Significance
Root waving, a growth response previously discussed predominantly in Arabidopsis, is reported in Medicago truncatula. Analogous to bacterial chemotaxis where Escherichia coli uses a “run-and-tumble” strategy to find sources of food, our experiments reveal a “grow-and-switch” gravitropic response in these root systems. This finding offers valuable insights into the strategies used for plants as they navigate heterogeneous environments in search of water and nutrient resources.
Keywords: plant biomechanics, root morphology, root waving, root coiling, pattern formation
Abstract
Experimental studies show that plant root morphologies can vary widely from straight gravity-aligned primary roots to fractal-like root architectures. However, the opaqueness of soil makes it difficult to observe how environmental factors modulate these patterns. Here, we combine a transparent hydrogel growth medium with a custom built 3D laser scanner to directly image the morphology of Medicago truncatula primary roots. In our experiments, root growth is obstructed by an inclined plane in the growth medium. As the tilt of this rigid barrier is varied, we find Medicago transitions between randomly directed root coiling, sinusoidal root waving, and normal gravity-aligned morphologies. Although these root phenotypes appear morphologically distinct, our analysis demonstrates the divisions are less well defined, and instead, can be viewed as a 2D biased random walk that seeks the path of steepest decent along the inclined plane. Features of this growth response are remarkably similar to the widely known run-and-tumble chemotactic behavior of Escherichia coli bacteria, where biased random walks are used as optimal strategies for nutrient uptake.
Plants are able to sense a wide variety of external stimuli, giving rise to actively controlled responses driven by gradients in light, gravity, touch, nutrient resources, and water. These responses, which include phototropism, gravitropism, thigmotropism, chemotropism, and hydrotropism, take input from the local environment and modulate phenotype development in a manner essential for survival (1–5). A number of plant growth responses, such as the popping of chiral seed pods (6) and the overwinded morphology of cucumber tendrils (7), are dominated by the mechanical properties of plant tissues and their passive response to physical forces. However, these special cases aside, growth patterns are more typically modulated by a combination of actively regulated biological processes and passive mechanical response. The snapping of a Venus fly trap (8–11) is a classic example, where cell turgor pressure and thin shell mechanics work in tandem to determine the plant’s phenotype. Continued studies of developmental morphology at the interface between mechanical and biological regulation play an essential role in bridging phenotypic and biomolecular points of view (12, 13), while offering a more complete understanding of plant biomechanics.
In the context of roots, the mechanical properties of the growth medium play a critical role in modulating root morphology, as evidenced by a variety of studies examining the role of soil impedance (14–19), granularity (20), the presence of cracks (21), and mechanical barriers (22–24). For example, experiments with Arabidopsis thaliana primary roots show that normal gravity-aligned morphologies interrupted by a horizontal barrier lead to an in-plane coiling of the root. As the barrier is tilted, a combination of active and passive growth responses drive root waving (25–30). In these conditions, the primary root exhibits sinusoidal growth that deviates from a strict downward direction along the plane. Early experimental work accounted for the waving morphology as a combination of positive gravitropism and a thigmotropic (growth response to touch) effect (25). This interpretation relied on the barrier to simultaneously prevent gravity-aligned growth and activate a thigmotropic twisting of the root tip; however, later experiments demonstrated a role for friction as an additional contributing factor (28). Although Arabidopsis mutants have been used to explore the underlying genetic regulatory pathways of root waving, the detailed mechanism coupling gravity sensing and the growth environment’s mechanical properties is still open to debate (27, 29, 30).
While these initial studies have proposed different mechanisms for root waving, it remains unknown whether the phenomenon is species-specific or a generic root growth strategy. Here, we perform experiments on Medicago truncatula, a model legume, and find growth patterns similar to root waving. This plant is larger than Arabidopsis and fast-growing, which makes it convenient for study. Our experiments combine 3D imaging with a controlled mechanical growth environment that interpolates between a horizontal physical barrier and normal unobstructed growth. This approach allows us to nondestructively examine the in situ root development and quantify the resulting morphology with a variety of geometric and statistical metrics. Whereas previous studies have focused on temporal dynamics and genetic components of root waving, we turn our attention to the growth barrier’s tilt angle and subsequently probe different aspects of the phenomenon. Ultimately, our analysis reveals a mechanism that produces root waving as a byproduct of gravitropic reorientation on the mechanical barrier, and the root’s measurement tolerance for the direction of gravity.
Experimental Procedures
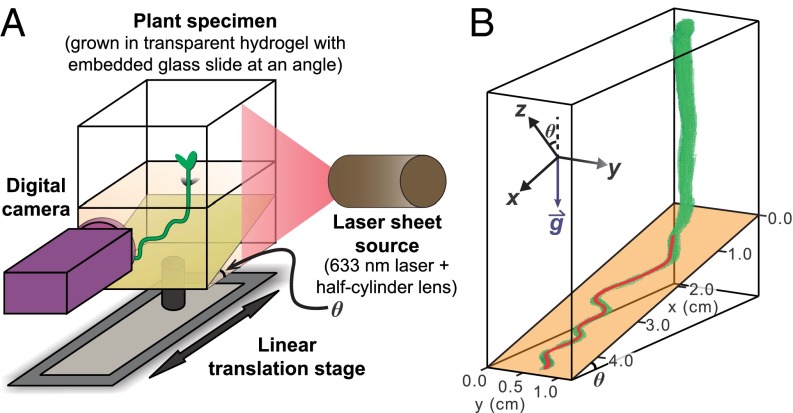
Medicago seedlings were germinated and transferred to transparent chambers containing growth media with Gelrite, which provided both moderate mechanical impedance to root growth and an abundant source of nutrients (see SI Appendix, SI Materials and Methods; also see ref. 31). In total, 92 plants were germinated, with each growth chamber containing one plant; 87 samples were analyzed in this work, whereas the remaining 5 exhibited atypical stunted growth and were excluded from our analysis. To systematically study root waving, we introduced a physical barrier in the growth medium tilted at an angle θ, measured from the horizontal (Fig. 1A). Initially, the primary root of each newly transferred seedling was ∼1 cm in length, and it grew vertically downward until encountering the physical barrier. It then grew almost exclusively on the surface of the barrier plane for 10–14 d. We used a translating stage moving perpendicular to a laser sheet to illuminate successive cross sections of the root and captured the resulting images with a digital camera (Fig. 1A) (24). The resulting image stack was then analyzed in MATLAB, and the 3D root morphology was reconstructed (Fig. 1B). Undulations of the root perpendicular to the barrier surface were generally not observed but, when they did occur, were 1 mm or less. Thus, we projected the 3D root path onto the 2D plane of the growth barrier and used this digitized trajectory in our analysis.
Fig. 1.
Schematic of experimental setup and definition of coordinate system. (A) Diagram of apparatus used to scan the full 3D root morphology of Medicago truncatula grown in a hydrogel medium. (B) Example 3D reconstruction of a Medicago root (green) and extracted centerline used for analysis (red line). In this specific example, the inclined glass plane (orange) is at an angle . For each angle, the coordinate system is defined on the tilted surface.
Results and Discussion
Coiling, Waving, and Skewing Morphologies.
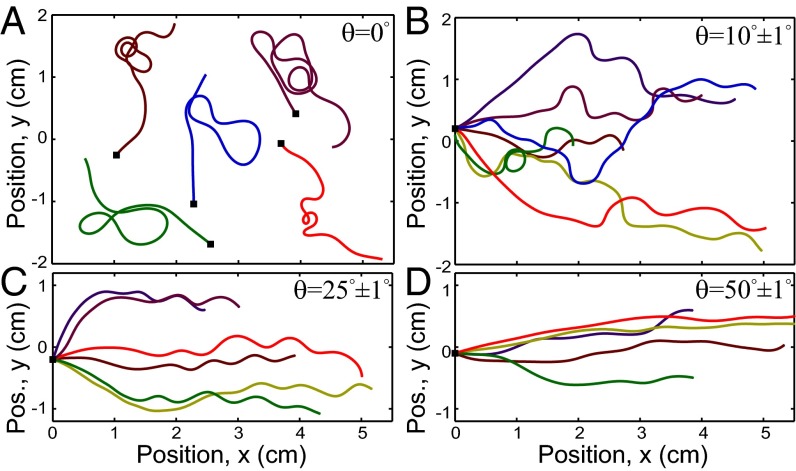
The morphology of Medicago exhibits distinct regimes as the tilt angle of the mechanical barrier θ is increased from to . When the plane is horizontal, the primary root meanders on the surface, with segments of alternating chirality that make incomplete planar coils before reversing their direction (Fig. 2A). As expected, these reversals exhibit no in-plane directional preference, which is consistent with the uniform gravitational signal across the horizontal growth plane. Owing to the random coils that dominate the morphology and similarity to growth response observed in Arabidopsis, this regime is referred to as “root coiling” (29, 30).
Fig. 2.
Overlays of several Medicago root centerlines grown on planes tilted at various angles. As the tilt θ increases, the root morphologies transition from (A) a random, meandering root path at to (B and C) a sinusoidal pattern around (B) to (C) and, ultimately, (D) a skewed trajectory with small undulations at . In each panel, there are multiple root paths shown in different colors, and the point where each root makes first contact with the tilted plane is marked with a black square. For clarity, A has the black squares spread out, and B−D have the squares starting at cm with growth generally proceeding toward the right.
When a nonzero tilt angle is introduced to the mechanical barrier (), the gravitational signal along the growth plane is no longer uniform, and the symmetry of the system is broken. The root now has a net growth directed downhill, a manifestation of its expected response to gravity (Fig. 2 B and C). We continue to observe root segments with alternating chirality of bending, but the length of each segment is increasingly shorter and more regular as θ increases. At a tilt of , we observe nearly periodic reversals of root bending that resemble the sinusoidal wave reported in Arabidopsis, where the morphology is called “root waving” (29, 30).
As the tilt angle is further increased to , the waving oscillations become less pronounced, and the root appears to grow in a more linear fashion along the downhill direction (Fig. 2D). This regime is known as “root skewing” due to the skewed growth trajectories (29, 30).
Thus far, the only experimental parameter varied is the tilt angle of the physical barrier. However, the root morphologies have changed from a random meandering root path to a regular sinusoidal wave and, finally, to a relatively straight skewed path. This observation suggests that the distinct morphologies have common underlying causal mechanisms and thus can be viewed in a unified fashion. To test this hypothesis, we first quantify the root morphologies and their dependence on the barrier tilt angle θ.
Curvature and Morphological Quantification.
Active regulation of root morphology by biomolecular processes manifests at the tissue scale by asymmetric elongation of new growth. This differential elongation enables spatial and temporal variations in the root’s curvature that can be measured experimentally, and may ultimately be useful for testing mathematical models of root development (SI Appendix, Fig. S1). To quantify Medicago root waving, we calculated the curvature κ as a function of arc length s (Fig. 3A). Defining the bearing angle as the angle between the root’s tangent vector and the x axis, is given by . Plotting the curvature as a function of arc length at consecutive times shows that as the root grows and the arc length increases, the curvature 3 mm behind the root tip maintains a constant morphology, as indicated by the vertical time-independent stripes (Fig. 3B, , 110 h of growth). These time-lapse data demonstrate that the root steadily elongates at about 200 μm/h and oscillates in the elongation zone, which is the region within mm of the root tip (Fig. 3B and Movie S1). Beyond this zone, the rest of the morphology remains static, and, consequently, the root’s curvature can be accurately studied by a single scan recorded after many hours of growth.
Fig. 3.
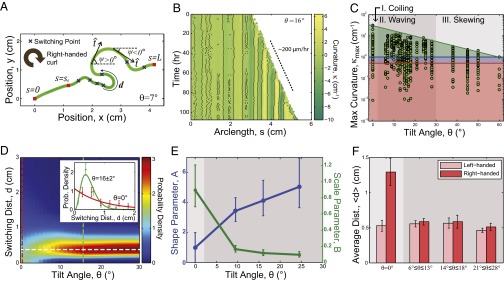
Quantification of Medicago root growth shows smooth transitions between root morphologies. (A) A 2D morphology of a typical Medicago primary root (green line). The three red squares represent the points where the root first encounters the glass plane (), the point where waving begins (), and the root tip (). Our analysis only includes root segments between and , where is defined as the first switching point of the segment with curvature greater than 1 cm−1. The angle between the tangent and the horizontal is the bearing angle, ψ. (B) A kymograph showing the curvature κ along the arclength s as a function of time. Black pixels indicate the reversal points measured for each time point. The color intensity denotes the magnitude of curvature in cm−1. The vertical nature of the striations indicates that the curvature remains steady in time. (C) The spread of from each segment of root between switching points at a given tilt angle θ. Data below the red line are below the measurement threshold and are not used in our analysis. The blue line corresponds to the initiation point where root patterns begin to emerge. The green shaded region that bounds all of the measurements has an upper limit that smoothly varies with θ, showing no obvious transitions between regions typically defined as coiling, waving, and skewing (gray bands). (D) An interpolated heat map representation of the switching distance probability density shows an intensity peak centered on cm (white dashed line). Taking cuts (red, green dashed lines) and plotting the measured distributions along with fits shows comparisons with a negative exponential distribution at (Inset, red), and a representative example of the gamma distribution for (Inset, green). (E) The maximum likelihood estimation of the shape parameter A and scale parameter B for the probability distribution of switching distance . Error bar represents 95% confidence interval. for as the distribution is fitted to a negative exponential distribution (SI Appendix, Fig. S4). Background shading is consistent with labeling in C. (F) Bias in the chirality of switching distance defined in A. The bar chart shows the average switching distance d of left- and right-handed segments at different θ, defined by the right-handed curl shown in Fig. 3A. Background shading is consistent with labeling in C. For D−F, each sample grouping has n between 60 and 90.
Although κ continuously varies along the root’s arc length, there are well-defined regions of positive and negative curvature. These regions are bound by switching points, positions where the root changes direction (Fig. 3A, blue crosses where cm−1). Noticing that the range of curvature values varies with θ (Fig. 2), we extracted the maximum curvature magnitude from each segment between switches as a simple means to characterize the morphology (Fig. 3C and SI Appendix, Fig. S2). The resolution of our 3D imaging and reconstruction technique set a lower limit of 0.5 cm−1 on the curvature values that can be reliably measured (Fig. 3C, red line and shaded region indicate below-threshold measurements, and SI Appendix, Fig. S3). Moreover, samples that clearly demonstrate root waving show an initial period of nearly straight growth (Fig. 3A, ). To eliminate this transient growth period from our analysis, we set a curvature threshold of 1 cm−1 for all samples to define the point where root patterns begin to emerge (Fig. 3C, blue line). A scatter plot shows that the upper limit in smoothly varies with decreasing θ, lending support to the hypothesis that different root morphologies share a common underlying mechanism (Fig. 3C, green line).
Distribution of Directional Switching.
To further characterize Medicago root growth responses on tilted barriers, we measured the arc length distance d between points of zero curvature on each sample (SI Appendix, Fig. S4). Phenomenologically, features in the data were captured by binning over θ and fit to a probability distribution for d (Fig. 3D). To determine an appropriate distribution, we consider that when , the root morphology is reminiscent of a random polymer coil (32), whereas, in the waving and skewing regimes, the morphology is far more regular (Figs. 2 and 3D, Inset). A simple two-parameter function that captures this full range of switching behavior is the gamma distribution. This distribution is characterized by the shape parameter A and the scale parameter B, and is described by the density function , where is the gamma function evaluated at A. Fits for (Fig. 3D, Inset, red) and (Fig. 3D, Inset, green) show how this function performs for two different root growth responses. Plotting an interpolated heat map of the fitted data shows a peak associated with root waving, around the cut where cm, that diminishes at lower tilt angles (Fig. 3D, dashed white line; SI Appendix, Fig. S4). This cut through distribution space demonstrates that transitions between root coiling (Fig. 3D, red dashed line) and root waving (Fig. 3D, green dashed line) are smooth, and suggests these morphologies arise from the same mechanical considerations, particularly because the characteristic half-oscillation length of 0.4 cm appears independent of θ. At higher tilt angles (), we find that the necessary curvature threshold for distinguishing transient growth behavior () eliminated half of the roots from consideration (SI Appendix, Fig. S3). Because these data may not be representative of the underlying growth response, we apply a cutoff at to our analysis that depends on the switching distance d.
While inspecting the fitted values for A and B along with their 95% confidence intervals (Fig. 3E and SI Appendix, Fig. S4), we found that the distribution with a horizontal barrier () has comparable fits whether we allowed A to vary () or fixed (; see SI Appendix, Fig. S4). This special case with fixed A simplifies the gamma distribution to a negative exponential distribution, indicating that reversals on a flat surface can be quantitatively described by a Poisson process. This property is found when each reversal event is independent of previous reversals, or, in other words, the reversals are memoryless. Memoryless behavior, however, is lost when the tilt angle θ is nonzero. The gravitational bias introduced by the tilted mechanical barrier breaks the symmetry in the system, bringing the root from a state of random coiling to a state of more regular waving. Additionally, this symmetry-breaking barrier drives a transition from chiral to achiral morphologies, which is most clearly evident by averaging the switching distance d for each binned value of θ for left- and right-handed coils (Fig. 3F). We find that in the symmetric case, there is a clear dominance of right-handed coils, whereas nonzero values of θ have equal amounts of left- and right-handed coiling behavior. This preference for right-handed chirality in Medicago roots was previously seen in observations of helical root buckling, where its origin was attributed to twisted growth in the root’s elongation region (24).
Interpretation of Root Morphologies by Analogy to Escherichia coli Chemotaxis.
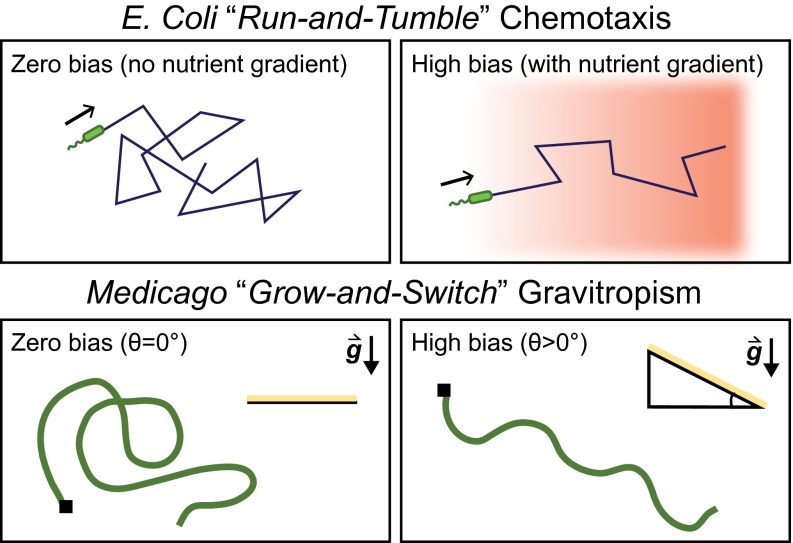
We have shown that the three distinct coiling, waving, and skewing morphologies of Medicago roots can be viewed in a unified fashion, where the different transitions are driven by changes in the growth barrier tilt angle θ. To understand the underlying mechanism, we look to bacterial foraging behaviors for a useful analogy. Specifically, E. coli uses chemotaxis to navigate its environment for food and nutrient resources. Broadly, the process is characterized by a series of straight-line motions punctuated by periods of random reorientation. In a chemically uniform environment, this “run-and-tumble” (33) motion exhibits the exponentially distributed run lengths indicative of a memoryless Poisson process (34). When nutrient resources are introduced and a chemical gradient is established, this unbiased random walk becomes asymmetric; runs along the direction of steepest gradient have a longer duration than runs in the transverse direction. Thus, despite the randomizing effect of tumbles, E. coli is able to swim in a favorable direction (Fig. 4).
Fig. 4.
A comparison between E. coli run-and-tumble chemotaxis and Medicago grow-and-switch gravitropism. When moving within an environment of uniform chemical gradient, E. coli executes a random walk. However, this random walk is biased when a nutrient gradient is established and the net displacement of E. coli is in the direction of high nutrient concentration. Similarly, the root path of Medicago growing on a horizontal plane () is random and has no directional preference. When a gravitational bias is introduced by tilting the growth plane such that , the root path has a net direction downhill driven by the gravitropic tendencies of root growth.
By inspecting the reversals of Medicago root trajectories, we see a behavior analogous to E. coli’s run-and-tumble motion. In essence, the tilted mechanical barrier establishes a gravitational gradient akin to the chemical gradient in E. coli chemotaxis. When the mechanical barrier is horizontal (), the root performs a random walk, in the sense that the switching distance d is exponentially distributed and the reversal events are a Poisson process (Fig. 3D, Inset). Because the root experiences uniform gravitational stimulus, it grows without any directional preference (Figs. 2A and 4). However, when the mechanical barrier is tilted (), the root is able to move through the gravitational gradient, yielding trajectories biased toward the downhill direction (Fig. 2 B and C). With increasing θ, the root becomes increasingly more biased, so that at , the root hardly deviates from the x axis (Fig. 2D). Hence, the unifying mechanism behind root coiling, waving, and skewing can be considered a form of root “grow-and-switch” gravitropism (Fig. 4). In this picture, Medicago’s root growth is like E. coli’s runs, whereas Medicago’s switching points are like E. coli’s tumbles. We therefore predict that just as E. coli’s rate of “run-and-tumbling” is dependent on the strength of the chemical gradient, Medicago’s rate of directional reversal will depend on the strength of the gravity gradient.
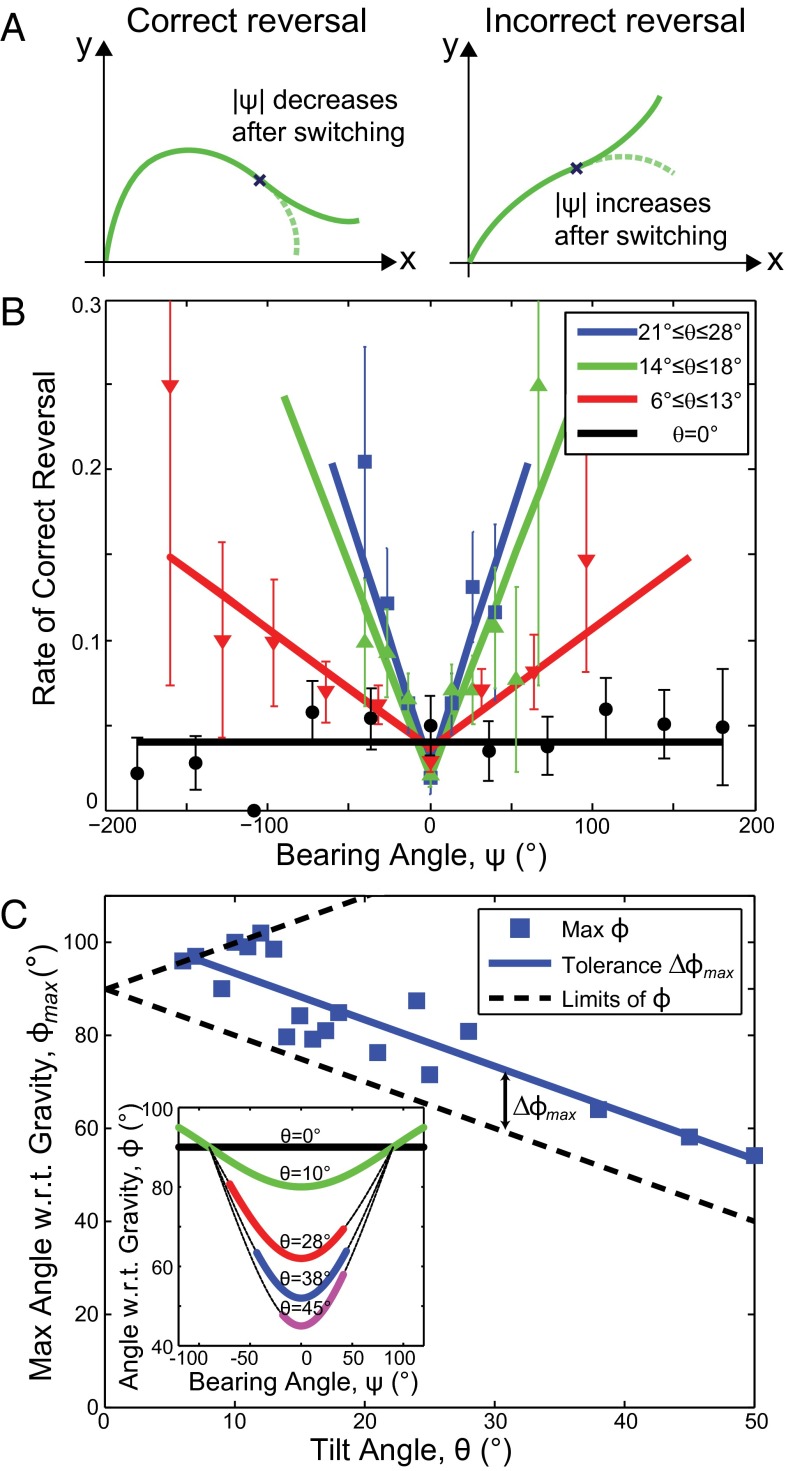
To test this prediction of the grow-and-switch gravitropic interpretation, we discretize the root into small segments of length 0.04 cm and compute the probability that a segment at a particular bearing ψ occurs at a reversal point. Although this probability is equal to the rate of reversal, each reversal event itself can be identified as either correct or incorrect depending on whether the subsequent root trajectory aligns toward either the downhill or transverse direction (Fig. 5A). Thus, by analogy to chemotaxis, we expect that the further the root deviates from , the more likely it is to make a correct reversal. Plotting the measured rate of correct reversals against ψ for shows that Medicago follows this expected behavior (Fig. 5B, red data and linear fits). Indeed, data for θ throughout the root waving regime exhibit such dependencies (Fig. 5B, green and blue data), and exhibit a V-like shape centered at with the slope becoming larger as the tilt θ increases. These trends demonstrate that gravitropism is biasing the statistical properties of the reversal events. In this analysis, we excluded outlier reversals occurring at extreme values of ψ by requiring at least two counts in each binned value for ψ (see SI Appendix, Fig. S5 for full histogram data). When , the choice of is arbitrary and reversal events are neither correct nor incorrect, because there is no gravitational gradient. Plotting the rate of all reversals for (Fig. 5B, black data and linear fit) shows no directional preference in the bearing angle ψ, consistent with expectations based on E. coli’s behavior in a chemically isotropic environment. Thus, the plots of reversal rate for varying θ further support the analogy between Medicago grow-and-switch gravitropism and E. coli run-and-tumble chemotaxis.
Fig. 5.
Extracting the principles of Medicago grow-and-switch gravitropism. (A) Illustrations of correct and incorrect reversals. A correct reversal immediately decreases the bearing ψ so that the root is more aligned with the downhill direction. An incorrect reversal sends the root in a transverse direction. (B) The rate of correct reversals plotted against bearing angle ψ shows that when the tilt angle θ increases, the root is able to find the downward direction more efficiently. In the special case where , there is no distinction between correct and incorrect reversals, and we therefore include all reversal events in the plot (black data and line). (C) Given a specific tilt angle θ, there are physical limits to the angle with respect to gravity ϕ (black dashed lines). Plotting the maximum angle with respect to gravity for each value of θ gives a scatter plot that shows a characteristic range of values that is greater then the physical limit. This angle can be considered Medicago’s tolerance for sensing the direction of steepest descent. Inset shows the angle with respect to gravity ϕ plotted against the bearing angle ψ for a few representative values of tilt angle θ. The dashed lines are calculated from a trigonometric analysis, and the upper limit for each colored line is used in the scatter plot of C.
We emphasize that for , of all reversals are correct reversals. This imbalance implies that in addition to sensing its bearing with respect to gravity, the root also has information about the sign of the root path curvature. Otherwise, reversal events would only be in the correct direction half of the time. Because curvature is determined by derivatives of the root’s trajectory, the root must have information that extends over some physical distance. Whether this distance is a few cells or a few centimeters remains unclear; however, the preference for correct reversals indicates that the underlying mechanism for root waving involves nonlocal information.
The data show that the range of observed bearing angle ψ decreases with increasing tilt θ (Fig. 5B). This reduced range suggests the presence of a θ-dependent threshold, beyond which the root will reverse its direction to navigate downhill. Because the root can only measure its orientation with respect to gravity (35–39), we use a trigonometric analysis to define the the angle ϕ between the root tip tangent and the gravity vector by . Plotting the root’s angle with respect to gravity ϕ versus the bearing angle ψ shows that there is a well-defined minimum value at (Fig. 5C, Inset, minimum of purple, blue, red, and green curves). As the barrier’s tilt angle is varied, however, there is a range of maximum angles the root tip makes with respect to gravity, (Fig. 5C, Inset, limits of colored lines). A scatter plot of for all tilt angles shows a linear trend (Fig. 5C, blue data and line; ) that parallels the line of minimum ϕ (Fig. 5C, lower dashed line). These data include roots grown on barriers with because the measurements of are insensitive to the threshold process applied to the switching distance d. Remarkably, the range of ϕ between its minimum and maximum values, , for different tilts is nearly constant (Fig. 5C), indicating that grow-and-switch gravitropism has a characteristic angle range, with respect to gravity, of (mean SD), at which point the root switches direction to move more directly downhill. This characteristic angle can therefore be construed as the measurement tolerance of Medicago’s gravity sensing abilities.
In our quantification of Medicago’s root growth, we found several indications of smooth transitions between different morphologies. For example, the upper bound on the maximum curvature smoothly varied with tilt θ and had no obvious transitions between root coiling and root waving or root waving and root skewing (Fig. 3C). Similarly, the switching distance d exhibited smooth behavior between root coiling and root waving (Fig. 3D), which is also evident in the gamma distribution fitting parameters A and B (Fig. 3E). This finding is consistent with our analysis of the reversal rates, where the wedge-shape trend in bearing angle ψ visibly narrows with increasing barrier tilt θ (Fig. 5B). Although the resolution of curvature measurements does not permit us to distinguish whether root skewing consists of low-amplitude root waving, we also note that the maximum angle with respect to gravity smoothly decreases across all three morphologies (Fig. 5C). Taken as a whole, these data reinforce the overall interpretation that divisions between phenotypes can be unified by the grow-and-switch mechanism proposed here.
Conclusion
Inspired by E. coli’s run-and-tumble approach to chemotaxis, our experiments and analysis indicate that Medicago’s coiling and root waving growth response arise from a grow-and-switch gravitropism. Whereas previous studies with Arabidopsis (25–30) have examined genetic and temporal properties of root waving, the interpretation proposed here is based on the observation of continuous transitions between root morphologies, as well as a statistical analysis of the root’s directional switching. The data show that switching events are related to the root’s growth direction with respect to gravity, and are governed by the root’s ability to measure the direction of gravity within some precision, which roughly corresponds to . Thus, just as bacterial chemotaxis is enabled by E. coli’s ability to measure a differential nutrient concentration along straight runs, Medicago’s ability to find the path of steepest descent is enabled by the root’s capacity to sense orientation relative to gravity. Because this growth strategy aids in navigating highly obstructed environments, we speculate that grow-and-switch gravitropism may have been an evolutionarily favorable trait for Medicago.
By casting root morphologies in a framework similar to E. coli chemotaxis, we offer a simplified model for phenotype regulation that can be further studied at genetic and biomolecular levels. The analogy to E. coli, however, was not unique, as a number of other cells, including amoeba, Dictyostelium discoideum, and mammalian neutrophils, show chemotactic behavior (40). In particular, Dictyostelium is known to execute directed drifts along nutrient gradients (41–43). This behavior is mediated by transient pseudopod formation fronts that drive changes in the direction of motion. Although there are multiple organisms that use this type of “try-and-correct” strategy, the microscopic origin for how directional switching is executed in Medicago remains to be discovered. One possibility is a time-delayed gravitropic signal measured by statoliths in the root tip (35–39) that propagate back to the elongation region. This proposal is consistent with time-lapse data (Fig. 3B and Movie S1) showing transient curvature reversal events, which may be related to the asymmetry between correct and incorrect reversals. Alternatively, differential elongation coupled to a touch-activated twisting mechanism previously reported in Medicago (24) may also generate the necessary switching. In either case, these hypothesized mechanisms may be distinguished by their ability to understand and predict the characteristic root waving length of cm. Ultimately, a set of detailed and in-depth experiments combining mechanical and biological approaches are required to further probe the origins of Medicago’s directional switching.
A benefit of the analogy between Medicago and E. coli proposed here is that studies already conducted in the context of bacterial chemotaxis can inspire new studies in root growth. For example, experiments that involve dynamically changing chemical gradients have helped probe the biochemical origins of chemotaxis. Inspired by such studies, we conceptually map these dynamic chemical gradients to dynamic gravity gradients, and consider the potential opportunities of a variably tilting barrier. This modification could be accomplished either with a barrier that has sections with different tilted angles or by attaching the sample box to a rotating stepper motor. In either case, one could explore the timescale for how long it takes roots to respond to changes in gravity gradients. Alternatively, taking inspiration from studies that examine bacterial quorum sensing, we could probe the analogous scenario of root−root interactions between same-species plants or species that are known to compete for resources (44). It would also be interesting to probe how roots respond to multiple conflicting tropisms by incorporating a nutrient gradient along a different direction than the gravity gradient. Such future studies aside, we expect that these efforts to better understand interactions between mechanical and biological regulation should enhance our understanding of root system architectures and the strategies plants use to navigate their environment.
Supplementary Material
Acknowledgments
We dedicate this work in memory of Chris Henley, whose tireless efforts have helped make this work possible. The authors thank A. Geitmann for insightful conversations. We also thank S. Gerbode and S. Imes for apparatus development. T.H.T. was supported by Cornell’s Engineering Learning Initiative. J.L.S. was supported by the National Science Foundation through a Graduate Research Fellowship. Research in the M.J.H. lab was supported by the National Science Foundation through Grants IOS-1127155 and IOS-1353367. C.L.H was supported by the US Department of Energy through Grant DE-FG02-89ER-45405. I.C. was supported by a National Science Foundation Grant DMR-1056662.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509942112/-/DCSupplemental.
References
- 1.Esmon CA, Pedmale UV, Liscum E. Plant tropisms: Providing the power of movement to a sessile organism. Int J Dev Biol. 2005;49(5-6):665–674. doi: 10.1387/ijdb.052028ce. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins WG, Hüner NPA. Introduction to Plant Physiology. Vol 355 Wiley; New York: 1995. [Google Scholar]
- 3.Hart JW. Plant Tropisms: And Other Growth Movements. Springer; New York: 1990. [Google Scholar]
- 4.Firn RD, Digby J. The establishment of tropic curvatures in plants. Annu Rev Plant Physiol. 1980;31(1):131–148. [Google Scholar]
- 5.Schrank AR. Plant tropisms. Annu Rev Plant Physiol. 1950;1:59–74. [Google Scholar]
- 6.Armon S, Efrati E, Kupferman R, Sharon E. Geometry and mechanics in the opening of chiral seed pods. Science. 2011;333(6050):1726–1730. doi: 10.1126/science.1203874. [DOI] [PubMed] [Google Scholar]
- 7.Gerbode SJ, Puzey JR, McCormick AG, Mahadevan L. How the cucumber tendril coils and overwinds. Science. 2012;337(6098):1087–1091. doi: 10.1126/science.1223304. [DOI] [PubMed] [Google Scholar]
- 8.Stuhlman O. A physical analysis of the opening and closing movements of the lobes of venus’ fly-trap. Bull Torrey Bot Club. 1948;75(1):22–44. [Google Scholar]
- 9.Williams SE, Bennett AB. Leaf closure in the venus flytrap: An acid growth response. Science. 1982;218(4577):1120–1122. doi: 10.1126/science.218.4577.1120. [DOI] [PubMed] [Google Scholar]
- 10.Hodick D, Sievers A. On the mechanism of trap closure of Venus flytrap (Dionaea muscipula Ellis) Planta. 1989;179(1):32–42. doi: 10.1007/BF00395768. [DOI] [PubMed] [Google Scholar]
- 11.Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433(7024):421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 12.Thitamadee S, Tuchihara K, Hashimoto T. Microtubule basis for left-handed helical growth in Arabidopsis. Nature. 2002;417(6885):193–196. doi: 10.1038/417193a. [DOI] [PubMed] [Google Scholar]
- 13.Ishida T, Kaneko Y, Iwano M, Hashimoto T. Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104(20):8544–8549. doi: 10.1073/pnas.0701224104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barley KP, Farrell DA, Greacen EL. The influence of soil strength on the penetration of a loam by plant roots. Soil Res. 1965;3(1):69–79. [Google Scholar]
- 15.Dexter AR. Mechanics of root growth. Plant Soil. 1987;98(3):303–312. [Google Scholar]
- 16.Bengough AG, Mullins CE. Mechanical impedance to root growth: A review of experimental techniques and root growth responses. J Soil Sci. 1990;41(3):341–358. [Google Scholar]
- 17.Bengough AG, Croser C, Pritchard J. A biophysical analysis of root growth under mechanical stress. In: Anderson HM, Barlow PW, Clarkson DT, Jackson M, Shewry PR, editors. Plant Roots—From Cells to Systems. Springer; New York: 1997. pp. 107–116. [Google Scholar]
- 18.Clark LJ, Whalley WR, Barraclough PB. How do roots penetrate strong soil? In: Abe JJ, editor. Roots: The Dynamic Interface Between Plants and the Earth. Springer; New York: 2003. pp. 93–104. [Google Scholar]
- 19.Bengough AG, McKenzie BM, Hallett PD, Valentine TA. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J Exp Bot. 2011;62(1):59–68. doi: 10.1093/jxb/erq350. [DOI] [PubMed] [Google Scholar]
- 20.Abdalla AM, Hettiaratchi DRP, Reece AR. The mechanics of root growth in granular media. J Agric Eng Res. 1969;14(3):236–248. [Google Scholar]
- 21.Whiteley GM, Dexter AR. The behaviour of roots encountering cracks in soil. Plant Soil. 1984;77(2-3):141–149. [Google Scholar]
- 22.Dexter AR, Hewitt JS. The deflection of plant roots. J Agric Eng Res. 1978;23(1):17–22. [Google Scholar]
- 23.Whiteley GM, Hewitt JS, Dexter AR. The buckling of plant roots. Physiol Plant. 1982;54(3):333–342. [Google Scholar]
- 24.Silverberg JL, et al. 3D imaging and mechanical modeling of helical buckling in Medicago truncatula plant roots. Proc Natl Acad Sci USA. 2012;109(42):16794–16799. doi: 10.1073/pnas.1209287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250(4978):274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- 26.Simmons C, Söll D, Migliaccio F. Circumnutation and gravitropism cause root waving in arabidopsis thaliana. J Exp Bot. 1995;46(1):143–150. [Google Scholar]
- 27.Migliaccio F, Piconese S. Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci. 2001;6(12):561–565. doi: 10.1016/s1360-1385(01)02152-5. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MV, Holbrook NM. Root-gel interactions and the root waving behavior of Arabidopsis. Plant Physiol. 2004;135(3):1822–1837. doi: 10.1104/pp.104.040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliva M, Dunand C. Waving and skewing: How gravity and the surface of growth media affect root development in Arabidopsis. New Phytol. 2007;176(1):37–43. doi: 10.1111/j.1469-8137.2007.02184.x. [DOI] [PubMed] [Google Scholar]
- 30.Migliaccio F, Fortunati A, Tassone P. Arabidopsis root growth movements and their symmetry: Progress and problems arising from recent work. Plant Signal Behav. 2009;4(3):183–190. doi: 10.4161/psb.4.3.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Floss DS, et al. Gene silencing in Medicago truncatula roots using RNAi. Methods Mol Biol. 2013;1069:163–177. doi: 10.1007/978-1-62703-613-9_12. [DOI] [PubMed] [Google Scholar]
- 32.Sperling LH. Introduction to Physical Polymer Science. Wiley; New York: 2005. [Google Scholar]
- 33.Berg HC, Brown DA. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 34.Berg HC. E. coli in Motion. Springer; New York: 2004. [Google Scholar]
- 35.Moulia B, Fournier M. The power and control of gravitropic movements in plants: A biomechanical and systems biology view. J Exp Bot. 2009;60(2):461–486. doi: 10.1093/jxb/ern341. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin KL, Strohm AK, Masson PH. Gravity sensing and signal transduction in vascular plant primary roots. Am J Bot. 2013;100(1):126–142. doi: 10.3732/ajb.1200318. [DOI] [PubMed] [Google Scholar]
- 37.Leitz G, Kang B-H, Schoenwaelder MEA, Staehelin LA. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. The Plant Cell Online. 2009;21(3):843–860. doi: 10.1105/tpc.108.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- 39.Yoder TL, Zheng HQ, Todd P, Staehelin LA. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 2001;125(2):1045–1060. doi: 10.1104/pp.125.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagorda A, Parent CA. Eukaryotic chemotaxis at a glance. J Cell Sci. 2008;121(Pt 16):2621–2624. doi: 10.1242/jcs.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerisch G. Chemotaxis in Dictyostelium. Annu Rev Physiol. 1982;44(1):535–552. doi: 10.1146/annurev.ph.44.030182.002535. [DOI] [PubMed] [Google Scholar]
- 42.Amselem G, Theves M, Bae A, Bodenschatz E, Beta C. A stochastic description of Dictyostelium chemotaxis. PLoS One. 2012;7(5):e37213. doi: 10.1371/journal.pone.0037213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graziano BR, Weiner OD. Self-organization of protrusions and polarity during eukaryotic chemotaxis. Curr Opin Cell Biol. 2014;30:60–67. doi: 10.1016/j.ceb.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paya AM, Silverberg JL, Padgett J, Bauerle TL. X-ray computed tomography uncovers root–root interactions: Quantifying spatial relationships between interacting root systems in three dimensions. Front Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.