Abstract
Introduction
In order to prevent falls, older people should exercise for at least 2 h per week for 6 months, with a strong focus on balance exercises. This article describes the design of a randomised controlled trial to evaluate the effectiveness of a home-based exercise programme delivered through a tablet computer to prevent falls in older people.
Methods and analysis
Participants aged 70 years or older, living in the community in Sydney will be recruited and randomly allocated to an intervention or control group. The intervention consists of a tailored, home-based balance training delivered through a tablet computer. Intervention participants will be asked to complete 2 h of exercises per week for 2 years. Both groups will receive an education programme focused on health-related information relevant to older adults, delivered through the tablet computer via weekly fact sheets. Primary outcome measures include number of fallers and falls rate recorded in weekly fall diaries at 12 months. A sample size of 500 will be necessary to see an effect on falls rate. Secondary outcome measures include concern about falling, depressive symptoms, health-related quality of life and physical activity levels (in all 500 participants); and physiological fall risk, balance, functional mobility, gait, stepping and cognitive performance (in a subsample of 200 participants). Adherence, acceptability, usability and enjoyment will be recorded in intervention group participants over 2 years. Data will be analysed using the intention-to-treat principle. Secondary analyses are planned in people with greater adherence. Economic analyses will be assessed from a health and community care provider perspective.
Ethics and dissemination
Ethical approval was obtained from UNSW Ethics Committee in December 2014 (ref number HC#14/266). Outcomes will be disseminated through publication in peer-reviewed journals and presentations at international conferences.
Trial registration number
Australian New Zealand Clinical Trials Registry (ACTRN)12615000138583.
Keywords: REHABILITATION MEDICINE, PUBLIC HEALTH, QUALITATIVE RESEARCH, PREVENTIVE MEDICINE, GERIATRIC MEDICINE
Strengths and limitations of this study.
This randomised controlled trial will investigate the effectiveness of an engaging home-based exercise programme delivered through a tablet computer in line with current evidence-based principles to prevent falls and maximise long-term adherence.
The use of technology makes it possible to provide tailored and progressive balance exercises and allows real-time data gathering of adherence.
The trial will establish predictors of adherence to the exercise programme for a 24-month follow-up period and estimate the cost-effectiveness of this approach.
Participant blinding is not possible.
Introduction
One-third of people aged 65 years and over fall every year, and falls and fractures account for over half of all injury-related healthcare costs. This impact will grow substantially in the near future due to the increased proportion of older people in the population. Furthermore, the personal and societal impact from falls is enormous due to mobility-related disability and loss of independence. Developing and implementing effective strategies that prevent falls and mobility problems among older people is, therefore, an urgent public health issue.
There is clear evidence that falls in older people can be prevented with appropriately designed intervention programmes. A Cochrane systematic review concluded that exercise is one of the single most effective strategies to reduce the rate of falls in older adults.1 There is also systematic review evidence that, to be effective in preventing falls, exercise programmes must include at least moderately challenging balance exercises and be performed frequently (ie, for more than 2 h a week over a 6-month period).2 If well-designed, exercise programmes can reduce falls by 42%, as demonstrated by pooled results from 44 trials (pooled rate ratio 0.58, 95% CI 0.48 to 0.69),2 and for up to 2 years in people who continue to exercise.3 However, sustained adherence in effective trials is poor with median reported adherence rates of 52% (range 43–61%) at 12 months,4 dropping down to 35% at 2 years.3 Therefore, in order to prevent falls at the public health level, strategies that optimise long-term adherence to balance training programmes are needed.
The incorporation of regular exercise as a consistent lifestyle behaviour (ie, more than 2 h per week) is not easy for many older people due to poor exercise tolerance and lack of enjoyment. Several factors create barriers to exercise uptake and adherence, including lack of interest in the exercise programme, low expectation of positive outcomes, fear of falling and adverse weather.5 A large survey in 5440 older adults indicated that home-based exercises were preferred over other fall prevention strategies and this was particularly so in older and more socially disadvantaged people who are a high risk of falling.6 A recent systematic review further highlighted that the inclusion of balance training was associated with the best adherence to home exercise programmes.4 This indicates that a well-designed home-based balance training programme has the potential to be widely adopted by older people as a fall prevention strategy.
Recent advances in smart phones and tablet computers offer viable, inexpensive and highly accessible means to deliver health messages, and to motivate and guide people to make lifestyle changes. Smart phone apps are currently used for management of chronic diseases, such as diabetes and cardiac rehabilitation, as well as to deliver exercise programmes. Using apps to deliver balance training to older people at risk of falls may enable greater choice in preferred exercise options, increased convenience and accessibility, and instil a greater level of engagement through immediate performance feedback. While many apps are available, few have been evaluated in rigorously conducted clinical trials.
We have developed an engaging home-based exercise programme delivered through a tablet computer in line with current evidence-based principles to prevent falls and maximise long-term adherence. The programme is called Standing Tall. It offers tailored and progressive balance exercises and an in built coach to encourage users to exercise at the desired frequency. This randomised controlled trial (RCT) aims to determine the effect of Standing Tall on fall rate and known fall risk factors (including balance, gait, concern about falling and physical activity levels) in older people over a 24-month follow-up period, when compared with a health promotion education ‘control’ programme. The trial will also establish predictors of adherence to the exercise programme and estimate the cost-effectiveness of this approach.
Methods and analysis
Trial design
An RCT will be conducted to examine the effectiveness of a home-based balance training programme delivered through a tablet computer. The intervention will involve balance training for at least 2 h per week for 2 years and a health promotion education programme. The control group will solely receive the health promotion education programme, also delivered through a tablet computer, in addition to usual care. Falls will be monitored in all participants for 24 months from their start in the trial. The trial protocol has been registered prior to starting the trial on the Australian and New Zealand Clinical Trial Registry (ACTRN12615000138583), as outlined in table 1. Important protocol modifications will be communicated to the trial registry.
Table 1.
Trial registration data
| Data category | Information |
|---|---|
| Primary registry and trial identifying number | Australian New Zealand Clinical Trial Registry ACTRN12615000138583 |
| Date of registration in primary registry | 13 February 2015 |
| Source(s) of monetary or material support | Government body; Charities/Societies/Foundations |
| Primary sponsor | National Health and Medical Research Council Sponsor's reference: APP1084739 |
| Secondary sponsor(s) | Gandel Philanthropy; NeuRA Foundation |
| Contact for public queries | KD (k.delbaere@neura.edu.au) |
| Contact for scientific queries | KD (k.delbaere@neura.edu.au) |
| Lead investigator | KD (k.delbaere@neura.edu.au) |
| Public title | Standing Tall—a home-based exercise programme using mobile technology for preventing falls in older people |
| Scientific title | Evaluating the effect of a home-based exercise programme delivered through mobile technology for preventing falls in older community-dwelling people over 2 years, compared with a health promotion education ‘control’ programme—the ‘Standing Tall’ randomised control trial |
| Countries of recruitment | Australia |
| Health condition(s) or problem(s) studied | Falls |
| Intervention(s) | Active comparator: home-based balance training (2 h per week) and health promotion education programme delivered through a tablet computer |
| Control comparator: health promotion education programme delivered through a tablet computer in people's homes | |
| Key inclusion and exclusion criteria | Ages eligible for study: ≥70 years; Sexes eligible: both; Accepts healthy volunteers: yes |
| Inclusion criteria: older adults (≥70 years), community-dwelling, independent in activities of daily living, able to walk household distances without the use of a walking aid, willing to give informed consent and comply with the study protocol, have sufficient English language skills to understand the assessment and intervention procedures | |
| Exclusion criteria: current participation in a fall prevention programme, cognitive impairment defined as a Pfeiffer SPMSQ score <8, progressive neurological disease or any other unstable or acute medical condition precluding exercise | |
| Study type | Interventional Allocation: randomised; Masking: single blind |
| Primary purpose: Prevention | |
| Date of first enrolment | 17 February 2015 |
| Target sample size | 500 |
| Recruitment status | Recruiting |
| Primary outcome(s) | Proportion of fallers in each group (time frame: 12 months after baseline assessment) |
| Rate of falling in each group (time frame: 12 months after baseline assessment) | |
| Key secondary outcomes | In a subsample of 200 participants (time points: 6 and 12 months): |
|
|
| In all participants (time points: 6, 12, 18 and 24 months): | |
|
|
| In intervention group participants (time points: 6, 12, 18 and 24 months): | |
|
SPMSQ, Short Portable Mental Status Questionnaire.
Participants and eligibility criteria
Five-hundred people aged 70 years or older, living in the community in the Sydney metropolitan area will be recruited. Eligibility criteria include English-speaking, living independently at home, able to walk in their home without the use of a walking aid, and willingness to give informed consent and comply with the study protocol. People will be ineligible to participate in the trial if they have an unstable or acute medical condition that precludes exercise participation, have a progressive neurological condition (such as Parkinson's disease, multiple sclerosis, Meniere disease), are cognitively impaired defined as a Pfeiffer Short Portable Mental Status Questionnaire (SPMSQ) score <8,7 or are currently participating in a fall prevention programme (ie, more than 30 min of balance or lower limb strength exercises per week).
Recruitment
Participants will be recruited primarily via presentation of the study to targeted senior audiences. The targeted groups (and individuals) are sourced from advertisements in community services newsletters, notice boards, local newspapers, social media, and retirement villages and community centres in Sydney, Australia. Potential participants will be screened for eligibility over the phone. Eligible participants will receive information sheets by post and will be able to have an informed discussion with trained research assistants regarding the information provided (see online supplementary appendix 1). Trained research assistants will obtain written consent prior to baseline assessment.
Randomisation
Following baseline assessment, participants will be randomly allocated to the intervention or control group using a web-based randomisation service. Permuted block randomisation using computer-generated random numbers is applied to form two groups of similar size (allocation ratio 1:1). People living in the same household are treated as one unit and randomised into their own blocks to ensure that equal numbers of couples are allocated to intervention and control groups. Allocation concealment will be ensured as the randomisation code will only be released after all baseline assessments have been completed.
Intervention
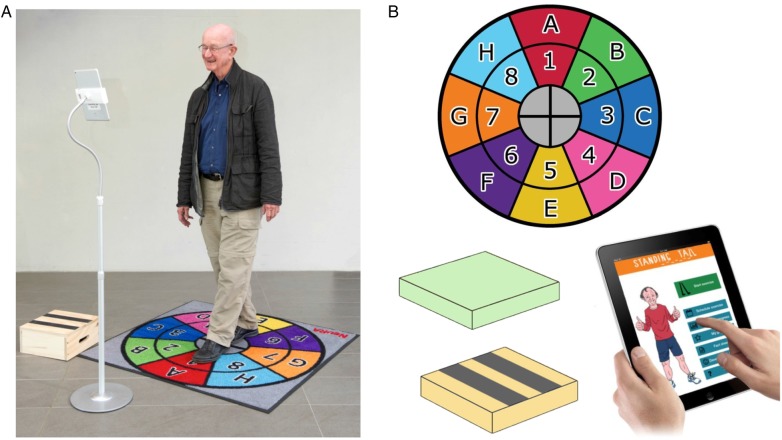
The intervention consists of balance training delivered through a tablet computer at home. Participants will receive a tablet computer with the balance training programme and exercise equipment (foam cushion, stepping box, exercise mat) as depicted in figure 1.
Figure 1.
Standing Tall set-up with computer stand (A), Standing Tall exercise equipment: exercise mat, foam cushion, stepping box and tablet computer with Standing Tall application (B).
During an initial home visit (approximately 2 h, 1–3 weeks after the baseline assessment), the exercise trainer (exercise physiologist or physiotherapist) will explain to the participant how to use the Standing Tall programme. The exercise trainer will perform an inbuilt initial balance assessment to ensure that the exercises are tailored to the participant's balance abilities. The exercise trainer will deliver exercise instructions and also explain how to use other features of the Standing Tall app (eg, scheduling exercises, tracking progress, setting goals), and use additional features of the tablet computer (eg, app store, browsing the internet, emails). Participants will receive a follow-up home visit at 1 month (approximately 1 h) to ensure safe use and progression of training, provide help with setting individual goals, and discuss any issues related to using the programme.
The exercises are designed to train static balance (semi-tandem, tandem, standing on one leg) and dynamic balance (leaning balance, walking), while also training on an unstable surface (ie, foam cushion), stepping in different directions on the exercise mat and stepping on a box. Instructions on how to perform each exercise will be provided with on-screen text, video demonstrations and voice-over instructions. The exercises will be tailored to the participant's balance abilities over the duration of the trial. The intensity of the balance exercises will be monitored continuously using a modified rate of perceived exertion (RPE) scale and adjusted as performance improves to ensure that exercises remain challenging. Progression of training intensity will be guided by an inbuilt coach that uses RPE scale data from their recent training activity. To maintain participant engagement and adherence, new exercises will be introduced based on progression rules.
The initial exercise duration is set at 40 min per week (weeks 1 and 2), and gradually increases to 60 min (weeks 3 and 4), 80 min (weeks 5 and 6), 100 min (weeks 7 and 8) and 120 min from week 9 onwards. Participants will be encouraged to exercise at least 120 min per week for 2 years in line with current evidence.2 The activity planner will help participants to schedule their exercises (with reminder notifications). The goal setting component will help participants to set and monitor personal goals. Participant adherence (training duration and frequency) will be monitored following automatic data transfer to a server and will be examined weekly. Participants will also be reminded of their training duration within the app. Participants not engaging in the minimum weekly training duration for two consecutive weeks will be contacted by phone to discuss any issues and to encourage adherence for the first 6 months. Phone support will be available and additional home visits will be offered as needed or requested for the duration of the study (ie, 2 years).
Participants in both intervention and control groups will receive the Standing Tall education programme containing health-related information relevant to older adults. The health education programme is also delivered through the tablet computer. A message will alert participants that a new ‘fact sheet’ can be viewed by automated weekly updates for the duration of the trial. The education content includes information on general health, medications, good sleep, diet, depression, how to talk to medical professionals, etc. It also includes fall-specific information on known risk factors for falls and evidence-based prevention strategies. Systematic review evidence has shown that falls cannot be reduced by increasing knowledge about fall prevention1; therefore, the provision of education material alone is considered a suitable control intervention. All participants are instructed on how to use the Standing Tall education programme during the baseline assessment as well as to provide data on falls and health service use, and complete the reassessment questionnaires. The control group is followed up by a phone call at 1 week and again at 1 month from their start in the trial, to discuss any issues with accessing the health education programme, using additional features of the tablet computer and data collection. Figure 2 shows the study design.
Figure 2.
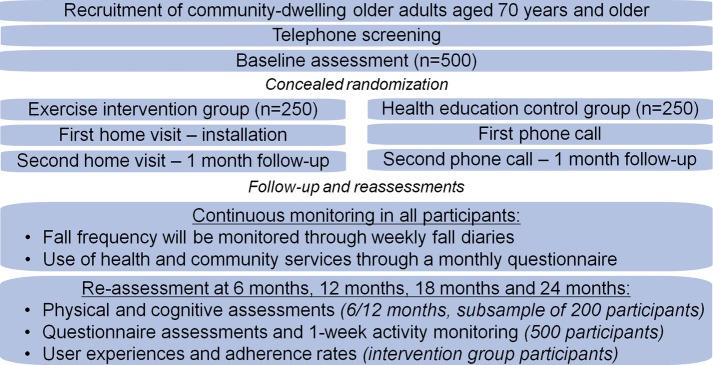
Study design.
Outcomes
Data collection
Assessments will be conducted by trained research assistants with a health science background and will involve questionnaires, and physical and cognitive performance assessments (table 1). Demographic data will be collected at baseline. This will include information on age, gender, living arrangements, education, computer use and medical history (presence of medical conditions, medication use and fall history). Each participant will undertake a questionnaire-based reassessment at 6, 12, 18 and 24 months from their start in the trial. A subsample of 200 participants will undergo a physical and cognitive reassessment at 6 and 12 months from their start in the trial. Each assessment will take about 2 h to complete. Assessors will be blinded to group allocation. To avoid unmasking of the assessors, participants will be instructed not to refer to their group allocation, the programme or their use of the tablet. Questionnaire assessments (6-monthly) and follow-up of falls (weekly) and health services use (monthly) will be collected through the tablet computer. If a survey is not returned, participants will be contacted by email or phone. Participants may withdraw from the study for any reason at any time or may be withdrawn by the study investigators if they are unwilling or unable to comply with the study procedures. Once a participant is randomised, study staff will make every effort to follow-up the participant on outcome measures for the entire study period. All data will be stored securely at the study site, and participant information will be stored in locked cabinets in areas with limited access.
Measures
The primary outcome measures include the number of fallers and falls rate at 12 months. A fall will be defined using the internationally derived consensus definition of “an unexpected event in which the participant comes to rest on the ground, floor, or lower level”.8 Fall frequency will be monitored in weekly fall diaries through the tablet for 24 months from their start in the trial. The number of fallers and falls rate at 24 month are included as secondary outcome measures. Participants who report falling will be asked to complete a short questionnaire detailing the circumstances and consequences of the fall, including medical interventions and hospitalisations. Fall diary data will be monitored by using data transfer to a secure server.
Secondary outcome measures will be assessed to elucidate mechanisms underlying any fall reduction observed in the trial and determine to what extent the training transfers to other important outcomes such as balance, gait and mobility; concern about falling; cognitive function; and overall physical activity levels. The secondary outcomes, listed in table 2, will be collected at the identified time points by a trained assessor blinded to group allocation. The order of measurements will be standardised.
Table 2.
List of measures collected at baseline, 6, 12, 18 and 24-month assessments
| Physical and cognitive assessments | BA | 6-month | 12-month | 18-month | 24-month | O |
|---|---|---|---|---|---|---|
| Physiological fall risk | ||||||
| The Physiological Profile Assessment (measures visual contrast sensitivity, proprioception, quadriceps strength, simple reaction time and postural sway) | Y | Y* | Y* | N | N | S* |
| Balance, functional mobility and gait | ||||||
| Static balance measures (floor and foam, in different feet positions) | Y | Y* | Y* | N | N | S* |
| Leaning balance measures (Maximal Balance Range test, Coordinated Stability test) | Y | Y* | Y* | N | N | S* |
| Functional mobility (timed up-and-go test, sit-to-stand test) | Y | Y* | Y* | N | N | S* |
| Walking speed (10 m walk test) | Y | Y* | Y* | N | N | S* |
| Stepping performance | ||||||
| Choice Stepping Reaction Time | Y | Y* | Y* | N | N | S* |
| Inhibitory Stepping Reaction Time | Y | Y* | Y* | N | N | S* |
| Stroop Stepping Reaction Time | Y | Y* | Y* | N | N | S* |
| Cognitive performance and executive function | ||||||
| Montreal Cognitive Assessment | Y | Y* | Y* | N | N | S* |
| Trail Making Tests, parts A and B | Y | Y* | Y* | N | N | S* |
| Victoria Stroop task | Y | Y* | Y* | N | N | S* |
| Questionnaire-based assessments | BA | 6-month | 12-month | 18-month | 24-month | O |
| Psychological | ||||||
| Depressive symptoms (Patient Health Questionnaire-9) | Y | Y | Y | Y | Y | S |
| Well-being (COMPAS-W scale) | Y | Y | Y | Y | Y | S |
| Concern about falling (Iconographical Falls Efficacy Scale) | Y | Y | Y | Y | Y | S |
| Readiness to exercise (Attitudes to Falls-Related Interventions Scale) | Y | Y# | Y# | Y# | Y# | S# |
| Exercise self-efficacy (Exercise Self-Efficacy Scale) | Y | Y# | Y# | Y# | Y# | S# |
| Personality (NEO Five-Factor Inventory: Neuroticism, Openness, Conscientiousness) | Y | N | N | N | N | N |
| Impact of daily life events (Daily Life Event scale) | N | N | Y | N | Y | N |
| Health-related quality of life | ||||||
| WHO Disability Assessment Schedule | Y | Y | Y | Y | Y | S |
| European Quality of Life—5 Dimensions | Y | Y | Y | Y | Y | S |
| Assessment of Quality of Life, 6 Dimensions | Y | Y | Y | Y | Y | S |
| Physical activity levels | ||||||
| Self-report physical activity (Incidental and Planned Exercise Questionnaire) | Y | Y | Y | Y | Y | S |
| Daily activity monitoring (activity monitor for 1 week) | Y | Y | Y | Y | Y | S |
| Usability and enjoyment to the intervention | ||||||
| Usability of the intervention (System Usability Scale) | N | Y# | Y# | Y# | Y# | S# |
| Enjoyment of the intervention (Physical Activity Enjoyment Scale) | N | Y# | Y# | Y# | Y# | S# |
| Continuous monitoring | BA | 6-month | 12-month | 18-month | 24-month | O |
| Falls and health service use | ||||||
| Falls and fall-related injuries (monitored weekly through the tablet) | N | N | Y(P) | N | Y(S) | P,S |
| Use of health services (monitored monthly through the tablet) | N | N | Y | N | Y | S |
| Adverse events due to system use | N | Y# | Y# | Y# | Y# | S# |
| Participant adherence to the intervention | ||||||
| Average weekly training duration (recorded by the app and monitored following data transfer to server) | N | Y# | Y# | Y# | Y# | S# |
| Total training duration (recorded by the app and monitored following data transfer to server) | N | Y# | Y# | Y# | Y# | S# |
*Assessments in a subsample of 200 participants. #Assessments only in intervention group participants. BA, baseline assessment; N, no; O, outcome measure; P, primary; S, secondary; Y, yes.
Physical and cognitive assessments will be assessed in all participants at baseline, and repeated at 6 and 12 months in a subsample of 200 participants.
Physiological fall risk will be assessed using the Physiological Profile Assessment (PPA).9 Five parameters of physiological performance are used to calculate an estimate of physiological fall risk, including visual contrast sensitivity, proprioception, quadriceps strength, simple reaction time and postural sway on a foam mat.9
Balance, functional mobility and gait will be assessed in additional tests. Balance will be assessed using tests of static balance (in different feet positions on floor and foam mat),10 maximum forward and backward leaning balance (Maximal Balance Range test), and controlled leaning balance (Coordinated Stability test).9 Functional mobility will be assessed with the timed sit-to-stand (five repetitions)11 and timed up-and-go tests.12 Walking speed will be assessed at participants’ self-selected walking pace, using a 10-m walk test.13
Stepping performance will be assessed with Choice, Stroop and Inhibitory Stepping Reaction Time (SRT) tests as composite measures of balance, executive function and reaction time. For Choice SRT, participants are required to step as fast as possible onto six target panels on an electronic step mat in response to arrows pointing to the front, sides and back presented in a random order on a display screen.14 Stroop SRT requires participants to step by the word written in the arrow and not the arrow direction, and inhibitory SRT includes a no-go task.15
Cognitive outcome measures will be assessed with the Montreal Cognitive Assessment (MoCA),16 Trail Making Tests and the Victoria Stroop task. The MoCA assesses several cognitive domains and executive function will be assessed separately using the Trail Making Tests (parts A and B)17 and the Victoria Stroop task.18
Questionnaire-based assessments will be conducted at baseline and at 6, 12, 18 and 24 months in all participants.
Psychological outcome measures are assessed through questionnaires. Concern about falling will be measured by the iconographical Falls Efficacy Scale,19 depressive symptoms by the nine-item Patient Health Questionnaire20 and well-being using the COMPAS-W scale.21 The Exercise Self-Efficacy Scale (ESES)22 and Attitudes to Falls-Related Interventions Scale (AFRIS)23 will assess exercise self-efficacy and readiness to exercise at baseline. Additional information on personality and daily life events will also be sought, using the NEO Five-Factor Inventory (NEO-FFI)24 and Daily Life Event scale,25 respectively.
Health-related quality of life will be assessed using the 12-item WHO Disability Assessment Schedule (WHODAS II),26 the European Quality of Life, 5 Dimensions (EQ-5D) questionnaire27 and the 20-item Assessment of Quality of Life, 6 Dimensions (AQoL-6D).28
Detailed self-report information on frequency and duration of physical activity will be assessed using the Incidental and Planned Exercise Questionnaire.29 Physical activity levels during daily activities and daily life gait will be monitored with a wearable accelerometer for 1 week.30
The use of health and community services will be recorded through a monthly questionnaire using the tablet computer for 24 months. Health service data will be monitored by using data transfer to a server, and followed up by email or phone if not returned.
Intervention group participants will be asked about their user experiences using questionnaires at 6,12, 18 and 24 months, and adverse events and adherence rates to the intervention will be monitored for 24 months.
Usability and enjoyment will be assessed in the intervention group with the System Usability Scale31 and Physical Activity Enjoyment Scale,32 respectively. The ESES and AFRIS will be repeated in the intervention group only.
Participant adherence rates to the exercise intervention will be automatically recorded by the tablet computer in the intervention group and monitored following data transfer to a secure server. The average weekly training duration and total training duration will be used as an outcome measure of short-term (6 months) and long-term adherence (12, 18 and 24 months). Interviews will be conducted at 6, 12 and 24 months to identify reasons for adherence and non-adherence in a subsample of participants.
Any adverse events from the intervention will be monitored, recorded and reported to the research institute's (NeuRA) safety monitoring committee. Serious adverse events will be reported to the ethics committee.
Statistical analysis
Trial data integrity will be monitored by regularly checking data files for omissions and errors. Analyses conducted will be masked to group allocation and will use an intention-to-treat approach, independent of the sponsor and competing interests. Number of falls per person-year will be analysed using negative binomial regression to estimate the difference in falls rates between the two groups. Proportion of fallers between groups will be compared using the incidence rate ratio statistic. Secondary analyses using causal modelling will be conducted to establish intervention effects in people with greater adherence. General linear models will be used to assess the effect of group allocation on continuously scored secondary outcome measures. Logistic regression models will be used to compare groups on dichotomous outcome measures. Predictors of uptake, acceptability and adherence will be established using multivariate modelling techniques.
Economic analyses will be assessed from a health and community care provider perspective. The outcome measure for the cost-effectiveness analysis will be cost per fall prevented over the study duration (time horizon: 12 and 24 months). In the cost-utility analysis, primary outcomes will be measured in terms of quality-adjusted life years (QALYs) gained. Resource use will include intervention costs, healthcare costs and community service costs. Utility-based quality of life (EQ-5D and AQoL-6D) will be measured at baseline and at 6, 12, 18 and 24 months, allowing calculation of QALYs over the 24-month study duration. The base QALY analysis will use the EQ-5D, with AQoL-6D values being used in a sensitivity analysis. By using mean costs in each trial arm and mean health outcomes in each arm, the incremental cost per fall prevented and incremental cost per QALY gained of the intervention group compared with control group will also be calculated. Sensitivity analyses will be performed to assess the robustness of our base-case findings. We will conduct one-way and multiway sensitivity analyses, and a probabilistic sensitivity analyses to estimate the joint uncertainty in cost and effect parameters. The probability that the intervention is cost-effective, given a decision maker's willingness to pay for each additional unit of health outcome achieved, will be estimated by bootstrapping of costs and health outcomes. As the underlying willingness to pay is unknown, the probability of the intervention being cost-effective will be presented as a function of a varying willingness to pay in cost-effectiveness acceptability curves.
Sample size
A sample size calculation (5% significance level, 80% power, 33% effect and 20% dropout rate) indicated a sample size of 500 will be necessary to see an effect on the fall rate. An α value (measure of overdispersion in negative binomial regression) of 1.2 was taken from a previous fall prevention trial of 500 older adults.33 Control group rate of falls was assumed to be 0.8 fall per person-year and an Incidence Rate Ratio (IRR) of 0.67 was based on metaregression results from 44 RCTs investigating the effect of balance challenging exercise on fall rates.2 An average follow-up period of 22 months (rather than the planned 24 months) was used to account for loss to follow-up. Power calculations of secondary outcome measures indicate that 500 participants will be sufficient to detect between-group differences in questionnaire-based measures. For example, we have 90% power to detect differences in concern about falling (effect size f=0.15, correlation ρ=0.75, repeated measures analysis of variance (RM ANOVA), α 5%, 2 groups, 4 measurements, 20% dropout).34 A subsample of 200 participants will be sufficient to detect between-group differences in physical outcome measures. For example, we have 95% power to detect differences in postural sway (effect size f=0.38, correlation ρ=0.76, RM ANOVA, α 5%, 2 groups, 4 measurements, 20% dropout).34
Discussion
Balance training has been shown to be effective in reducing falls in older people. However, poor compliance with the recommended duration of 2 h of exercise per week calls for new alternatives. This trial involves the use of a tablet computer for delivering an innovative home-based balance training programme that has the potential to be effective, cost-effective and sustainable. The Standing Tall programme incorporates evidence-based exercises to prevent falls and behavioural techniques to enhance long-term exercise adherence, using a person-centred approach while embracing new and emerging technologies. Delivery of the intervention through a tablet computer allows real-time data gathering and is particularly appropriate for providing the tailored support that is needed towards effective fall prevention in older people. It may also enable greater choice in preferred exercise options, greater level of engagement, increased convenience and increased accessibility of health services to large populations. With growing popularity of tablet computers, it is anticipated that this mode of healthcare delivery will become increasingly widespread.
This project will be the first RCT to evaluate the effectiveness of a home-based exercise programme delivered through a tablet computer for preventing falls in older community-dwelling people. The 2-year duration of the trial will allow the evaluation of long-term adherence to the programme and predictors of adherence to the programme. The project will also include an economic analysis to provide information for future implementation. Outcomes will be disseminated through publication in peer-reviewed journals and presentations at international conference meetings. The completion of this trial is expected by the end of 2018.
Footnotes
Contributors: KD conceived the idea for the trial. KD oversaw the development of the Standing Tall intervention programme and was closely involved in each of the following: TV and AW were responsible for the content of the programme; TD and JY were responsible for the software design of the programme; LM was responsible for the development of the weekly fact sheets; and GARZ assisted with the goal setting. The trial was designed by KD and TV; assisted by SRL; and implemented by AW, DS, LP and LM. The economic analyses were designed by KH. Funding was obtained by KD, LC, GARZ and JCTC. KD and TV prepared the first draft of the manuscript. All authors contributed to the refinement of the study protocol and approved the final version of the manuscript.
Funding: This work is supported by NHMRC grant number APP1084739, Gandel Philanthropy and individual donations through the NeuRA Foundation. KD and LC are supported by NHMRC as Career Development Fellows and SRL as a Senior Principal Research Fellow. TV is supported by a doctoral scholarship at UNSW.
Competing interests: None declared.
Ethics approval: Human Research Ethics Committee, University of New South Wales, Sydney, Australia (HREC 14/266).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1.Gillespie L, Robertson M, Gillespie W et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;(2):CD007146 10.1002/14651858.CD007146.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherrington C, Whitney JC, Lord SR et al. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 2008;56:2234–43. 10.1111/j.1532-5415.2008.02014.x [DOI] [PubMed] [Google Scholar]
- 3.Campbell AJ, Robertson MC, Gardner MM et al. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age Ageing 1999;28:513–18. 10.1093/ageing/28.6.513 [DOI] [PubMed] [Google Scholar]
- 4.Simek EM, McPhate L, Haines TP. Adherence to and efficacy of home exercise programs to prevent falls: a systematic review and meta-analysis of the impact of exercise program characteristics. Prev Med 2012;55:262–75. 10.1016/j.ypmed.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Yardley L, Donovan-Hall M, Francis K et al. Older people's views of advice about falls prevention: a qualitative study. Health Educ Res 2006;21:508–17. 10.1093/her/cyh077 [DOI] [PubMed] [Google Scholar]
- 6.Yardley L, Kirby S, Ben-Shlomo Y et al. How likely are older people to take up different falls prevention activities? Prev Med 2008;47:554–8. 10.1016/j.ypmed.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23:433–41. 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 8.Lamb SE, Jørstad-Stein EC, Hauer K et al. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618–22. 10.1111/j.1532-5415.2005.53455.x [DOI] [PubMed] [Google Scholar]
- 9.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther 2003;83: 237–52. [PubMed] [Google Scholar]
- 10.Guralnik JM, Simonsick EM, Ferrucci L et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 11.Csuka M, McCarty D. Simple method for measurement of lower extremity muscle strength. Am J Med 1985;78:77–81. 10.1016/0002-9343(85)90465-6 [DOI] [PubMed] [Google Scholar]
- 12.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 13.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing 1997;26:15–19. 10.1093/ageing/26.1.15 [DOI] [PubMed] [Google Scholar]
- 14.Schoene D, Wu SMS, Mikolaizak AS et al. Discriminative ability and predictive validity of the timed up and go test in identifying older people who fall: systematic review and meta-analysis. J Am Geriatr Soc 2013;61:202–8. 10.1111/jgs.12106 [DOI] [PubMed] [Google Scholar]
- 15.Schoene D, Smith ST, Davies TA et al. A Stroop Stepping Test (SST) using low-cost computer game technology discriminates between older fallers and non-fallers. Age Ageing 2014;43: 285–9. 10.1093/ageing/aft157 [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bédirian V et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–99. 10.1093/ageing/aft157 [DOI] [PubMed] [Google Scholar]
- 17.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary. 3rd edn New York: Oxford University Press, 2006. [Google Scholar]
- 18.Trenerry M, Crosson B, DeBoe J et al. Stroop neuropsychological screening test. Odessa, FL: Psychological Assessment Resources, Inc., 1988. [Google Scholar]
- 19.Delbaere K, Smith ST, Lord SR. Development and initial validation of the Iconographical Falls Efficacy Scale. J Gerontol A Biol Sci Med Sci 2011;66:674–80. 10.1093/gerona/glr019 [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16: 606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatt JM, Burton KLO, Schofield PR et al. The heritability of mental health and wellbeing defined using COMPAS-W, a new composite measure of wellbeing. Psychiatry Res 2014;219:204–13. 10.1016/j.psychres.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 22.Everett B, Salamonson Y, Davidson PM. Bandura's exercise self-efficacy scale: Validation in an Australian cardiac rehabilitation setting. Internat J Nurs Stud 2015;46: 824–9. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PubMed] [Google Scholar]
- 23.Yardley L, Donovan-Hall M, Francis K et al. Attitudes and beliefs that predict older people's intention to undertake strength and balance training. J Gerontol B Psychol Sci Soc Sci 2007;62:P119–25. 10.1093/geronb/62.2.P119 [DOI] [PubMed] [Google Scholar]
- 24.Costa PT, McCrae RR. The NEO™ Inventories: NEO™ Five Factor Inventory-3 (NEO™-FFI-3). PAR, 2010. [Google Scholar]
- 25.Gatt JM, Korgaonkar MS, Schofield PR et al. The TWIN-E project in emotional wellbeing: study protocol and preliminary heritability results across four MRI and DTI measures. Twin Res Hum Genet 2012;15:419–41. 10.1017/thg.2012.12 [DOI] [PubMed] [Google Scholar]
- 26.Üstün TB, Chatterji S, Kostanjsek N et al. Developing the world health organization disability assessment schedule 2.0. Bulletin of the World Health Organization 2010;88:815–23. 10.1017/thg.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viney R, Norman R, King M et al. Time trade-off derived EQ-5D weights for Australia. Value Health 2011;14:928–36. 10.1016/j.jval.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 28.Richardson J, Peacock S, Hawthorne G et al. Construction of the descriptive system for the assessment of quality of life AQoL-6D utility instrument. Health Qual Life Outcomes 2012; 10:1–9. 10.1186/1477-7525-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delbaere K, Hauer K, Lord SR. Evaluation of the incidental and planned exercise questionnaire (IPEQ) for older people. Br J Sports Med 2010;44:1029–34. 10.1136/bjsm.2009.060350 [DOI] [PubMed] [Google Scholar]
- 30.van Schooten KS, Pijnappels M, Rispens SM et al. Ambulatory fall-risk assessment: amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci 2015;70:608–15. 10.1093/gerona/glu225 [DOI] [PubMed] [Google Scholar]
- 31.Borsci S, Federici S, Lauriola M. On the dimensionality of the System Usability Scale: a test of alternative measurement models. Cogn Process 2009;10:193–7. 10.1007/s10339-009-0268-9 [DOI] [PubMed] [Google Scholar]
- 32.Mullen S, Olson E, Phillips S et al. Measuring enjoyment of physical activity in older adults: invariance of the physical activity enjoyment scale (paces) across groups and time. Int J Behav Nutr Phys Act 2011;8:103 10.1186/1479-5868-8-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delbaere K, Close JC, Heim J et al. A multifactorial approach to understanding fall risk in older people. J Am Geriatr Soc 2010;58:1679–85. 10.1111/j.1532-5415.2010.03017.x [DOI] [PubMed] [Google Scholar]
- 34.Schoene D, Lord SR, Delbaere K et al. A randomized controlled pilot study of home-based step training in older people using videogame technology. PLoS ONE 2013;8:e57734 10.1371/journal.pone.0057734 [DOI] [PMC free article] [PubMed] [Google Scholar]