nisin, the His6-TEV-FtsL fusion protein was efficiently secreted into the culture medium of L. lactis (Fig. 3). Upon removal of cells by centrifugation, dialysis against PBS and incubation with the TurboTEV protease, cleavage of His6-TEV-FtsL was analysed by LDS-PAGE and SimplyBlue staining. In the samples incubated in the absence of TurboTEV protease, the presumably complete fusion protein was detectable upon LDS-PAGE and SimplyBlue staining (Fig. 3). Of note, the apparent molecular weight (Mw) of the fusion protein judged by its mobility on LDS-PAGE was ~12 kDa, while the predicted Mw is only 9.5 kDa. Upon incubation in the presence of the TEV protease, an additional protein band with an apparent Mw of ~10 kDa was detectable. This is in agreement with the predicted mass reduction of the His6-TEV-FtsL fusion by ~1.7 kDa upon cleavage at the TEV site. The cleavage product was not detected in the control sample, solely TurboTEV protease, indicating the product is His6-TEV-FtsL derived (Fig. 3). Together, these findings show that efficient extracellular protein production can be achieved with pNG4111 and that the His6-tag can be removed from the secreted fusion product by cleavage with the TEV protease.
Fig. 3.
TEV cleavage of His6-FtsL. Production of the His6-TEV-FtsL protein by L. lactis PA1001 pNG4111-ftsL was induced overnight with nisin. Growth medium containing His6-TEV-FtsL was dialysed against PBS and then the TurboTEV protease (52 kDa) was added. Fractions with or without TurboTEV were separated by LDS-PAGE and stained with SimplyBlue. The positions of Mw marker proteins are indicated
Functional expression of ClfB in L. lactis
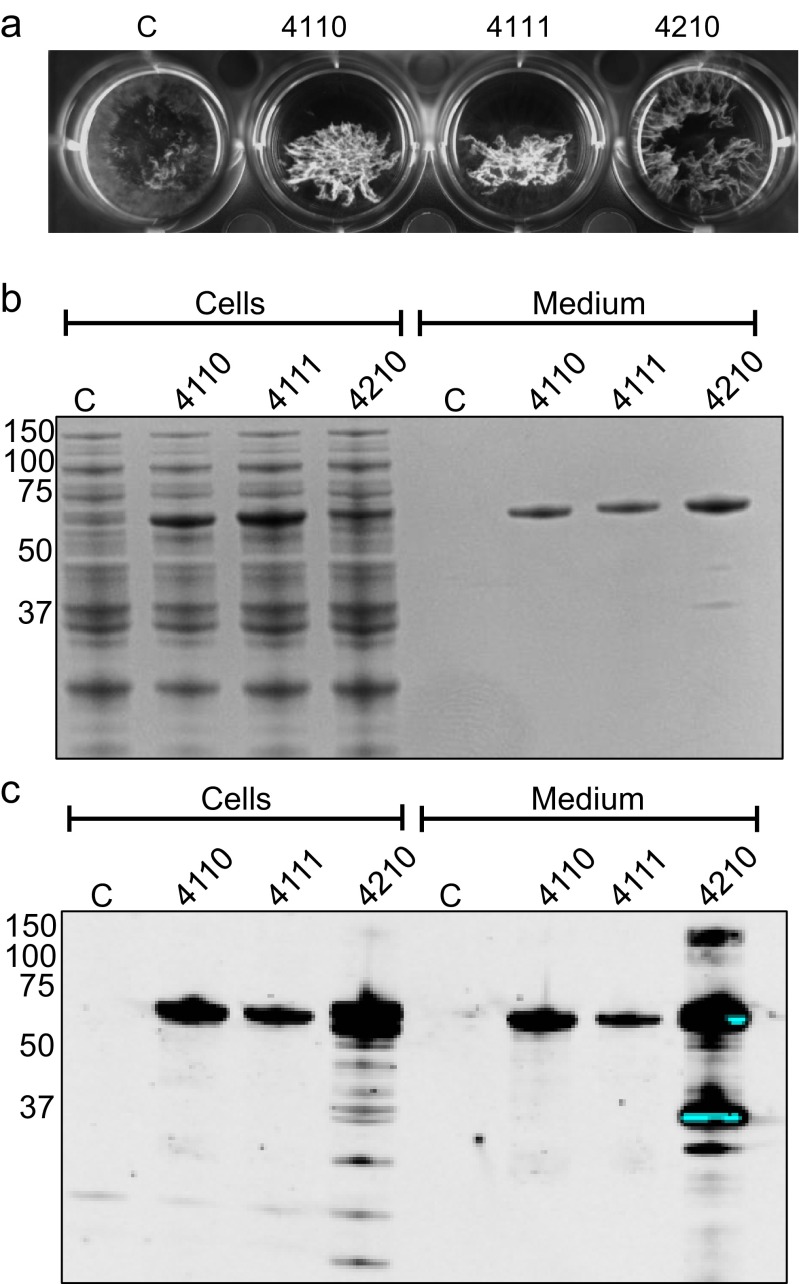
To assess whether pNG4110, pNG4111 and pNG4210 facilitate the expression of proteinaceous antigens in L. lactis that are covalently bound to the cell surface of S. aureus, the ClfB protein was used. Specifically, the clfB gene was introduced into the pNG expression vectors without the 3′ sequences that encode the LPxTG motif for sortase recognition and cleavage and the C-terminal transmembrane domain. Furthermore, the original signal peptide of ClfB was replaced with the Usp45 signal peptide. Remarkably, clumping of ClfB-expressing L. lactis strains was detectable (Fig. 4a) in cultures upon overnight induction with nisin. Interestingly, this clumping phenotype was not observed for control cells not expressing ClfB, which suggests that ClfB lacking the LPxTG motif still associates to the cell surface. To verify ClfB production and possible secretion, the cells were separated from the growth medium and both fractions were analysed by LDS-PAGE. Gels were then either stained with SimplyBlue (Fig. 4b) or used for Western blotting and immunodetection using anti-His6 antibodies (Fig. 4c). His6-tagged proteins were detectable both in the cellular and growth medium fractions (Fig. 4b, c) indicating ClfB was efficiently expressed from all three vectors (pNG4110, pNG4111 and pNG4210). Notably, several degradation products of the C-terminally His6-tagged ClfB were detectable in the cell and growth medium fractions, while this was not the case for the N-terminally His6-tagged forms of ClfB. This suggests that C-terminal His6-tagging makes ClfB more prone to degradation by as yet unknown proteases of L. lactis. Furthermore, the results shown in Fig. 4 suggest that the location of the His6-tag may influence ClfB-mediated clumping of L. lactis cells, since cells expressing ClfB-His6 from pNG4210 showed a milder clumping phenotype than cells expressing His6-ClfB or His6-TEV-Clfb from pNG4110 or pNG4111, respectively. Whether this relates to differences in the produced amounts of His6-tagged ClfB is difficult to say since the position of the His6-tag seems to influence either the efficiency of SimplyBlue protein staining or of immunodetection with His6-specific antibodies.
Fig. 4.
Functional expression of the S. aureus ClfB protein in L. lactis. a Clumping of L. lactis PA1001 cells producing His6-ClfB (4110), His6-TEV-ClfB (4111) or ClfB-His6 (4210) upon overnight induction with nisin. No clumping was observed in the absence of nisin, as shown under (C) for a non-induced control culture of L. lactis PA1001 harbouring pNG4110-clfB. b and c Cell and growth medium fractions of L. lactis PA1001 producing His6-ClfB (4110), His6-TEV-ClfB (4111) or ClfB-His6 (4210) were analysed by LDS-PAGE. Gels were either stained with SimplyBlue SafeStain (b) or used for Western blotting (c) with anti-histidine antibodies. As a control, non-induced L. lactis PA1001 pNG4110-clfB was included in the analysis (C). The positions of Mw marker proteins are indicated
Peptidoglycan cleavage activity of SA2100
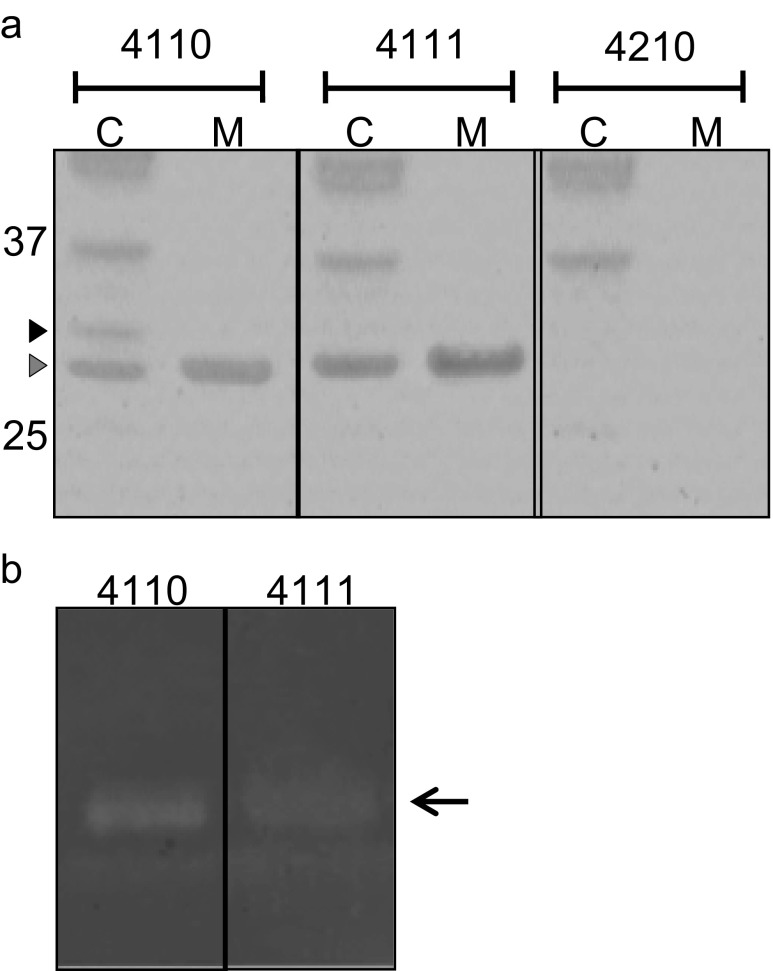
To investigate whether non-covalently cell wall-bound proteins of S. aureus can be produced in L. lactis using pNG4110, pNG4111 and/or pNG4210, the gene encoding the S. aureus SA2100 protein was cloned in these vectors. In the process, the native signal sequence of SA2100 was replaced with the Usp45 signal sequence. As shown in Fig. 5, cell-associated and secreted mature forms of SA2100 were only detectable by expression from pNG4110 or pNG4111, i.e. with N-terminal His6-tags, and not when expressed from the pNG4210 vector that encodes the C-terminally His6-tagged protein. Furthermore, a precursor from of His6-SA2100 was detectable in cells expressing this protein from pNG4110. Together, these observations support the view that the location of the His6-tag can be critical for effective protein production in L. lactis.
Fig. 5.
Functional expression of the S. aureus SA2100 protein in L. lactis. a Cells (C) and growth medium (M) fractions of L. lactis PA1001 producing His6-SA2100 (4110), His6-TEV-SA2100 (4111) or SA2100-His6 (4210) were analysed by LDS-PAGE and stained with SimplyBlue SafeStain. The black arrowhead indicates a potential precursor form of His6-SA2100, and the grey arrowhead indicates matured SA2100. The positions of Mw marker proteins are indicated. b The cell wall hydrolyzing activity of His6-SA2100 (4110) and His6-TEV-SA2100 (4111) was analysed by zymography upon SDS-PAGE in gels containing M. lysodeikticus cell wall extract. Upon electrophoresis and renaturation of separated proteins, the gel was stained with methylene blue as described in the “Materials and methods” section. A zone of cell wall-degrading activity, which corresponds to the position of mature SA2100 in the gel upon electrophoresis is indicated with an arrow
Notably, SA2100 is homologous to the autolysin E protein of S. aureus and contains a C-terminal domain with similarity to the so-called lysozyme subfamily 2 (LYZ2, smart00047; see the CCD database of NCBI at http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). The latter protein family has a peptidoglycan-hydrolyzing activity that is comparable to muramidase activity (Buist et al. 1995). To investigate whether the heterologously expressed and secreted form of SA2100 displays such an activity, a zymogram assay for the degradation of cell wall fragments from M. lysodeikticus was employed. As shown by zymographic analysis, the purified His6-SA2100 and His6-TEV-SA2100 were both capable of degrading cell wall fragments of M. lysodeikticus (Fig. 5b), which demonstrates that SA2100 is indeed a cell wall-degrading enzyme.
Expression of the Atl pro-peptide in L. lactis
In a recent study, the N-terminal pro-peptide of the bifunctional autolysin Atl of S. aureus is reported to be recognized by antibodies in the plasma of patients with the genetic blistering disease epidermolysis bullosa (van den Berg et al. 2015). This intriguing finding initiated an effort to express the pro-Atl (amino acids 29–199) in L. lactis using the three vectors, pNG4110, pNG4111 and pNG4210. As shown in Fig. 6a, the N-terminally His6-tagged pro-Atl as expressed from pNG4110 and pNG4111 was efficiently produced and secreted (Fig. 6a). In fact, most of these His6-tagged forms of pro-Atl were detected in the growth medium from which they were readily purified by metal affinity chromatography (Fig. 6b). Compared to the N-terminally His6-tagged pro-Atl, only a minute amount of C-terminally His6-tagged pro-Atl as expressed from pNG4210 was detectable by Western blotting (Fig. 6a).
Fig. 6.
Production and purification of the S. aureus pro-Atl pro-peptide. a Cells and growth medium fractions of L. lactis PA1001 producing His6-Pro-Atl (4110), His6-TEV-Pro-Atl (4111) or Pro-Atl-His6 (4210) were analysed by LDS-PAGE and subsequent Western blotting using anti-His6 antibodies. As a control, non-induced L. lactis PA1001 pNG4110-pro-atl was included in the analysis (C). Pro-Atl is indicated with an arrow. b Purification of His6-Pro-Atl and His6-TEV-Pro-Atl from the supernatant of cells that were induced with nisin overnight. The LDS-PAGE shows (1) induced cells producing His6-Pro-Atl, (2) the culture supernatant fraction of cells producing His6-Pro-Atl, (3) the culture supernatant fraction of cells producing His6-TEV-Pro-Atl, (4/5) the first two elution fractions of His6-Pro-Atl upon metal affinity chromatography and (6/7) the first two elution fractions of His6-TEV-Pro-Atl upon metal affinity chromatography. The position of His6-tagged Pro-Atl is marked with an arrow, and the positions of Mw marker proteins are indicated
Secretion of phosphorylated IsdB by L. lactis
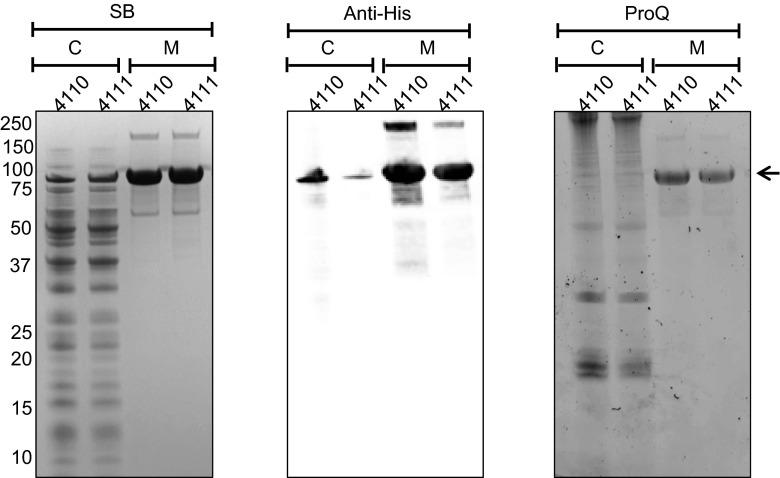
Recent studies by Basell et al. have shown that residues Tyr440 or Tyr444 of the covalently cell wall-attached IsdB protein of S. aureus are phosphorylated (Basell et al. 2014). To determine whether IsdB produced and secreted by L. lactis PA1001 is also phosphorylated, a 3′-truncated isdB gene lacking the sequences encoding the sortase recognition site was expressed from pNG4110 and pNG4111. As shown in Fig. 7, the N-terminally His6-tagged forms of IsdB were efficiently produced by L. lactis, and in fact, most of the protein is secreted into the growth medium. Importantly, phosphorylated His6-tagged IsdB was clearly detectable by gel staining with the Pro-Q® Diamond Phosphoprotein Gel Stain, showing L. lactis facilitates this post-translational modification. Whether the cytoplasmic forms of His6-IsdB and His6-TEV-IsdB as observed by Western blotting are also phosphorylated is presently unclear due to limited sensitivity of the Pro-Q staining in these extracts.
Fig. 7.
Extracellular production of phosphorylated S. aureus IsdB in L. lactis. a Cytosolic (C) and growth medium (M) fractions of L. lactis PA1001 producing His6-IsdB (4110) and His6-TEV-IsdB (4111) were analysed by LDS-PAGE and stained with SimplyBlue SafeStain (SB) or Pro-Q Diamond staining (Pro-Q). As a control, Western blotting was performed, using anti-His6 antibodies (α-His). The arrow marks the position of mature IsdB, and the positions of Mw marker proteins are indicated
To pinpoint the site of IsdB phosphorylation in L. lactis, the secreted His6-TEV-IsdB was purified by metal affinity chromatography and applied to an LDS-PAA gel. The His6-TEV-IsdB band was subsequently excised from the gel, destained, washed and cleaved overnight with trypsin. Liberated peptides were analysed by MS/MS, which showed that His6-TEV-IsdB was phosphorylated on the Tyr311(*) residue in the peptide KYMVMETTNDDY*WKDFMVEGQR (Supplementary Fig. S1). The same Tyr residue was also shown to be phosphorylated when TCA-precipitated His6-TEV-IsdB from the culture supernatant of L. lactis PA1001 containing pNG4111-IsdB was used for the MS/MS analysis.
Discussion
In the present study we describe a set of three cloning vectors for heterologous protein expression in L. lactis. These vectors enable easy exchange of the gene of interest for expression of protein variants with (i) a N-terminal His6-tag, (ii) a C-terminal His6-tag and (iii) a TEV protease cleavable C-terminal His6 protein. Examples of the successful use of the three vectors are presented for expression of six extracytoplasmic proteins from S. aureus, two of which were subsequently purified in one step by metal affinity chromatography.
Importantly, the position of the His6-tag purification label, either at the N- or the C-terminus, can have a major impact on the production level of S. aureus originated proteins expressed in L. lactis. This was particularly evident for SA0620, SA2100 and pro-Atl, which were only detectable when expressed as N-terminal His6-tagged protein. In contrast, no effect of the His6-tag position was observed for the production of FtsL, ClfB and IsdB (data not shown for FtsL and IsdB). The reason(s) for the negative impact of a C-terminal His6-tag on the production of SA0620, SA2100 and pro-Atl is presently not clear. For the SA0620 protein, the tag appears to interfere with the protein stability/folding as indicated by the presence of a degradation product. Similar degradation products are observed for the C-terminally His6-tagged ClfB, whereas these are not observed for the N-terminally His6-tagged ClfB. Similarly, the TEV cleavage site as encoded by pNG4111 may influence the protein production level. This was most evident for SA0620, although effects of the TEV cleavage site on the levels of detected product were also observed for ClfB and pro-Atl. However, for the latter two proteins, it is presently not entirely clear whether the TEV site interfered with protein production per se or with the immunodetection of the His6-tag with anti-His6-tag antibodies. The latter idea would be supported by the experiments on ClfB production, where there was a discrepancy between the ClfB levels detected by SimplyBlue gel staining and immunodetection.
With the exception of SA0620, all S. aureus proteins expressed in L. lactis were secreted into the growth medium with the help of the vector-encoded Usp45 signal peptide. The cause of the deviating behaviour of SA0620, which is part of the core exoproteome of S. aureus (Sibbald et al. 2006; Ziebandt et al. 2010), is unknown. Apparently, this protein has a particular unidentified feature that interferes with its secretion in L. lactis. This could, for example, relate to its targeting to the Sec secretion machinery, pre-translocational control of folding by chaperones or efficient post-translocational folding by dedicated folding catalysts that could be present in S. aureus but absent from L. lactis.(Sarvas et al. 2004; Bolhuis et al. 1999; Tjalsma et al. 2004)
The expression host strain used for the present studies was L. lactis PA1001, which lacks the major lactococcal proteases PrtP and HtrA (Poquet et al. 2000; Liu et al. 2010). Despite the absence of these two proteases, our results show that proteolysis of expressed proteins can still occur. This focuses attention on the remaining proteases that could be responsible for this unwanted effect. Conceivably, the responsible protease could be located in the cytoplasm (e.g. ClpP; Frees et al. 2001), the membrane (e.g. FtsH or a RseP-like protease; Dalbey et al. 2012) or an as yet unidentified protease in the cell wall. The elimination of this remnant protease activity could be beneficial for further production strain improvement, similar to what was shown for heterologous protein production in B. subtilis where the deletion of multiple protease genes resulted in large improvements in the production of heterologous proteins (Pohl et al. 2013; Krishnappa et al. 2014).
Lastly, our analysis of the production of S. aureus IsdB in L. lactis shows that this heterologous expression host does phosphorylate the IsdB protein as is the case in S. aureus (Basell et al. 2014). However, while in S. aureus either Tyr440 or Tyr444 are phosphorylated, the IsdB protein expressed in L. lactis was found to be phosphorylated on Tyr311. At present, the molecular basis for this apparently different choice of phosphorylation sites in S. aureus and L. lactis is not clear. One possible reason for the different results could be that the sample preparation was performed differently. While Basell et al. (2014) applied a gel-free proteomics analysis where phosphopeptides were enriched with TiO2, in the present analyses the IsdB protein was extracted from gel slices. Another perhaps more plausible reason could be that we expressed in L. lactis a 3′-truncated isdB gene lacking the sequences encoding the sortase recognition site and encoding a TEV protease cleavable N-terminal His6-tag. This may have resulted in an aberrant presentation of IsdB to the as yet unknown kinase responsible for phosphorylation of this protein. In contrast, the study in S. aureus addressed the authentic IsdB protein synthesized with the sortase cleavage site and, therefore, covalently anchored to the cell wall. A third possibility would be that the kinases responsible for IsdB phosphorylation in S. aureus and L. lactis have somewhat different specificities. A careful comparative analysis of the mechanisms of IsdB phosphorylation in S. aureus and L. lactis may pinpoint possible host-specific differences.
In conclusion, based on our present findings, we are confident that the newly developed vectors combined with the L. lactis expression host PA1001 have the potential to become very useful tools for the extracytoplasmic production and purification of bacterial antigens. Such a tool facilitates structural and functional studies on previously hard-to-produce proteins and may provide a starting point for vaccine development.
Electronic supplementary material
(PDF 580 kb)
Acknowledgments
We like to thank Sabrina Jacobs, Ilona Schepel and Alain Dekker for their experimental contribution.
Funding
This research was supported by the Top Institute Pharma projects T4-213 and T4-502.
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Arnau J, Lauritzen C, Petersen GE, Pedersen J (2011) Reprint of: Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr Purif [DOI] [PubMed]
- Bardoel BW, Van Kessel KPM, Van Strijp JAG, Milder FJ. Inhibition of Pseudomonas aeruginosa virulence: characterization of the AprA-AprI interface and species selectivity. J Mol Biol. 2012;415:573–583. doi: 10.1016/j.jmb.2011.11.039. [DOI] [PubMed] [Google Scholar]
- Basell K, Otto A, Junker S, Zuhlke D, Rappen GM, Schmidt S, Hentschker C, Macek B, Ohlsen K, Hecker M, Becher D. The phosphoproteome and its physiological dynamics in Staphylococcus aureus. Int J Med Microbiol. 2014;304:121–132. doi: 10.1016/j.ijmm.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Bolhuis A, Tjalsma H, Smith HE, de Jong A, Meima R, Venema G, Bron S, van Dijl JM. Evaluation of bottlenecks in the late stages of protein secretion in Bacillus subtilis. Appl Environ Microbiol. 1999;65:2934–2941. doi: 10.1128/aem.65.7.2934-2941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn F, Bartel J, Buttner K, Hecker M, Otto A, Becher D. Picking vanished proteins from the void: how to collect and ship/share extremely dilute proteins in a reproducible and highly efficient manner. Anal Chem. 2014;86:7421–7427. doi: 10.1021/ac501189j. [DOI] [PubMed] [Google Scholar]
- Bosma T, Kanninga R, Neef J, Audouy SAL, Van Roosmalen ML, Steen A, Buist G, Kok J, Kuipers OP, Robillard G, Leenhouts K. Novel surface display system for proteins on non-genetically modified gram positive bacteria. Appl Environ Microbiol. 2006;72:880–889. doi: 10.1128/AEM.72.1.880-889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P, Gerritse G, van Dijl JM, Quax WJ. Improving protein secretion by engineering components of the bacterial translocation machinery. Curr Opin Biotechnol. 1999;10:376–381. doi: 10.1016/S0958-1669(99)80068-8. [DOI] [PubMed] [Google Scholar]
- Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G, Haandrikman AJ. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist G, Karsens H, Nauta A, Van Sinderen D, Venema G, Kok J. Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol. 1997;63:2722–2728. doi: 10.1128/aem.63.7.2722-2728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnol Adv. 2012;30:1102–1107. doi: 10.1016/j.biotechadv.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Cortes-Perez NG, Poquet I, Oliveria MN, Gratadoux JJ, Madsen SM, Miyoshi A, Corthier G, Azevedo V, Langella P, Bermudez-Humaran LG. Construction and characterization of a Lactococcus lactis strain deficient in intracellular ClpP and extracellular HtrA proteases. Microbiology. 2006;152:2611–2618. doi: 10.1099/mic.0.28698-0. [DOI] [PubMed] [Google Scholar]
- Dalbey RE, Wang P, van Dijl JM. Membrane proteases in the bacterial protein secretion and quality control pathway. Microbiol Mol Biol Rev. 2012;76:311–330. doi: 10.1128/MMBR.05019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ruyter PGGA, Kuipers OP, De Vos WM. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- Frees D, Varmanen P, Ingmer H. Inactivation of a gene that is highly conserved in Gram-positive bacteria stimulates degradation of non-native proteins and concomitantly increases stress tolerance in Lactococcus lactis. Mol Microbiol. 2001;41:93–103. doi: 10.1046/j.1365-2958.2001.02503.x. [DOI] [PubMed] [Google Scholar]
- Jones C, Patel A, Griffin S, Martin J, Young P, O’Donnell K, Silverman C, Porter T, Chaiken I. Current trends in molecular recognition and bioseparation. J Chromatogr A. 1995;707:3–22. doi: 10.1016/0021-9673(95)00466-Z. [DOI] [PubMed] [Google Scholar]
- Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, Novick RP. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Krishnappa L, Dreisbach A, Otto A, Goosens VJ, Cranenburgh R, Harwood CR, Becher D, Van Dijl JM. Extracytoplasmic proteases determining the cleavage and release of secreted proteins, lipoproteins, and membrane proteins in Bacillus subtilis. J Proteome Res. 2013;12:4101–4110. doi: 10.1021/pr400433h. [DOI] [PubMed] [Google Scholar]
- Krishnappa L, Monteferrante CG, Neef J, Dreisbach A, Van Dijl JM. Degradation of extracytoplasmic catalysts for protein folding in Bacillus subtilis. Appl Environ Microbiol. 2014;80:1463–1468. doi: 10.1128/AEM.02799-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers OP, Beerthuyzen MM, Siezen RJ, De Vos WM. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem. 1993;216:281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- Kuipers OP, De Ruyter PGGA, Kleerebezem M, De Vos WM. Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol. 1997;15:135–140. doi: 10.1016/S0167-7799(97)01029-9. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/S0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- Leenhouts KJ, Venema G. Lactococcal plasmid vectors. In: Hardy KG, editor. Plasmids, a practical approach. Oxford: Oxford University Press; 1993. pp. 65–94. [Google Scholar]
- Li W, Zhou X, Lu P. Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Res Microbiol. 2004;155:605–610. doi: 10.1016/j.resmic.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Liu M, Bayjanov JR, Renckens B, Nauta A, Siezen RJ. The proteolytic system of lactic acid bacteria revisited: a genomic comparison. BMC Genomics. 2010;11:36–2164. doi: 10.1186/1471-2164-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierau IKM. 10 Years of the nisin controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol. 2005;68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- Miyoshi A, Poquet I, Azevedo V, Commissaire J, Bermudez-Humaran LG, Domakova E, Le Loir Y, Oliveira SC, Grusse A, Langella P. Controlled production of stable heterologous proteins in Lactococcus lactis. Appl Environ Microbiol. 2002;68:3141–3146. doi: 10.1128/AEM.68.6.3141-3146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello E, Bermudez-Humaran LG, Llull D, Sole V, Miraglio N, Langella P, Poquet I. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J Mol Microbiol Biotechnol. 2008;14:48–58. doi: 10.1159/000106082. [DOI] [PubMed] [Google Scholar]
- Neef J, Koedijk DG, Bosma T, van Dijl JM, Buist G. Efficient production of secreted staphylococcal antigens in a non-lysing and proteolytically reduced Lactococcus lactis strain. Appl Microbiol Biotechnol. 2014;98:10131–10141. doi: 10.1007/s00253-014-6030-y. [DOI] [PubMed] [Google Scholar]
- Pel MJ, van Dijken AJ, Bardoel BW, Seidl MF, van der Ent S, van Strijp JA, Pieterse CM. Pseudomonas syringae evades host immunity by degrading flagellin monomers with alkaline protease AprA. Mol Plant Microbe Interact. 2014;27:603–610. doi: 10.1094/MPMI-02-14-0032-R. [DOI] [PubMed] [Google Scholar]
- Petsch D, Anspach FB. Endotoxin removal from protein solutions. J Biotechnol. 2000;76:97–119. doi: 10.1016/S0168-1656(99)00185-6. [DOI] [PubMed] [Google Scholar]
- Pohl S, Bhavsar G, Hulme J, Bloor AE, Misirli G, Leckenby MW, Radford DS, Smith W, Wipat A, Williamson ED, Harwood CR, Cranenburgh RM. Proteomic analysis of Bacillus subtilis strains engineered for improved production of heterologous proteins. Proteomics. 2013;13:3298–3308. doi: 10.1002/pmic.201300183. [DOI] [PubMed] [Google Scholar]
- Poquet I, Saint V, Seznec E, Simoes N, Bolotin A, Gruss A. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol. 2000;35:1042–1051. doi: 10.1046/j.1365-2958.2000.01757.x. [DOI] [PubMed] [Google Scholar]
- Sarvas M, Harwood CR, Bron S, Van Dijl JM. Post-translocational folding of secretory proteins in Gram-positive bacteria. Biochim Biophys Acta. 2004;1694:311–327. doi: 10.1016/j.bbamcr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sibbald MJJB, Ziebandt AK, Engelmann S, Hecker M, De Jong A, Hamsen HJM, Raangs GC, Stokroos I, Arends JP, Dubois JYF, Van Dijl JM. Mapping the pathway to staphylococcal pathogenesis by comparative secretomics. Microbiol Mol Biol Rev. 2006;70:755–788. doi: 10.1128/MMBR.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2003;60:523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of bacterial expression system for heterologous protein production from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- Tjalsma H, Koetje EJ, Kiewiet R, Kuipers OP, Kolkman M, Van der Laan J, Daskin R, Ferrari E, Bron S. Engineering of quorum-sensing systems for improved production of alkaline protease by Bacillus subtilis. J Appl Microbiol. 2004;96:569–578. doi: 10.1111/j.1365-2672.2004.02179.x. [DOI] [PubMed] [Google Scholar]
- van den Berg S, Koedijk DG, Back JW, Neef J, Dreisbach A, van Dijl JM, Bakker-Woudenberg IA, Buist G. Active immunization with an octa-valent Staphylococcus aureus antigen mixture in models of S. aureus bacteremia and skin infection in mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westers L, Westers H, Quax WJ. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim Biophys Acta. 2004;1694:299–310. doi: 10.1016/j.bbamcr.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Woestenenk EA, Hammarstrom M, van den Berg S, Hard T, Berglund H. His tag effect on solubility of human proteins produced in Escherichia coli: a comparison between four expression vectors. J Struct Funct Genomics. 2004;5:217–229. doi: 10.1023/B:jsfg.0000031965.37625.0e. [DOI] [PubMed] [Google Scholar]
- Zerbs S, Frank AM, Collart FR. Bacterial systems for production of heterologous proteins. Methods Enzymol. 2009;463:149–168. doi: 10.1016/S0076-6879(09)63012-3. [DOI] [PubMed] [Google Scholar]
- Zhou XX, Li WF, Ma GX, Pan YJ. The nisin-controlled gene expression system: construction, application and improvements. Biotechnol Adv. 2006;24:285–295. doi: 10.1016/j.biotechadv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ziebandt AK, Kusch H, Degner M, Jaglitz S, Sibbald MJ, Arends JP, Chlebowicz MA, Albrecht D, Pantucek R, Doskar J, Ziebuhr W, Broker BM, Hecker M, Van Dijl JM, Engelmann S. Proteomics uncovers extreme heterogeneity in the Staphylococcus aureus exoproteome due to genomic plasticity and variant gene regulation. Proteomics. 2010;10:1634–1644. doi: 10.1002/pmic.200900313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 580 kb)