Abstract
The aim of this study is to evaluate the diagnostic value of shear wave elastography (SWE) added to conventional ultrasound (US) in the diagnosis of small (≤2 cm) breast cancer.
Among 410 patients who underwent SWE before US-guided biopsy from June 2012 to June 2013, 171 patients (mean age: 45.17 ± 9.37 years) with 177 small (≤2 cm) breast lesions were enrolled in this study. Diagnostic performances of each quantitative SWE parameters were calculated by receiver operating characteristic (ROC) curves. Performances of conventional US and US combined to SWE was also compared. Histologic diagnosis was used as a reference standard.
Of the 177 lesions, 22 lesions (12.4%) were malignant and 155 (87.6%) were benign. With respect to conventional US, when a cutoff point between category 3 and 4a was used, the Az value was 0.915 (100% sensitivity, 36.8% specificity, 18.3% positive predictive value (PPV), and 100% negative predictive value (NPV)). All average quantitative elastography values were significantly higher in malignant lesions compared to benign lesions (P = 0.001).
The Emax value with a cutoff of 87.5 kPa had the highest Az value of 0.796 (68.2% sensitivity and 87.1% specificity, 42.9% PPV, and 95.1% NPV). Az value of combined data (0.861, 95% CI: 0.801, 0.909) was significantly lower than that of conventional US alone (P = 0.02). By using an Emax value for downgrading Breast Imaging Reporting and Data System (BI-RADS) category 4a lesions to category 3, 76/94 category 4a lesions (80.9%) were downgraded. After downgrading, 5 cancers were missed and the malignancy rate of category 3 lesions increased from 0% (0/55) to 3.8% (5/133) (P = 0.01).
In conclusion, combined use of SWE and conventional US increased the specificity by reducing the number of unnecessary biopsies in differential diagnosis of small breast lesions. However, we propose that the application of conservative strategy for downgrading of soft category 4a lesions would be appropriate to minimize false-negative cases.
INTRODUCTION
Screening for breast cancer focuses on detecting occult small cancer at an early stage, negative lymph node status without distant metastasis. Nowadays, breast ultrasound (US) has become an invaluable method for the detection of lesions, especially in women with dense breasts. Numerous independent studies have demonstrated that adding US to screening in women with dense breast tissue at mammography will yield additional 2.3 to 4.6 mammographically occult cancers per 1000 women.1,2 Mammographically occult cancers detected on breast US are generally small node-negative invasive cancers.3 However, there is a considerable overlap of sonographic features between benign and malignant masses. Therefore, a large number of biopsies are still performed for benign abnormalities.
Elastography is an interesting imaging tool that reflects the tissue stiffness, which enables characterization of lesions. Among the various methods for performing elastography, the quantitative technique of shear wave elastography (SWE) depends less on the individual operator and is highly reproducible.4–6 Many recent studies suggest that SWE improves the diagnostic accuracy and the specificity of conventional US alone in the diagnosis of breast lesions.7–10 However, several studies have observed that small breast cancers are not as stiff as larger cancers, indicating that tumor size as well as the specific histological type can also affect the stiffness value.11,12 In contrast, it has been reported that the diagnostic performance of static elastography was better than that of conventional US in the characterization of small masses (1 cm), since tissue stiffness is an intrinsic material property and should not depend on the mass size.13–15
To our knowledge, there are no reports on the determination of the diagnostic performance of SWE added to conventional US, especially focused on small breast lesions. Because tumor size is one of the most important prognostic factors for breast cancer, we evaluated whether adding shear wave elastographic features could improve the accuracy of the sonographic assessment of small (≤2 cm) lesions.
METHODS
Patients and Inclusion Criteria
From June 2012 to June 2013, 410 consecutive patients underwent SWE before US-guided core needle biopsy (CNB) or surgical excision for breast lesions visible on conventional US. An Institutional Review Board (IRB) approved our retrospective study and neither patient approval nor informed consent was required for the review of medical records or radiological images.
After excluding the patients who had breast lesions larger than 2 cm, we assessed 171 patients aged 21 to 88 years (mean, 45.17 ± 9.37 years) with 177 breast lesions which size was smaller than or equal to 2 cm. Forty-one (24.0%) of the patients were symptomatic, presenting with symptoms such as palpable breast mass (n = 36), breast pain (n = 2), or nipple discharge (n = 3). The remaining 130 patients (76%) were asymptomatic. One hundred thirty two patients had performed mammograms simultaneously with breast US examinations, among which 122 patients had dense breasts (93.1%). According to the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS), the 177 breast lesions were categorized as follows: 57 (32.2%) lesions were category 3, 94 (53.1%) lesions were category 4a, 13 (7.3%) lesions were category 4b, 8 (4.5%) lesions were category 4c, and 5 (2.8%) lesions were category 5.
US Examinations
Conventional US and SWE images were obtained using the Aixplorer® system (Supersonic Imagine, Aix en Provence, France), equipped with a 4–15 MHz linear-array transducer by 1 of 3 board-certified radiologists each with 5 to 10 years’ experience in breast US and at least 1 month experience performing SWE on solid breast lesions before enrolling their first participant. All radiologists were well informed of the clinical information or mammographic findings of the patient before US examinations. Lesion size and location were recorded by the radiologist. After conventional US, SWE imaging was obtained by the same radiologist. The transducer was applied very lightly to the skin above the lesion with a generous amount of transducer jelly. And it was held still for 5 to 10 seconds to let the SWE image stabilize, and an elastography image displaying abnormal stiffness clearly without pressure artifacts was frozen and saved. The built-in-region-of-interest (ROI) (Q-box; Supersonic Imagine) of the system was set to include the mass and the surrounding breast parenchyma tissue, which demonstrated a semitransparent color map of tissue stiffness overlaid on the B-mode image with a range of dark blue, indicating lowest stiffness, to red, indicating the highest stiffness (0–180 kPa). Quantitative elasticity values were measured in all cases using two 2-mm-diameter circular quantification ROIs. One was placed by an investigator on the stiffest part of the mass, and included some tissue adjacent to the stiffest part. And the other ROI was placed on the normal fatty tissue. The system automatically calculated and visualized the maximum elasticity (Emax), mean elasticity (Emean), standard deviation, and elasticity ratio (Eratio), which is the ratio of Emean value in the stiffest portion of the mass to the Emean value of normal fatty tissue.
Statistical Analysis
The histopathological report was regarded as standard reference. Of the BI-RADS US final assessment based on conventional US, category 3 was considered negative, while category 4a and higher was considered positive, since masses of these categories warrant biopsy. Statistical analyses were performed using the SPSS 20.0 software package (SPSS, Chicago, IL) and MedCalc version 10.1.6 (MedCalc software, Mariakerke, Belgium). An independent two sample t test was used for comparisons of continuous variables between benign and malignant groups. For evaluating the diagnostic performance of each quantitative SWE parameter, analyses of the receiver operating characteristic (ROC) curves were applied. Optimal cutoff values for each quantitative SWE parameter were calculated. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy using the cutoff values were measured. Area under the ROC curves (Az) using the calculated cutoff for each parameters were obtained to compare the diagnostic performances of conventional US and US combined to SWE.
RESULTS
General Characteristics of Small Breast Lesions
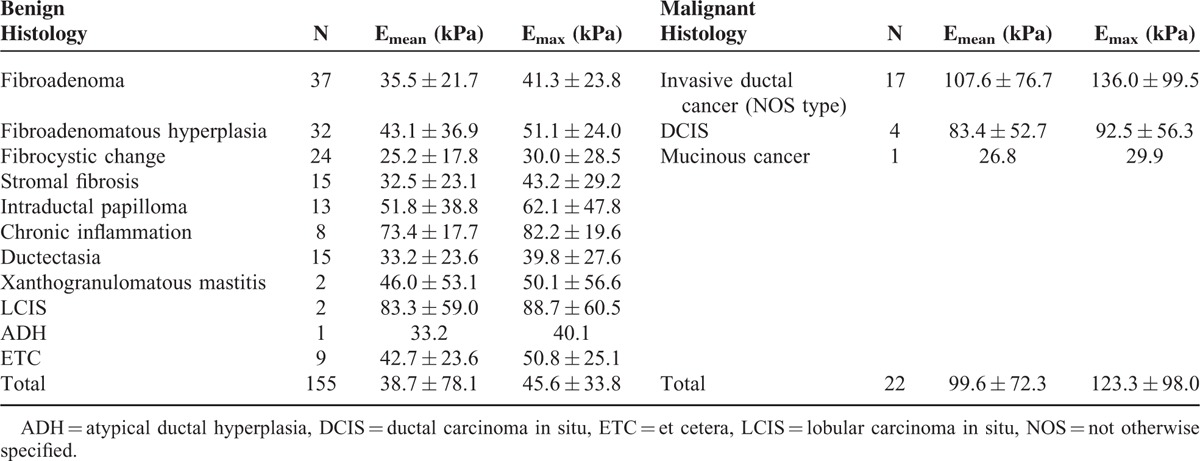
Of the 177 lesions, there were 88 lesions (49.7%) smaller than or equal to 1 cm and 89 lesions (50.3%) between 1 and 2 cm in diameter. Twenty-two (12.4%) were malignant and 155 (87.6%) were benign, as confirmed by US-guided CNB (14-gauge automated gun or 11-gauge vacuum assisted biopsy) (n = 145) or surgical excision (n = 32). Malignant masses included invasive ductal cancer (IDC) (n = 17), ductal carcinoma in situ (DCIS) (n = 4), and mucinous carcinoma (n = 1). The most common histologic findings in 155 benign breast lesions were fibroadenoma (n = 37) (Table 1). The mean size of malignant masses was 1.1 ± 0.44 cm, and that of benign masses was 1.1 ± 0.42 cm (P = 0.1). All benign lesions remained stable during the follow-up period (mean 18.5 months, range 13–20 months).
TABLE 1.
Histopathologic Diagnosis and Mean Quantitative Elastography Values of 177 Small Breast Lesions
Comparing the SWE Values of Benign and Malignant Breast Lesions
All average quantitative SWE values were significantly higher in malignant lesions comparing with benign lesions (P = 0.001). Malignant lesions had an average Emax of 123.28 kPa ± 98.03, whereas benign lesions demonstrated an average Emax of 45.56 kPa ± 33.75 (P = 0.001). The highest average Emax was noted in IDC (136.0 kPa ± 99.5). DCIS showed lower elasticity values than IDC with an average Emax of 92.5 kPa ± 56.3 and mucinous carcinoma showed an extremely low Emax of 29.9 kPa.
There was 1 malignancy among 46 lesions with Emax of 20 kPa or less. There were 4 cancers among 19 lesions with Emax of greater than 20 to 30 kPa or less. Among the 8 stiffest lesions with Emax of 160 kPa or greater, 6 (75%) were cancers and all of them were IDC. Histopathological results and SWE quantitative values including Emax and Emean are listed in Table 1 Most benign lesions had an average Emax less than 60 kPa. But, the average Emax was 82.7 kPa ± 19.6 for chronic inflammation, 88.7 kPa ± 60.5 for lobular carcinoma in situ (LCIS), and 62.1 kPa ± 47.8 for intraductal papilloma, respectively.
Subcentimeter-sized (≤1 cm) breast lesions showed lower mean Emax (40.2 kPa ± 31.5) than the mean Emax (70.6 kPa ± 63.1) for the larger lesions (>1 cm) (P = 0.001). There was no statistical significant difference of the mean Emax between benign (40.6 kPa ± 32.0) and malignant lesions (36.4 kPa ± 26.8) for subcentimeter-sized group (P = 0.1).
Diagnostic Performance of SWE Based on ROC
Malignancy rates for each BI-RADS US categories are as follows; 0.0% (0/57) for category 3, 5.3% (5/94) for category 4a, 30.8% (3/14) for category 4b, 100.0% (8/8) for category 4c, and 100.0% (5/5) for category 5. With respect to conventional US, when a cutoff point between category 3 and 4a was used, BI-RADS US showed 100% (22/22) sensitivity, 36.8% (57/155) specificity, 18.3% (22/120) PPV, and 100% NPV (57/57). The Az value was 0.915 (95% CI: 0.864, 0.951).
The Emax value with a cutoff of 87.5 kPa had the highest Az value of 0.796 (95% CI: 0.668, 0.925) compared to other quantitative SWE measurements. With this cutoff value, SWE showed 68.2% (15/22) sensitivity and 87.1% (135/155) specificity, 42.9% (15/35) PPV, and 95.1% (135/142) NPV. After combining SWE to conventional US the specificity increased from 36.8% (57/155) to 82.6%(128/155) and the accuracy increased from 44.6% to 81.9% (P = 0.01). The sensitivity significantly decreased from 100% (22/22) to 77.3% (17/22) (P = 0.01). Az value of combined data (0.861, 95% CI: 0.801, 0.909) was significantly lower than that of conventional US alone (P = 0.02) (Figure 1).
FIGURE 1.
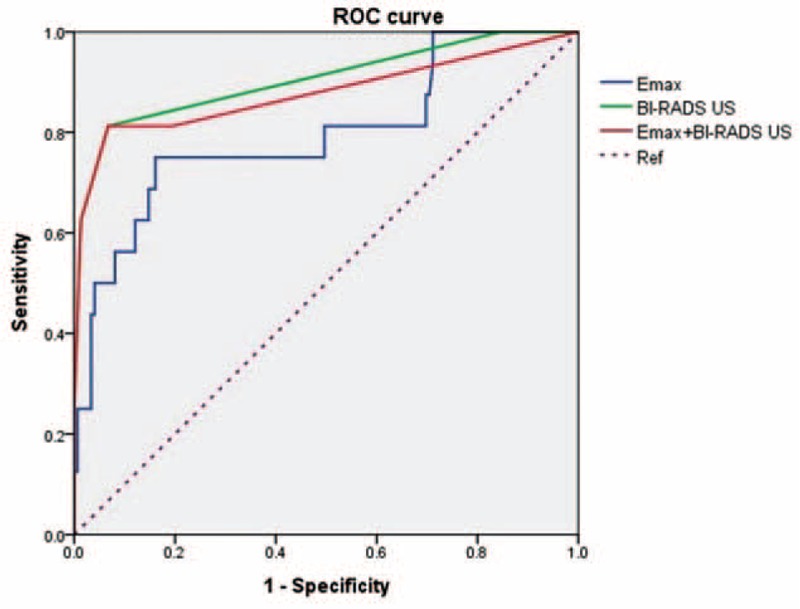
Receiver operating characteristic curves for BI-RADS US, SWE and combining BI-RADS US with SWE.
False-Positive and False-Negative Lesions on SWE
When applying an optimal cutoff of Emax 87.5 kPa, 20 out of 155 benign breast lesions (12.9%) were falsely positive, with Emax values ranging from 90.1 to 192.2 kPa. The average size of these lesions was 12.33 ± 3.95 mm. One mass was categorized as BI-RADS category 3, 16 as category 4a, and 3 as category 4b. Among the 22 malignant lesions, 7 (31.8%) were determined to be falsely negative by SWE, with Emax values ranging from 15.7 to 42.9 kPa. They included 5 IDC, 1 DCIS, and 1 mucinous cancer. Among 5 IDCs, 3 were minimally invasive cancers. The average size of these lesions was 7.71 ± 1.92 mm. All but one was smaller than 1 cm. Characteristics of false-negative breast lesions are summarized in Table 2 and an example is shown in Figure 2.
TABLE 2.
False-Negative Lesions According to Emax of SWE With a Cutoff Value of 87.5 kPa
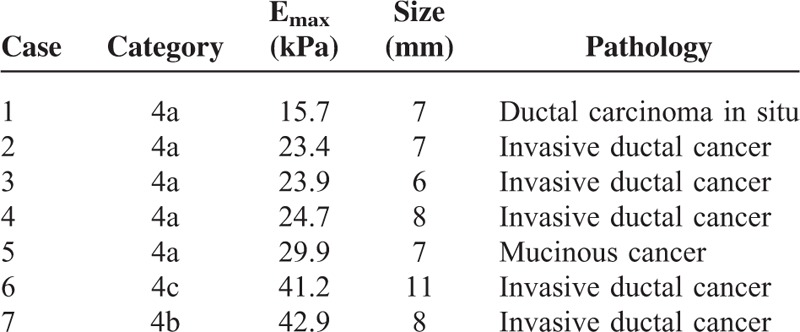
FIGURE 2.
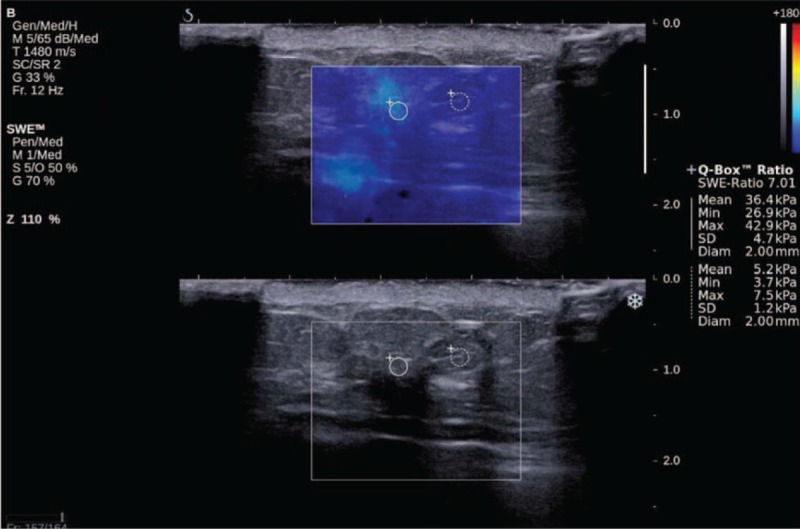
Minimally invasive ductal cancer in a 54-year-old woman. Conventional US (superior) shows a 5-mm-sized hypoechoic irregular mass with spiculated margin, classified as category 4b. SWE shows (inferior) low stiffness (light blue color on a visual scale) at the margin of the small breast mass (Emax of 42.9 kPa).
Effects of Combining of SWE Features to BI-RADS Category 4a Lesions
Various Emax values were selectively added to small breast lesions with a BI-RADS category 4a to determine whether SWE could increase the specificity of conventional US and decrease the benign biopsy rate. By applying an Emax ≤87.5 kPa for downgrading soft category 4a lesions to category 3, 76 from the 94 category 4a lesions (80.9%) were downgraded to category 3. We could reduce 75.5% (71/94) unnecessary biopsies from the 94 category 4a lesions, but 5 cancers were missed. After downgrading, the malignancy rate of category 3 lesions increased from 0% (0/55) to 3.8% (5/133) (P = 0.01). When we downgraded category 4a lesions with an Emax ≤ 50 kPa to category 3, 57 cases including 5 cancers were downgraded (60.6%). As a result, the malignancy rate of reclassified category 3 increased from 0% to 4.4% (5/114) (P = 0.05). By using the most conservative strategy of an Emax ≤ 20 kPa for downgrading, 19 soft category 4a lesions were downgraded to category 3 (20.2%). One cancer was also downgraded. The malignancy rate of category 3 was 1.3% (1/76) which was within 2%.
DISCUSSION
As expected, we found that the quantitative SWE values were significantly higher in malignant masses than in benign masses.7,12,16 Mean values of Emean and Emax were 38.7 and 45.6 kPa for benign masses and 99.6 and 123.3 kPa for malignant masses (P = 0.001). Chang et al12 reported that these significant differences were noted in all subcategorized groups classified by lesions size. However, in this study, there was no statistical significant difference of the mean Emax between benign (40.6 kPa ± 32.0) and malignant lesions (36.4 kPa ± 26.8) in subcentimeter-sized group (P = 0.1). Our results might be because the characteristic of histolopathologic subtypes of benign and malignant groups. Subcentimeter-sized malignant masses included DCIS, mucinous cancer, and several minimally invasive low-grade invasive cancers which showed the low elasticity value. Early stage of breast cancers and specific tumor types such as mucinous cancer were reported to be the causes of false-negative elastography.17,18 In contrast, the mean elasticity values of chronic inflammation and intraductal papillomas were relatively high. These findings are in agreement with the results of previous study by Scaperrotta et al.17 They found the cases of chronic mastitis, adenosis, and intraductal papilloma in the subset of false-positive elastography but further studies with large population should be performed to find out the factors causing false-positive findings.
According to our study, the Emax value with a cutoff of 87.5 kPa had the highest Az value compared with other quantitative SWE parameters, which was similar to results by Berg et al16 and Yoon et al.9 For SWE, relatively high sensitivity and specificity (68.1%, 87.1%) were achieved with Emax, and they were in the range of previously published data.12,16,19 After combining SWE with conventional US, the sensitivity decreased from 100% to 77.3%, and the specificity increased from 36.8% to 82.6% as we expected. However, the areas under the ROC curves (Az value) significantly decreased from 0.913 to 0.861 (P = 0.02). These results are in conflict with those of many studies that show that SWE improves the performance of conventional US when combined with it. Several studies15,20 have reported that the sensitivities of conventional US and strain elastography were similar for diagnosis of small breast cancers and the sensitivity of the 2 modalities combined improved remarkably. They thought that strain elastography is valuable in detecting small malignant lesions which are difficult to diagnose with conventional US. Although, their studies were focused on small masses of less than 2 cm, the proportion of the masses less than 1 cm, and between 1 and 2 cm was different from our study population. While 49.7% (88/177) of subcentimeter-sized lesions were included in our study, they included only 22% (70/308) of subcentimeter-sized lesions. In addition, as we know, static elastography is operator dependent and a substantial amount of interobserver variability can occur during data acquisition.
In addition, the Az value of conventional US alone was quite high, probably because of the long-term experience of the breast radiologists in our institution. From this reason, it might be difficult for new imaging technique like SWE to improve the overall diagnostic performance.
Most recent studies have concluded that US elastography might be useful for further characterization of the lesions with low suspicion and thereby reducing the number of biopsies in this subset, leading to substantial cost savings.21 Because the elastography evaluation should not override the more predictive morphologic features of malignancy for patient management, we calculated the effect of downgrading with SWE only for BI-RADS category 4a lesions. In this study, more than half of lesion (53.1%) was classified as BI-RADS category 4a and malignancy rate was 5.3%. We agree that the additive role of SWE is important in minimizing the number of benign category 4a lesions. However, we favored the conservative strategy that no malignancies would be downgraded to category 3. While we could reduce 75.5% of unnecessary biopsies in BI-RADS category 4a lesions by downgrading to BI-RADS category 3 with optimal cutoff value, 5 malignancies were unfortunately missed. All of them were smaller than 1 cm and 3 of them were minimally invasive breast cancers. Although missed cancers might be found to still carry a favorable prognosis that is equivalent to that of cancers detected by screening, the malignancy rate of category 3 lesions increased from 0% to 3.8% by this application, which is more than the recommended rate for BI-RADS category 3. Only after we used the most conservative strategy of an Emax ≤ 20 kPa for downgrading to category 3, the malignancy rate of reclassified BI-RADS category 3 was 1.3%, which was within 2%. It is not simple which cutoff value we should choose for downgrading to reduce false-positive rate while not downgrading cancers. As Vinnicombe et al22 documented, soft invasive cancers are frequently small (≤10 mm), low grade and screen detected, “softness” on SWE should not raise the threshold for biopsy when assessing small masses.
Besides intrinsic soft tissue characteristics of small breast cancers, we should be aware of the limitations of SWE. Based on previous reports, 10.3% to 15.1% of benign or malignant masses show SWE features that do not fit with the histopathological diagnoses, leading to false-positive or false-negative SWE results.12 The factors that have an effect on false-positive or false-negative elastography results were reported in a study by Chang et al.23 They found clinical factors such as dense breast parenchyma on mammography, breast thickness at the location of the lesion, lesion size and image quality showed significance in discordant images of elastography. Considering that 75.1% of our study population was asymptomatic and most of them had dense breast tissue on mammography, our SWE performance also could be affected by such clinical factors. As parenchymal tissue is known to attenuate shear waves, it would be difficult to differentiate small breast cancer from adjacent dense breast parenchyma on SWE. Therefore, in clinical settings using supplemental screening US in women in dense breasts, radiologists need to consider these clinical factors when performing and interpreting SWE examinations.
This study has several limitations. First, we did not assess the interobserver and intraobserver variability in data acquisition and interpretation. Second, radiologists could not evaluate conventional US and SWE images in an independent manner, as the SWE image acquisition and measuring of quantitative values were performed by the same radiologist. Third, the study population was relatively small and thus this result did not provide a complete representation of all histologic types of benign and malignant breast lesions. In addition, a multivariate analysis for evaluating various factors was not performed. Therefore, for this result to be clinically useful, a large prospective study should be performed in the future.
In conclusion, SWE might be useful for increasing the specificity and reducing the number of unnecessary biopsy for the diagnosis of small breast cancers. However, we should be careful before deciding to recommend follow-up or biopsy for a small breast lesion on the basis of SWE features, to minimize false-negative cases.
Footnotes
Abbreviations: ACR = American College of Radiology, BI-RADS = Breast Imaging Reporting and Data System, CNB = core needle biopsy, DCIS = ductal carcinoma in situ, IDC = invasive ductal cancer, IRB = Institutional Review Board, NPV = negative predictive value, PPV = positive predictive value, ROC = receiver operating characteristic, ROI = region-of-interest, SWE = shear wave elastography, US = ultrasound.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008; 299:2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooley RJ, Greenberg KL, Stackhouse RM, et al. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology 2012; 265:59–69. [DOI] [PubMed] [Google Scholar]
- 3.Bae MS, Han W, Koo HR, et al. Characteristics of breast cancers detected by ultrasound screening in women with negative mammograms. Cancer Sci 2011; 102:1862–1867. [DOI] [PubMed] [Google Scholar]
- 4.Gweon HM, Youk JH, Son EJ, et al. Clinical application of qualitative assessment for breast masses in shear-wave elastography. Eur J Radiol 2013; 82:e680–e685. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove DO, Berg WA, Dore CJ, et al. Shear wave elastography for breast masses is highly reproducible. Eur Radiol 2012; 22:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mun HS, Choi SH, Kook SH, et al. Validation of intra- and interobserver reproducibility of shearwave elastography: Phantom study. Ultrasonics 2013; 53:1039–1043. [DOI] [PubMed] [Google Scholar]
- 7.Athanasiou A, Tardivon A, Tanter M, et al. Breast lesions: quantitative elastography with supersonic shear imaging—preliminary results. Radiology 2010; 256:297–303. [DOI] [PubMed] [Google Scholar]
- 8.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006; 239:341–350. [DOI] [PubMed] [Google Scholar]
- 9.Lee EJ, Jung HK, Ko KH, et al. Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur Radiol 2013; 23:1803–1811. [DOI] [PubMed] [Google Scholar]
- 10.Au FW, Ghai S, Moshonov H, et al. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. AJR Am J Roentgenol 2014; 203:W328–W336. [DOI] [PubMed] [Google Scholar]
- 11.Evans A, Whelehan P, Thomson K, et al. Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology 2012; 263:673–677. [DOI] [PubMed] [Google Scholar]
- 12.Chang JM, Moon WK, Cho N, et al. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 2011; 129:89–97. [DOI] [PubMed] [Google Scholar]
- 13.Zhu QL, Jiang YX, Liu JB, et al. Real-time ultrasound elastography: its potential role in assessment of breast lesions. Ultrasound Med Biol 2008; 34:1232–1238. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim SH, Kang BJ, et al. Role and clinical usefulness of elastography in small breast masses. Acad Radiol 2011; 18:74–80. [DOI] [PubMed] [Google Scholar]
- 15.Fu LN, Wang Y, Wang Y, et al. Value of ultrasound elastography in detecting small breast tumors. Chin Med J 2011; 124:2384–2386. [PubMed] [Google Scholar]
- 16.Berg WA, Cosgrove DO, Dore CJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012; 262:435–449. [DOI] [PubMed] [Google Scholar]
- 17.Scaperrotta G, Ferranti C, Costa C, et al. Role of sonoelastography in non-palpable breast lesions. Eur Radiol 2008; 18:2381–2389. [DOI] [PubMed] [Google Scholar]
- 18.Raza S, Odulate A, Ong EM, et al. Using real-time tissue elastography for breast lesion evaluation: our initial experience. J Ultrasound Med 2010; 29:551–563. [DOI] [PubMed] [Google Scholar]
- 19.Evans A, Whelehan P, Thomson K, et al. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 2010; 12:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhi H, Xiao XY, Ou B, et al. Could ultrasonic elastography help the diagnosis of small (≤2 cm) breast cancer with the usage of sonographic BI-RADS classification? Eur J Radiol 2012; 81:3216–3221. [DOI] [PubMed] [Google Scholar]
- 21.Cho N, Moon WK, Park JS, et al. Nonpalpable breast masses: evaluation by US elastography. Korean J Radiol 2008; 9:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinnicombe SJ, Whelehan P, Thomson K, et al. What are the characteristics of breast cancers misclassified as benign by quantitative ultrasound shear wave elastography? Eur Radiol 2014; 24:921–926. [DOI] [PubMed] [Google Scholar]
- 23.Chang JM, Moon WK, Cho N, et al. Breast mass evaluation: factors influencing the quality of US elastography. Radiology 2011; 259:59–64. [DOI] [PubMed] [Google Scholar]


