Abstract
Hepatitis B virus (HBV) infection is the leading cause for liver disorders and can lead to hepatocellular carcinoma, cirrhosis and liver damage which in turn can cause death of patients. HBV DNA Polymerase is essential for HBV replication in the host and hence is used as one of the most potent pharmacological target for the inhibition of HBV. Chronic hepatitis B is currently treated with nucleotide analogues that suppress viral reverse transcriptase activity and most of them are reported to have viral resistance. Therefore, it is of interest to model HBV DNA polymerase to dock known phytochemicals. The present study focuses on homology modeling and molecular docking analysis of phytocompounds from the traditional antidote Phyllanthus niruri and other nucleoside analogues against HBV DNA Polymerase using the software Discovery studio 4.0. 3D structure of HBV DNA Polymerase was predicted based on previously reported alignment. Docking studies revealed that a few phytochemicals from Phyllanthus niruri had good interactions with HBV DNA Polymerase. These compounds had acceptable binding properties for further in vitro validation. Thus the study puts forth experimental validation for traditional antidote and these phytocompounds could be further promoted as potential lead molecule.
Keywords: Hepatitis B, Phyllanthus niruri, Phytochemicals, Homology modeling, Molecular Docking
Background
Hepatitis B virus (HBV) causes chronic hepatitis infection to over 350 million people worldwide and it is estimated that over 2 billion people have been exposed to HBV worldwide [1, 2]. The risk of developing hepatocellular carcinoma associated with HBV is higher when compared to non- carriers worldwide and accounts for about 31% of cases [3, 4]. HBV, small doublelayered virus in the family hepadnaviridae, is a 42 nm partially double stranded circular DNA virus [5]. Hepadnaviruses are known to exhibit limited tissue tropisms and host range, confined to their innate host and a few closely related species [6]. Chronic hepatitis B is now treated with interferon-α-2a, interferon-α-2b, lamivudine and nucleotide analogue such as adefovir dipivoxil which all aim in suppressing viral replication thereby hindering the progression of disease [7, 8]. Unfortunately currently available drugs have not shown beneficial effects on the treatment of chronic hepatitis B to a vast range of patients and are coupled with severe side effects. Moreover nucleoside or nucleotide analogues induce the suppression of viral replication during the course of treatment but have limited long term efficacy [9]. Prolonged use of these drugs may lead to liver failure, acute infections and are also associated with a high rate of resistance to the drug [10].
Ayurvedic herbs and formulations have wide spectrum of therapeutical or biological activity that can be exploited in pharmaceutical drug discovery and drug design. Traditional medicines such as Ayurveda, Unani and Chinese are preferred for the treatment of chronic hepatitis B due to lesser side effects and low cost. Herbal extracts of the genus, Phyllanthus is composed of various combinations of secondary metabolites that have shown hepatoprotective effect, the most widely used ones are Phyllanthus niruri and Phyllanthus amarus [11, 12]. Many active compounds were identified from the genus, Phyllanthus which possess anti HBV activities. Phytocompounds isolated from Phyllanthus niruri has shown inhibitory effect on endogenous DNA polymerase and surface antigen binding property thereby suppressing the replication of Hepatitis B virus in vitro [13, 14]. Phyllanthus niruri extracts was also found to inhibit the replication of wood chuck hepatitis virus (WHV) in vivo and reduce the pathological effects of WHV in woodchucks (Marmota monax) [15].
DNA polymerase of HBV has been considered as a promising target for the treatment of HBV infections in the past few years. Targeting DNA polymerase can inhibit viral replication by affecting necessary cellular regulatory components that are coupled with HBV replication and viral nucleo capsid formation [16]. The present study employs an in silico method to analyze the interaction of HBV DNA polymerase and phytochemicals from phyllanthus niruri using molecular docking studies.
Methodology
Homology Modeling of HBV DNA polymerase:
The X-ray structure of Hepatitis B virus DNA polymerase (HBV-DP) has not yet been successfully determined. HIV-1 reverse transcriptase (HIV -1 RT) shares ample structural similarity with HBV-DP to serve as a good template to predict the three dimensional structure [17]. HIV-1 RT (template) (PDB ID: 1RTD) structure was obtained from protein data bank (http://www.rcsb.org/pdb). Sequence alignment of HBV-DP (target) and HIV -1 RT (template) provided by Daga et al; 2010 was utilized for constructing the 3D structure of the target. 3D model of HBV-DP was generated from the aligned sequence using “Build homology model” protocol in Discovery Studio. A total of 20 models were generated. Out of generated models, one with least Discrete Optimized Protein Energy (DOPE) score was utilized for docking studies.
Protein Structure Validation and Active Site Prediction:
Quality of generated model was assessed using “Verify Protein (Profiles-3D)” protocol of Discovery studio. Validation of the model was performed using Procheck [18] by analyzing Ramachandran plot. Structural evaluation and stereochemical analyses were performed using ProSA-web Z-scores (https://prosa.services.came.sbg.ac.at/prosa.php) [19]. Energy minimization of the protein structure was performed by applying “prepare protein” protocol of DS. This protocol cleans the protein molecule by adding missing atoms, inserting missing loops, assigning charges and fixing CHARMm forcefields. Active site of HBV-DP was predicted using “Define and Edit Binding Site” protocol of Discovery studio.
Ligand Identification, Preparation and Screening:
A total of 35 phytocompounds from Phyllanthus niruri was collected through literature survey. The 3D structure of 32 compounds was downloaded in .sdf format from PubChem database (https://pubchem.ncbi.nlm.nih.gov/). 3D structure of 3 phytocompounds was generated using Marvin Sketch and exported in .sdf format for docking studies. All the compounds were then imported to DS and prepare ligands protocol was applied in order to add missing hydrogen bonds and energy minimization using CHARMm force fields. Prepared ligands were further filtered by applying Lipinski‟s properties such as Molecular weight, XLog P, number of hydrogen bond donors and acceptors.
Molecular Docking:
Possible binding modes between the filtered ligands and HBVDP model were studied by CDOCKER (CHARMm-based DOCKER) protocol incorporated within DS. The algorithm offers full ligand flexibly and employs CHARMm force fields. Ligand binding affinity was calculated using CDOCKER energy, CDOCKER Interaction energy, Hydrogen bonds, binding energies, protein energy and ligand protein complex energy.
Results & Discussion
Homology Model of HBV-DP:
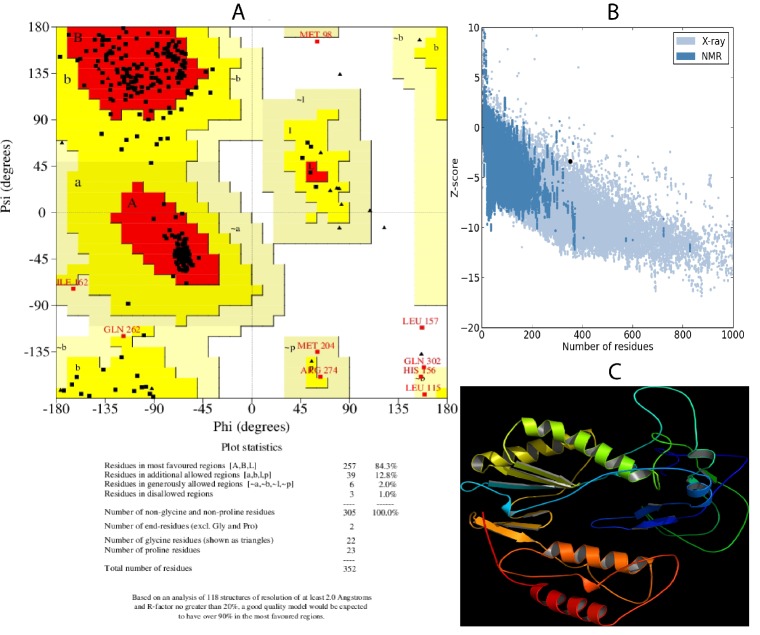
Sequence alignment between HIV -1 RT and HBV -DP in Clustal format was imported to DS and homology modeling was performed. Out of 20 models generated, fifth model was selected for further studies based on minimum DOPE score (- 32517.9). Verify model using profiles 3D was applied to the model and the verify score was observed as 85.87 that lie between expected high score of 160.379 and expected low score of 72.1706. The verified model was then validated using PROCHECK. 84.3% of residues were found in the most favorable regions, 12.8% in additional allowed region, 2% in generously allowed region and 1% residues in disallowed region of Ramachandran Plot (Figure 1A). Protein structure analysis using PROSA showed a Z score of -3.39 (Figure 1B). Selected model produced reliable results in protein verification steps and hence fourth one was chosen as the model for further docking analysis. The final structure of HBV-DP is given in (Figure 1C).
Figure 1.
Homology modeling and validation of model: A) Validation using Ramachandran plot. Summary of the plot is as follows: Residues in favored regions- 257 (84.3%), Residues in additionally allowed regions- 39 (12.5%), Residues in generously allowed regions-6 (2.0%) and Residues in disallowed regions- 3 (1.0%); B) PROSA Z score for model. The Z score for the model was -3.39; C) Homology model of HBV-DP created using Pymol software.
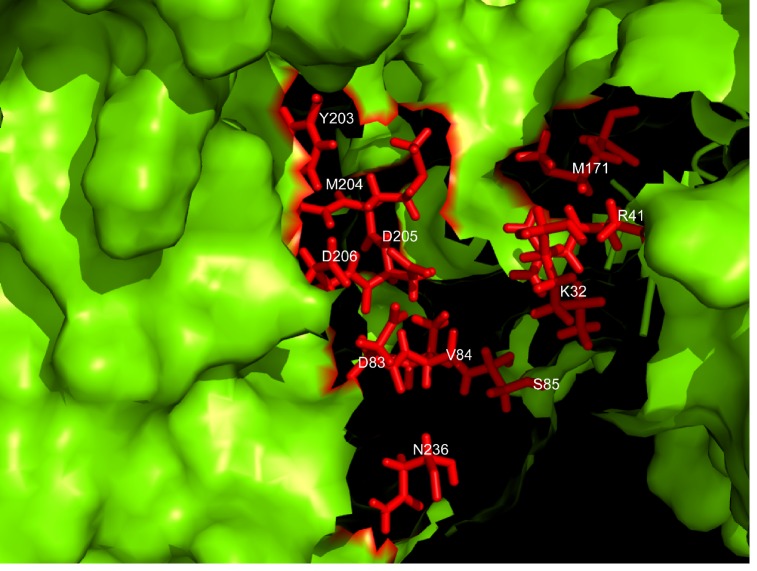
Molecular Docking:
The most critical requirement for interaction of HBV-DP protein and ligands was the proper orientation and conformation of ligands into the HBV-DP active site. The active site prediction protocol of DS produced 15 binding sites from which 1st active site grid box with dimensions of 52.074 ×22.24 ×32.556A° was used. Important amino acids present in active site of HBV- DP as previously reported is labeled in the structure and shown in (Figure 2). The active site cleft contains amino acids LYS32, ARG41, AP83, VAL84, SER85, MET171, ASP203, MET204, ASP205, ASP206 and ASN236. 35 phytocompounds from Phyllanthus niruri generated 582 conformers of which 60 ligands passed Lipinski's rule. Docking simulations of HBV-DP active site and ligands were performed using the CDOCKER algorithm. The binding mode, hydrogen bond interactions and docking scores for 60 compounds identified through virtual screening were ranked based on the different scoring constraints. Based on CDOCKER energy and CDOCKER interaction energy scores, the binding energy (ΔGbindkacl/mol) for 11 inhibitors were calculated and represented in Table 1 (see supplementary material). The binding energies were observed in the range of -53 to -283. Phyllanthose (binding energy –132.3977 kcal/mol) showed maximum number of hydrogen bond interactions (9nos) with the target of which most of them (7 bonds) were with active site residues.
Figure 2.
Residues involved in the binding cavity of HBV-DP. The figure shows the residues in the active site of the target protein (HBV-DP). The figure was made in Pymol software by implementing ‘surface’ and ‘stick’ representations and was colored accordingly
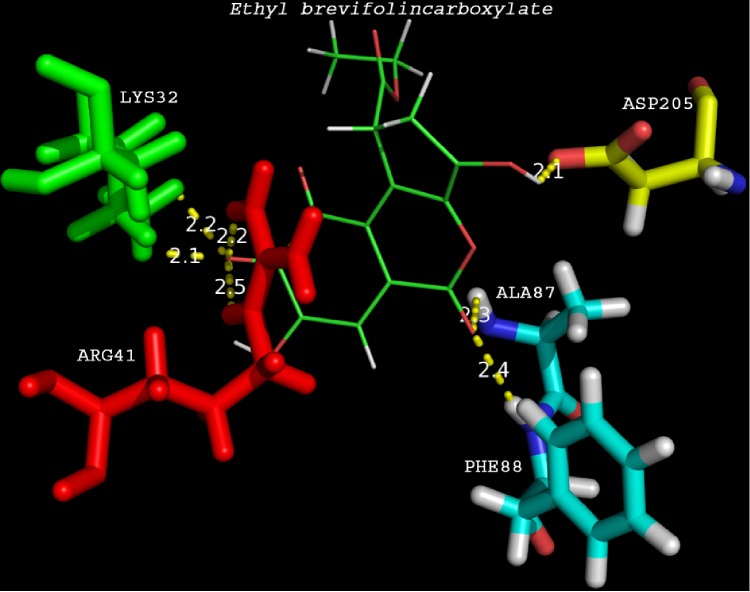
Ethyl brevifolincarboxylate (binding energy -195.409kcal/mol) and astragalin (binding energy -195.431kcal/mol) showed 7 hydrogen bonds with HBV-DP. Active site residues involved in hydrogen bond interactions of astragalin was less compared to ethyl brevifolincarboxylate. However the compound found to have the least binding energy (–283.757 kcal/mol) was quercitrin. The compound showed hydrogen bond interactions with Lys32, Asn36, Val84 and Asp205, residues in the binding pocket of HBV-DP. The second ranked compound based on binding energy was quercetin (-263.645) which showed hydrogen bond interactions with Lys32, Asn36 and Arg41. Comparative docking studies were performed using commercial nucleoside analogues such as lamivudine, tenofovir, telbivudine, and entecavir with the modeled protein. Result showed that analogue tenofovir ranked one among these 6 compounds with minimum binding energy of -225.652 kcal/mol and four hydrogen bonds Table 2 (see supplementary material). ADME-Toxicity for the top docking hits was predicted using ADMET descriptors of DS. ADME/Toxicity properties for compounds ranked on the basis of binding energies and number of hydrogen bonds were predicted using DS toxicity prediction module Table 3 (see supplementary material). All the phytocompounds except ethyl brevifolincarboxylate displayed hepatotoxicity, and nucleoside analogue tenofovir was found to be hepatotoxic in our study. Docking results showing interaction between active site residues of HBV-DP and ethyl brevifolincarboxylate was depicted in Figure 3. Molecular docking studies using Phyllanthus niruri secondary metabolites and nucleoside analogues against the binding cavity of HBV-DP revealed that phytochemicals are having more favorable interactions with the target.
Figure 3.
Ethyl brevifolincarboxylate docked to the active site residues in the protein. The figure shows the hydrogen bond distances between Ethyl brevifolincarboxylate and the active site residues (LYS32, ARG41, ALA87, PHE88 and ASP205).The distances are within 2.5Å distance which indicates strong binding.
Conclusion
Conventional medicines that are both safe and easily affordable have not yet been developed for the treatment of chronic Hepatitis B. Phyllanthus niruri is a medicinal herb used in traditional Indian medicine for the treatment of Hepatitis B. Therefore; it is of interest to relate molecular properties to its medicinal properties using molecular docking of the plant's phytochemicals with HBV DNA polymerase. In the present work we show the binding interactions of phytochemicals such as Ethyl brevifolincarboxylate, Tenofovir, Quercetin and Quercitrin from Phyllanthus niruri with the modeled structure of Hepatitis B Virus DNA polymerase using CDOCKER protocol in Discovery studio. In silico molecular docking studies clearly demonstrated binding activity of ligands with HBV-DP which warrants further studies for the development of potent inhibitors in the treatment of Hepatitis B. These results clearly indicate that the phytochemicals from Phyllanthus niruri have similar binding sites and better interactions with Hepatitis B Virus DNA polymerase compared to the nucleoside analogues at present utilized for treatment. Using a combination of in silico approaches such as homology modeling, virtual screening and molecular docking, we successfully validated Phyllanthus niruri phytochemicals as HBV-DP inhibitors. Hence we conclude that secondary metabolites from Phyllanthus niruri could be potential lead molecules against Hepatitis B which can be further evaluated through in vivo studies.
Supplementary material
Acknowledgments
The financial grant (No. BT/BI/03/014/2002) from the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, New Delhi is acknowledged.
Footnotes
Citation:Mohan et al, Bioinformation 11(9): 426-431 (2015)
References
- 1.Li Y, et al. Antivir Chem Chemother. 2005;16:193. doi: 10.1177/095632020501600305. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E, et al. Viruses. 2010;2:1279. doi: 10.3390/v2061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fattovich G, et al. Am J Gastroenterol. 2002;97:2886. doi: 10.1111/j.1572-0241.2002.07057.x. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. Int J Cancer. 2006;118:3030. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 5.Block TM, et al. Clin Liver Dis. 2007;11:685. doi: 10.1016/j.cld.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prassolov A, et al. J Virol. 2003;77:1964. doi: 10.1128/JVI.77.3.1964-1976.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon H, Lok AS. Nat Rev Gastroenterol Hepatol. 2011;8:275. doi: 10.1038/nrgastro.2011.33. [DOI] [PubMed] [Google Scholar]
- 8.Perrillo RP. Semin Liver Dis. 2004;1:23. doi: 10.1055/s-2004-828675. [DOI] [PubMed] [Google Scholar]
- 9.Borgia G, Gentile I. Curr Med Chem. 2006;13:2839. doi: 10.2174/092986706778521995. [DOI] [PubMed] [Google Scholar]
- 10.Gitlin N. Clin Chem. 1997;43:1500. [PubMed] [Google Scholar]
- 11.Sarin B, et al. ScientificWorldJournal. 2014;3:1155. [Google Scholar]
- 12.Pal SK, Shukla Y. Asian Pac J Cancer Prev. 2003;4:281. [PubMed] [Google Scholar]
- 13.Thyagarajan SP, et al. Lancet. 1988;2:764. doi: 10.1016/s0140-6736(88)92416-6. [DOI] [PubMed] [Google Scholar]
- 14.Venkateswaran PS, et al. Proc Natl Acad Sci USA. 1987;84:274. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unander DW, et al. J Ethnopharmacol. 1995;45:1. doi: 10.1016/0378-8741(94)01189-7. [DOI] [PubMed] [Google Scholar]
- 16.Chang JM, Huang KL. Hepat B Annu. 2007;4:72. [Google Scholar]
- 17.Daga PR, et al. Protein Sci. 2010;19:796. doi: 10.1002/pro.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskowski RA, et al. J Biomol NMR. 1996;8:477. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 19.Wiederstein M, Sippl MJ. Nucleic Acids Res. 2007;35:W407. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.