Abstract
The basic architecture and functionality of ribbon synapses of mechanosensitive hair cells are well conserved among vertebrates. Forward and reverse genetic methods in zebrafish (Danio rerio) have identified components that are critical for the development and function of ribbon synapses. This review will focus on the findings of these genetic approaches, and discuss some emergent concepts on the role of the ribbon body and calcium in synapse development, and how perturbations in synaptic vesicles lead to a loss of temporal fidelity at ribbon synapses.
1. Preface
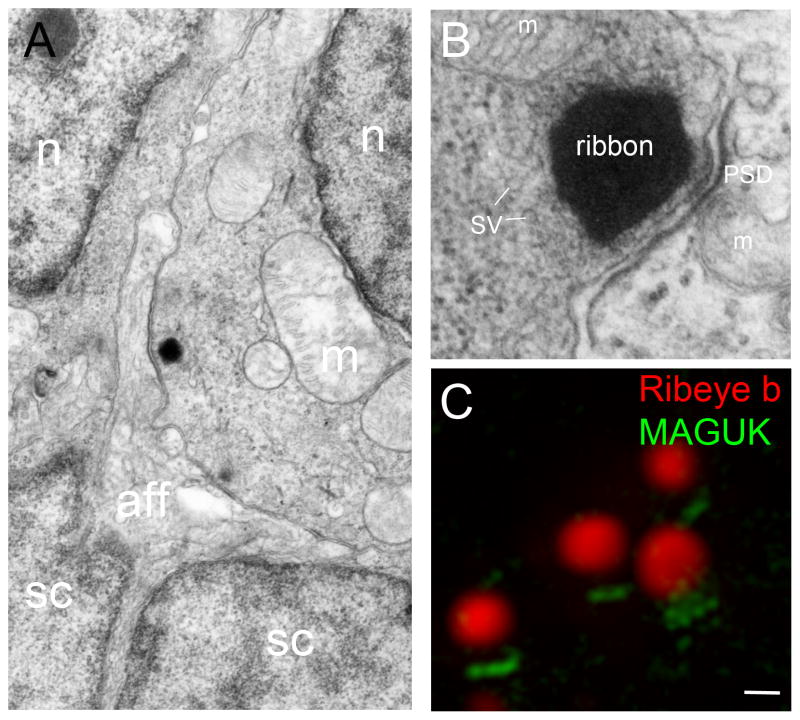
The ribbon synapse of sensory receptor cells is an ancient presynaptic structure that predates the evolution of jawed vertebrates, being present in craniates (jawless fish) such as lampreys and hagfish (Holmberg, 1971; Khonsari et al., 2009). The presynaptic active zones of sensory hair cells are typically associated with one or two ribbons (also known as dense bodies), to which are tethered glutamate-filled synaptic vesicles (reviewed in Matthews and Fuchs, 2010; Moser et al., 2006; Fig. 1). The ribbons are anchored to the basolateral membrane, directly adjacent to clusters of L-type voltage-gated calcium channels. The afferent postsynaptic compartment contains the characteristic density commonly seen in synaptic boutons (Fig. 1B). Voltage-dependent activation of the presynaptic calcium channels leads to the fusion of synaptic vesicles, releasing glutamate into the synaptic cleft, and postsynaptic action potentials are driven by the activation of glutamatergic AMPA receptors.
Fig. 1.
Ribbon synapses in zebrafish hair cells. A, Transmission electron microscopy (TEM) micrograph of the basal end of an inner ear hair cell located within the anterior macula. A prominent ribbon with vesicles is seen juxtaposed to an afferent fiber that appears to contact a large surface area (a second faint ribbon outside of the sectioning plane is seen to right). B, High magnification view of a ribbon along with the postsynaptic density. C, Super resolution structured illumination microscopy (SR-SIM) image of several ribbon synapses labeled with Ribeye b (red) and pan-MAGUK (green) antibodies (Image: Lavinia Sheets). Abbreviations: aff, afferent; m, mitochondria; n, nucleus; PSD, postsynaptic density; sc, supporting cell; SV, synaptic vesicle. Scale bar, 200 nm in A; 75 nm in B; C, 250 nm.
Zebrafish hair cells of the lateral line organ and inner ear have a similar appearance to type II vestibular hair cells in terms of overall shape and innervation, although the postsynaptic contacts of the afferent fibers can be extensive and calyx-like (Nicolson, 2005; Fig. 1). Studies using electron microscopy have shown that synaptic ribbons in frog and zebrafish hair cells are mainly spherical, unlike the ribbon-shaped dense bodies found in photoreceptors (Lenzi et al., 1999; Fig. 1A–C). Typically, hair cells in zebrafish larvae contain 3–5 single ribbons (Obholzer et al., 2008); double ribbon synapses where two dense bodies are located within 500 nm are also observed (Sheets et al., 2012; Sidi et al., 2004). The presynaptic region surrounding the ribbons in zebrafish hair cells are rich with microtubules, endoplasmic reticulum, mitochondria, and synaptic vesicles. Electron dense contacts between the ribbons and the active zone are sometimes present and look like the equivalent of pedicles, however, it cannot be unambiguously said whether these densities are synaptic vesicles or actual protein anchors.
The basolateral contacts between hair cells and afferent neurons vary in appearance; some are fairly discrete boutons while other contacts extend outside of the presumed active zone and can be described as calyx–like (Fig. 1A). In contrast to the presynaptic zone, the bouton’s most noticeable features are mitochondria and a thin, web-like postsynaptic density (Fig. 1A–C). In mammals, the patterns of innervation differ according to the hair-cell type, with either a one to one ratio as seen in the cochlea, or an afferent neuron may form contacts with multiple hair cells, which is the case in vestibular organs (Moser et al., 2006). In zebrafish, an afferent neuron of the lateral line ganglion will create synapses with hair cells possessing the same polarity of response within a single neuromast (Nagiel et al., 2008; Obholzer et al., 2008). The pattern of innervation in the zebrafish inner ear has not been described, however, innervation begins as early as 1 day postfertilization, within eight hours of differentiation of the first hair cells (Tanimoto et al., 2009).
2. Molecular and developmental aspects of ribbon synapses
Recent work has begun to elucidate the basic framework of how synaptogenesis in zebrafish hair cells occurs and has identified key molecules that play critical roles during development. The transparency of the zebrafish inner ear and lateral line organ, along with the ease of genetic approaches, has enabled the study of early events and how mutations or gene knockdown can disrupt biogenesis of ribbons and innervation of hair cells.
2.1 Subdomain organization of Ribeye on the dense body
RIBEYE was first identified biochemically from synaptosomal preparations of cow retinas (Schmitz et al., 2000) and has since been determined to be a major protein constituent of ribbons (Zenisek et al., 2004). All vertebrate RIBEYE proteins are isoforms of the CTBP2 gene where a unique N-terminal ‘A’ domain is linked to a C-terminal ‘B’ domain that is identical to C-terminal binding protein 2 (Ctbp2). For this reason, antibodies against CTBP2 are often used to immunolabel synaptic ribbons (Khimich et al., 2005; Schmitz, 2009; tom Dieck et al., 2005). Interestingly, CTBP1, another related family member of this transcription factor family, is also present within ribbons (tom Dieck et al., 2005; Uthaiah and Hudspeth, 2010). The Ribeye-specific A-domain does not share homology with any known protein. However, the B domain contains known motifs such as an NAD(H) binding site, and a lipid synthesis motif, lysophosphatidic acid acyltransferase (LPAAT) at the C-terminus (Magupalli et al., 2008; Schwarz et al., 2011). In the retina, the lipid product of LPAAT, phosphatidic acid, is enriched at ribbon synapses and may influence the trafficking of synaptic vesicles (Schwarz et al., 2011). Vesicle trafficking may also be modulated by the NAD(H) binding site of RIBEYE as protein-protein interactions of RIBEYE with regulators of vesicle formation, ArfGAP3 and Arf1, are dependent on NAD(H) (Dembla et al., 2014).
Although models of how mammalian RIBEYE assembles have been proposed based on yeast two-hybrid interactions (Magupalli et al., 2008), the in vivo orientation of RIBEYE within the spherical structure of hair cell ribbons is not known. Recent evidence obtained by exogenously expressing each subdomain of either Ribeye a, Ribeye b or Ctbp1 in zebrafish hair cells reveals that incorporation of the B domain and Ctbp1 into the dense body occurs mainly at the region of the dense body that faces the active zone (Sheets et al., 2014). Whether the surface of the ribbon body facing the plasma membrane is the site of ribbon growth, or whether the various motifs of the B domain like the LPAAT motif or Ctpb1 have an effect on the lipid composition of tethered vesicles remains to be determined.
2.2 Ribeye is a key organizer of hair-cell synapses
In both fish and mammalian nascent hair cells, Ribeye can be detected as small particles throughout the cytoplasm (Sheets et al., 2011; Sobkowicz et al., 1986; Yu and Goodrich, 2014). During maturation, these particles coalesce into much larger structures that are distributed along the basolateral membrane (Fig. 1C). Knockdown of Ribeye (ribeye a and ribeye b paralogs) in zebrafish larvae results in a severe reduction in the size or the absence of synaptic ribbons, confirming that Ribeye is a major component and organizer of these electron dense structures (Sheets et al., 2011; Wan et al., 2005). Knockdown also has far reaching effects on other aspects of synaptic development, namely that L-type Cav1.3a channels no longer cluster at the active zone, and innervation fails (Sheets et al., 2011; Fig. 2A–B). In contrast, overexpression of Ribeye b in zebrafish hair cells has a profound effect on calcium channels; ectopic dense bodies are accompanied by calcium channels, even at the apical end of the cell body (Sheets et al., 2011). A role for mammalian RIBEYE in clustering of CAV1.3 was also described in cochlear inner hair cells (Frank et al., 2010), suggesting that the genesis and organization of the presynaptic active zone is conserved in vertebrates. Presumably the organizing activity requires bassoon, a large scaffolding protein that is essential for the maintenance of anchored ribbons in cochlear hair cells (Jing et al., 2013; Khimich et al., 2005). The physical distance of the ribbon to the active zone suggests that a molecular bridge or complex may interconnect the ribbon to calcium channels. One possibility is that bassoon acts as a bridging component (Jing et al., 2013; Khimich et al., 2005; Rutherford, 2015). Another possibility is that tethered synaptic vesicles associated with the GTP-binding protein Rab3 may interact with Rab3-interacting molecules (RIMs) that couple to RIM binding proteins, which in turn interact with the alpha subunit of Cav1.3 channels (Hibino et al., 2002). The precise mechanism for the clustering of calcium channels beneath the ribbon has yet to be resolved, nevertheless, the above studies reveal an unexpected role for Ribeye in synaptogenesis and in organizing calcium channels within membranes.
Fig. 2.
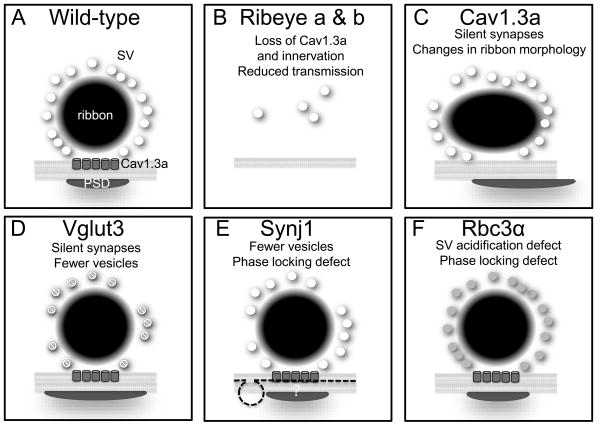
Phenotypes of ribeye morpholino-injected larvae and zebrafish auditory/vestibular mutants isolated from forward genetic screens. A, Diagram of the ribbon synapse in wild-type hair cells. B, In ribeye a/b morphants, Ribeye protein and associated clusters of calcium channel are not detectable and innervation fails. C, Mechanically-evoked responses in afferent neurons are absent and morphological changes in the size, shape and number of ribbons is apparent in cav1.3a mutants. In addition, the PSD is enlarged and with time, shifts away from the position of the ribbon. Lasting effects on the size of ribbons vary according to allele. D, Like cav1.3a mutants, synapses are silent in vglut3 mutants. In addition, fewer vesicles are tethered to the ribbon and the PSD is enlarged. E, In synj1 mutants, endocytic or recycling defects such as blebbing of the presynaptic membrane and fewer vesicles are observed near the ribbon. Phase locking of afferent spiking with respect to deflection of the hair bundle is defective at higher frequencies or upon prolonged mechanical stimulation of hair cells. Effects on the PSD have not been determined, however blebbing of the presynaptic membrane is prevalent (indicated by dotted line). F, Defects in the acidification of synaptic vesicles are present in mutant rbc3α hair cells. Firing rate and phase locking are also defective. See text for details. Abbreviations: PSD, postsynaptic density; SV, synaptic vesicle.
2.3 Effects of calcium on ribbons
Upon clustering by Ribeye, Cav1.3a channels influence the size, shape and number of dense bodies. Ribbons are initially enlarged and less spherical in cav1.3a mutants, and there is a higher percentage of double or triple ribbon synapses (Sheets et al., 2012; Fig. 2C). In addition, the postsynaptic density (visualized with pan-MAGUK antibodies) is expanded, and with time, appears to slip away from the position of the ribbons. Some of the effects are due to changes in calcium influx because acute block of Cav1.3a results in a similar increase in size and number, along with a more aspherical shape, whereas increasing Cav1.3a activity results in a shrinkage or loss of ribbons in hair cells (Sheets et al., 2012). These changes occur at immature stages and the role of Cav1.3a-dependent calcium influx appears to be universal as zebrafish pineal ribbons also respond in similar fashion to acute block or activation of L-type calcium channels (Sheets et al., 2012). The requirement for calcium influx with regard to ribbon size, shape and number is not clear, however, one explanation is that calcium acts on the assembly process of Ribeye. When local calcium concentrations at the synapse are abnormally low, the self-assembly of Ribeye may proceed unchecked during synapse maturation. The Ribeye protein itself does not appear to contain calcium-binding sites, therefore the assembly process likely requires other components that may be modulated by calcium.
Like zebrafish, Cav1.3−/− inner hair cells in mice also form afferent synapses (Brandt et al., 2003; Nemzou N et al., 2006). However, changes in the size, shape and number of ribbons during early developmental stages were not reported. Although calcium currents have not been measured in zebrafish cav1.3a mutant hair cells, one difference may be that other types of calcium channels exist at the hair-cell presynapse in mice, particularly with respect to vestibular hair cells. Vestibular function appears to be normal in Cav1.3−/− mice, whereas zebrafish cav1.3a mutants have a severe defect in balance (Nicolson et al., 1998; Sidi et al., 2004). Calcium currents in mouse vestibular hair cells are reduced only by half (Dou et al., 2004). Indeed, the remaining calcium current in both vestibular and cochlear Cav1.3−/− inner hair cells (10–15% according to Brandt et al., 2003 and Dou et al., 2004) may be sufficient to prevent detectable changes in RIBEYE assembly. Thus, the molecular composition of voltage gated calcium channels at the hair-cell presynapse is more complex in mice and multiple knockouts would be required to determine whether calcium regulation of ribbons occurs to a similar extent in mouse hair cells. Due to the severe defect in balance, zebrafish cav1.3a mutants do not survive into adulthood. In contrast, Cav1.3−/− mice are viable but show a decrease in ribbon number and a loss of afferent innervation after several months (Nemzou et al, 2006). This phenotype suggests that CAV1.3 activity is required for maintenance of afferent synapses.
Larger and more numerous Ribeye puncta were also reported in zebrafish larvae in which clarin-1 was knocked down (Ogun and Zallocchi, 2014). In morpholino-injected fish, kinocilia were shorter and Pcdh15a was absent from hair bundles. It is not clear how the lack of Clarin-1 could cause an affect on ribbons as Clarin-1 is most abundant near the kinocilium and was shown to interact with Pcdh15a (Ogun and Zallocchi, 2014). One potential mechanism is through calcium influx, that is, without Pcdh15a, which is essential for mechanotransduction and subsequent opening of Cav1.3a channels, one might expect an increase in the size of ribbons as seen in cav1.3a mutants. On the other hand, Clarin-1 may be directly involved in ribbon assembly. Further study may clarify how Clarin-1 affects ribbon biogenesis.
2.4 Lack of glutamate-filled synaptic vesicles
The changes seen in cav1.3a mutants could in part be due to the absence of synaptic transmission. Synaptic activity may be important for generating and maintaining synaptic contacts. vesicular glutamate transporter 3 (vglut3 / slc17a8) mutants exhibit normal levels of calcium influx, but have silent synapses presumably due to a lack of glutamate loading into synaptic vesicles (Obholzer et al., 2008). In addition, fewer tethered vesicles were observed in TEM micrographs of vglut3 mutant ribbons (Obholzer et al., 2008). The reason for the decrease in vesicle tethering is not clear, but one possibility is that a checkpoint for the attachment of synaptic vesicles to the dense body requires the presence of Vglut3, which is likely to exist in low copy number in individual vesicles (Takamori et al., 2006). In contrast to cav1.3a mutants, there is only a slight enlargement of ribbons and the postsynaptic density (Sheets et al., 2012; Figure 2D), and the postsynaptic density does not shift away from the presynaptic site in vglut3 mutant hair cells (Sheets et al., 2012). This phenotype suggests that calcium influx, and not just synaptic transmissions itself, is critically important for afferent synapse formation in hair cells. Similar findings were reported for Vglut3−/− mice in that the overall appearance and number of anchored ribbon synapses were normal in cochlear inner hair cells at P14 (Ruel et al., 2008). Although the levels of AMPA receptors were not quantified, immunolabel of GluR2/3 was aligned with the ribbons (RIBEYE-positive puncta) in Vglut3−/− mice (Ruel et al., 2008). At three months of age, however, there were fewer ribbons and spiral ganglion neurons in Vglut3−/− mice, indicating that partial degeneration occurs in the absence of synaptic transmission.
2.5 Wiring of afferent synapses
Afferent synapses in hair cells can form in the absence of synaptic transmission as is the case with both fish and mouse cav1.3 or vglut3 mutants. This uncoupling of synaptogenesis and synaptic activity suggests that the hair-cell synapse is hard-wired or mostly dependent on molecular cues such as adhesion proteins or receptor signaling cascades. However, whether synaptic activity plays a role in the selection of targets by afferent neurons during development is not clear. In the lateral line organ, each afferent neuron innervates one or two neuromasts, and synapses with only a subset of hair cells sharing the same planar polarity (Nagiel et al., 2008). Such a selective pattern of connectivity can be detected in live fish using either electrophysiology (Obholzer et al., 2008) or live imaging (Nagiel et al., 2008). In experiments using larvae expressing red fluorescent proteins in afferent neurons, it was found that hair cells that either lacked mechanotransduction (pcdh15a and tmie mutants, or chronic amiloride treatment) or synaptic transmission (cav1.3a, vglut3) were still selectively innervated according to the planar polarity of the hair bundles (Nagiel et al., 2009). However, the results with the tmie mutant are controversial, as a second study found that a large percentage of fibers were positioned near Ribeye-positive puncta of hair cells with opposite planar polarity (Faucherre et al., 2009). This result suggests that activity does play a role in determining the specificity of innervation. In both studies, varicosities or the proximity of neurite endings were interpreted as synaptic contacts and labeling of molecular markers of the postsynaptic synapse such as MAGUK was not performed. As the fibers form a fairly complex web beneath the hair cell bodies, it can be difficult to discern the actual site of synaptic contact using morphological cues alone. Nevertheless, the differences between these findings remain to be resolved.
2.6 Survival factors for innervation
Regardless of synaptic activity, the secretion of trophic factors is critical for synaptic innervation of hair cells in the auditory/vestibular system. In mice, four factors are required for trophic signaling to afferent neurons: brain derived growth factor (BDNF), neurotrophin-3 (NT-3) and the tyrosine kinase receptors TrkB and TrkC (reviewed in Fritzsch et al., 2004; Singer et al., 2014). The role of trophic factors in the development or maintenance of zebrafish afferent synapses is less well understood. Both TrkB and BDNF are expressed in zebrafish hair cells and afferent neurons of the lateral line organ (Germanà et al., 2010). Secretion of BDNF requires the activity of N-ethylmaleimide sensitive factor (Nsf) in afferent neurons (Mo and Nicolson, 2011). Nsf also plays a role in hair cells, likely mediating the secretion of BDNF or other neurotrophic factors that act on afferent fibers to maintain synaptic contacts. If Nsf is absent, then afferent synaptic contacts are lost during development (Mo and Nicolson, 2011). The nsf mutant phenotype can be partially rescued by injection of BDNF (Mo and Nicolson, 2011). In a similar context, reintroduction of BDNF in Pou4f3−/− mutant mice that lack hair cells caused robust sprouting of nerve fibers and better survival of afferent neurons (Fukui et al., 2012). A role for Nsf in secretion of BDNF from hair cells is of interest in light of the evidence suggesting that exocytosis at ribbon synapses of mouse hair cells occurs independently of the canonical SNARE machinery (Nouvian et al., 2011). Nevertheless, the cumulative results are consistent with the notion that trophic factors promote and support the presence of afferent fibers within the neuroepithelium and these findings may have implications for cochlear implants that require the survival of the auditory nerve.
3. Functional aspects of ribbon synapses
The study of ribbon synapses in zebrafish hair cells has profited from recently developed methods that enable quantification of deficits in synaptic transmission. In particular, the development of a loose patch method of recording spiking activity in afferent neurons of the lateral line organ has been instrumental in describing defects in various mutants (Trapani and Nicolson, 2010). Moreover, new methods for quantifying calcium transients in zebrafish hair cells (Kindt et al., 2012) have made valuable contributions to understanding mutant phenotypes, and finally, the ability to directly measure hair-cell currents and capacitance using patch clamp recordings in larvae (Olt et al., 2014; Ricci et al., 2013) opens the door to more detailed analysis in future studies.
3.1 Cav1.3 and Otoferlin
Presynaptic Cav1.3 channels are critical for mediating voltage-gated calcium influx and subsequent neurotransmitter release in lateral-line and auditory/vestibular hair cells (Beutner et al., 2001; Dou et al., 2004; Platzer et al., 2000; Sheets et al., 2012; Sidi et al., 2004; Trapani and Nicolson, 2011). Outer hair cells of the cochlea also have ribbon synapses and display Cav1.3-dependent calcium currents (Dou et al., 2004; Knirsch et al., 2007; Michna et al., 2003), although afferent signaling by outer hair cells may require much higher intensity sounds (Weisz et al., 2014). In cav1.3a zebrafish mutants, the microphonic or extracellular potentials of lateral line hair cells are reduced by two thirds, indicating that these channels contribute to the sum of microphonic currents (Sidi et al., 2004). The impact on synaptic transmission is devastating; evoked action currents in afferent neurons are completely absent in cav1.3a mutants (Trapani and Nicolson, 2011; Fig. 2C). Interestingly, spontaneous release occurs at a glacial pace of about one event per minute with extended interspike intervals of greater than several minutes. These results suggest that on occasion the intracellular calcium level may be high enough to allow for spontaneous release, but otherwise evoked activity is absent in cav1.3a mutant synapses. Reductions in voltage-gated calcium currents in vestibular and cochlear hair cells have been described for Cav1.3−/− mice (Brandt et al., 2003; Dou et al., 2004; Michna et al., 2003) and mutations in CAV1.3 (aka CACNAD1) are associated with human deafness (Baig et al., 2011), indicating that the function for this particular L-type calcium channel in hair-cell synaptic transmission is conserved across species.
Upon calcium influx, exocytosis of synaptic vesicles in hair cells is dependent on otoferlin, a C2 domain transmembrane protein (Dulon et al., 2009; Roux et al., 2006). Otoferlin has been detected in hair cells of multiple species including zebrafish (Goodyear et al., 2010). A recent study using morpholinos against otoferlin a and otoferlin b showed that double knockdown resulted in defective balance and hearing in zebrafish larvae (Chatterjee et al., 2015). Rescue experiments using various deletions of the C2 domains revealed that the C-terminal C2 domains are critical for function (Chatterjee et al., 2015). Aside from a severe reduction of exocytosis in hair cells, which is presumably the case in the zebrafish knockdowns, the deletion of otoferlin in mice results in a significant decrease in the number of ribbons (Roux et al., 2006). The effect of otoferlin knockdown on ribbons and other components of the ribbon synapses in zebrafish remains to be determined.
3.2 Vglut3 in synaptic vesicles
Ribbon synapses have been long known to be glutamatergic, but the actual glutamate transporter in hair cells had not been identified until 2008. For unknown reasons, hair cells in both mammals and zebrafish employ the least abundant type of glutamate transporter Vglut3 (Obholzer et al., 2008; Ruel et al., 2008; Seal et al., 2008). This transporter is seen in very few areas of the brain and mutations cause synaptic transmission in hair cells to cease. Unlike the cav1.3a mutant, vglut3 zebrafish mutant hair cells have normal microphonic potentials, and do not produce any observable spontaneous activity in afferent fibers (Obholzer et al., 2008; Fig. 2D). Due to a lack of glutamate loading into synaptic vesicles, these synapses are truly silent. However, synaptic development is relatively normal, with only a modest increase in the size of the postsynaptic compartment at a later stage of development (Sheets et al., 2012). Enlargement of the postsynaptic density may occur as a compensatory response to the lack of synaptic activity in vglut3 mutant hair cells.
3.3 Rabconnectin3α (Rbc3α) as a key regulator of the vesicular proton pump
As an adaptor or scaffold for modulators of Rab G-proteins, the interaction of Rbc3α with the vacuolar ATPase (V-ATPase) was initially discovered in Drosophila ovaries (Yan et al., 2009). The proton gradient created by the V-ATPase is required for glutamate loading into synaptic vesicles (Liguz-Lecznar and Skangiel-Kramska, 2007). Interestingly, each synaptic vesicle is thought to contain a single V-ATPase, making this holoenzyme a rare, yet critical component (Takamori et al., 2006). Mutations in zebrafish rbc3α affect the stability of the holoenzyme in synaptic vesicles as the cytosolic component of the holoenzyme is no longer enriched in the basal half of mutant hair cells (Einhorn et al., 2012). As a result, acidification of synaptic vesicles is defective. Other synaptic constituents such as Vglut3 and Rab3 proteins are unchanged in rbc3α mutants. The loss of Rbc3α leads to a reduction in evoked afferent spiking and a loss of synchronized firing with the mechanical stimulus or ‘phase locking’, presumably the synaptic vesicles are not fully loaded with glutamate (Einhorn et al., 2012; Fig. 2E). Decreased afferent responses could account for the vestibular dysfunction and the raised auditory thresholds in Rbc3α mutants. The loss of Rbc3α is likely to affect synaptic transmission in other regions of the nervous system because rbc3α mutants appear to be blind and unable to move their eyes in response to vestibular stimulation. The interaction of Rbc3α with Rab3 and the V-ATPase suggests that this scaffold protein coordinates G protein signaling with synaptic vesicle function. Interestingly, the gross morphology of the hair-cell ribbon and postsynaptic density in rbc3α mutants is normal, suggesting that such a role of coupling G protein signaling and acidification is not critical during development.
3.4 Synjanin1 (Synj1) in synaptic vesicle recycling
Similar to the rbc3α phenotype, synj1 mutants also show a reduction in evoked spiking rate and a loss of phase-locking (Trapani et al., 2009; Fig. 2F). Unlike rbc3α mutants, prolonged mechanical stimulation of synj1 mutant hair cells results in significantly decreased evoked spiking rates and dramatic increases the length of time before the resumption of spontaneous release (Trapani et al., 2009). These changes in synaptic activity correlate with an observed reduction in the number of synaptic vesicles. A reduction in vesicle numbers is consistent with the role of synj1 in removing the clathrin coat of endocytic vesicles as a necessary step in the recycling of synaptic membranes (Cremona and De Camilli, 2001). In addition, extrusions of the plasma membrane near the active zones of hair cells are observed in synj1 zebrafish mutants (Trapani et al., 2009). Blebbing of the presynaptic membrane is dependent upon Cav1.3a activity, which is required to trigger the exocytosis of synaptic vesicles; double synj1 cav1.3a mutants no longer exhibit abnormal blebs near hair-cell ribbons (Trapani et al., 2009). Such a phenotype is consistent with a role for synj1 in the recycling of exocytosed vesicles. The phase locking phenotype seen in both rbc3α and synj1 mutants suggests that when glutamate levels or vesicle numbers are less than adequate for normal synaptic transmission, then fidelity of the ribbon synapse decreases.
3.5 Spontaneous release at hair afferent synapses
Unevoked release of glutamate from hair cells generates spontaneous action potentials in afferent neurons (Glowatzki and Fuchs, 2002; Keen and Hudspeth, 2006; Li et al., 2009; Moser and Beutner, 2000; Rutherford et al., 2012). Spontaneous activity is thought to play an important role in the development of the neural circuitry within brain nuclei that process auditory information (Kennedy, 2012) and may modulate sensitivity of auditory or vestibular synapses (Liberman, 1978; Peusner et al., 2012; Taberner and Liberman, 2005). As in auditory/vestibular hair cells, spontaneous release of glutamate occurs in zebrafish lateral-line hair cells (Trapani and Nicolson, 2011). The rate of spontaneous spiking of lateral-line afferent neurons is depressed in mutants that lack mechanotransduction (cadherin 23 and pcdh15a), and requires Cav1.3a (Trapani and Nicolson, 2011). A similar requirement for L-type calcium channels in spontaneous release was reported for guinea pig hair cells (Robertson and Paki, 2002). In addition, HCN1 channels that set the resting potential of the cell also contribute to spontaneous spiking of afferent neurons, presumably by slightly depolarizing hair cells and thereby leading to greater activity of Cav1.3a channels at rest (Trapani and Nicolson, 2011). The role of spontaneous release of glutamate during development of the inner ear or lateral line organ in zebrafish is not clear. The overall range in spontaneous rates among zebrafish afferent neurons is relatively narrow in comparison to the rates reported for mammalian auditory nerve fibers. Whether the rate of spontaneous activity in the zebrafish afferent neurons correlates to sensitivity or thresholds as seen in other vertebrates, or whether this type of activity is involved in the generation of maps within the hindbrain needs further investigation.
4. Conclusions
Our understanding of the molecular basis of development and function of hair-cell afferent synapses has benefitted greatly from genetic and imaging methods in animal models, particularly in zebrafish. Collectively, studies of the development of zebrafish ribbon synapses have revealed an unexpected interdependence of the ribbon and calcium channels. On the one hand, the major protein components of the ribbon Ribeye a & b are required for synaptogenesis with afferent fibers and establishment of an active zone clustered with calcium channels. Hair-cell ribbons act as a synaptic organizer, bringing together the basic pre- and postsynaptic components that enable communication with the brain. On the other hand, calcium influx through those very same calcium channels at the presynapse exerts an effect on the size, number and shape of ribbons. How calcium influences the assembly of Ribeye subunits and subsequent morphology of dense bodies is not clear and requires further study.
Calcium influx coupled to exocytosis also appears to be critical for the stabilization of the postsynaptic density. Indeed, in cav1.3a and vglut3 mutants that lack synaptic activity, the postsynaptic density increases in size. This response of the afferent neuron may be an attempt to compensate or is a type of default response in the absence of glutamate release from the hair cell. With regard to innervation, the role of hair-cell activity in determining specific contacts between afferent neurons and hair cells of the lateral line organ is still unresolved. The complete absence of synaptic transmission in the auditory/vestibular or lateral line organ does not, however, have a dramatic impact on innervation during development. Nevertheless, how specificity between afferent neurons and planar polarized hair cells of the lateral line organ is achieved is still an open question.
In zebrafish mutants where neurotransmitter release is attenuated rather than absent, the effects on ribbon synapse morphology are more subtle in comparison. Instead, the main defects appear to be in the timing of synaptic vesicle release. An increase in jitter or a loss of temporal fidelity is a common phenotype to those mutants that affect either the numbers or content of synaptic vesicles. Jitter in the system leads to behavioral deficits in vestibular function and a reduction in auditory sensitivity, which highlights the importance of the precision of phase locking for normal hearing and balance in vertebrates.
Highlights.
Ribeye acts as an organizer of afferent synapses in hair cells
calcium influx through presynaptic L-type calcium channels influences ribbon size, shape and number
lack of neurotransmitter signaling leads to expansion of the postsynaptic density
decreases in phase locking with mechanical stimuli occur under conditions of reduced synaptic transmission
Acknowledgments
I thank Timothy Erickson for his comments on the manuscript. Funding for related work of our laboratory was provided by Howard Hughes Medical Institute and by the National Institutes of Health (R01 DC006880 and P30 DC005983).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nürnberg P, Striessnig J, Bolz HJ. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–90. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–40. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Padmanarayana M, Abdullah N, Holman CL, LaDu J, Tanguay RL, Johnson CP. Otoferlin deficiency in zebrafish results in defects in balance and hearing: rescue of the balance and hearing phenotype with full-length and truncated forms of mouse otoferlin. Mol Cell Biol. 2015;35:1043–1054. doi: 10.1128/MCB.01439-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- Dembla M, Wahl S, Katiyar R, Schmitz F. ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms an NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis. J Neurosci Off J Soc Neurosci. 2014;34:5245–5260. doi: 10.1523/JNEUROSCI.3837-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Vazquez AE, Namkung Y, Chu H, Cardell EL, Nie L, Parson S, Shin HS, Yamoah EN. Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice. J Assoc Res Otolaryngol JARO. 2004;5:215–226. doi: 10.1007/s10162-003-4020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci Off J Soc Neurosci. 2009;29:10474–10487. doi: 10.1523/JNEUROSCI.1009-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn Z, Trapani JG, Liu Q, Nicolson T. Rabconnectin3α promotes stable activity of the H+ pump on synaptic vesicles in hair cells. J Neurosci Off J Soc Neurosci. 2012;32:11144–11156. doi: 10.1523/JNEUROSCI.1705-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A, Pujol-Martí J, Kawakami K, López-Schier H. Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS One. 2009;4:e4477. doi: 10.1371/journal.pone.0004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršič T, Khimich D, Fejtova A, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fukui H, Wong HT, Beyer LA, Case BG, Swiderski DL, Di Polo A, Ryan AF, Raphael Y. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci Rep. 2012;2:838. doi: 10.1038/srep00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanà A, Laurà R, Montalbano G, Guerrera MC, Amato V, Zichichi R, Campo S, Ciriaco E, Vega JA. Expression of brain-derived neurotrophic factor and TrkB in the lateral line system of zebrafish during development. Cell Mol Neurobiol. 2010;30:787–793. doi: 10.1007/s10571-010-9506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–54. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Christiansen JR, Xia B, Korchagina J, Gale JE, Warchol ME, Corwin JT, Richardson GP. Identification of the hair cell soma-1 antigen, HCS-1, as otoferlin. J Assoc Res Otolaryngol JARO. 2010;11:573–586. doi: 10.1007/s10162-010-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca(2+) channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K. The hagfish retina: electron microscopic study comparing receptor and epithelial cells in the Pacific hagfish, Polistotrema stouti, with those in the Atlantic hagfish, Myxine glutinosa. Z Für Zellforsch Mikrosk Anat Vienna Austria 1948. 1971;121:249–269. doi: 10.1007/BF00340676. [DOI] [PubMed] [Google Scholar]
- Jing Z, Rutherford MA, Takago H, Frank T, Fejtova A, Khimich D, Moser T, Strenzke N. Disruption of the presynaptic cytomatrix protein bassoon degrades ribbon anchorage, multiquantal release, and sound encoding at the hair cell afferent synapse. J Neurosci Off J Soc Neurosci. 2013;33:4456–4467. doi: 10.1523/JNEUROSCI.3491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell’s afferent synapse. Proc Natl Acad Sci U S A. 2006;103:5537–5542. doi: 10.1073/pnas.0601103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ. New developments in understanding the mechanisms and function of spontaneous electrical activity in the developing mammalian auditory system. J Assoc Res Otolaryngol JARO. 2012;13:437–445. doi: 10.1007/s10162-012-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–94. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Khonsari RH, Li B, Vernier P, Northcutt RG, Janvier P. Agnathan brain anatomy and craniate phylogeny. Acta Zool. 2009;90:52–68. doi: 10.1111/j.1463-6395.2008.00388.x. [DOI] [Google Scholar]
- Kindt KS, Finch G, Nicolson T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev Cell. 2012;23:329–341. doi: 10.1016/j.devcel.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirsch M, Brandt N, Braig C, Kuhn S, Hirt B, Münkner S, Knipper M, Engel J. Persistence of Ca(v)1.3 Ca2+ channels in mature outer hair cells supports outer hair cell afferent signaling. J Neurosci Off J Soc Neurosci. 2007;27:6442–6451. doi: 10.1523/JNEUROSCI.5364-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci Off J Soc Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Li GL, Keen E, Andor-Ardó D, Hudspeth AJ, von Gersdorff H. The unitary event underlying multiquantal EPSCs at a hair cell’s ribbon synapse. J Neurosci. 2009;29:7558–68. doi: 10.1523/JNEUROSCI.0514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters (VGLUTs): the three musketeers of glutamatergic system. Acta Neurobiol Exp (Warsz) 2007;67:207–218. doi: 10.55782/ane-2007-1649. [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci. 2008;28:7954–67. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna M, Knirsch M, Hoda JC, Muenkner S, Langer P, Platzer J, Striessnig J, Engel J. Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice. J Physiol. 2003;553:747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Brandt A, Lysakowski A. Hair cell ribbon synapses. Cell Tissue Res. 2006;326:347–59. doi: 10.1007/s00441-006-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo W, Nicolson T. Both pre- and postsynaptic activity of Nsf prevents degeneration of hair-cell synapses. PloS One. 2011;6:e27146. doi: 10.1371/journal.pone.0027146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiel A, Andor-Ardó D, Hudspeth AJ. Specificity of afferent synapses onto plane-polarized hair cells in the posterior lateral line of the zebrafish. J Neurosci. 2008;28:8442–53. doi: 10.1523/JNEUROSCI.2425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiel A, Patel SH, Andor-Ardó D, Hudspeth AJ. Activity-independent specification of synaptic targets in the posterior lateral line of the larval zebrafish. Proc Natl Acad Sci U A. 2009;106:21948–53. doi: 10.1073/pnas.0912082106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzou NRM, Bulankina AV, Khimich D, Giese A, Moser T. Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels. Neuroscience. 2006;141:1849–60. doi: 10.1016/j.neuroscience.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–83. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Neef J, Bulankina AV, Reisinger E, Pangršič T, Frank T, Sikorra S, Brose N, Binz T, Moser T. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. Nat Neurosci. 2011;14:411–413. doi: 10.1038/nn.2774. [DOI] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Söllner C, Duncan RN, Boehland A, Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28:2110–8. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogun O, Zallocchi M. Clarin-1 acts as a modulator of mechanotransduction activity and presynaptic ribbon assembly. J Cell Biol. 2014;207:375–391. doi: 10.1083/jcb.201404016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olt J, Johnson SL, Marcotti W. In vivo and in vitro biophysical properties of hair cells from the lateral line and inner ear of developing and adult zebrafish. J Physiol. 2014;592:2041–2058. doi: 10.1113/jphysiol.2013.265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peusner KD, Shao M, Reddaway R, Hirsch JC. Basic Concepts in Understanding Recovery of Function in Vestibular Reflex Networks during Vestibular Compensation. Front Neurol. 2012;3 doi: 10.3389/fneur.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Bai JP, Song L, Lv C, Zenisek D, Santos-Sacchi J. Patch-clamp recordings from lateral line neuromast hair cells of the living zebrafish. J Neurosci Off J Soc Neurosci. 2013;33:3131–3134. doi: 10.1523/JNEUROSCI.4265-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Paki B. Role of L-type Ca2+ channels in transmitter release from mammalian inner hair cells. II Single-neuron activity. J Neurophysiol. 2002;87:2734–2740. doi: 10.1152/jn.2002.87.6.2734. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJH, Lesperance MM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–92. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA. Resolving the structure of inner ear ribbon synapses with STED microscopy. Synap N Y N. 2015 doi: 10.1002/syn.21812. [DOI] [PubMed] [Google Scholar]
- Rutherford MA, Chapochnikov NM, Moser T. Spike encoding of neurotransmitter release timing by spiral ganglion neurons of the cochlea. J Neurosci Off J Soc Neurosci. 2012;32:4773–4789. doi: 10.1523/JNEUROSCI.4511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F. The making of synaptic ribbons: how they are built and what they do. Neuroscientist. 2009;15:611–24. doi: 10.1177/1073858409340253. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Königstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–72. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Natarajan S, Kassas N, Vitale N, Schmitz F. The synaptic ribbon is a site of phosphatidic acid generation in ribbon synapses. J Neurosci Off J Soc Neurosci. 2011;31:15996–16011. doi: 10.1523/JNEUROSCI.2965-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L, Hagen MW, Nicolson T. Characterization of Ribeye subunits in zebrafish hair cells reveals that exogenous Ribeye B-domain and CtBP1 localize to the basal ends of synaptic ribbons. PloS One. 2014;9:e107256. doi: 10.1371/journal.pone.0107256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L, Kindt KS, Nicolson T. Presynaptic CaV1.3 channels regulate synaptic ribbon size and are required for synaptic maintenance in sensory hair cells. J Neurosci Off J Soc Neurosci. 2012;32:17273–17286. doi: 10.1523/JNEUROSCI.3005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L, Trapani JG, Mo W, Obholzer N, Nicolson T. Ribeye is required for presynaptic Ca(V)1.3a channel localization and afferent innervation of sensory hair cells. Dev Camb Engl. 2011;138:1309–1319. doi: 10.1242/dev.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24:4213–23. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Panford-Walsh R, Knipper M. The function of BDNF in the adult auditory system. Neuropharmacology. 2014;76(Pt C):719–728. doi: 10.1016/j.neuropharm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GL, Levenick CV. Distribution of synaptic ribbons in the developing organ of Corti. J Neurocytol. 1986;15:693–714. doi: 10.1007/BF01625188. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–569. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P. Molecular Anatomy of a Trafficking Organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Ota Y, Horikawa K, Oda Y. Auditory input to CNS is acquired coincidentally with development of inner ear after formation of functional afferent pathway in zebrafish. J Neurosci Off J Soc Neurosci. 2009;29:2762–2767. doi: 10.1523/JNEUROSCI.5530-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtová A, Bracko O, Gundelfinger ED, Brandstätter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–36. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, Nicolson T. Mechanism of spontaneous activity in afferent neurons of the zebrafish lateral-line organ. J Neurosci Off J Soc Neurosci. 2011;31:1614–1623. doi: 10.1523/JNEUROSCI.3369-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, Nicolson T. Physiological recordings from zebrafish lateral-line hair cells and afferent neurons. Methods Cell Biol. 2010;100:219–231. doi: 10.1016/B978-0-12-384892-5.00008-6. [DOI] [PubMed] [Google Scholar]
- Trapani JG, Obholzer N, Mo W, Brockerhoff SE, Nicolson T. Synaptojanin1 is required for temporal fidelity of synaptic transmission in hair cells. PLoS Genet. 2009;5:e1000480. doi: 10.1371/journal.pgen.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, Obholzer N, Mo W, Brockerhoff SE, Nicolson T. Synaptojanin1 is required for temporal fidelity of synaptic transmission in hair cells. PLoS Genet. 2009;5:e1000480. doi: 10.1371/journal.pgen.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaiah RC, Hudspeth AJ. Molecular anatomy of the hair cell’s ribbon synapse. J Neurosci Off J Soc Neurosci. 2010;30:12387–12399. doi: 10.1523/JNEUROSCI.1014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Almers W, Chen W. Two ribeye genes in teleosts: the role of Ribeye in ribbon formation and bipolar cell development. J Neurosci. 2005;25:941–9. doi: 10.1523/JNEUROSCI.4657-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJC, Glowatzki E, Fuchs PA. Excitability of type II cochlear afferents. J Neurosci Off J Soc Neurosci. 2014;34:2365–2373. doi: 10.1523/JNEUROSCI.3428-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Denef N, Schüpbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell. 2009;17:387–402. doi: 10.1016/j.devcel.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Goodrich LV. Morphological and physiological development of auditory synapses. Hear Res. 2014;311:3–16. doi: 10.1016/j.heares.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004;24:9752–9. doi: 10.1523/JNEUROSCI.2886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]