Abstract
Traditional therapies against cancer, chemo- and radiotherapy, have multiple limitations that lead to treatment failure and cancer recurrence. These limitations are related to systemic and local toxicity, while treatment failure and cancer relapse are due to drug resistance and self-renewal, properties of a small population of tumor cells called cancer stem cells (CSCs). These cells are involved in cancer initiation, maintenance, metastasis and recurrence. Therefore, in order to develop efficient treatments that can induce a long-lasting clinical response preventing tumor relapse it is important to develop drugs that can specifically target and eliminate CSCs. Recent identification of surface markers and understanding of molecular feature associated with CSC phenotype helped with the design of effective treatments. In this review we discuss targeting surface biomarkers, signaling pathways that regulate CSCs self-renewal and differentiation, drug-efflux pumps involved in apoptosis resistance, microenvironmental signals that sustain CSCs growth, manipulation of miRNA expression, and induction of CSCs apoptosis and differentiation, with specific aim to hamper CSCs regeneration and cancer relapse. Some of these agents are under evaluation in preclinical and clinical studies, most of them for using in combination with traditional therapies. The combined therapy using conventional anticancer drugs with CSCs-targeting agents, may offer a promising strategy for management and eradication of different types of cancers.
Keywords: Cancer stem cells, Targeted therapy, Anticancer drugs, Biomarkers, Signaling pathways, Apoptosis
Core tip: Cancer stem cells (CSCs) play important roles in tumor formation, metastasis and cancer relapse. In this article, we review the literature on the recent progress in developing anti-cancer stem cell strategies based on improved understanding of CSCs properties and molecular features. These novel therapeutic systems are designed with the aim of eradicating CSCs, by targeting surface specific markers and altering signaling pathways or mechanisms involved in CSCs maintenance and drug resistance, and also to disturb microenvironmental signals that sustain CSCs growth, with specific aim of impede CSCs regeneration and cancer relapse.
INTRODUCTION
Tumor progression is explained by two models: The clonal (stochastic) evolution model and cancer stem cell model. The first one sustain that all transformed cell within a tumor have carcinogenic potential, with unlimited proliferation capacity, and the disease healing requires as therapy the elimination of all tumor cells[1,2]. This hypothesis is supported by several studies demonstrating that a large number of cancer cells sustain tumor growth when are transplanted into histocompatible mice and the relevant results in this area depends on the xenotransplantation model used. Thereby, modifying xenotransplantation assay conditions can increase the detection of all tumorigenic cells[3,4]. The second cancer evolution model sustain that tumors evolve from a small population of cells with self-renewal ability and high resistance to chemotherapy and radiotherapy. These cells were called CSCs or cancer initiating cells, due to their capacity to auto-regenerate, proliferate and induce tumor formation. Among their properties, resistance to standard oncology treatments is responsible for ineffectiveness of traditional cancer therapies and lead to tumor recurrence and metastasis[5].
The CSCs model gained wide acceptance over the last years, based on continuous observations. Recent data suggests that CSCs might evolve from normal stem cells, progenitors or more differentiated cells with whom they share many resemblance like: Self-renewing, differentiation to progenitor cells, expression of surface markers, common signaling pathways and a close association with microenvironment[2].
There are two hypothesis regarding CSCs formation: (1) transformation of normal stem cells or progenitor cells into CSCs, process that occurs through multiple gene mutations as result of genetic and epigenetic instability[6]; and (2) tumor cells progressively acquire stem cell properties through reversal of ontogeny based on oncogene-induced plasticity[7].
Several studies suggest that epithelial-mesenchymal transition (EMT) process characterized by the repression of epithelial markers (e.g., E-cadherin) and up-regulation of mesenchymal markers (e.g., vimentin, fibronectin and N-cadherin) can also generates cells with stem-like properties[8]. Mani et al[9] using immortalized human mammary epithelial cells demonstrated that EMT induction results in the enrichment of cells with stem-like properties, an increased expression of stem-cell markers and an increased capacity of cells to form mammospheres. Gupta et al[10] showed that E-cadherin inhibition and EMT induction, respectively leads to an increase number of CSCs in breast cancer cell populations, characterized by an increased resistance to chemotherapy.
CSCs were first described in acute myeloid leukemia, in which a population of CD34+CD38- was noticed to possess stem cells capacities of proliferation, self-renewal and differentiation, and to reconstitute a heterogeneous cell population in nonobese diabetic/severe combined immunodeficiency mice[11]. Later, CSCs were identified in various solid tumors including glioma[12], as well as breast[13], head and neck[14], lung[15], pancreatic[16], liver[17], stomach[18], colon cancer[19], etc.
Current review presents basic information about CSCs and discusses targeted therapeutic strategies developed for cancer eradication.
CSC: DEFINITION, CHARACTERISTICS, MARKERS
CSCs are auto-regenerating cells, able to proliferate and differentiate through symmetrical and asymmetrical cell divisions, with tumorigenic potential and specific surface markers useful for CSC identification and isolation. Additionally, several other properties like sphere forming capacity in serum-free medium or soft agar, dye exclusion ability based on over-expression of drug-efflux pumps (ATP binding cassette or multidrug resistance transporters), enzymatic activity of aldehyde dehydrogenase 1, are used to identify CSCs. However, the most important property of CSCs can be verified only by in vivo assay: Tumorigenicity in animal model, maintained even after serial transplantation.
The surface markers for CSCs vary according to tumor type. Main surface markers for CSCs from solid tissue are CD133, CD44 and CD24. To these, several other more specific markers might be added, according to tumor tissue origin (Table 1). Thus, the phenotype epithelial specific antigen (ESA+) together with CD44+CD24+ was described in pancreatic CSCs[16], while ESA+CD44+CD24-/low was the phenotype identified in breast CSCs[13]. For liver CSCs the following combination ESA+CD133+CD90+CD44+CD24+ was proposed[17]. For hematological malignancy CD34 and CD38 are main surface antigens[11]. Subsequently novel markers have been found to be highly expressed on leukemia stem cells than normal hematopoietic stem cells. These include CD47[20], C-type lectin-like molecule-1 (CLL-1)[21], CD96[22], TIM3[23], CD32 and CD25[24].
Table 1.
Cell surface antigens present on cancer stem cells
| Cancer type | Surface antigens | Ref. |
| Leukemia | CD34+CD38-CD47+CCL-1+CD96+ TIM3+CD32+CD25+ | [11,20-24] |
| Brain | CD133+ | [12] |
| Head and neck | CD44+CD24+ | [14] |
| Breast | ESA+CD44+CD24- | [13,16] |
| Pancreatic | ESA+CD44+CD24+ | [13,40] |
| Lung | CD133+CD44+ | [15] |
| Liver | ESA+CD133+CD90+CD44+CD24+ | [17] |
| Gastric | CD44+ | [18,38] |
| Colorectal | ESA+CD133+CD166+CD44+CD24+ | [19,26] |
Although recent studies have contributed to a better understanding of CSCs surface molecules, the picture is not complete. It is often observed that CSCs do not express the same markers, or that normal cells also express these surface antigens. Therefore, it is not possible yet to certainly isolate CSCs, but only to identify a CSCs-enriched population. Consequently, identification of CSCs must be based on additional functional assays such as the ability to form spheres in serum-free medium and to initiate tumor growth after serial transplantation in immunocompromised animal models, based on their self-renewal capacity. However, these assays also have limitations due to microenvironment. Thus, the in vitro assay may not detect quiescent stem cells that are not capable to develop spheres due to lack of additional extrinsic signals needed for their activation. Moreover, in this type of assay there is a selection pressure imposed by specific culture conditions in presence of exogenous growth factors. Serial transplantation as well might have limitations, since considerable number of cells is required to induce tumor growth in vivo, due to insertion in a foreign microenvironment deficient in specific signals for survival and development[25].
Therefore, to specifically address CSCs in further experiments, it is necessary to sort cells based on surface markers and subsequently to assess their functional abilities by in vitro and in vivo specific assays.
THERAPIES TARGETING CANCER STEM CELLS
Traditional therapies against cancer, chemo- and radiotherapy, have multiple limitations that result in treatment failure and cancer recurrence. These limitations are related to systemic and local toxicity because the agents are not selective enough and may affect also healthy tissue. An additional restriction is drug resistance due to CSCs specific properties like: Slow rate of division, high expression of drug-efflux pumps, high capacity for DNA repairing, and also to microenvironment characteristics: hypoxia and acidosis. Therefore, targeting CSCs became essential in treating cancer and preventing tumor relapse.
Recently, multiple strategies have been conceived with the specific aim of destroying CSCs and their niche. These include targeting specific surface markers, modulation of signaling pathways, adjustment of the microenvironment signals, inhibiting of drug-efflux pumps, manipulation of miRNA expression, induction of CSCs apoptosis and differentiation. A summary of these therapeutic strategies is presented in the Figure 1. At the moment, some of them are successfully used in clinic, mainly in combination with traditional therapies, and others are still under evaluation.
Figure 1.
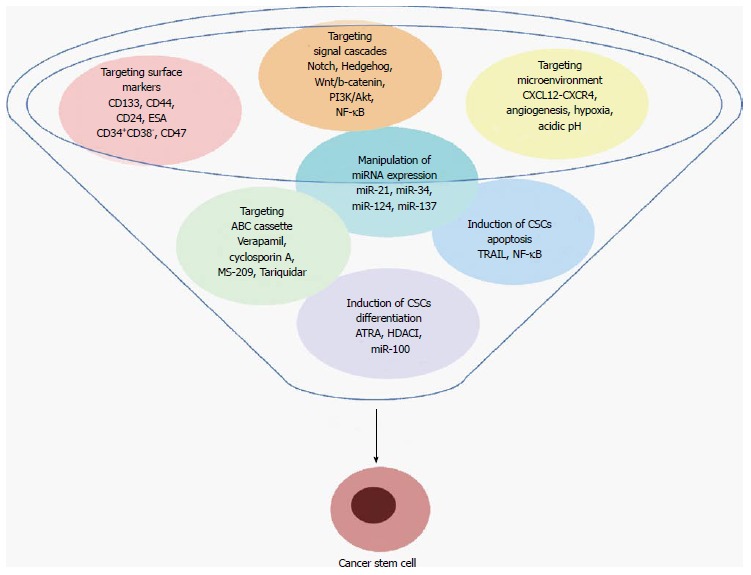
Therapies targeting cancer stem cells. Numerous therapies aiming to eradicate cancer stem cells have been developed during last year’s. Here we highlighted most common seven approaches: targeting surface biomarkers, signaling pathways that regulate cancer stem cells (CSCs) self-renewal and differentiation, drug-efflux pumps involved in apoptosis resistance, microenvironmental signals that sustain CSCs growth, manipulation of miRNA expression, and induction of CSCs apoptosis and differentiation, with specific aim of hamper CSCs regeneration and cancer relapse. ATRA: All trans retinoic acid; HDACI: Histone deacetylase inhibitors; PI3K: Phosphatidylinositol 3-kinase; NF-κB: Nuclear factor kappa B; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand.
Targeting surface markers
The surface markers used for identification and isolation of CSCs are also important targets for therapy. Immunotherapy that involves antibodies targeting CSCs specific markers is often used as an adjunct to chemotherapy, radiotherapy and surgery. The most important CSCs associated markers together with strategies for targeting them are mentioned below.
CD133 (prominin-1) is a cell surface glycoprotein widely expressed on CSCs in solid tumors such as glioma[12], lung[15], liver[17] and, colorectal cancer[19,26]. Cancers with large CD133 subpopulation have a drug resistant phenotype and poor prognosis. For this reason, several strategies for anti-CD133 therapy have been generated. The polymeric nanoparticles loaded with paclitaxel (CD133NPs) targeting CD133 were tested on colorectal adenocarcinoma Caco-2 cells and proved to efficiently decrease the cell number and colonies formed. On the other hand, in xenograft model, the CD133NPs improved therapeutic efficacy compared to free paclitaxel treatment[27]. Another anti-CD133 antibody constructed through the fusion with pseudomonas exotoxin 38, inhibited the progression of tumor growth after multiple intraperitoneal injections of drug over a period of 4-6 wk in xenografted mice with ovarian cancer. The elimination of CD133+ ovarian cancer cells resulted in long-term disease free tumor survivors[28]. These studies suggest that anti-CD133 therapy might be associated with drug delivery, forming antibody-drug conjugates that enhance the effect on CD133+ CSCs and eliminate them. Anti-CD133+ cell therapy was also tested on sarcoma CSCs, reducing proliferative capacity and resulting in decreased sarcoma tumor-initiating ability[29]. Similar results were obtained for pancreatic and hepatic CSCs[30,31].
However CD133 expression is not restricted to CSCs. Shmelkov et al[32] demonstrated that both CD133+ and CD133-metastatic colon cancer cells can initiate tumors. Also, Beier et al[33] demonstrated that CD133+ and CD133-glioblastoma cancer cells meet stem cell criteria, but they might reflect two biologically distinctive glioblastoma subtypes. The authors concluded that primary glioblastomas might develop either from different cells of origin or from related cell types that further acquired different molecular alterations.
CD44 is a transmembrane protein that mediates cell to cell adhesion and cell to extracellular matrix interactions, being a receptor for hyaluronic acid, selectin, collagen, osteopontin, fibronectin and laminin[34]. CD44 is involved in cell proliferation, survival, migration, differentiation, apoptosis, self-renewal, niche preparation, epithelial-mesenchymal transition, and resistance to apoptosis[35,36]. It was found overexpressed in many tumor cells: breast[13], bladder[37], gastric[38], prostate[39], pancreas[40], ovar[41], colorectal[26,42], hepatocellular[43], head and neck[44], acute myeloid leukemia (AML) CSCs[45], etc. Targeting CD44 with monoclonal antibodies appears as a good strategy to eliminate CSCs. Treatment with anti-human CD44 monoclonal antibody can induce myeloid differentiation in patient-derived AML blasts, inhibits homing to the microenvironmental niche and alters stem cell fate[46]. Marangoni et al[47] used an anti-human CD44 monoclonal antibody in combination with doxorubicin and cyclophosphamide to prevent relapse of aggressive breast cancer. Moreover, a mouse IgG1 anti-human CD44 receptor that inhibits CD44-STAT3 signaling pathways was used on human pancreatic cancer stem-like cells or MiaPaCa-2 cells and was found to decrease in vitro tumor sphere formation of CSCs, inhibiting pancreatic tumor growth, metastasis and tumor recurrence in xenografted nude mice[48]. Similar results were obtained in colorectal, lung, bladder, larynx and breast cancers[49,50].
CD47 is a transmembrane protein, receptor for thrombospondin family members and for signal regulatory protein alpha (SIRPα)[51]. It was found widely expressed on AML CSCs[20] and almost all human solid tumor cells[51,52]. Two anti-CD47 mAbs such as B6H12.2 and B6H12 were developed as strategy for cancer therapy. In a xenograft mouse model, B6H12.2 antibodies prevented the engraftment of human AML cancers stem cells and completely eradicated them[20]. Administration of B6H12.2 prevented and inhibited growth of tumors derived from glioblastoma, ovarian, breast, colon, bladder cancer[52], human non-Hodgkin lymphoma[53], acute lymphoblastic leukemia[54] and multiple myeloma CSCs[55]. B6H12, a fully humanized anti-CD47, effectively inhibits aggressive leiomysarcoma growth and metastasis in xenograft mice model[56].
Other antibodies approved by FDA for the treatment of solid and haematological tumours, e.g., rituximab (anti-CD20), cetuximab (anti-EGFR), trastuzumab (anti-HER2), bevacizumab (anti-VEGF-A), ipilimumab (anti-CTLA-4), pembrolizumab (anti-PD-1) are currently used in immunotherapy against tumor cells[57].
Targeting signal cascades
One of the mechanisms by which CSCs manage to avoid or to survive cancer treatments seems to be represented by signals generated within the tumor microenvironment, due to dysregulation of signaling pathway networks[58]. Like normal stem cells, CSCs use signaling pathways that are essential for self-renewal, proliferation and differentiation in order to preserve stem cell properties but the final result is carcinogenesis. Many studies have focused on signaling pathways dysregulation in CSCs attempting to find new strategy for cancer therapy; this line of research is promising mainly because many cancers present up- or down-regulation of the same signaling cascades. In this regard, CSCs can be identified by surface markers but also by the signals they send in tumor microenvironment[59]. The major signaling pathways involved in the regulation of self-renewal and differentiation of normal and cancer stem cells are Notch, Hedgehog, Wnt/b-catenin, NF-κB, phosphatidylinositol 3-kinase (PI3K)/Akt, PTEN; sustained by aberrant activation of these pathways, CSCs have the capacity to initiate cancer and promote recurrence after the surgical removal of tumor[60].
Notch signaling cascade is one major pathway involved in numerous critical cellular processes, including stem cell maintenance, progenitor cell proliferation and differentiation, and determination of cell fate during embryonic development[61,62]. Notch signaling involvement in carcinogenesis and tumor progression seems to depend on tissue/cell-type. Thereby, Notch signaling was identified as oncogenic due to an increased activation in T-cell acute lymphoblastic leukemia, medulloblastoma, colorectal cancer, non–small cell lung carcinoma, hepatocellular carcinoma, melanoma, and breast cancer while in myeloid malignancies, head and neck squamous cell carcinoma, Notch displays tumor suppressor functions[63,64]. Notch signaling pathway comprises four Notch receptors (Notch 1, Notch 2, Notch 3 and Notch 4) and five ligands (Delta-like 1, Delta-like 3, Delta-like 4, Jagged 1 and Jagged 2); depending on the cellular type, many studies have been focused on activation or inhibition of these proteins[65,66]. In vitro and in vivo studies showed that human lung cancer lines presented an overexpression of Notch3; downregulation of Notch signaling, using a specific inhibitor was associated with decrease of tumor cell proliferation, induction of apoptosis, and inhibition of in vivo growth. Notch3 was also found to be overexpressed in resected human lung cancers and was associated with poor overall survival[67,68]. Moreover, activation of Notch4 and Notch1 was noted breast CSCs, and inhibition of these receptors, especially Notch4, leads to decreased activity of breast CSCs in vitro, and reduced tumor development in vivo[69]. In hepatocellular carcinoma, inhibition of Notch signaling pathway also reduced the invasion of tumor cells by downregulation of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor[70]. In colorectal cancers, Notch1 signaling seems to have a dual role; it can increase the tumor progression but also counteracts β-catenin signaling whose activation is an important factor in human colorectal carcinogenesis[71]. Many studies reported an overexpression of Notch signaling proteins in head and neck squamous cell carcinoma, sustaining a pro-oncogenic role of this signaling but recent exome sequencing analyses reported loss-of-function mutations in the Notch1 gene in a significant proportion of patients with this malignancy[72].
Notch signaling inhibitors tested in clinical trials include inhibitors of γ-secretase complex involved in Notch activation and antibodies against DLL-4 and Notch-1, -2 or -3 receptors[43]. Results for phase 1 clinical trial using BMS-906024, one of these γ-secretase inhibitors, for patients with relapsed T-cell acute lymphoblastic leukemia showed at least 50% reduction in BM blasts in 8 of the 25 patients (32%)[73].
Recently was reported the development of an antibody OMP-59R5 (tarextumab) which alone or together with chemotherapeutic agents can inhibit Notch2 and Notch3 function. The antitumor effect of OMP-59R5 was observed on xenograft tumors representing different types of epithelial cancers like breast, small-cell lung, ovarian, and pancreatic; and was associated with down-regulation of Notch target genes in tumor cells and with suppression of Notch3, HeyL, and Rgs5, expression in tumor stroma[74].
Hedgehog is another pathway that plays an important role in stem cells maintenance and embryonic development, being involved in various cellular processes such as proliferation and differentiation; this cascade was also aberrant activated in several human tumors, including glioblastoma, breast cancer, pancreatic adenocarcinoma, multiple myeloma and chronic myeloid leukemia[75,76]. Furthermore, Hedgehog signaling contributes to development and maintenance of CSCs and acquisition of epithelial-to-mesenchymal transition, highlighting the involvement of Hedgehog cascade in cancer cell invasion, metastasis, chemotherapeutic resistance and tumor recurrence[77]. Canonical Hedgehog signaling involves three ligands: Sonic (SHH), Indian (IHH), and Desert (DHH) with different expression depending on cell type; SHH is widely expressed, mostly during embryogenesis, IHH is found in hematopoietic cells, bone, and cartilage, while DHH is expressed in the peripheral nervous system and testes. Hedgehog signaling implies Hedgehog ligands binding to Patched receptor, Smoothened activation, and activation of transcriptional effectors that belong to the GLI family[78,79]. Aberrant activation of Hedgehog signaling may be due to gene mutations, resulting in ligand-independent pathway activation, or by interaction with other molecular signaling pathways, such as Ras/Raf/MEK/Erk, PI3K/Akt/mTOR, and Notch. Thus, there are many studies sustaining that the combination therapies targeting more than one signaling pathway improves antitumor efficacy and survival in animal models[80]. There are also several preclinical studies showing that in several cancers, Hedgehog signaling inhibition using specific inhibitors of Smoothened leads to the blocking of drug resistance, relapse, and metastasis. In basal cell carcinoma and medulloblastoma patients, Smoothened inhibitors such as vismodegib, BMS-833923, saridegib (IPI-926), sonidegib/erismodegib (LDE225), PF-04449913, LY2940680, LEQ 506, and TAK-441 were used as monotherapy with promising results[81]. A recent study reported a significant negative association between GLI1 and GLI2 expression and overall survival, and also an increase of DHH plasma levels in acute myeloid leukemia patients. By in vitro and in vivo experiments the authors demonstrated that GLI1/2 inhibition induces apoptosis, and reduces proliferation, and colony formation in acute myeloid leukemia cells, and also increases the survival in murine models[82]. Another study focused on biliary tract cancer showed that inhibition of Hedgehog and mTOR signaling pathways with rapamycin and vismodegib specific inhibitors results in decrease of Nanog and Oct-4 expressions and also in decrease of CSCs and ALDH-positive cells proliferation[83]. In pancreatic cancer, the combination of focal radiation with Hedgehog inhibitors reduces lymph node metastasis, sustaining the involvement of this signaling pathway in carcinogenesis[84].
Wnt/β-catenin signaling pathway is one of the most deregulated pathways in many cancers, including leukemia, colon, breast and skin cancers. For instance, in human colon carcinoma, mutations in adenomatous polyposis coli (APC) result in aberrant activation of Wnt signaling and induce transformation of epithelial cells[60]. There are also studies that support the involvement of Wnt signaling in self-renewal and maintenance of stem cells and CSCs, in several tissues like skin, intestine and mammary gland[85]. A minor subpopulation of breast tumor-initiating cells associated with drug resistance was identified in human breast cancer; in vitro and in vivo study showed that suppression of Wnt signaling inhibited sphere- and colony-formation of primary breast tumor cells and blocked tumor growth in murine models[86].
Wnt/β-catenin signaling pathway includes Wnt ligands, Frizzled receptors and a complex, composed of APC, Axin1, Glycogen synthase kinase 3-β, and CK1 (casein kinase 1) which stabilizes β-catenin[87]. Wnt/β-catenin inhibitors include small-molecule inhibitors (e.g., nonsteroidal anti-inflammatory drugs and natural compounds: aspirin, indomethacin, vitamins A and D derivatives) and biologic inhibitors (monoclonal antibodies, small interfering RNAs and recombinant proteins)[2].
OMP-54F28 is an example of Wnt signaling inhibitor with interesting results in clinical trials, in several advanced solid tumors, such as ovarian, pancreatic and hepatocellular cancers[88]. OMP-18R5 - an antibody targeting several Frizzled receptors - reduces tumor cell proliferation and tumor-initiating cell number in lung, breast, colon, and pancreatic tumors[89]. In vitro study on lung CSCs showed that β-catenin nuclear transfer inhibitor PP significantly decreased colony formation and downregulated pluripotent stem cell signaling pathway, being a promising therapeutic approach in lung adenocarcinoma patients[90]. The studies performed until now showed that Wnt inhibitors and modulators can eliminate drug-resistant CSCs and tumor-initiating cells but more studies are needed regarding the safety of this therapeutic approach, given the essential role of Wnt signaling in tissue homeostasis and repair[91].
PI3K/Akt/mTOR signaling pathway is an important cascade of phosphorylation reactions, comprising several key molecules involved in carcinogenesis processes: PI3K - presents activating mutations in several human cancers; Akt - overexpression/activation of this protein kinase is associated with tumor metastasis and invasion; mTOR - protein kinase critical for cancer cell growth, cell proliferation, survival and angiogenesis[59].
Many studies suggest the involvement of this signaling pathway in maintaining CSCs features. In breast cancer, inhibition of PI3K or Akt activity reduced generation and growth of CD44/CD24 mammospheres, leading to stem cell/mesenchymal phenotype loss and recovery of epithelial-like markers[92]. In prostate cancer, PI3K/Akt/mTOR pathway deregulation is associated with CSCs quiescence and maintenance; moreover, prostate CSCs seem to present resistance to selective mTOR inhibitors[93]. Another study showed that combination between PI3K/mTOR inhibitor (BEZ235) and radiotherapy increased radiosensitivity and apoptosis, and also reduced CSCs marker expressions in prostate cancer radioresistant cell lines[94]. In endometrial tumors, PTEN/PI3K/Akt/mTOR pathway aberrant activation by miRNAs is a common event, being involved in epithelial-mesenchymal transition and CSC maintenance[95].
PTEN (phosphatase and tensin homolog) is a tumor suppressor and an inhibitor of PI3K and ERK activities, being one of the most inactivated tumor suppressor genes in cancers; many tumors present loss of PTEN function by mutations, deletions, transcriptional silencing, or protein instability affecting important cell processes such as survival, proliferation, energy metabolism and cellular architecture. Loss of PTEN activity was also linked to CSC development and proliferation in several cancers, including prostate, lung, intestinal, and pancreatic cancer[95]. Moreover, PTEN loss and Akt activation lead to increase activity of β-catenin in hematopoietic stem cells while Notch signaling activation results in reduced PTEN expression in human T-cell acute lymphoblastic leukemia[96].
Nuclear factor kappa B (NF-κB) is a transcription factor constitutively activated in several tumors, being also associated with self-renewal and expansion of CSCs[97]. NF-κB interacts with many apoptosis–related proteins, including Bcl-xL, Bcl-2, survivin, cellular inhibitors of apoptosis (cIAPs), TRAF and cell cycle regulatory components, and NF-κB aberrant expression was related to cancer development and progression, chemoresistance, chronic inflammation and autoimmune diseases[60].
In hepatocellular carcinoma cell lines, one of the most activated signaling pathways is NF-κB cascade; NF-κB inhibition using SN50, suppressed tumor cell growth and the authors suggested that targeting NF-κB signaling might specifically inhibit CSCs populations and it could be considered a new therapeutic strategy for hepatocellular carcinoma patients with poor prognosis[97]. Pancreatic ductal adenocarcinoma characterized by a pronounced hypoxic tumor microenvironment presents epithelial-mesenchymal transition and stem-like features related to NF-κB signaling activation. An in vitro study demonstrated that inhibition of NF-κB with triptolide can reverse epithelial-mesenchymal transition and reduced migration, self-renewal activity, stem cell-related signaling[98]. NF-κB signaling is also considered an important therapeutic target in breast cancer; HER2-NF-κB-HER2 pro-survival pathway seems to be activated in breast CSCs upon radiation therapy[99].
Numerous studies have focused on identifying the molecular mechanisms and signaling pathways characteristic for CSCs, in solid and hematological malignancies. Notch, Hedgehog, Wnt/b-catenin, NF-κB, PI3K/Akt, and PTEN cascades present aberrant activation in cancers and they have been associated with high proliferative and metastatic capacity, self-renewal and differentiation of CSCs and also multi-drug resistance, being considered attractive targets for CSCs specific eradication. However, more studies are required to demonstrate the safety of these targeted therapies, considering the crucial role of these signaling pathways for normal stem cell maintenance[97].
Targeting microenvironment
The tumor microenvironment provides necessary signals for CSCs maintenance, regulation of self-renewal and homeostatic processes such as angiogenesis, hypoxia and weakly acidic pH.
Interaction between CSCs and tumor stroma is ensured by CXCL12-CXCR4 axis. CXCL12 [also called stromal-derived factor-1 (SDF-1)] is a chemokine that binds its receptor CXCR4 and it is involved in migration, invasion and survival of normal and malignant cells[100]. CXCR4 is highly expressed on several stem cells mainly haematopoietic, but also on endothelial, neural and embryonic stem cells, facilitating their response to gradients of CXCL12 produced in case of tissue damage and hypoxia. On the other hand, CXCL12 ligand has a constitutive expression on stromal cells in many tissues. It has an increased level in bone marrow, lymph nodes, lung and liver, and a low level in small intestine, skin skeletal muscle[101].
Many studies have emphasized that under physiological conditions CXCL12-CXCR4 axis plays a crucial role in processes like normal development, tissue regeneration and repair. With respect to tumor growth, it was shown that CXCL12-CXCR4 axis ensures a close contact between cells and tumor stroma and consequently activates various signals related to cell growth, metastasis and chemoresistance[102]. In human breast cancer CXCL12 generated by stromal fibroblasts exerts two types of effects on tumor growth: an endocrine effect by promoting angiogenesis via recruiting endothelial progenitor cells into the tumor mass, as well as a paracrine effect by acting directly on tumor cells through CXCR4[103].
The important roles of CXCL12-CXCR4 axis in cancer led to an intense research for developing drugs that inhibit signaling through this axis. A series of inhibitors have been investigated, targeting either CXCR4 or CXCL12. Among CXCR4 inhibitors, AMD3100, also known as plerixafor, induces the rapid mobilization of hematopoietic stem and progenitor cells into the blood in mice and humans[104], inhibits growth of intracranial glioblastoma and medulloblastoma xenografts by increasing apoptosis and decreasing the proliferation of tumor cells[105], and can reduce the intraperitoneal dissemination of epithelial ovarian cancer[106]. On the other hand, in a recent study it was shown that treatment with the AMD3100 diminished metastatic growth, but it didn’t affect significantly the frequency of metastases or overall survival in a murine model of metastatic human non-small cell lung cancer[107].
CTCE-9908 is another CXCR4 antagonist that proved to be effective in reducing both tumor growth and metastasis in xenograft mouse models of inflammatory breast cancer[108] and also in an orthotopic model of esophageal carcinoma[109]. A recent study highlighted the role of CTCE-9908 in decreasing the tumor invasivity and angiogenesis in prostate cancer[110].
One of the most studied CXCL12 inhibitor is NOX-A12 that seems to suppress CXCL12-induced chemotaxis of chronic lymphocytic leukemia cells and to cause chemosensitization[111]. In addition, Liu et al[112] observed that this compound had the potential to improve tumor response in glioblastoma multiforme animal model after irradiation.
CXCL12-CXCR4 axis can also promote angiogenesis. Liang et al[113] have shown that CXCR4 induces an increased expression of vascular-endothelial growth factor (VEGF) at both the mRNA and protein levels through the activation of PI3K/Akt pathway.
Tumor angiogenesis is another mechanism promoted by microenvironment that has been related with CSCs survival and tumor growth, since targeting VEGF can lead to normalization of the vasculature, decrease in tumor growth and disruption of the CSCs niche[60,114]. Inhibitors against the VEGF/VEGFR-system are already in clinical use. Among the approved drugs that target VEGF/VEGFR-system are bevacizumab, anti-VEGF blocking antibody, and pazopanib, sorafenib and sunitinib, VEGFR-2 pathway inhibitors. Moreover, a series of compounds are tested in clinical trials or they are just in experimental phase[115]. From those, fruquintinib (HMPL-013), a small molecule inhibitor very potent and highly selective against VEGFR family, is currently in phase II clinical studies[115].
Another feature of the tumor microenvironment is hypoxia that is regulated by inducible transcription factors HIF-1 and HIF-2. Many studies correlated tumor hypoxia with tumor growth, cancer progression, metastasis, resistance to chemo- and radiotherapy. Therefore targeting HIF activity might represent an effective method of inhibit tumor metastasis and improve the outcome of chemo- and radiotherapy[116,117].
In breast cancer, inhibition HIF activity in BrCa cells by using RNA interference or digoxin treatment prevent primary tumor growth and also reduce breast cancer dissemination in lungs by down-regulating the expression of angiopoietin-like 4 (ANGPTL4) and L1 cell adhesion molecule (L1CAM) 21860410. In a recent study, Gillespie et al[118] investigated the inhibition of HIF-1α by using small interfering RNA in an orthotopic mouse model for glioblastoma. In vivo treatment reduced tumor growth and increased survival.
Another important therapeutic target can be represented by acidic extracellular pH, a major feature of tumor tissue that is the result of cancer cells increased metabolic activity and of the poor vascular perfusion of tumors[119,120]. As it was shown in previous studies, tumor acidity can offer a selective advantage of cancer cells over the normal tissues and contribute to drug resistance[119,121].
The acidity of tumor environment can be managed either by using delivery drugs that have specificity for acid environment, or can be reversed directly with systemic buffers or indirectly by using inhibitors for pH-regulatory pathways like carbonic anhydrase IX (CAIX).
Developing compounds that are tumoral acidic pH-responsive is a dynamic area of research; liposomes, micelles, polymers and inorganic nanoparticles have been tested for anticancer therapy[122]. An improved drug-delivery method is to encapsulate drugs into silica matrices like camptothecin and doxorubicin[122,123].
Sodium bicarbonate is one of the systemic buffers that can be used to alkalinize the microenvironment. In intraductal tumors it have been shown that sodium bicarbonate can prevent or slow down the transition from in situ to invasive cancer[124].
One approach, more captivating is to regulate cellular pH with drugs that inhibit CAIX, a hypoxia and HIF-1-inducible protein, overexpressed in many cancers. Data from phase III clinical trial suggest that CAIX score can be also used as statistically prognostic biomarker for survival in patients with high-risk nonmetastatic renal cell carcinoma (ccRCC)[125].
Targeting the tumor microenvironment might affect communication with stroma and self-regulating process, the vasculature as the main nutrients supply, and essential features like oxygen level and acidity needed by CSCs, all these being fundamentals for CSCs maintenance. However these strategies are not specific enough and might affect also normal stem cells and their stroma. Consequently, a more particular approach is needed that addresses only tumor stroma.
Targeting ATP-binding cassette transporters
The ability to isolate and characterize CSCs from different tumors facilitated a more specific investigation of the therapeutic possibilities based on another distinctive feature of the CSCs, chemoresistance[126]. CSCs identification by augmented efflux of Hoechst 33342 dye through ATP-binding cassette (ABC) transporters (defined as side population, SP cells) in flow cytometry analysis, proved to be very useful in isolating hematopoietic stem cells, and a variety of CSCs from solid tumors, including ovarian, breast, colon and hepatic cancers[127-131]. Aberrant expression of ABC transporters is a major mechanism of chemoresistance in cancers cells, including cancer CSCs[132] but may be generated by other mechanisms involved directly or indirectly in the process, from transcription to protein expression. All the mechanisms involved in ABC transporter modulation might be potential targets for overcoming chemoresistance in CSCs.
ABC transporter proteins are members of the ABC superfamily that have as most important physiologic role prevention of the accumulation of xenobiotics and toxic compounds in normal cells. These efflux pumps consist of a single or multiple sets of transmembrane domains and nucleotide binding domains. Nucleotide binding domains hydrolyze ATP giving power for the efflux while a variety of structurally unrelated substrates, including drugs, sugars, proteins, and metabolites are “pumped-out” through the transmembrane domains.
Among the numerous members of the ABC-transporters described to date, only few were well documented to be expressed in human CSCs: Multidrug resistance 1 (MDR1) or P-glycoprotein (Pgp)/ABCB1, multidrug resistance protein 1 (MRP1)/ABCC1, and breast cancer resistance protein (BCRP)/ABCG2/MXR/ABCP. These proteins differ in structure and in substrate specificity. MDR1 is a protein that confers cross-resistance to many antitumor agents, including anthracyclines, mitoxantrone, epipodophyllotoxins, and taxanes. ABCC1 is structurally similar to ABCB1 but shares only 15% amino acid sequence identity; it also generates resistance to anthracyclines, mitoxantrone, and epipodophyllotoxins but differs from MDR1 in the level of resistance to taxanes. BCRP also confers resistance to mitoxantrone, but anthracyclines resistance was found to depend on mutations at the codon 482[133].
The new concept of targeting ABC transporters considers inhibitors as “CSC sensitizing agents”. Clinical studies have attempted to overcome drug resistance by combination therapies in which a cytotoxic drug was given along with an ABC-transporter inhibitor. However, ABCB1 inhibitors have shown very limited effectiveness in clinical trials despite the debate on the fact that the clinical trials on the first generation of inhibitors have not been targeting CSCs but only the reduction of the tumors that express a particular drug transporter (usually ABCB1). If the stem cells are considered the principal culprits of drug resistance, the efficacy of ABC inhibitors would be better evaluated monitoring the relapse instead of tumor size reduction[134]. However, ABC inhibitors would be most effective if are combined with an anticancer agent that specifically targets the stem cells.
Three generations of ABC transporter blockers were investigated until now and the fourth generation, based on natural compounds, is under development[135].
First generation of inhibitor drugs were already in use for different conditions and also were able to block MDR1 (ABCBC1), such as calcium channel inhibitors like verapamil, immuno-supressants like cyclosporin A, anti-arrhythmics and neuroleptics like quinidine, reserpine, and yohimbine, and antiestrogens like tamoxifen and toremifene. The efficacy of these drugs was limited by their toxicity, which urged the development of drugs with less important side effects as first generation compounds.
Second generation of MDR1 modulators like R-verapamil, elacridar (GF120918), dofequidar (MS-209), valspodar (PSC833), biricodar (INCEL, VX-710), or timcodar (VX-853) were derived from first generation P-gp modulators. Although second generation modulators are less toxic than first generation modulators, they still induce important side effects due to nonselective inhibition of multiple cell ABC transporters and unpredictable pharmacokinetic interactions[136].
Third generation modulators such as Zosuquidar (LY335979), oc144093 (ONT-093), laniquidar (R101933), and tariquidar (XR-9576), which are more selective inhibitors of ABCB1, ABCC1, ABCG2, are still in different phases of investigation as CSCs sensitizing agents.
Although the expression of ABC transporters could render CSCs resistant to drugs, it is not the unique cause of resistance by ABC proteins, as multiple levels of regulation between transcription and protein expression might be susceptible to modifications. Future trends of drug development should consider such levels of regulation of ABC transporters that might be speculated in cancer therapy. One such direction should evaluate, for example, the potential of using miRNAs targeting specific RNA that results in its degradation. MiR-328, miR 519c, miR-520h, miR-487a, miR-181a are only few of the miRNAs reported to be involved in ABC transporter regulation.
Recently, was observed that the presence of side population (SP) cells in tumors is highly dependent on the driving genetic alterations of the tumor. For example, hepatic tumors driven by Myc, but not Akt and RAS, had a significant number of SP cells that show the same properties as chemoresistant tumor-initiating CSCs. These studies show that even the common mechanisms of chemoresistance may change under genomic alterations conditions that are very frequent in cancer. There are increasing evidence that small-molecule tyrosine kinase inhibitors (TKIs), such as AST1306, lapatinib, linsitinib, masitinib, motesanib, nilotinib, telatinib and WHI-P154, can inhibit ABC transporters, suggesting that these TKI might have also a direct effect on ABC proteins inhibitors[137]. Effect of Akt, PI3K and mTOR inhibitors on ABC transporters trafficking and/or expression has been well documented[138]. Particularly, LY294002, a derivative of quercetin, is able to inhibit PI3Ks, mTOR and ABCG2 and has been recently proposed as a new candidate to target efficiently ABCG2-expressing CSCs that depend on Akt signaling for survival. ABCG2 expression and the SP might be regulated by PTEN through the PI3K/Akt pathway, which was proposed as a potentially effective strategy for targeting CSCs[139].
Using nanomedicines as delivery vehicles can enhance the therapeutic response in resistant tumors by bypassing efflux pumps or by increasing the concentration of drugs in CSCs at a given time point[140].
Simultaneous targeting of genomic alterations that are responsible for different types of cancer and resistance phenotypes together with new strategies of delivery and retention of the drugs inside the CSCs, will allow the development of more selective therapy.
Manipulation of miRNA expression
MicroRNAs (miRs) are small non-coding RNAs (20 to 24 nucleotides in length) that negatively regulate post-transcription by binding to the 3′UTR of target messenger RNA, having a broad range of effects over self-renewal, differentiation and division of cells[141]. MiRNA are involved not only in maintaining normal cell functions, but also might modify several signaling pathways that could transform stem cells into cancer stem cells. Aberrant expression of miRNA in cancer stem cells was noted by many studies. MiRNAs differentially regulate the key properties of CSCs, including cell-cycle exit and differentiation, prosurvival and antistress mechanisms and EMT, migration and invasion, which increase tumor initiation and metastatic potential[142]. They can act either as oncogenes or tumor suppressors.
Targeting oncogenic miRNAs can be achieved by antisense oligonucleotide inhibition. Studies have shown that miR-21 has been found to be frequently up-regulated in different CSCs[143]. Thus, knockdown of miR-21 inhibits cell proliferation, migration and tumor growth in breast cancers[144,145], ovarian[146], and lung cancer[147].
Several studies have exposed the potential of miRNA based therapeutics as a novel strategy towards cancer stem cells. MiR-34 is a target of tumor suppressor gene p53 and is down-regulated in many cancers[148]. Liu et al[149] showed that miR-34a, was underexpressed in CD44+ prostate cancer cells and increased expression of miR-34a in CD44+ prostate cancer cells inhibited clonogenic expansion, tumor regeneration, and metastasis. Moreover, Shi et al[150] demonstrated that transfection of synthetic miR-34a in CD44+ non-small cell lung cancer cell lines inhibited clonogenic expansion, and tumor regeneration in vivo. Consistently, forced expression of miR-124 and miR-137 in human derived glioblastoma stem cells leads to loss of their self-renewal and oncogenic capacity, leaving normal stem and precursor cells unharmed[151].
Abnormal miRNA expressions lead to the initiation, development, and progression of cancer. Thus, miRNA based therapy that can correct abnormal transcripts at CSCs level show great potential for cancer cure.
Induction of CSCs apoptosis
Apoptosis is a critical mechanism that mediates death and survival through a complex signaling mechanism. Escape from this system is the precondition for cancer initiating cells. Usually, the apoptotic mechanisms are impaired during cancer development and progression.
Induction of apoptosis in CSC holds great promise for cancer therapy. Therefore, many compounds have been developed to target intrinsic and extrinsic apoptotic pathways. For example, activating of the death receptors [CD95 and trimeric human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)], part of extrinsic apoptotic pathway, leads to caspase-8 activation. Once activated, caspase-8 either directly cleaves and activates effector caspase-3 or, alternatively, processes Bid into the active fragment tBid, which translocates to mitochondrial membranes to initiate mitochondrial outer membrane permeabilization[152]. Treatment with TRAIL in combination with various anticancer agents was reported to be effective in removing cancer stem cells. Thus, co-treatment with cisplatin was described to be very efficient in reducing triple negative breast cancer stem cells through inhibition of Wnt signaling and increasing of apoptosis[153]. Likewise, combined treatment with TRAIL and cytarabine or daunorubicin, has been shown to suppress the growth of acute myeloid progenitors[154]. Furthermore, TRAIL in addition to Bortezomib, a proteasome inhibitor, was recently shown trigger apoptosis in glioblastoma stem cells[155]. Moreover, mesenchymal stem cells (MSC) engineered to express TRAIL though transduction with a lentiviral vector, were used to activate apoptosis in of squamous and lung cancer stem cell population. Experiments were done by injecting TRAIL- secreting MSC subcutaneously into nude mice that hold tumors. Results showed that MSC migrate and localized near the cancer site and inhibited tumor growth through apoptosis induction[156].
Another target in inducing CSCs death might be NF-κB, a transcription factor linked to the control of apoptosis signaling. Mainly, NF-κB inhibits apoptosis and promotes cell proliferation, inflammation, tumor promotion, angiogenesis and metastasis[157-159]. In breast cancer, pharmacological inhibition of NF-κB with small-molecules like parthenolide, pyrrolidinedithiocarbamate and its analog diethyldithiocarbamate preferentially target breast cancer stem cells. These results underline that NF-κB activity is critical to maintain the survival of tumor-initiating cancer stem cells[160]. Moreover, inhibition of NF-κB by the proteasome inhibitor MG-132 together with the anticancer drug idarubicin induced apoptosis preferentially in the leukemic stem cells population but considerably less in normal hematopoietic stem cells[161].
Targeting CSC population by reactivation of apoptosis programs has been shown in preclinical studies to offer the possibility to eradicate cancers. Future challenges should include the increasing of specificity and efficiency in targeting cancer stem cells and avoid toxicity of normal tissue stem cells.
Induction of CSCs differentiation
Traditional anti-cancer therapies successfully manage differentiated cancer cells but did not affect CSCs. This observation leads to another method to restrain cancer progression, induction of CSCs differentiation that became favored over self-renewal programs, diminishing CSC population. Many studies are currently in progress proposing various differentiation agents like: retinoic acid, histone deacetylase inhibitors, miRNAs, tyrosine-kinase and signaling pathways inhibitors.
Retinoic acid and its analogs (ATRA) are currently used to treat acute promyelocytic leukemia. Retinoic acid regulates several chromatin remodeling factors due to its interaction with retinoid receptors[162]. Campos et al[163] demonstrated that ATRA induced differentiation of glioma CSCs, and showed that the anti-tumor effect is present both in vitro and in vivo experiments. Moreover, Ginestier et al[164] showed that modulation of retinoid signaling may might promote self-renewal or induce differentiation of breast CSCs, and suggested that ATRA may be considered as targeted therapy for breast CSC population.
Other agents that can affect cancer stem cell differentiation are histone deacetylase inhibitors (HDACI). To date, HDACI are used as differentiation therapy in several hematologic malignancies. Recently, this type of therapy was proposed for breast CSCs[165] and head and neck CSCs[166]. In the first study HDACI (abexinostat) induced CSC differentiation. Moreover, in the second study, HDACI (suberoylanilide hydroxamic acid and trichostatin A) altered the CSCs phenotype and also induced cell cycle arrest and apoptosis.
Petrelli et al[167] showed that miR-100 favors breast CSCs differentiation, converting a basal like phenotype into luminal. It induces the expression of a functional estrogen receptor and renders basal-like BrCSCs responsive to hormonal therapy. Vaz et al[168] suggest that pancreatic differentiation 2 (PD2) is a novel CSC maintenance protein, loss of which renders the CSCs more susceptible to drug-induced cell death. Knockdown of PD2 protein in CSCs decreased cell viability, enhanced apoptosis and diminish expression of CD133 and MDR2. Dong et al[169] demonstrated that long-term culture of glioblastoma cancer stem cells with imatinib mesylate, a tyrosine-kinase inhibitor, induced cell differentiation, confirmed by decreased expression of stem cell markers, CD133, Oct-3/4, nestin, and Bmi1, and increased expression of GFAP (astrocyte marker) and class III β-tubulin isotype (Tuj1, neuron marker), associated with reduced ability to form aggregates and colonies in vitro and tumorigenicity in vivo. Another strategy targeting signaling pathways in order to induce CSCs differentiation is directed against hyperactivated Akt/mTOR and the inhibited wild-type p53 pathways in glioma CSCs. Daniele et al[170] uses two inhibitors (FC85 and ISA27) that blocked proliferation and promoted the differentiation of GSCs by reducing Akt/mTOR signalling and reactivating p53 functionality.
Inducing CSC differentiation combined with CSC-targeted therapies and traditional therapies may bring real benefits in depleting cancer cells by increasing the efficacy.
CONCLUSION
In this review we have summarized recent advances in identification of CSCs molecular markers that might became targets for developing new therapies, aiming to hamper CSCs regeneration and cancer relapse. These novel therapeutic strategies are designed to target directly CSCs or to interrupt signals from the microenvironment that regulate important properties like self-renewal, differentiation and apoptosis resistance.
All these alternative therapies are very promising, but some of them not specific and might affect also healthy tissue since CSCs niche is similar and close to normal stem cell niche that can also be affected by compounds. There are also many ways by which CSCs could evade treatment. They reside is an area of low oxygen and vascularity, preventing efficient delivery of the drugs.
Future challenges should include the increasing of specificity and efficiency in targeting CSCs, avoiding toxicity of normal tissue stem cells, and also new strategies of delivery and retention of the drugs inside the CSCs. These new therapies should increase the efficacy of existing drugs against aggressive cancers, and thus should prevent tumor relapse and enhance patient survival.
Footnotes
Supported by The European Social Fund; and the Romanian Government (Laura G Necula), No. POSDRU/159/1.5/S/137390.
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 28, 2015
First decision: July 3, 2015
Article in press: September 8, 2015
P- Reviewer: Holan V, Kolonin MG, Sarkadi B, Thomas X, Yao CL S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Podlaha O, Riester M, De S, Michor F. Evolution of the cancer genome. Trends Genet. 2012;28:155–163. doi: 10.1016/j.tig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 3.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 4.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Borodyansky L, Yang Y. Genomic instability en route to and from cancer stem cells. Cell Cycle. 2009;8:1000–1002. doi: 10.4161/cc.8.7.8041. [DOI] [PubMed] [Google Scholar]
- 7.Rapp UR, Ceteci F, Schreck R. Oncogene-induced plasticity and cancer stem cells. Cell Cycle. 2008;7:45–51. doi: 10.4161/cc.7.1.5203. [DOI] [PubMed] [Google Scholar]
- 8.Wu KJ, Yang MH. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Biosci Rep. 2011;31:449–455. doi: 10.1042/BSR20100114. [DOI] [PubMed] [Google Scholar]
- 9.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 12.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 13.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamgeer M, Peacock CD, Matsui W, Ganju V, Watkins DN. Cancer stem cells in lung cancer: Evidence and controversies. Respirology. 2013;18:757–764. doi: 10.1111/resp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123:1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30:363–371. doi: 10.1002/stem.1031. [DOI] [PubMed] [Google Scholar]
- 20.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Stigter-van Walsum M, Zweegman S, Ossenkoppele GJ, Jan Schuurhuis G. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 22.Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, Krensky AM, Weissman IL. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104:11008–11013. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jan M, Chao MP, Cha AC, Alizadeh AA, Gentles AJ, Weissman IL, Majeti R. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci USA. 2011;108:5009–5014. doi: 10.1073/pnas.1100551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone A, Najima Y, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17ra9. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherciu I, Bărbălan A, Pirici D, Mărgăritescu C, Săftoiu A. Stem cells, colorectal cancer and cancer stem cell markers correlations. Curr Health Sci J. 2014;40:153–161. doi: 10.12865/CHSJ.40.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swaminathan SK, Roger E, Toti U, Niu L, Ohlfest JR, Panyam J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J Control Release. 2013;171:280–287. doi: 10.1016/j.jconrel.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, Panoskaltsis-Mortari A, Vallera DA. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol. 2013;130:579–587. doi: 10.1016/j.ygyno.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratford EW, Bostad M, Castro R, Skarpen E, Berg K, Høgset A, Myklebost O, Selbo PK. Photochemical internalization of CD133-targeting immunotoxins efficiently depletes sarcoma cells with stem-like properties and reduces tumorigenicity. Biochim Biophys Acta. 2013;1830:4235–4243. doi: 10.1016/j.bbagen.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, Wicha MS, Chang AE, Li Q. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clin Immunol. 2013;149:156–168. doi: 10.1016/j.clim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Pan Q, Li Q, Liu S, Ning N, Zhang X, Xu Y, Chang AE, Wicha MS. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells. 2015;33:2085–2092. doi: 10.1002/stem.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 34.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negi LM, Talegaonkar S, Jaggi M, Ahmad FJ, Iqbal Z, Khar RK. Role of CD44 in tumour progression and strategies for targeting. J Drug Target. 2012;20:561–573. doi: 10.3109/1061186X.2012.702767. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh SC, Neslihan Alpay S, Klostergaard J. CD44: a validated target for improved delivery of cancer therapeutics. Expert Opin Ther Targets. 2012;16:635–650. doi: 10.1517/14728222.2012.687374. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin Jinesh G, Willis DL, Kamat AM. Bladder cancer stem cells: biological and therapeutic perspectives. Curr Stem Cell Res Ther. 2014;9:89–101. doi: 10.2174/1574888x08666131113123051. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Feng F, Zhou YN. Stem cells in gastric cancer. World J Gastroenterol. 2015;21:112–123. doi: 10.3748/wjg.v21.i1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo V, Valenzuela R, Huidobro C, Contreras HR, Castellon EA. Functional characteristics of cancer stem cells and their role in drug resistance of prostate cancer. Int J Oncol. 2014;45:985–994. doi: 10.3892/ijo.2014.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Hao X, Qin J, Tang W, He F, Smith A, Zhang M, Simeone DM, Qiao XT, Chen ZN, et al. Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology. 2014;146:1108–1118. doi: 10.1053/j.gastro.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garson K, Vanderhyden BC. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction. 2015;149:R59–R70. doi: 10.1530/REP-14-0234. [DOI] [PubMed] [Google Scholar]
- 42.Dotse E, Bian Y. Isolation of colorectal cancer stem-like cells. Cytotechnology. 2014:Dec 23; Epub ahead of print. doi: 10.1007/s10616-014-9806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng D, Wang N, Hu J, Li W. Surface markers of hepatocellular cancer stem cells and their clinical potential. Neoplasma. 2014;61:505–513. doi: 10.4149/neo_2014_061. [DOI] [PubMed] [Google Scholar]
- 44.Athanassiou-Papaefthymiou M, Shkeir O, Kim D, Divi V, Matossian M, Owen JH, Czerwinski MJ, Papagerakis P, McHugh J, Bradford CR, et al. Evaluation of CD44 variant expression in oral, head and neck squamous cell carcinomas using a triple approach and its clinical significance. Int J Immunopathol Pharmacol. 2014;27:337–349. doi: 10.1177/039463201402700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majeti R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene. 2011;30:1009–1019. doi: 10.1038/onc.2010.511. [DOI] [PubMed] [Google Scholar]
- 46.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 47.Marangoni E, Lecomte N, Durand L, de Pinieux G, Decaudin D, Chomienne C, Smadja-Joffe F, Poupon MF. CD44 targeting reduces tumour growth and prevents post-chemotherapy relapse of human breast cancers xenografts. Br J Cancer. 2009;100:918–922. doi: 10.1038/sj.bjc.6604953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang W, Hao X, He F, Li L, Xu L. Anti-CD44 antibody treatment inhibits pancreatic cancer metastasis and post-radiotherapy recurrence. Cancer Res. 2011;71:565. [Google Scholar]
- 49.Masuko K, Okazaki S, Satoh M, Tanaka G, Ikeda T, Torii R, Ueda E, Nakano T, Danbayashi M, Tsuruoka T, et al. Anti-tumor effect against human cancer xenografts by a fully human monoclonal antibody to a variant 8-epitope of CD44R1 expressed on cancer stem cells. PLoS One. 2012;7:e29728. doi: 10.1371/journal.pone.0029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringnér M, Vallon-Christersson J, Jönsson G, Holm K, Lövgren K, et al. CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer. 2011;11:418. doi: 10.1186/1471-2407-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naujokat C. Targeting human cancer stem cells with monoclonal antibodies. J Clin Cell Immunol. 2012;S5:7. [Google Scholar]
- 52.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118:4890–4901. doi: 10.1182/blood-2011-02-338020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 56.Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, Liu J, Majeti R, West RB, Fletcher JA, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA. 2012;109:6656–6661. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwiatkowska-Borowczyk EP, Gąbka-Buszek A, Jankowski J, Mackiewicz A. Immunotargeting of cancer stem cells. Contemp Oncol (Pozn) 2015;19:A52–A59. doi: 10.5114/wo.2014.47129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salem ML, El-Badawy AS, Li Z. Immunobiology and signaling pathways of cancer stem cells: implication for cancer therapy. Cytotechnology. 2015;67:749–759. doi: 10.1007/s10616-014-9830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clayton S, Mousa SA. Therapeutics formulated to target cancer stem cells: Is it in our future? Cancer Cell Int. 2011;11:7. doi: 10.1186/1475-2867-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao L. NOTCH mutations: multiple faces in human malignancies. Cancer Prev Res (Phila) 2015;8:259–261. doi: 10.1158/1940-6207.CAPR-15-0063. [DOI] [PubMed] [Google Scholar]
- 62.Greife A, Hoffmann MJ, Schulz WA. Consequences of Disrupted Notch Signaling in Bladder Cancer. Eur Urol. 2015;68:3–4. doi: 10.1016/j.eururo.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 63.Lobry C, Oh P, Mansour MR, Look AT, Aifantis I. Notch signaling: switching an oncogene to a tumor suppressor. Blood. 2014;123:2451–2459. doi: 10.1182/blood-2013-08-355818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aithal MG, Rajeswari N. Role of Notch signalling pathway in cancer and its association with DNA methylation. J Genet. 2013;92:667–675. doi: 10.1007/s12041-013-0284-5. [DOI] [PubMed] [Google Scholar]
- 67.Osanyingbemi-Obidi J, Dobromilskaya I, Illei PB, Hann CL, Rudin CM. Notch signaling contributes to lung cancer clonogenic capacity in vitro but may be circumvented in tumorigenesis in vivo. Mol Cancer Res. 2011;9:1746–1754. doi: 10.1158/1541-7786.MCR-11-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, Kalemkerian GP, Wicha MS. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res. 2013;19:1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y, et al. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 71.Kim HA, Koo BK, Cho JH, Kim YY, Seong J, Chang HJ, Oh YM, Stange DE, Park JG, Hwang D, et al. Notch1 counteracts WNT/β-catenin signaling through chromatin modification in colorectal cancer. J Clin Invest. 2012;122:3248–3259. doi: 10.1172/JCI61216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yap LF, Lee D, Khairuddin A, Pairan MF, Puspita B, Siar CH, Paterson IC. The opposing roles of NOTCH signalling in head and neck cancer: a mini review. Oral Dis. 2015;21:850–857. doi: 10.1111/odi.12309. [DOI] [PubMed] [Google Scholar]
- 73.Gavai AV, Quesnelle C, Norris D, Han WC, Gill P, Shan W, Balog A, Chen K, Tebben A, Rampulla R, et al. Discovery of Clinical Candidate BMS-906024: A Potent Pan-Notch Inhibitor for the Treatment of Leukemia and Solid Tumors. ACS Med Chem Lett. 2015;6:523–527. doi: 10.1021/acsmedchemlett.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res. 2015;21:2084–2095. doi: 10.1158/1078-0432.CCR-14-2808. [DOI] [PubMed] [Google Scholar]
- 75.Cohen DJ. Targeting the hedgehog pathway: role in cancer and clinical implications of its inhibition. Hematol Oncol Clin North Am. 2012;26:565–588, viii. doi: 10.1016/j.hoc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Merchant AA, Matsui W. Targeting Hedgehog--a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Maitah MY, Ahmad A, Kong D, Bao B, Sarkar FH. Targeting the Hedgehog signaling pathway for cancer therapy. Expert Opin Ther Targets. 2012;16:49–66. doi: 10.1517/14728222.2011.617367. [DOI] [PubMed] [Google Scholar]
- 78.Campbell V, Copland M. Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells Cloning. 2015;8:27–38. doi: 10.2147/SCCAA.S58613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aberger F, Kern D, Greil R, Hartmann TN. Canonical and noncanonical Hedgehog/GLI signaling in hematological malignancies. Vitam Horm. 2012;88:25–54. doi: 10.1016/B978-0-12-394622-5.00002-X. [DOI] [PubMed] [Google Scholar]
- 80.Brechbiel J, Miller-Moslin K, Adjei AA. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat Rev. 2014;40:750–759. doi: 10.1016/j.ctrv.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Justilien V, Fields AP. Molecular pathways: novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin Cancer Res. 2015;21:505–513. doi: 10.1158/1078-0432.CCR-14-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wellbrock J, Latuske E, Köhler J, Wagner K, Stamm H, Vettorazzi E, Vohwinkel G, Klokow M, Uibeleisen R, Ehm P, et al. Expression of Hedgehog Pathway Mediator GLI Represents a Negative Prognostic Marker in Human Acute Myeloid Leukemia and Its Inhibition Exerts Antileukemic Effects. Clin Cancer Res. 2015;21:2388–2398. doi: 10.1158/1078-0432.CCR-14-1059. [DOI] [PubMed] [Google Scholar]
- 83.Zuo M, Rashid A, Churi C, Vauthey JN, Chang P, Li Y, Hung MC, Li D, Javle M. Novel therapeutic strategy targeting the Hedgehog signalling and mTOR pathways in biliary tract cancer. Br J Cancer. 2015;112:1042–1051. doi: 10.1038/bjc.2014.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gu D, Liu H, Su GH, Zhang X, Chin-Sinex H, Hanenberg H, Mendonca MS, Shannon HE, Chiorean EG, Xie J. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther. 2013;12:1038–1048. doi: 10.1158/1535-7163.MCT-12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wend P, Holland JD, Ziebold U, Birchmeier W. Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol. 2010;21:855–863. doi: 10.1016/j.semcdb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Hallett RM, Kondratyev MK, Giacomelli AO, Nixon AM, Girgis-Gabardo A, Ilieva D, Hassell JA. Small molecule antagonists of the Wnt/β-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS One. 2012;7:e33976. doi: 10.1371/journal.pone.0033976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amado NG, Predes D, Moreno MM, Carvalho IO, Mendes FA, Abreu JG. Flavonoids and Wnt/β-catenin signaling: potential role in colorectal cancer therapies. Int J Mol Sci. 2014;15:12094–12106. doi: 10.3390/ijms150712094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, et al. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports. 2015;4:569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang X, Lou Y, Zheng X, Wang H, Sun J, Dong Q, Han B. Wnt blockers inhibit the proliferation of lung cancer stem cells. Drug Des Devel Ther. 2015;9:2399–2407. doi: 10.2147/DDDT.S76602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gargini R, Cerliani JP, Escoll M, Antón IM, Wandosell F. Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells. 2015;33:646–660. doi: 10.1002/stem.1904. [DOI] [PubMed] [Google Scholar]
- 93.Marhold M, Tomasich E, El-Gazzar A, Heller G, Spittler A, Horvat R, Krainer M, Horak P. HIF1α Regulates mTOR Signaling and Viability of Prostate Cancer Stem Cells. Mol Cancer Res. 2015;13:556–564. doi: 10.1158/1541-7786.MCR-14-0153-T. [DOI] [PubMed] [Google Scholar]
- 94.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong P, Konno Y, Watari H, Hosaka M, Noguchi M, Sakuragi N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J Transl Med. 2014;12:231. doi: 10.1186/s12967-014-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill R, Wu H. PTEN, stem cells, and cancer stem cells. J Biol Chem. 2009;284:11755–11759. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D, Breuhahn K, Conner EA, et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661–669. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Salnikov AV, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Schemmer P, Werner J, et al. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation. Int J Cancer. 2014;134:2489–2503. doi: 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duru N, Candas D, Jiang G, Li JJ. Breast cancer adaptive resistance: HER2 and cancer stem cell repopulation in a heterogeneous tumor society. J Cancer Res Clin Oncol. 2014;140:1–14. doi: 10.1007/s00432-013-1494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Panneerselvam J, Jin J, Shanker M, Lauderdale J, Bates J, Wang Q, Zhao YD, Archibald SJ, Hubin TJ, Ramesh R. IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. PLoS One. 2015;10:e0122439. doi: 10.1371/journal.pone.0122439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 102.Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S, Jin Q, Li B, Yao F, Jin F. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol. 2014;35:7765–7773. doi: 10.1007/s13277-014-1816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 104.Welschinger R, Liedtke F, Basnett J, Dela Pena A, Juarez JG, Bradstock KF, Bendall LJ. Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp Hematol. 2013;41:293–302.e1. doi: 10.1016/j.exphem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 107.Singla AK, Downey CM, Bebb GD, Jirik FR. Characterization of a murine model of metastatic human non-small cell lung cancer and effect of CXCR4 inhibition on the growth of metastases. Oncoscience. 2015;2:263–271. doi: 10.18632/oncoscience.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh B, Cook KR, Martin C, Huang EH, Mosalpuria K, Krishnamurthy S, Cristofanilli M, Lucci A. Evaluation of a CXCR4 antagonist in a xenograft mouse model of inflammatory breast cancer. Clin Exp Metastasis. 2010;27:233–240. doi: 10.1007/s10585-010-9321-4. [DOI] [PubMed] [Google Scholar]
- 109.Drenckhan A, Kurschat N, Dohrmann T, Raabe N, Koenig AM, Reichelt U, Kaifi JT, Izbicki JR, Gros SJ. Effective inhibition of metastases and primary tumor growth with CTCE-9908 in esophageal cancer. J Surg Res. 2013;182:250–256. doi: 10.1016/j.jss.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 110.Wong D, Kandagatla P, Korz W, Chinni SR. Targeting CXCR4 with CTCE-9908 inhibits prostate tumor metastasis. BMC Urol. 2014;14:12. doi: 10.1186/1471-2490-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoellenriegel J, Zboralski D, Maasch C, Rosin NY, Wierda WG, Keating MJ, Kruschinski A, Burger JA. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123:1032–1039. doi: 10.1182/blood-2013-03-493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, Merchant M, Zboralski D, Zöllner S, Kruschinski A, et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol. 2014;16:21–28. doi: 10.1093/neuonc/not149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burkhardt JK, Hofstetter CP, Santillan A, Shin BJ, Foley CP, Ballon DJ, Pierre Gobin Y, Boockvar JA. Orthotopic glioblastoma stem-like cell xenograft model in mice to evaluate intra-arterial delivery of bevacizumab: from bedside to bench. J Clin Neurosci. 2012;19:1568–1572. doi: 10.1016/j.jocn.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 115.Prager GW, Poettler M, Unseld M, Zielinski CC. Angiogenesis in cancer: Anti-VEGF escape mechanisms. Transl Lung Cancer Res. 2012;1:14–25. doi: 10.3978/j.issn.2218-6751.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burroughs SK, Kaluz S, Wang D, Wang K, Van Meir EG, Wang B. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem. 2013;5:553–572. doi: 10.4155/fmc.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J, Du F, Shen G, Zheng F, Xu B. The role of hypoxia-inducible factor-2 in digestive system cancers. Cell Death Dis. 2015;6:e1600. doi: 10.1038/cddis.2014.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gillespie DL, Aguirre MT, Ravichandran S, Leishman LL, Berrondo C, Gamboa JT, Wang L, King R, Wang X, Tan M, et al. RNA interference targeting hypoxia-inducible factor 1α via a novel multifunctional surfactant attenuates glioma growth in an intracranial mouse model. J Neurosurg. 2015;122:331–341. doi: 10.3171/2014.10.JNS132363. [DOI] [PubMed] [Google Scholar]
- 119.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8:2032–2038. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci USA. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 122.Xu Z, Liu S, Kang Y, Wang M. Glutathione- and pH-responsive nonporous silica prodrug nanoparticles for controlled release and cancer therapy. Nanoscale. 2015;7:5859–5868. doi: 10.1039/c5nr00297d. [DOI] [PubMed] [Google Scholar]
- 123.Xu Z, Wang D, Xu S, Liu X, Zhang X, Zhang H. Preparation of a camptothecin prodrug with glutathione-responsive disulfide linker for anticancer drug delivery. Chem Asian J. 2014;9:199–205. doi: 10.1002/asia.201301030. [DOI] [PubMed] [Google Scholar]
- 124.Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, Gatenby RA. Systemic buffers inhibit carcinogenesis in TRAMP mice. J Urol. 2012;188:624–631. doi: 10.1016/j.juro.2012.03.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chamie K, Klöpfer P, Bevan P, Störkel S, Said J, Fall B, Belldegrun AS, Pantuck AJ. Carbonic anhydrase-IX score is a novel biomarker that predicts recurrence and survival for high-risk, nonmetastatic renal cell carcinoma: Data from the phase III ARISER clinical trial. Urol Oncol. 2015;33:204.e25–204.e33. doi: 10.1016/j.urolonc.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 126.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 129.Christgen M, Ballmaier M, Bruchhardt H, von Wasielewski R, Kreipe H, Lehmann U. Identification of a distinct side population of cancer cells in the Cal-51 human breast carcinoma cell line. Mol Cell Biochem. 2007;306:201–212. doi: 10.1007/s11010-007-9570-y. [DOI] [PubMed] [Google Scholar]
- 130.Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 131.Chow EK, Fan LL, Chen X, Bishop JM. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56:1331–1341. doi: 10.1002/hep.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 133.Minderman H, O’Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826–1834. doi: 10.1158/1078-0432.ccr-0914-3. [DOI] [PubMed] [Google Scholar]
- 134.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 135.Karthikeyan S, Hoti SL. Development of Fourth Generation ABC Inhibitors from Natural Products: A Novel Approach to Overcome Cancer Multidrug Resistance. Anticancer Agents Med Chem. 2015;15:605–615. doi: 10.2174/1871520615666150113103439. [DOI] [PubMed] [Google Scholar]
- 136.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 137.Wang YJ, Zhang YK, Kathawala RJ, Chen ZS. Repositioning of Tyrosine Kinase Inhibitors as Antagonists of ATP-Binding Cassette Transporters in Anticancer Drug Resistance. Cancers (Basel) 2014;6:1925–1952. doi: 10.3390/cancers6041925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Imai Y, Yoshimori M, Fukuda K, Yamagishi H, Ueda Y. The PI3K/Akt inhibitor LY294002 reverses BCRP-mediated drug resistance without affecting BCRP translocation. Oncol Rep. 2012;27:1703–1709. doi: 10.3892/or.2012.1724. [DOI] [PubMed] [Google Scholar]
- 139.Huang FF, Zhang L, Wu DS, Yuan XY, Yu YH, Zhao XL, Chen FP, Zeng H. PTEN regulates BCRP/ABCG2 and the side population through the PI3K/Akt pathway in chronic myeloid leukemia. PLoS One. 2014;9:e88298. doi: 10.1371/journal.pone.0088298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ganoth A, Merimi KC, Peer D. Overcoming multidrug resistance with nanomedicines. Expert Opin Drug Deliv. 2015;12:223–238. doi: 10.1517/17425247.2015.960920. [DOI] [PubMed] [Google Scholar]
- 141.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–460. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 142.Garg M. MicroRNAs, stem cells and cancer stem cells. World J Stem Cells. 2012;4:62–70. doi: 10.4252/wjsc.v4.i7.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 144.Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ, Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011;13:R2. doi: 10.1186/bcr2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song B, Wang C, Liu J, Wang X, Lv L, Wei L, Xie L, Zheng Y, Song X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lou Y, Yang X, Wang F, Cui Z, Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int J Mol Med. 2010;26:819–827. doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- 147.Shi SJ, Zhong ZR, Liu J, Zhang ZR, Sun X, Gong T. Solid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cells. Pharm Res. 2012;29:97–109. doi: 10.1007/s11095-011-0514-6. [DOI] [PubMed] [Google Scholar]
- 148.Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P, Tassone P, et al. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi Y, Liu C, Liu X, Tang DG, Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9:e90022. doi: 10.1371/journal.pone.0090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Papagiannakopoulos T, Kosik KS. MicroRNAs: regulators of oncogenesis and stemness. BMC Med. 2008;6:15. doi: 10.1186/1741-7015-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19:325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 153.Yin S, Xu L, Bandyopadhyay S, Sethi S, Reddy KB. Cisplatin and TRAIL enhance breast cancer stem cell death. Int J Oncol. 2011;39:891–898. doi: 10.3892/ijo.2011.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Plasilova M, Zivny J, Jelinek J, Neuwirtova R, Cermak J, Necas E, Andera L, Stopka T. TRAIL (Apo2L) suppresses growth of primary human leukemia and myelodysplasia progenitors. Leukemia. 2002;16:67–73. doi: 10.1038/sj.leu.2402338. [DOI] [PubMed] [Google Scholar]
- 155.Unterkircher T, Cristofanon S, Vellanki SH, Nonnenmacher L, Karpel-Massler G, Wirtz CR, Debatin KM, Fulda S. Bortezomib primes glioblastoma, including glioblastoma stem cells, for TRAIL by increasing tBid stability and mitochondrial apoptosis. Clin Cancer Res. 2011;17:4019–4030. doi: 10.1158/1078-0432.CCR-11-0075. [DOI] [PubMed] [Google Scholar]
- 156.Loebinger MR, Sage EK, Davies D, Janes SM. TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br J Cancer. 2010;103:1692–1697. doi: 10.1038/sj.bjc.6605952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 158.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 159.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111:419–427. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matsushita H, Scaglioni PP, Bhaumik M, Rego EM, Cai LF, Majid SM, Miyachi H, Kakizuka A, Miller WH, Pandolfi PP. In vivo analysis of the role of aberrant histone deacetylase recruitment and RAR alpha blockade in the pathogenesis of acute promyelocytic leukemia. J Exp Med. 2006;203:821–828. doi: 10.1084/jem.20050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res. 2010;16:2715–2728. doi: 10.1158/1078-0432.CCR-09-1800. [DOI] [PubMed] [Google Scholar]
- 164.Ginestier C, Wicinski J, Cervera N, Monville F, Finetti P, Bertucci F, Wicha MS, Birnbaum D, Charafe-Jauffret E. Retinoid signaling regulates breast cancer stem cell differentiation. Cell Cycle. 2009;8:3297–3302. doi: 10.4161/cc.8.20.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Salvador MA, Wicinski J, Cabaud O, Toiron Y, Finetti P, Josselin E, Lelièvre H, Kraus-Berthier L, Depil S, Bertucci F, et al. The histone deacetylase inhibitor abexinostat induces cancer stem cells differentiation in breast cancer with low Xist expression. Clin Cancer Res. 2013;19:6520–6531. doi: 10.1158/1078-0432.CCR-13-0877. [DOI] [PubMed] [Google Scholar]
- 166.Chikamatsu K, Ishii H, Murata T, Sakakura K, Shino M, Toyoda M, Takahashi K, Masuyama K. Alteration of cancer stem cell-like phenotype by histone deacetylase inhibitors in squamous cell carcinoma of the head and neck. Cancer Sci. 2013;104:1468–1475. doi: 10.1111/cas.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Petrelli A, Carollo R, Cargnelutti M, Iovino F, Callari M, Cimino D, Todaro M, Mangiapane LR, Giammona A, Cordova A, et al. By promoting cell differentiation, miR-100 sensitizes basal-like breast cancer stem cells to hormonal therapy. Oncotarget. 2015;6:2315–2330. doi: 10.18632/oncotarget.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vaz AP, Ponnusamy MP, Rachagani S, Dey P, Ganti AK, Batra SK. Novel role of pancreatic differentiation 2 in facilitating self-renewal and drug resistance of pancreatic cancer stem cells. Br J Cancer. 2014;111:486–496. doi: 10.1038/bjc.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong Y, Han Q, Zou Y, Deng Z, Lu X, Wang X, Zhang W, Jin H, Su J, Jiang T, et al. Long-term exposure to imatinib reduced cancer stem cell ability through induction of cell differentiation via activation of MAPK signaling in glioblastoma cells. Mol Cell Biochem. 2012;370:89–102. doi: 10.1007/s11010-012-1401-0. [DOI] [PubMed] [Google Scholar]
- 170.Daniele S, Costa B, Zappelli E, Da Pozzo E, Sestito S, Nesi G, Campiglia P, Marinelli L, Novellino E, Rapposelli S, et al. Combined inhibition of AKT/mTOR and MDM2 enhances Glioblastoma Multiforme cell apoptosis and differentiation of cancer stem cells. Sci Rep. 2015;5:9956. doi: 10.1038/srep09956. [DOI] [PMC free article] [PubMed] [Google Scholar]


