Abstract
Type 2 diabetes mellitus (DM) is the most common single cause of end-stage renal disease. Albuminuria is the most commonly used marker to predict onset of diabetic nephropathy (DN) without enough sensitivity and specificity to detect early DN. This is the first study to identify urinary cyclophilin A (CypA) as a new biomarker for early DN.
We recruited DM outpatients and healthy control subjects from January 2014 to December 2014. In this cross-sectional study, patients’ urine samples were collected to determine the expression of urinary CypA. We also treated mesangial (MES-13) and tubular (HK-2) cells with glucose or free radicals to observe the expression of secreted CypA in Western blot analysis.
A total of 100 DN patients and 20 healthy control subjects were enrolled. All variables were matched. In univariate analysis, the concentration of urinary CypA correlated well with the progression of renal function. A significant increase in urinary CypA was noted in stage 2 DN and persisted in later stages. We could diagnose stage 2 DN using urinary CypA with a sensitivity of 90.0% and specificity of 72.7%. The area under curve was up to 0.85, indicating a good discriminatory power. In cellular models, MES-13 and HK-2 cells can both release CypA.
Urinary CypA is a good biomarker for early DN detection in humans and it can be released from either mesangial or tubular cells. The underlying molecular mechanisms still need further clarification in cellular and animal studies.
INTRODUCTION
Type 2 diabetes mellitus (DM) is the most common single cause of end-stage renal disease (ESRD).1 ESRD in almost half of patients is due to diabetic nephropathy (DN), and these cases have the worst outcome compared to patients with other causes of ESRD. Although there are many novel drugs for DM, there are no specific curative treatments yet for DN. Reasons for poor outcome include inadequate markers and the complicated mechanisms of DN.2 Currently, the stage of severity is determined according to the levels of albuminuria. Albuminuria is the most commonly used marker to predict onset and progression of DN clinically. However, this traditional marker for DN lacks both sensitivity and specificity to detect early stage of DN.3 Furthermore, some DN patients with ESRD do not present with significant albuminuria.4–6 The lack of a strong association between glomerular filtration rate (GFR) and albuminuria suggests that an alternative to this albuminuria-based staging system is needed. Some studies have noted the existence of pathological change before microalbuminuria.4 Therefore, even if microalbuminuria can be regarded as the earliest manifestation of DN, it is possible that a new biomarker for DN exists. Recently, different markers of DN were reviewed4,7,8 including fibroblast growth factor 23,9 tubular markers10 (kidney injury molecule 1, neutrophil gelatinase-associated lipocalin, and liver-type fatty acid-binding protein [L-FABP]),11 inflammatory markers (interleukin 6 [IL-6], IL-8, monocyte chemoattractant protein 1, and interferonγ–inducible protein),12 urinary 8-hydroxy-20-deoxyguanosine,13 serum cystatin C,14 and so on. Among these, genetic susceptibility almost always leads to irreversible DN, and detection of the clinical markers mostly occurs too late to diagnose and monitor the progression of DN. As such, it is crucial to find an earlier and reliable marker for DN. Earlier diagnosis and intervention may provide an opportunity to stop the permanent damage caused by DN.
Cyclophilin A (CypA) is an 18-kDa protein with ubiquitous characteristics.15 It is mostly distributed in the cytoplasm and facilitates protein folding and protein trafficking. It also acts as a cellular receptor for cyclosporine A (CsA). The expression of CypA is relatively high in the kidney,16 where proximal tubular epithelial cells (PTECs) are reported to contain considerably more CypA than other kidney tissues.17 With respect to kidney diseases, the majority of research has been on the cellular relationship between CypA and CsA, which is used as an immunosuppressant, and leaves behind its secreted form. This secreted CypA (sCypA) was reported to be correlated with cardiovascular disease (CVD), asthma, rheumatoid arthritis (RA), and lung and liver injury.18 sCypA has been suggested to be a potential biomarker and mediator in CVD.19
In addition, sCypA is associated with inflammatory or infectious diseases such as RA, asthma, and periodontitis.20 Interestingly, sCypA was also detected in diabetic patients’ plasma21 and was shown to be secreted by monocytes in response to hyperglycemia,22 indicating that sCypA could be a potential secretory marker in type 2 DM.22 Furthermore, a relatively high expression level of CypA in normal kidneys16 has led to speculation that sCypA may be associated with solid organ damage. As a product directly produced by kidney, urine could be best measure for renal injury detection. Therefore, we postulated that CypA level in urine would be the most suitable indicator of DN.
Research Design and Methods
Study Population
We recruited all the DM outpatients and healthy control groups with informed consent. In the group of DM patients, the different stages of DN were screened for the concentrations of urinary CypA. All subjects in this cross-sectional study were 20 years of age and older. Patients were free from infectious disease, inflammatory disease, liver disease, or malignancy, and all were nonsmokers. Only metabolic syndrome and/or CVD were noted. Patients who took drugs for hypertension, DM, hyperlipidemia, hyperuricemia, CVD, hyperuricemia, and gout were not excluded. Patients who took drugs for any other disease or condition were excluded. These data were collected in the outpatient department of metabolism and nephrology at Taichung Veterans General Hospital between January 2014 and December 2014. All of the study procedures were conducted in accordance with the ethical standards of Taichung Veterans General Hospital and were approved by the institutional review committee (CE14077, TCVGH).
Data Collection
All DM patients were diagnosed according to the DM guidelines of the American Diabetes Association in 2013.23 We collected the participants’ clinical parameters including gender, age (years old), and duration after diagnosis of DM (years). The stages of DN were categorized according to the previous literature24 where stage 1 is associated with hyperfiltration and a measured GFR exceeding the upper limit of the normal range (120 mL/min per 1.73 m2) or beyond +2 standard deviation from mean GFR. Stage 2 DN develops silently over many years and is characterized by morphologic lesions without signs of clinical disease. Thus, it is usually called the silent stage. Stage 3 DN is characterized by “microalbuminuria” where urinary albumin excretion is between 30 and 300 mg/day or between 30 and 300 mg/g creatinine on a spot urine sample. Patients with normal GFR (no > 2SD of GFR) and without microalbuminuria were defined as stage 2 DN. More importantly, some patients with normal GFR (no > 2SD of GFR) and without microalbuminuria do not have DN. Patients included in our study should fit the above criteria and should have increased GFR (>2 SD of GFR) before timing of recruitment (progression of stage 1 DN) to make sure they really had DN and they were in the stage 2 of DN. Stage 4 DN is defined by severely increased albuminuria, also known as the “macroalbuminuria” (urinary albumin excretion above 300 mg/day or above 300 mg/g creatinine on a spot urine sample). The final stage, stage,5 is known as ESRD. Blood samples were tested for fasting sugar (mg/dL), glycated hemoglobin (%), SCr (mg/dL), GFR (mL/min per 1.73 m2),25 total cholesterol (mg/dL), triglyceride (mg/dL), and low density lipoprotein cholesterol (mg/dL). Spot urine test was used to measure the concentration of CypA (ng/mL) and albumin creatinine ratio (ACR) (mg/g). The index estimated glomerular filtrate rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) equation:25 eGFR (mL/min per 1.73 m2) = 186∗SCr−1.154∗Age−0.203∗0.742 (if female). Patients were screened for CVD (hypertension, stroke, coronary artery disease, heart failure, and aortic aneurysm). Hypertension was defined as an average home systolic blood pressure greater than 140 mmHg and a diastolic blood pressure greater than 90 mmHg before medication according to the definition for stage I/II hypertension set forth in the JNC-7 guidelines.26 Patients currently receiving antihypertensive agents were deemed to have hypertension. Stroke was confirmed by neurologists or brain images. Coronary artery disease (CAD) was defined according to arterial angiography. Some were diagnosed according to cardiologists, who made diagnosis of CAD according to if patients with typical angina pectoris, myocardial infarction, or silent myocardial ischemia. They used electrocardiogram, cardiac enzyme, coronary calcium score, and stress test to diagnose CAD. Heart failure was confirmed by cardiac sonography or the guidelines of the Framingham study.27 Drugs such as angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, dipeptidyl peptidase 4 inhibitor sulfonylurea, metformin, dipyridamole, pentoxifylline, and statin were also recorded to analyze possible correlations with urinary CypA concentration. We would also like to point out how we select patients. All data including medication, laboratory, and clinical parameters are without significant changes within 6 months or between 2-time outpatient department visits. We checked all parameters during the period of recruitment. If they remain similar to the previous data, we include them in the study. If there are significant changes, we will follow up this patient 3 months later and choose the data (the ones after 3 months) if it became similar to the previous data.
Urine Collection and Analysis
Urine was collected in the morning from the outpatient subjects and stored in an ice package immediately. Within 4 hours, it was then restocked under −80 °C until analysis. The expression of urinary CypA was examined using an enzyme-linked immunosorbent assay kit (SEA979Hu, Uscn Life Science Inc., Texas, USA). All data of urinary CypA were double-checked at least twice.
Materials and methods of cell study
Cell Culture
Mesangial cell (MES-13 cells, glomerular mesangial cells from an SV40 transgenic mouse) were obtained from the American Type Culture Collection (CRL-1927; Manassas, VA). MES-13 cells were cultured in a 3:1 mixture of M199 (Invitrogen, Carlsbad, CA) and Ham F-12 (Invitrogen), supplemented with 5% FBS, 1% penicillin–streptomycin, 1% l-glutamine, and 14 mM HEPES, and maintained at 37 °C in an incubator with 5% CO2. All culture supplies were acquired from Life Technologies (Gaithersburg, MD). HK-2 cells (human PTEC) were obtained from American Type Culture Collection (CRL-2190; Manassas, VA). HK-2 cells were maintained in DMEM/F12 and supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin/amphotericin B, and 1% glutamine (Invitrogen, Carlsbad, CA).
Western Blotting
The cell lysates were collected from MES-13 and HK-2 cells, and Western blot analysis was performed as described previously.28 Western blot reagents were obtained from Pierce (Rockford). Primary antibodies included polyclonal anti-CypA (1:10,000, Millipore, MA) and β-actin (Novus, Colorado) overnight at 4 °C followed by incubation with a horseradish peroxidase conjugated secondary antibody. Proteins were visualized using enhanced chemiluminescence (Amersham Biosciences, Amersham, UK). Protein bands of Western blot analysis were quantified using Quantity One software (BioRad). All other chemical supplies were acquired from Sigma (St Louis, MO).
Glucose Treatment
MES-13 and HK-2 cells were seeded in a 6 cm cell culture plate with 4 × 105 and 3 × 105 cells/plate, respectively. They were incubated in M199:F12 (3:1) and DMEM:F12 (1:1) complete medium, respectively, for 1 day. Then culture media were replaced by 0.5% FBS (M199/low glucose:F12 = 3:1) for 2 days. The cells were incubated in the serum-free media supplemented with 0, 10, 25, and 50 mM of glucose. The procedure was conducted according to the methods described in a previous study (Su et al, unpublished data). After 24 hours of treatment, the secreted cellular proteins were collected for immunoblotting analysis.
H2O2 Treatment
MES-13 and HK-2 cells were seeded in a 6 cm cell culture plate with 5 × 105 and 3 × 105 cells/plate, respectively. They were incubated in M199:F12 = 3:1 complete medium for 1 day and were then replaced by serum-free medium (M199:F12 = 3:1) and incubated for another 2 days. After 30 minutes of 0, 20 μM, or 40 μM of H2O2 stimulation, the secreted and cellular proteins were collected for immunoblotting analysis. To confirm the role of H2O2 in sCypA upregulation, mesangial cells and tubular cells were treated for 30 minutes with 20, and 40 μM of H2O2 in the presence or absence of 300 U/mL catalase (Sigma, St Louis, MO) according to a previously described method.29
Statistical Analyses
Data were expressed as the mean ± SD in continuous variables. Mann–Whitney U test was used for continuous variables and the Chi-square test was used for categorical variables. A general linear model was used for categorical variables, and simple linear regression was used for continuous variables. The results from Western blot were expressed as mean ± SEM and were analyzed by Student's t-test. All statistical procedures were performed using the SPSS statistical software package, version 17.0 (Chicago, IL). A value of P < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of Cohorts
A total of 100 DN patients and 20 healthy control subjects were enrolled in this study (Table 1). The DN patients were categorized according to their stages of DN with matched basic variables. The control individuals were healthy subjects without any metabolic syndrome or medical drug treatment. Among all 100 DN patients, there were no significant differences in gender distribution (P = 0.553), age (P = 0.469), fasting sugar (P = 0.403), glycated hemoglobin (P = 0.352), total cholesterol (P = 0.447), triglyceride (P = 0.324), or low density lipoprotein cholesterol (P = 0.199). Prevalence rates of other metabolic syndromes and CVD were both similar, including hypertension (P = 0.668), stroke (P = 0.480), coronary artery disease (P = 0.724), heart failure (P = 0.712), aortic aneurysm (P = 1.000), and hyperlipidemia (P = 0.075). All included drugs were matched (except metformin) as well, such as angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (P = 0.144), insulin (P = 0.625), dipeptidyl peptidase 4 inhibitor (P = 0.710), sulfonylurea (P = 0.276), dipyridamole (P = 0.740), pentoxifylline (P = 0.121), and statin (P = 0.095). It was not possible to match the usage of metformin because it is contraindicated in advanced DN. Among all the basic characteristics, it was difficult to match duration of DM (P = 0.009) because progression of DN is highly time-dependent. Patients with more severe DN had higher serum creatinine (SCr) (P < 0.001), lower GFR (P < 0.001), higher ACR (P < 0.001), and higher urinary CypA (P < 0.001). Taken together, all basic variables were matched (except metformin), and with later stage of DN, patients had worse renal function parameters, including SCr, GFR, and ACR. Importantly, the concentrations of urinary CypA were statistically different among the different stages of severity of DN.
TABLE 1.
Demography of Different Stages of Diabetic Nephropathy

Correlation Between Urinary CypA and Other Clinical Variables
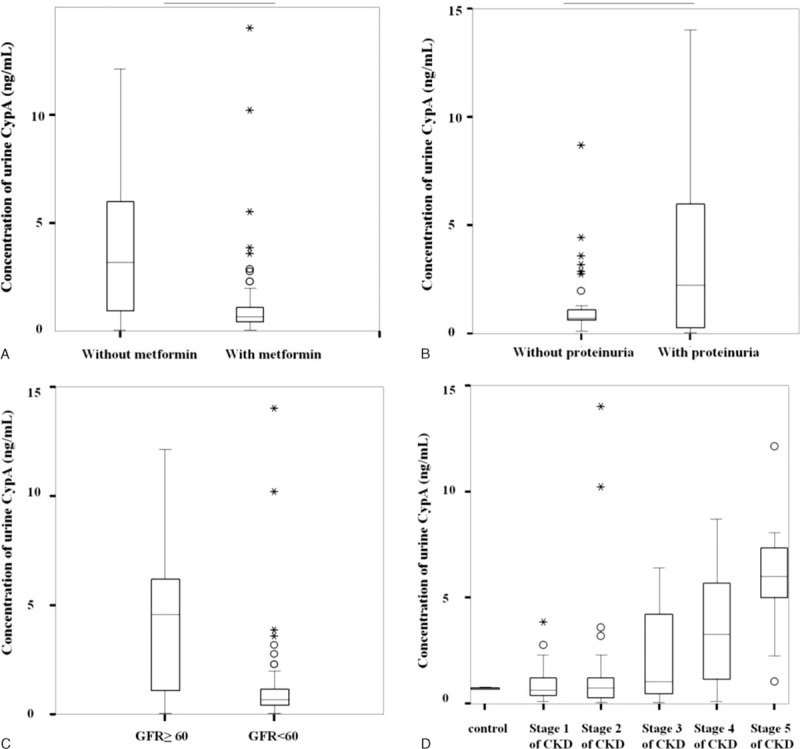
Because some variables are clinically associated with renal functions, we performed univariate analysis to verify the associations between these variables and urinary CypA (Table 2). Our analysis showed that if patients did not use metformin, the concentration of urinary CypA would increase by 3.281 ng/mL (Fig. 1A). The concentration of urinary CypA increased by 0.395 ng/mL for each 1 mg/dL increase of SCr. With each 1 mL/min decrease in GFR, the concentration of urinary CypA increased by 0.030 ng/mL. Without proteinuria, the concentration of urinary CypA decreased by 3.095 ng/mL (Fig. 1B). Even though there were no statistically significant differences among the different stages of chronic kidney disease (CKD) (Fig. 1D), there seemed to be a trend of increasing urinary CypA in the later stages of CKD. Also, there was a trend of higher urinary CypA in the group with GFR less than 60 mL/min per 1.73 m2 as compared with the GFR group with more than 60 mL/min per 1.73 m2 (Fig. 1C). For each 1 mg/g increase in ACR, the concentration of urinary CypA increased by 0.001 ng/mL (Fig. 2A and Table 2). All of the abovementioned variables were renal function-related or renal function-dependent. In summary, the concentration of urinary CypA correlated well with the progression of renal function in DN patients, based on the albuminuria-based model.
Table 2.
Univariate Analysis and Multivariate Analysis
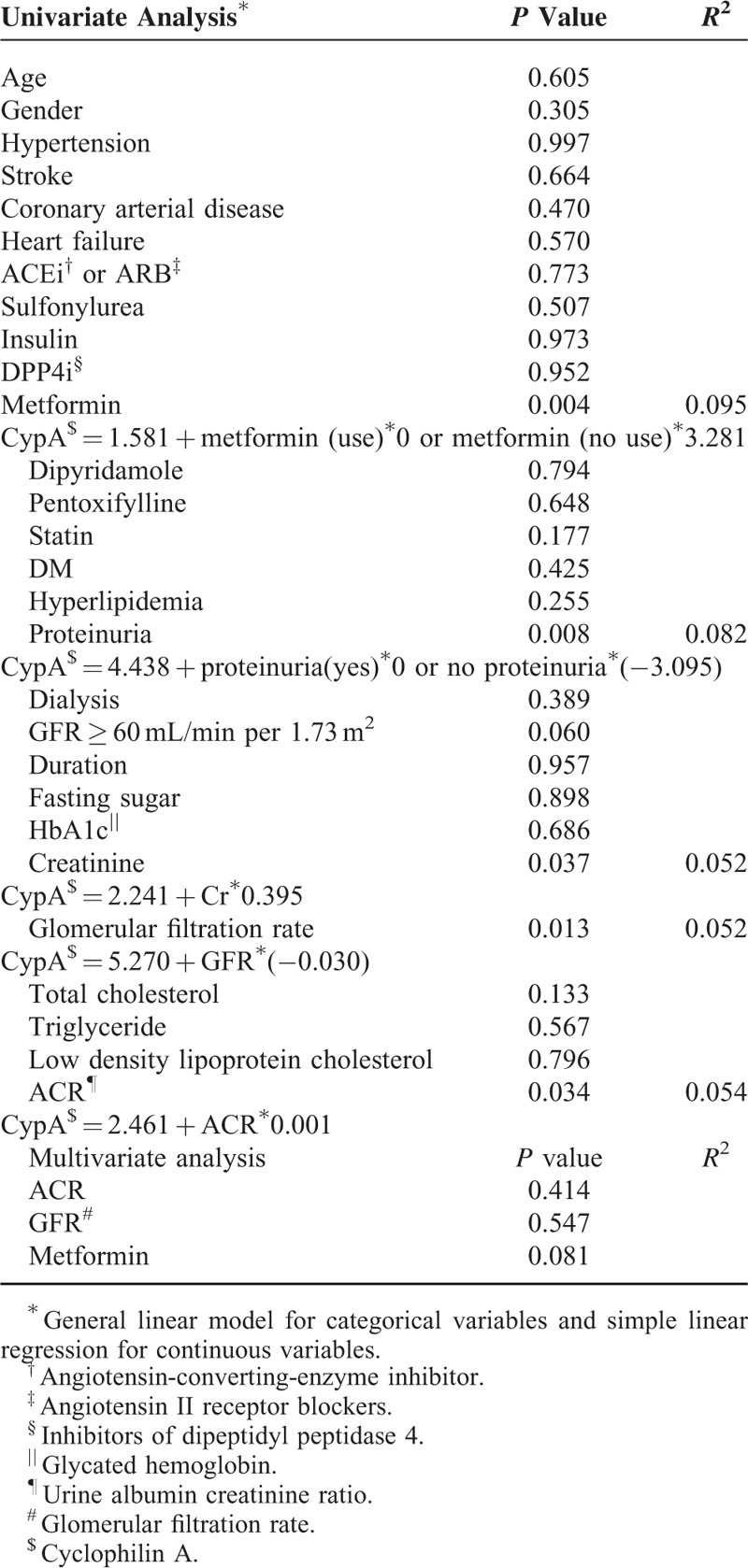
FIGURE 1.
Univariate analysis between clinical parameters and urinary CypA. P value for A was <0.001, for B was 0.007, and for C was <0.060. P value between stages 1 and 2, 2 and 3, 3 and 4, 4 and 5 of CKD were 0.511, 0.633, 0.365, 0.203, and 0.061, respectively. CKD = chronic kidney disease, CypA = cyclophilin A.
FIGURE 2.
Concentrations of urinary CypA in different stages of DN. (A) Concentration of urinary CypA and ACR were plotted. When ACR increased by 1 mg/g, the concentration of urinary CypA increased 0.030 ng/mL (CypA = 2.461 + ACR∗0.001). R2 linear was 0.054. (B) No difference in concentration of urinary CypA was found between stage 1 DN and healthy control groups (P = 0.117). However, there were statistically significant differences between stages 1 and 2, stages 2 and 3, stages 3 and 4, and stages 4 and 5 DN (P = 0.012, 0.003, <0.001, and 0.005, respectively). (C) The differences between stage 1 DN and stages 2 to 5 DN were statistically significant (P = 0.006). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. ACR = albumin creatinine ratio, CypA = cyclophilin A, DN = diabetic nephropathy.
Urinary CypA Correlated With the Severity of DN Stages
The relationship between urinary CypA and ACR is summarized in Figure 2A. The R2 was 0.054 with a statistically significant correlation between urinary CypA and ACR. Moreover, we analyzed the correlation of urinary CypA among all 6 groups, including 5 stages of DN. The concentrations of urinary CypA were not different between the control group and stage 1 of DN (P = 0.117) (Fig. 2B). However, with progression of DN, urinary CypA significantly increased in stage 2 DN compared to that in stage 1 DN (P = 0.012). Most importantly, the concentration of urinary CypA increased as DN stages progressed (P = 0.003, <0.0001, and 0.005 between stage 2 and 3, stages 3 and 4, stages 4 and 5, respectively). Consistently, compared to patients with DN stage 1, the CypA concentrations in patients with DN stages 2 to 5 were significantly increased (P = 0.006) (Fig. 2C).
Diagnosis of Silent Stage of DN via Urinary CypA
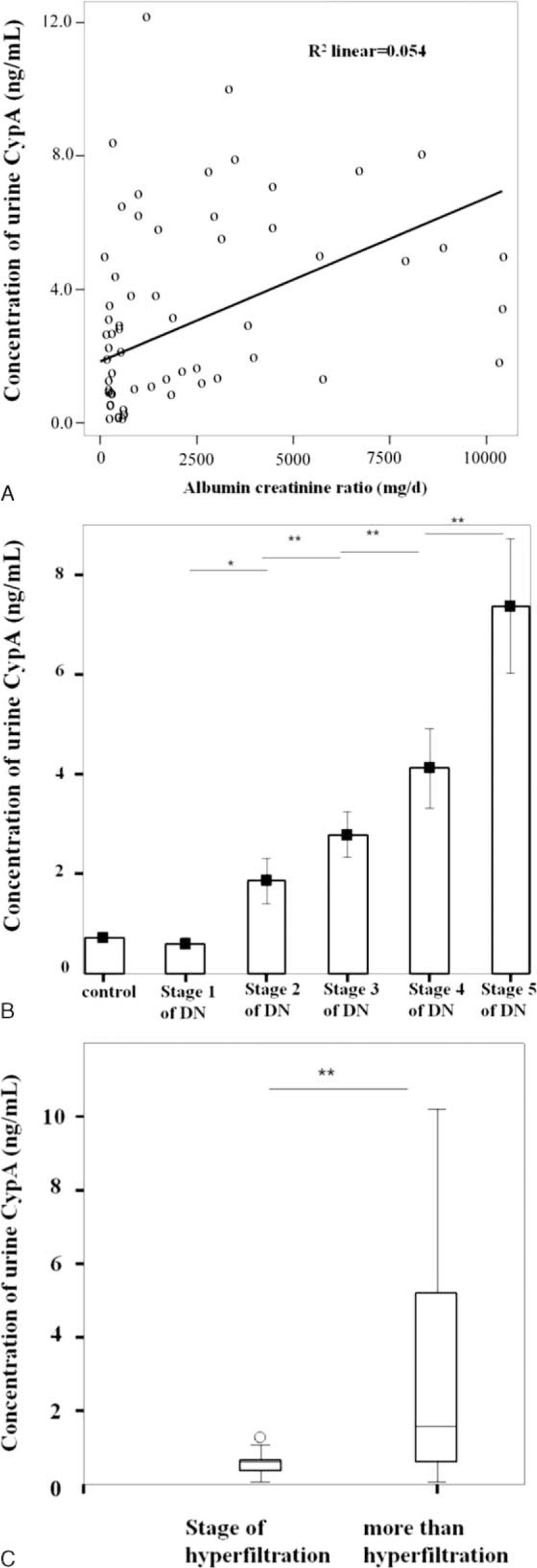
Since the concentration of urinary CypA significantly increased in stage 2 DN (silent stage) and it persistently increased significantly with the progression of DN, we performed an analysis of the receiver operating characteristic curve (Fig. 3). Our analysis demonstrated that when the concentration of urinary CypA was more than 0.7250 ng/mL, we could diagnose the silent stage of DN with a sensitivity of 90.0% and specificity of 72.7%. The area under curve (AUC) was up to 0.85, indicating that the use of urinary CypA for the diagnosis of silent stage of DN had a moderately good discriminatory power.
FIGURE 3.
ROC curve for diagnosing silent stage of DN via urinary CypA. The concentration of urinary CypA to diagnose silent stage of DN was 0.7250 ng/mL with a sensitivity of 0.900 and a specificity of 0.727. The area under the ROC curve was 0.850. CypA = cyclophilin A, DN = diabetic nephropathy, ROC = receiver operating characteristic.
Secreted CypA in Mesangial Cells Treated With High Concentration of Glucose or Free Radicals
At the microscopic level, there are 3 major histological changes in the glomeruli in DN: mesangial expansion, glomerular basement membrane thickening, and glomerular sclerosis.30 A hyperglycemic state stimulates mesangial cell matrix production31 and mesangial cell apoptosis.32 Hence, we examined whether sCypA was secreted from mesangial cells following high glucose treatment. As shown in Figure 4A, glucose increased sCypA level in a dose-dependent manner (10, 25, and 50 mM). Statistical analysis showed that the increased expression of sCypA was found at 25 mM versus control (P = 0.037) and 50 mM versus control (P = 0.037). Expression of sCypA was much higher at 50 versus 10 mM (P = 0.018) (Fig. 4B). Meanwhile, it is known that reactive oxidative stress also plays an important role in DN. NADPH oxidase-mediated renal reactive oxidative stress promotes mesangial expansion and albuminuria.33 We found that the expression of sCypA was significantly increased after 20 or 40 μM H2O2 treatment for 30 minutes (Fig. 4C). Quantitative assessment showed that either 20 or 40 μM H2O2 treatment significantly increased the expression of sCypA, which could be reversed by 300 U/mL of catalase (scavenger of free radicals) (Fig. 4D), which was used to counteract the effects of H2O2. It is worth noting that all the experiments were carefully performed with proper controls to eliminate CypA released from cell death. Taken together, free radicals or high concentrations of glucose stimulate the secretion of CypA from mesangial cells, suggesting that there is a link between sCypA and pathogenesis of DN.
FIGURE 4.
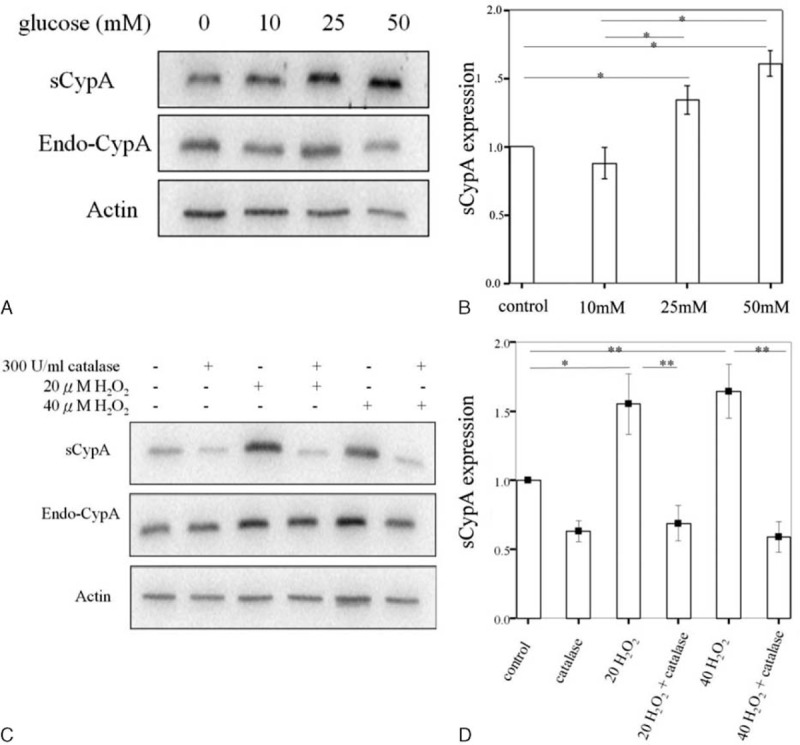
Western blotting of sCypA expression in MES-13 cells treated with different concentrations of glucose and H2O2. (A) Glucose increased the expression of sCypA, which was dose-dependent (10, 25, and 50 mM). (B) Statistical analysis showed that the increased expression of sCypA was found at 25 mM versus control (P = 0.037), and 50 mM versus control (P = 0.037). Increased expression of sCypA was observed at 50 versus 10 mM (P = 0.018). (C) The expression of sCypA was increased in cells with 20 or 40 μM H2O2 treatments. They could both be counteracted by 300 U/mL catalase. (D) Quantitative assessment showed that 20 μM H2O2 increased the expression of sCypA (P < 0.05) (n = 6), which could be reversed by 300 U/mL catalase (P < 0.01) (n = 6). The sCypA expression was also stimulated by 40 μM H2O2 (P < 0.01) (n = 6), which could be counteracted by 300 U/mL catalase (P < 0.01) (n = 6). (n = 4) ∗P < 0.05, ∗∗P < 0.01. Endo-CypA = endogenous cyclophilin A, MES-13 = mesangial, sCypA = secreted cyclophilin A.
sCypA Released From HK-2 Cells Upon High Glucose Or Free Radical Treatment
Mesangial cell injury is the classical expression of DN, but recent studies suggested that DN is also a tubular disease. Early changes in tubular epithelial cells may be an essential factor in the development of progressive kidney diseases.34 HK-2 cells, human PTEC, have been used as a cell model to study tubular diseases. Therefore, Western blotting was used to disclose the expression of sCypA whereby various concentrations of glucose and H2O2 were applied to HK-2 cells. As expected, different concentrations of glucose (10, 25, and 50 mM) could effectively increase the expression of sCypA (Fig. 5A), indicating that hyperglycemia can also induce sCypA release from tubular cells. In addition, either 20 or 40 μM H2O2 treatment significantly increased the expression of sCypA, which could be reversed by 300 U/mL of catalase (Fig. 5B).
FIGURE 5.
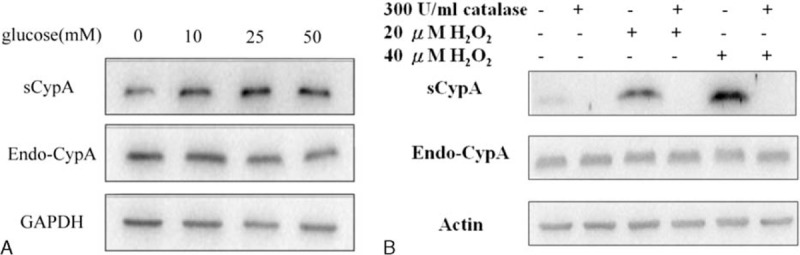
Western blotting of sCypA expression in HK-2 cells treated with different concentrations of glucose and H2O2. (A) HK-2 cells were treated with different concentrations (10, 25, and 50 mM) of glucose. All concentrations of glucose increased the expression of sCypA. (B) The expression of sCypA was increased in cells with 20 or 40 μM H2O2 treatments. They could both be counteracted by 300 U/mL catalase. Endo-CypA = endogenous cyclophilin A, sCypA = secreted cyclophilin A.
DISCUSSION
The current clinical markers for DN are GFR and microalbuminuria. SCr is routinely measured for GFR, which can be used to stage CKD regardless of DM association. Since all renal diseases will progress to CKD and the cause and progression of CKD are heterogeneous, every cause related to CKD should theoretically have its own staging or detection criteria. Specific markers allow physicians to target and treat the definite cause, thereby potentially preventing further renal function deterioration. Albuminuria or proteinuria is the typical marker used for staging DN progression. However, it has become evident that there exists a subpopulation of patients with discrepant classifications of DN (albuminuria-based) and CKD (GFR-based).4–6 Therefore, some committees are trying to develop a new classification of DN,35 combining both GFR and albuminuria systems.
In this study, we measured renal function parameters and demonstrated that urinary CypA was significantly associated with SCr, GFR, proteinuria, ACR, stages of DN, and stages of CKD. In addition, either GFR-based or albuminuria-based classifications of DN correlated significantly with urinary CypA. When comparing different stages of DN or CKD, there was only a trend of higher CypA in higher CKD stages, but truly statistically significant difference existed among the different DN stages. This finding supports the notion that urinary CyA is better correlated using the albuminuria-based classification, which is the better and earlier detection method for monitoring DN compared with the GFR-based system in clinical practice. Although the albuminuria-based system is better than GFR, it is far from ideal for a number of reasons. First, increased albuminuria is actually a relatively late manifestation of early-stage DN, so it is not sensitive enough to detect early stages of DN. Second, only one-third of patients with microalbuminuria present with persistent macroalbuminuria according to 1 cohort study,36 indicating a poor predictive power for outcome of DN. Third, some patients have renal pathological changes without microalbuminuria.37 Finally, albuminuria is not specific enough for DN because it can be detected in other non-DM related nephropathy, such as retinopathy and congestive heart failure.38 Therefore, urinary CypA could have enormous value as an earlier marker than albuminuria for identifying DN.
In this well-matched cohort of DN patients, urinary CypA correlated well to the different severity of DN according to the albuminuria-based classification. Compared with the control group, urinary CypA indeed increased significantly in stage 2 DN and this increase persisted throughout the later stages. The increment was more significant with worsening DN stage. In stage 1 DN, kidneys become dilated and glomerular capillary hydrostatic pressure increased in DN.39 There was a hemodynamic change without any ultrastructure abnormality. Stage 2 DN is a silent stage but, to date, no useful markers for detection have been identified. No microalbuminuria can be measured in clinical practice. However, hyperglycemic effects are initiated in this stage. The glomerular basement membrane becomes thicker, followed by an increase in mesangial volume, and interstitial expansion.24 The above structural changes do not become significant until stage 3 DN. If stage 2 DN could be detected early, intensive blood sugar monitoring, timely diet restriction, and exercise education would be useful to avoid further silent deterioration of DN. In this study, we propose that urinary CypA can be used as an early marker for identifying stage 2 DN with a high sensitivity (90%) and high diagnostic power (AUC = 0.885). Detection of urinary CypA is also very convenient because it is noninvasive. Now that urinary CypA appears to be capable of identifying DN in the silent stage, perhaps the term “silent” can be considered redundant. In an extensive review conducted by Lee et al,40 urinary CypA was not mentioned as a potential biomarker for DN. This is the first study to use urinary CypA in early DN detection. CypA was mostly studied in CVD and lung or liver injury.18 Asthma and RA are associated with this new marker.16 According to an extensive review of CypA in human disease,20 its association with DM was only mentioned once by Ramachandran et al.22 They examined proteomic profiling of high glucose primed monocytes and found that CypA could be a potential secretory marker of inflammation in type 2 DM.21 The present investigation is the 3rd study to identify a correlation between CypA and DM. Furthermore, this is the 1st study to verify the association between urinary CypA and DN with strong statistical significance in this well-designed human cohort.
It has been noted that urinary podocalyxin41 and podocalyxin-positive element,42 which increases after podocyte injury, could be useful as new biomarkers for early DN. However, podocalyxin also increases in other diseases with podocyte injury,41 including IgA nephropathy, focal segmental glomerulosclerosis, membranous nephropathy, and lupus nephritis, indicating that urinary podocalyxin is not specific to DN. In addition, urinary podocalyxin level or podocalyxin-positive element was not reported for early detection of stage 2 DN. Moreover, another biomarker, urinary L-FABP, expressed in the proximal tubules of the human kidney, was recently found to be associated with DN.43 L-FABP increased in a stepwise manner with progression of DN.7 In a study of type 1 DM,44 urinary L-FABP was an independent predictor of progression of DN irrespective of disease stage. The AUC to predict the progression to stage 3 DN by measuring both urinary L-FABP and urinary albumin was up to 0.786. In another study of type 2 DM,45 when the urinary L-FABP level was more than 8.4 μg/g creatinine, clinicians could predict the progression of DN to stage 3 DN with a sensitivity of 0.700 and specificity of 0.781. Compared to L-FABP as a marker for predicting stage 3 DN, urinary CypA is the first marker to be proposed for predicting progression to stage 2 DN with a much higher sensitivity (0.900 vs 0.700) and larger AUC (0.850 vs 0.786). In a recent extensive review of urinary biomarkers for early DN beyond albuminuria,40 it was found that all of the studied biomarkers were limited to predicting microalbuminuria (stage 3 DN). Therefore, our data demonstrate that urinary CypA may have value as a novel biomarker for predicting DN as early as stage 2.
In kidneys, CypA is mostly distributed in tubules, followed by glomeruli.17 Therefore, it is reasonable to hypothesize that urinary CypA could be secreted by tubular cells or mesangial cells. Because mesangial matrix expansion is a typical pathological finding of DN30 and a high glucose state evokes an intrinsic proapoptotic signaling pathway in mesangial cells,32 we first studied the expression of sCypA from mesangial cells. As shown in Figure 4, there was a significant release of CypA following glucose or free radical treatment. Even though DN has been traditionally considered as a glomerular disease, increasing evidence has shown that renal dysfunction correlates earlier and in association with the degree of tubular injury.46 A novel mechanism for albuminuria from PTEC revealed that tubular epithelial cell injury occurs relatively earlier than glomerular injury. There are many chemokines released from PTEC which stimulate certain physiological signals and whose effects culminate in progressive tubular injury, interstitial inflammation, and fibrosis in DN.47 Therefore, we next examined whether CypA can also be secreted by tubular epithelial cells as well. In Figure 5, after treatment of HK-2 cells with various concentrations of glucose or free radicals, sCypA was clearly increased in the conditioned medium. In the cell study using MES-13 and HK-2 cells, our results demonstrated that CypA was secreted after glucose or free radical stimulation, indicating that CypA could be secreted by either mesangial cell or PTEC into urine in early DN. Our results are consistent with those of previous studies that showed earlier renal dysfunction was associated with tubular change46,47 and the later but typical change was related to mesangial cell dysfunction in the glomerulus.31,32 Therefore, sCypA could be considered as both tubular and mesangial cell injury markers in DN.
In summary, we have demonstrated in this well-designed study of DN patients that urinary CypA is a good biomarker for early DN. Even though we cannot exclude the possibility that urinary CypA is released by renal cell lysis, our results from cellular models indicate it is very likely that CypA is secreted from either mesangial or PTEC in early DN. In addition to its role as a novel biomarker of early DN, sCypA may also play a pathological role in the development of DN and may be involved in the interplay between the tubulointerstitial and glomerular compartments. The underlying molecular mechanisms need to be elucidated in further cellular and animal studies.
Acknowledgements
The authors thank grant TCVGH-T1037804 from Taichung Veterans General Hospital and Tunghai University (Taichung, Taiwan) and grants from the Ministry of Science and Technology of the Republic of China (MOST102–2311-B-029–002; NSC101–2311-B-029–001).
Footnotes
Abbreviations: AUC = area under curve, CypA = cyclophilin A, CKD = chronic kidney disease, CAD = coronary arterial disease, CVD = cardiovascular disease, DM = diabetes mellitus, DN = diabetic nephropathy, ESRD = end-stage renal disease, GFR = glomerular filtration rate, L-FABP = liver-type fatty acid-binding protein, MES-13 = mesangial, PTEC = proximal tubule epithelial cell, RA = rheumatoid arthritis, sCypA = secreted cyclophilin A.
Urinary cyclophilin A is a new and earlier biomarker for diabetic nephropathy.
S-F T and C-W S carried out the molecular studies. S-F T, M-J W, C-H C, and MH drafted the manuscript. S-F T and C-W S carried out the immunoassays. S-F T, M-J W, C-H C, C-P F, C-S L, and MH participated in the design of the study. S-F T performed the statistical analysis. S-F T, M-J W, C-H C, C-S L, and MH conceived of the study. S-F T and MH participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
This study was supported by grant TCVGH-T1037804 from Taichung Veterans General Hospital and Tunghai University, Taichung, Taiwan and grants from the Ministry of Science and Technology of the Republic of China (MOST102-2311-B-029-002; NSC101-2311-B-029-001).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hostetter TH. Prevention of end-stage renal disease due to type 2 diabetes. N Engl J Med 2001; 345:910–912. [DOI] [PubMed] [Google Scholar]
- 2.Arora MK, Singh UK. Molecular mechanisms in the pathogenesis of diabetic nephropathy: an update. Vascul Pharmacol 2013; 58:259–271. [DOI] [PubMed] [Google Scholar]
- 3.Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab 2012; 38:291–297. [DOI] [PubMed] [Google Scholar]
- 4.Tramonti G, Kanwar YS. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine 2013; 43:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer HJ, Nguyen QD, Curhan G, et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289:3273–3277. [DOI] [PubMed] [Google Scholar]
- 6.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004; 27:195–200. [DOI] [PubMed] [Google Scholar]
- 7.Kamijo-Ikemori A, Sugaya T, Kimura K. Novel urinary biomarkers in early diabetic kidney disease. Curr Diabetes Rep 2014; 14:513. [DOI] [PubMed] [Google Scholar]
- 8.Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 2014; 63 (2 Suppl 2):S39–S62. [DOI] [PubMed] [Google Scholar]
- 9.Titan SM, Zatz R, Graciolli FG, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol 2011; 6:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen SE, Andersen S, Zdunek D, et al. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int 2011; 79:1113–1118. [DOI] [PubMed] [Google Scholar]
- 11.Tramonti G, Kanwar YS. Tubular biomarkers to assess progression of diabetic nephropathy. Kidney Int 2011; 79:1042–1044. [DOI] [PubMed] [Google Scholar]
- 12.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 2008; 19:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinokio Y, Suzuki S, Hirai M, et al. Urinary excretion of 8-oxo-7, 8-dihydro-2′-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia 2002; 45:877–882. [DOI] [PubMed] [Google Scholar]
- 14.Krolewski AS, Warram JH, Forsblom C, et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care 2012; 35:2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohman RJ, Hultsch T. Cyclosporin A: new insights for cell biologists and biochemists. New Biol 1990; 2:663–672. [PubMed] [Google Scholar]
- 16.Ryffel B, Woerly G, Greiner B, et al. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology 1991; 72:399–404. [PMC free article] [PubMed] [Google Scholar]
- 17.Demeule M, Laplante A, Sepehr-Arae A, et al. Association of cyclophilin A with renal brush border membranes: redistribution by cyclosporine A. Kidney Int 2000; 57:1590–1598. [DOI] [PubMed] [Google Scholar]
- 18.Dear JW, Simpson KJ, Nicolai MP, et al. Cyclophilin A is a damage-associated molecular pattern molecule that mediates acetaminophen-induced liver injury. J Immunol 2011; 187:3347–3352. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K, Shimokawa H, Berk BC. Cyclophilin A: promising new target in cardiovascular therapy. Circ J 2010; 74:2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: a key player for human disease. Cell Death Dis 2013; 4:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran S, Venugopal A, Kutty VR, et al. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc Diabetol 2014; 13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran S, Venugopal A, Sathisha K, et al. Proteomic profiling of high glucose primed monocytes identifies cyclophilin A as a potential secretory marker of inflammation in type 2 diabetes. Proteomics 2012; 12:2808–2821. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A. Standards of medical care in diabetes-2013. Diabetes Care 2013; 36 Suppl 1:S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983; 32 Suppl 2:64–78. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145:247–254. [DOI] [PubMed] [Google Scholar]
- 26.Verdecchia P, Angeli F. [The Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the weapons are ready]. Rev Esp Cardiol 2003; 56:843–847. [DOI] [PubMed] [Google Scholar]
- 27.Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22 (4 Suppl A):6A–13A. [DOI] [PubMed] [Google Scholar]
- 28.Tsai KD, Chang WW, Lin CC, et al. Differential effects of LY294002 and wortmannin on inducible nitric oxide synthase expression in glomerular mesangial cells. Int Immunopharmacol 2012; 12:471–480. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi N, Urushihara M, Satou R, et al. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species – ERK/JNK pathways. Hypertens Res 2010; 33:1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler S. Diabetic nephropathy: linking histology, cell biology, and genetics. Kidney Int 2004; 66:2095–2106. [DOI] [PubMed] [Google Scholar]
- 31.Floege J, Johnson RJ, Gordon K, et al. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int 1991; 40:477–488. [DOI] [PubMed] [Google Scholar]
- 32.Mishra R, Emancipator SN, Kern T, et al. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int 2005; 67:82–93. [DOI] [PubMed] [Google Scholar]
- 33.Asaba K, Tojo A, Onozato ML, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 2005; 67:1890–1898. [DOI] [PubMed] [Google Scholar]
- 34.Garg V, Kumar M, Mahapatra HS, et al. Novel urinary biomarkers in pre-diabetic nephropathy. Clin Exp Nephrol 2015; 30: [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Haneda M, Utsunomiya K, Koya D, et al. A new classification of diabetic nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Invest 2015; 6:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004; 328:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zachwieja J, Soltysiak J, Fichna P, et al. Normal-range albuminuria does not exclude nephropathy in diabetic children. Pediatr Nephrol 2010; 25:1445–1451. [DOI] [PubMed] [Google Scholar]
- 38.Haffner SM, Stern MP, Gruber MK, et al. Microalbuminuria Potential marker for increased cardiovascular risk factors in nondiabetic subjects? Arteriosclerosis 1990; 10:727–731. [DOI] [PubMed] [Google Scholar]
- 39.Hannedouche TP, Delgado AG, Gnionsahe DA, et al. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int 1990; 37:1126–1133. [DOI] [PubMed] [Google Scholar]
- 40.Lee SY, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol 2014; 25:1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hara M, Yamagata K, Tomino Y, et al. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 2012; 55:2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye H, Bai X, Gao H, et al. Urinary podocalyxin positive-element occurs in the early stage of diabetic nephropathy and is correlated with a clinical diagnosis of diabetic nephropathy. J Diabetes Complications 2014; 28:96–100. [DOI] [PubMed] [Google Scholar]
- 43.Kamijo-Ikemori A, Sugaya T, Ichikawa D, et al. Urinary liver type fatty acid binding protein in diabetic nephropathy. Clin Chim Acta 2013; 3: 424:104–108. [DOI] [PubMed] [Google Scholar]
- 44.Panduru NM, Forsblom C, Saraheimo M, et al. Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care 2013; 36:2077–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamijo-Ikemori A, Sugaya T, Yasuda T, et al. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 2011; 34:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol 2000; 11:1667–1673. [DOI] [PubMed] [Google Scholar]
- 47.Baines RJ, Brunskill NJ. Tubular toxicity of proteinuria. Nat Rev Nephrol 2011; 7:177–180. [DOI] [PubMed] [Google Scholar]


