Significance
Despite advances, a significant proportion of patients with Hodgkin’s lymphoma (HL) will not respond or will relapse. We demonstrated that up to seven infusions of 90Y-daclizumab, an anti-CD25–directed monoclonal antibody, provided responses in 50% of patients with relapsed HL. The daclizumab was directed primarily not at tumor cells themselves but toward nonmalignant T cells rosetting around the Reed–Sternberg cells. 90Y provided strong β emissions that killed antigen-nonexpressing tumor cells at a distance by a crossfire effect. Furthermore, the strong β irradiation killed normal cells in the tumor microenvironment that nurture the malignant cells in the lymphomatous mass. Therefore 90Y-daclizumab infusions provide meaningful therapy for select HL patients.
Keywords: radioimmunotherapy, 90Y-daclizumab, Hodgkin's lymphoma, myelodysplastic syndrome
Abstract
Despite significant advances in the treatment of Hodgkin’s lymphoma (HL), a significant proportion of patients will not respond or will subsequently relapse. We identified CD25, the IL-2 receptor alpha subunit, as a favorable target for systemic radioimmunotherapy of HL. The scientific basis for the clinical trial was that, although most normal cells with exception of Treg cells do not express CD25, it is expressed by a minority of Reed–Sternberg cells and by most polyclonal T cells rosetting around Reed–Sternberg cells. Forty-six patients with refractory and relapsed HL were evaluated with up to seven i.v. infusions of the radiolabeled anti-CD25 antibody 90Y-daclizumab. 90Y provides strong β emissions that kill tumor cells at a distance by a crossfire effect. In 46 evaluable HL patients treated with 90Y-daclizumab there were 14 complete responses and nine partial responses; 14 patients had stable disease, and nine progressed. Responses were observed both in patients whose Reed–Sternberg cells expressed CD25 and in those whose neoplastic cells were CD25− provided that associated rosetting T cells expressed CD25. As assessed using phosphorylated H2AX (γ-H2AX) as a bioindicator of the effects of radiation exposure, predominantly nonmalignant cells in the tumor microenvironment manifested DNA damage, as reflected by increased expression of γ-H2AX. Toxicities were transient bone-marrow suppression and myelodysplastic syndrome in six patients who had not been evaluated with bone-marrow karyotype analyses before therapy. In conclusion, repeated 90Y-daclizumab infusions directed predominantly toward nonmalignant T cells rosetting around Reed–Sternberg cells provided meaningful therapy for select HL patients.
Treatment with combination chemotherapy, radiation, and hematopoietic stem cell transplantation has increased the disease-free survival in Hodgkin’s lymphoma (HL) from less than 5% in 1963 to more than 80% at present (1–6). Recently the US Food and Drug Administration approved brentuximab vedotin for the treatment of relapsed HL (7). Furthermore the anti-PD1 agent pembrolizumab has shown promising results in classic HL (8). Nevertheless, a significant fraction of patients do not respond to treatment or subsequently relapse. To date more than 30 different mAb preparations directed toward antigens expressed by malignant Reed–Sternberg cells have been studied (6). These include mAbs linked to drugs or toxins targeting CD25 or CD30 expressed on Reed–Sternberg cells (6–11). Brentuximab vedotin, an anti-CD30 antibody drug conjugate, has induced a significant number of responses in refractory HL (7, 11). Although other antibody immunotoxins have demonstrated some clinical efficacy, they have yielded few complete responses (CRs) (6, 9, 10). An alternative strategy has been to arm mAbs with radionuclides. Radioimmunotherapy using 90Y–anti-ferritin and 131I–anti-CD30 antibodies has resulted in partial (PRs) and CRs in HL (12–15). Deficiencies with these approaches reflect the lack of tumor specificity of ferritin-targeted antibodies and the small number of CD30-expressing Reed–Sternberg cells in the tumor.
As an alternative, we identified CD25, the IL-2 receptor alpha subunit (IL-2Rα), as a more favorable target for systemic radioimmunotherapy of HL (16–22). The scientific rationale is that, with the exception of Treg cells, CD25 is not expressed by normal resting lymphoid cells, but it is expressed on both a minority of Reed–Sternberg cells and, critically, on T cells rosetting around Reed–Sternberg cells in HL (6, 23, 24). 90Y, an energetic β particle emitter with a mean tissue path length of 5 mm and a maximal path length of 11 mm, acts through “crossfire” throughout tumor masses, providing a strategy for killing tumor cells at a distance of several cell diameters, including Reed–Sternberg cells that lack CD25 expression provided that T cells in their vicinity express the target antigen (16, 23, 24). In the current phase II trial we treated 46 patients with recurrent or refractory HL with 90Y-daclizumab every 6–10 wk for up to seven doses, depending on hematological recovery. The activity of 90Y used in the present trial was determined on the basis of three previous phase I/II dose-escalation trials of 90Y–anti-CD25 performed in patients with lymphoproliferative disorders (16).
Results
Patient Characteristics.
Thirty consecutive patients with refractory or relapsed HL were studied between April 2003 and October 2007; an additional 16 consecutive patients were studied between November 2009 and June 2014. The patients had a median age of 35 y (range 22–77 y); 29 were men, and 17 were women (Table S1). Patients had received a median of five prior chemotherapy regimens (range, 1–21). Twenty-five had undertone autologous stem-cell transplantation alone, seven patients had both an autologous and an allogeneic transplant, and 14 did not have a transplant. Using immunohistochemical analysis, in only 10 biopsies (in 44 patients) did >10% of the HL neoplastic cells express CD25 (Fig. 1A), whereas the associated rosetting T cells expressed CD25 in all the 44 cases examined (Fig. 1B). Patients were treated with an initial dose of 15 mCi 90Y-daclizumab, with the exception that an initial dose of 10 mCi was used for patients who had received a prior stem cell transplant (Table S2). Patients without progressive disease and with hematological recovery received up to seven cycles of 15 mCi 90Y-labeled daclizumab. There was a 6-wk interval between cycles. A total of 189 cycles of treatment were administered to 46 patients with a median aggregate radiation dose of 39 mCi (range, 10–90 mCi).
Table S1.
Patient demographics
| Patient | Type of HL | Radiation | Transplant | Number of prior therapies | Age, y | Sex | Karnofsky score | Serum-soluble IL-2 receptor | Malignant cells positive for CD25 | CD25 expression by rosetting T cells |
| 1 | Classic | No | No | 4 | 34 | Male | 70 | 3,418 | Negative | Positive |
| 2 | Classic | Yes | No | 7 | 38 | Male | 90 | 644 | <10% | Positive |
| 3 | Classic | No | Allo/auto | 7 | 34 | Male | 90 | 3,454 | <10% | Positive |
| 4 | Classic | Yes | No | 4 | 58 | Female | 90 | <600 | >50% | Positive |
| 5 | Classic | Yes | Auto | 3 | 40 | Female | 90 | <1,200 | <10% | Positive |
| 6 | Classic | Yes | No | 2 | 24 | Male | 90 | 1,710 | Negative | Positive |
| 7 | Classic | Yes | Auto | 5 | 43 | Male | 90 | <1,200 | >50% | Positive |
| 8 | Classic | Yes | Auto | 3 | 24 | Male | 90 | 625 | Negative | Positive |
| 9 | Classic | Yes | Allo/auto | 8 | 41 | Female | 90 | <1,250 | Negative | Positive |
| 10 | Classic | Yes | No | 1 | 36 | Male | 90 | <620 | <10% | Positive |
| 11 | Classic | Yes | Allo/auto | 4 | 34 | Female | 80 | <1,230 | <10% | Positive |
| 12 | Classic | Yes | Auto | 3 | 64 | Male | 90 | <1,250 | >90% | Positive |
| 13 | Classic | Yes | Auto | 7 | 38 | Male | 90 | 700 | <10% | Positive |
| 14 | Classic | Yes | Auto | 5 | 24 | Male | 80 | <1,270 | Negative | Positive |
| 15 | Classic | Yes | No | 5 | 66 | Male | 80 | 4,460 | <10% | Positive |
| 16 | Classic | Yes | Allo/auto | 7 | 35 | Male | 90 | Not done | >25% | Positive |
| 17 | Classic | Yes | Auto | 6 | 27 | Male | 100 | <1,140 | Negative | Positive |
| 18 | Classic | Yes | Auto | 4 | 32 | Male | 90 | <1,135 | Negative | Positive |
| 19 | Classic | No | Auto | 3 | 22 | Male | 80 | 4,000 | Negative | Positive |
| 20 | Classic | No | Auto | 6 | 36 | Female | 90 | 1,550 | Negative | Positive |
| 21 | Classic | Yes | Auto | 3 | 41 | Male | 90 | <1,145 | >25% | Positive |
| 22 | Classic | No | Auto | 6 | 49 | Male | 90 | <1,135 | Negative | Positive |
| 23 | Classic | Yes | No | 5 | 32 | Female | 70 | 11,900 | <10% | Positive |
| 24 | Classic | Yes | Auto | 6 | 22 | Female | 90 | 755 | <10% | Positive |
| 25 | Classic | No | Auto | 4 | 67 | Male | 100 | 700 | Negative | Positive |
| 26 | Classic | Yes | No | 5 | 23 | Male | 100 | 580 | Negative | Positive |
| 27 | Classic | Yes | Auto | 3 | 31 | Female | 90 | <1,125 | Negative | Positive |
| 28 | Classic | Yes | Auto | 4 | 20 | Female | 90 | 2,200 | Negative | Positive |
| 29 | Classic | No | Auto | 6 | 31 | Female | 80 | 2,532 | Negative | Positive |
| 30 | Classic | Yes | Allo/auto | 21 | 28 | Male | 80 | 2,380 | Negative | Positive |
| 31 | Classic | Yes | No | 3 | 42 | Female | 80 | 2,946 | Positive | Positive |
| 32 | Classic | Yes | No | 5 | 77 | Female | 90 | 5,813 | Negative | Positive |
| 33 | Classic | No | Auto | 13 | 29 | Male | 2,744 | Positive | Positive | |
| 34 | Classic | Yes | Auto | 11 | 29 | Female | 90 | 4,504 | Negative | Positive |
| 35 | Classic | Yes | Auto | 8 | 36 | Female | 80 | 16,714 | Positive | Not done |
| 36 | Classic | Yes | No | 3 | 32 | Female | 100 | 348 | Weak focal | Positive |
| 37 | Classic | No | Auto | 7 | 29 | Male | 70 | 7,504 | Positive | Positive |
| 38 | Classic | Yes | Auto/allo | 17 | 30 | Male | 70 | 9,220 | Not done | Not done |
| 39 | Classic | Yes | Auto | 4 | 24 | Male | 100 | 511 | Negative | Positive |
| 40 | Classic | No | Auto | 5 | 41 | Male | 70 | 2,715 | Positive | Positive |
| 41 | NLP | Yes | No | 2 | 30 | Male | 90 | 1,250 | Negative | Positive |
| 42 | NLP | No | No | 2 | 36 | Male | 100 | <1,140 | Negative | Positive |
| 43 | NLP | No | Allo/auto | 16 | 38 | Male | 80 | 8,046 | Negative | Positive |
| 44 | NLP | Yes | Auto | 8 | 47 | Female | 90 | 2,487 | Negative | Positive |
| 45 | NLP | Yes | No | 7 | 27 | Female | 90 | 8,899 | Negative | Positive |
| 46 | NLP | No | Auto | 11 | 41 | Male | 70 | 7,452 | Positive for rare RS cells | Not done |
Allo, allogeneic transplant; Auto, autologous transplant; RS, Reed–Sternberg.
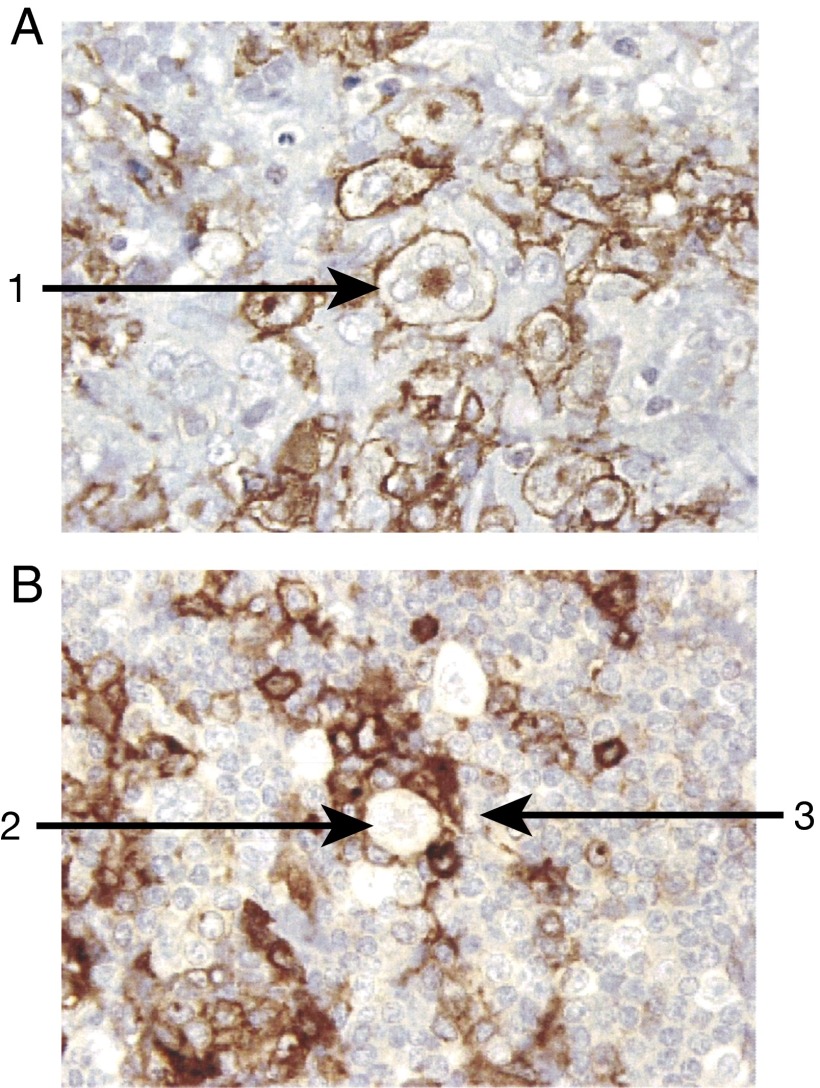
Fig. 1.
Histochemical analysis of CD25 expression was performed in patients with HL. (A) Ten patients manifested CD25+ expression by both the Reed–Sternberg HL cells and the associated rosetting polyclonal T cells (1). (B) Thirty-four patients manifested no expression of CD25 on the Reed–Sternberg cells (2) but expression of CD25 on the polyclonal T cells rosetting around the Reed–Sternberg cells (3).
Table S2.
Effect of 90Y-daclizumab therapy
| Patient | Total mCi per cycle, doses of 90Y-daclizumab administered | Maximum toxicity greater than grade 3 | Development of human antibodies to daclizumab | Response | Response duration, d | Progression-free survival, d | Overall survival, d |
| 1 | 15 × 4 | Anemia, myelodysplasia | No | CR | 314 | 400 | 1,289 |
| 2 | 15 × 6 | None | No | SD | NA | 280 | 735 |
| 3 | 10, 15 × 3 | None | No | CR | 230 | 298 | 947+ |
| 4 | 15 × 2 | Neutropenia | No | PD | NA | 75 | 259 |
| 5 | 10, 15 × 2 | Thrombocytopenia | No | PR | 163 | 240 | 864+ |
| 6 | 15, 5, 15 × 4, 10 | Neutropenia | Yes | CR | 323 | 441 | 824+ |
| 7 | 10, 15 × 5 | None | No | PR | 392 | 459 | 850+ |
| 8 | 10, 15 × 3 | Lymphocytopenia | No | PR | 114 | 140 | 488+ |
| 9 | 10 × 2 | Neutropenia | Yes | CR | 28 | 152 | 801+ |
| 10 | 10, 15 | Neutropenia | No | PD | NA | 97 | 97+ |
| 11 | 10, 15 × 4 | None | No | CR | 117 | 347 | 347+ |
| 12 | 10, 15 × 2 | Myelodysplasia | No | CR | 222 | 476 | 546+ |
| 13 | 10, 15 × 3 | Myelodysplasia | No | PR | 176 | 201 | 546+ |
| 14 | 10 | None | No | PD | NA | 27 | 81 |
| 15 | 10 | None | No | PD | NA | 68 | 138+ |
| 16 | 10, 15 × 4 | None | No | CR | 217 | 328 | 446+ |
| 17 | 10, 15 × 4 | Lymphocytopenia, pneumonia | No | CR | 245 | 355 | 421+ |
| 18 | 10, 15 × 5 | None | No | CR | 182 | 348 | 441+ |
| 19 | 10, 15 × 2 | Lymphocytopenia | No | SD | NA | 161 | 377 |
| 20 | 10, 15 × 2, 10, 5 | None | No | CR | 134 | 243 | 381+ |
| 21 | 10, 15 | Thrombocytopenia | No | SD | NA | 111 | 374+ |
| 22 | 10, 15, 15 | Lymphocytopenia | No | PR | 119 | 146 | 291+ |
| 23 | 15 | Thrombocytopenia, anemia | Yes | PD | NA | 26 | 85 |
| 24 | 10, 15, 10 | Lymphocytopenia, anemia, myelodysplasia | Yes | PR | 94 | 119 | 161 |
| 25 | 10, 15 | None | No | CR | 43+ | 110 | 143+ |
| 26 | 15 | None | No | PD | NA | 54 | 115+ |
| 27 | 10, 10 | Thrombocytopenia | No | PR | 40+ | 66 | 68+ |
| 28 | 10, 15 | Lymphocytopenia | No | SD | NA | 38 | 40+ |
| 29 | 10, 15 × 2 | None | No | SD | NA | 110 | 110+ |
| 30 | 10 | Pneumonitis/pulmonary infiltrates | No | PD | NA | 29 | 29+ |
| 31 | 15 × 3 | None | No | SD | NA | 112+ | 112+ |
| 32 | 15 × 3 | None | No | CR | 129 | 173 | 173+ |
| 33 | 10 × 3 | Thrombocytopenia | No | PR | 149 | 175 | 175+ |
| 34 | 10, 15 × 2 | None | No | SD | NA | 152 | 152+ |
| Anemia, lymphocytopenia | |||||||
| Neutropenia | |||||||
| 35 | 10 | Neutropenia | Yes | SD | 155 | ||
| 36 | 15 | None | No | SD | NA | 147 | 147+ |
| 37 | 10, 15 × 4 | None | No | SD | NA | 207 | 207+ |
| 38 | 10 × 2, 15 × 2 | None | No | SD | NA | 195 | 195+ |
| 39 | 10, 15 | None | No | SD | NA | 91 | 91+ |
| 40 | 10 | Anemia, lymphocytopenia, neutropenia | No | SD | NA | 31 | 31+ |
| 41 | 15 × 5 | Lymphocytopenia | No | CR | 720 | 788 | 788+ |
| 42 | 15 | Lymphocytopenia | Yes | SD | NA | 76 | 325+ |
| 43 | 10 × 2, 15 | Myelodysplasia | No | CR | 105 | 105 | 105+ |
| 44 | 10 | None | No | PD | NA | 28 | 28+ |
| 45 | 10, 15 × 4 | None | Yes | PR | 147 | 280 | 280+ |
| 46 | 10 | None | No | PD | NA | 32 | 32+ |
CR, complete remission; PD, progressive disease; PR, partial remission; SD, stable disease.
111In-Labeled Daclizumab Imaging.
Simultaneous with the administration of therapeutic 90Y-daclizumab, 111In-labeled daclizumab was administered to identify biodistribution and tumor targeting (Fig. 2). All patients, including those whose Reed–Sternberg cells did not express CD25, had positive localization of 111In-daclizumab at disease sites. The estimated tissue radiation dose per 15 mCi of 90Y-daclizumab was 173 cGy to bone marrow, 263 cGy to liver, 1,062 cGy to spleen, and 33 cGy to the whole body (Table S3). The estimated tumor dose with 15 mCi ranged from 210 to 365 cGy.
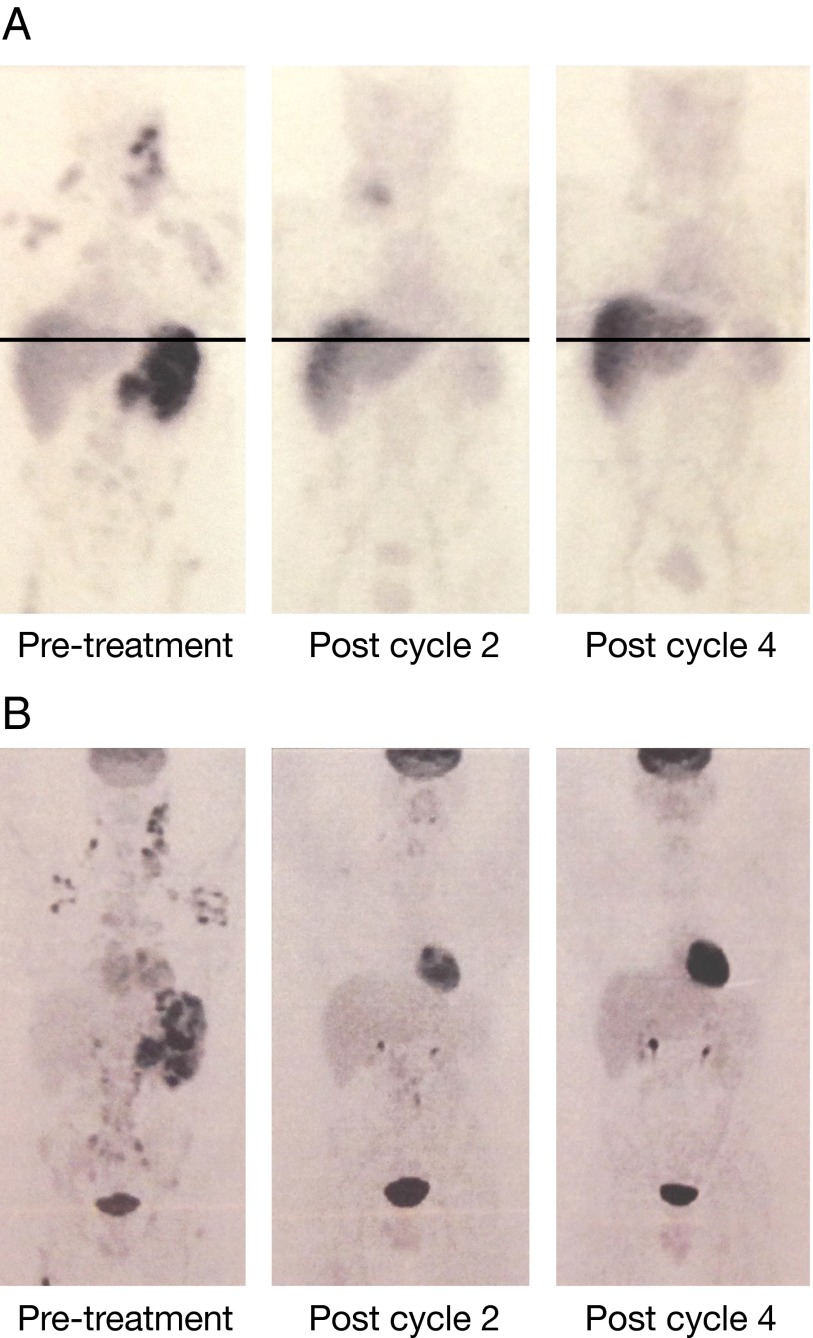
Fig. 2.
Clinical response of a patient with HL was demonstrated with 111In-daclizumab and FDG-PET. (A) 111In-daclizumab maximal intensity projection images (MIP) SPECT imaging studies of patient 1. (Left) At the time of the initial treatment with 90Y-daclizumab, there is localization in lymph nodes, bone, and spleen. (Center) After two treatments a decrease is seen in spleen, bone, and nodal uptake (the new uptake in the right base of the neck is related to central line placement). (Right) There is resolution of all abnormal uptake after four cycles. Findings with 111In-daclizumab were congruent with FDG findings. Note that the chest and abdomen/pelvis views were obtained in two separate acquisitions with slight overlap and then spliced together manually for display purposes. (B) Corresponding FDG-PET scans. (Left) Scan before treatment showing involvement in lymph nodes, spleen, and bone. (Center) At the time of the second treatment most of disease has resolved, with the exception of some nodes below the diaphragm. (Right) The images at the time of fourth treatment show complete resolution of disease with a progression-free survival of 400 d.
Table S3.
Tissue radiation dosimetry of 90Y-daclizumab humanized mAb
| Organ | 90Y-daclizumab radiation dose per number of mCi indicated delivered, REM | |||||
| 15 mCi | 30 mCi | 45 mCi | 60 mCi | 75 mCi | 90 mCi | |
| Red marrow | 172.5 | 345 | 517.5 | 690 | 862.5 | 1,035 |
| Lens of eye | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Ovaries | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Testes | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Breast | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Lung | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Thyroid | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Bone | 115.8 | 231.6 | 347.4 | 463.2 | 579 | 694.8 |
| Bladder wall | 30.15 | 60.3 | 90.45 | 120.6 | 150.8 | 180.9 |
| Heart | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Brain | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Spleen | 1,062 | 2,124 | 3,186 | 4,248 | 5,310 | 6,372 |
| Kidneys | 18.6 | 37.2 | 55.8 | 74.4 | 93 | 111.6 |
| Liver | 262.5 | 525 | 787.5 | 1,050 | 1,312.5 | 1,575 |
| Total body | 32.85 | 65.7 | 98.55 | 131.4 | 162.3 | 197.1 |
REM (Roentgen equivalent man), the dosage in rads that will cause the same amount of biological injury as one rad of X rays or gamma rays.
Toxicity.
There were no acute infusion reactions. Isolated patients had the following grade 3 or greater nonhematopoietic toxicities: grade 3 hypoalbuminemia, serum glutamic pyruvic transaminase (SGPT; alanine aminotransferase) elevation, hyperglycemia, hypocalcemia, lipase elevation, and grade 3 or 4 pneumonitis/pulmonary infiltrates. Thrombocytopenia and neutropenia were predominant toxicities initiating at weeks 4–5 after 90Y-daclizumab infusion, with nadirs usually occurring during weeks 5–7 (Table S4). Thrombocytopenia became more profound with the cumulative toxicity of multiple dosing. In seven patients, hematocytopenia, especially thrombocytopenia, persisted for more than 10 wk after treatment, requiring removal from the study. In all patients hematological values recovered to baseline values.
Table S4.
Toxicities of CTC grade 3 or higher
| Toxicity | No. patients (n = 46) |
| Lymphopenia | 10 |
| Neutropenia | 7 |
| Myelodysplastic syndrome (MDS) | 6 |
| Thrombocytopenia | 5 |
| Anemia | 5 |
| Pneumonia | 2 |
| Infusion reaction | 0 |
Six of the original 30 patients observed over a 7- to 11-y period following 90Y-daclizumab administration developed myelodysplastic syndrome (MDS) (Table S2). These six patients had had a mean of 6.2 prior therapies. Five had received an autologous hematopoietic stem cell transplant, and one had received an allogeneic transplant as well. Four had prior radiation to the lesions. These six patients received a mean of 41 mCi 90Y-daclizumab in a mean of 3.2 cycles of therapy. The mean time from diagnosis of HL to diagnosis of MDS was 99 mo, and the median time from the initiation of 90Y-daclizumab to the diagnosis of MDS was 7 mo (range, 6–63 mo). Three of these patients received chemotherapy between the last dose of 90Y-daclizumab and the diagnosis of MDS. In five of the six patients there was a disorder of chromosome 7, with other associated chromosomal abnormalities. The sixth patient had a translocation ± interstitial deletion of chromosome 6 and chromosome 9. A retrospective review revealed that one patient had cytogenetic aberrations on chromosomes 5 and 7 following chemotherapy before any systemic radioimmunotherapy with 90Y. The other five patients had not had a bone-marrow karyotype analysis before 90Y-daclizumab administration. On the basis of this observation the clinical trial protocol was amended to require pretreatment marrow cytogenetic studies before the initiation of systemic radioimmunotherapy and the exclusion of patients with chromosomal aberrations. Two patients found to have such aberrations at postchemotherapy evaluation were excluded from the study. None of the 16 patients entered with this criterion developed MDS over a mean 42-mo period of observation.
Immune Response to Daclizumab.
Five patients, including three responding patients, developed nonsymptomatic human anti-human antibody (HAHA) titers to daclizumab after one or more treatments.
Clinical Responses.
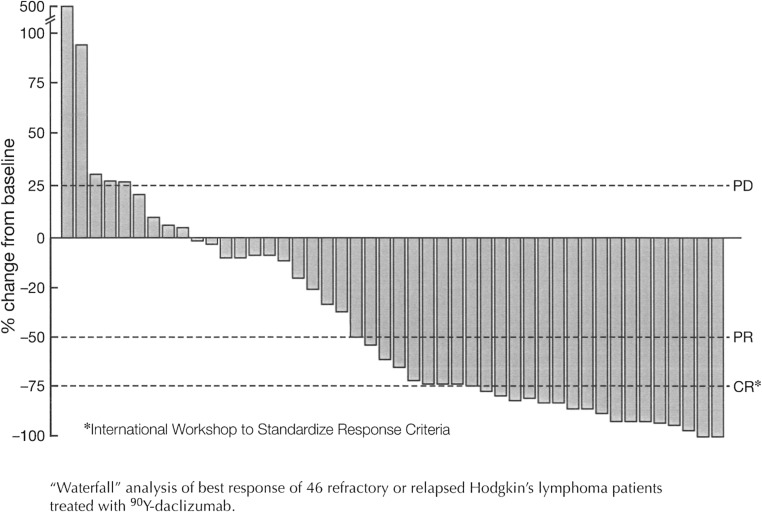
Of 46 patients with refractory or relapsed HL, as evaluated by Response Evaluation Criteria in Solid Tumors, who were treated with 90Y-daclizumab, 14 achieved a CR, nine had a PR, 14 had stable disease, and nine progressed (Table S2). Among 40 patients with classical HL there were eight PRs and 12 CRs; 13 had stable disease, and seven progressed. Among the six patients with the nodular lymphocyte-predominant (NLP) form of HL there were two CRs and one PR; one patient had stable disease, and two patients progressed. Tumor responses were evaluated by 111In-daclizumab single photon-emission computed tomography (SPECT) imaging (Fig. 2A), fluorodeoxyglucose (FDG) PET scans (Fig. 2B), and computed tomography (CT) scans (Fig. 3). The CRs were documented after two to five treatments. PRs or CRs were observed in 4 of the 10 patients (40%) with classical HL whose Reed–Sternberg cells expressed CD25 and in 19 of 36 patients (53%) whose malignant cells were CD25− but whose associated infiltrating T cells expressed CD25 (Fig. 1 and Tables S1 and S2). Freedom from progression ranged from 28 to 788 d with a median response duration of 151 d (range, 28–720 d). The median CR duration was 328 d, not counting the period in a PR (Figs. S1 and S2).
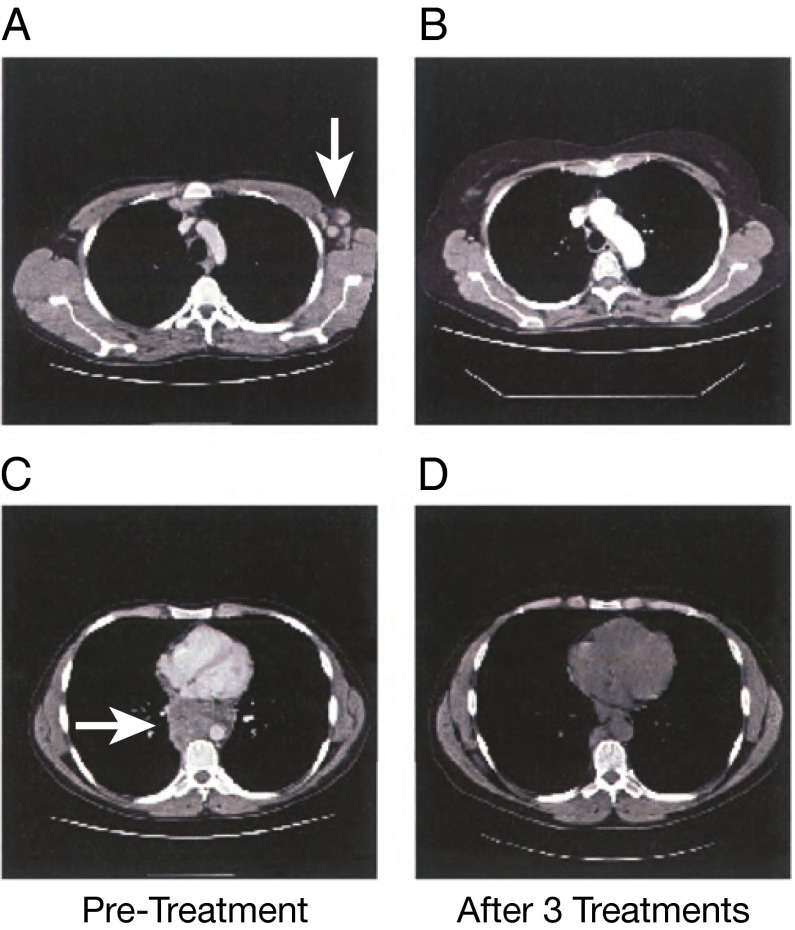
Fig. 3.
CT scans demonstrating clinical response of a patient with HL. (A and C) CT scans of the thorax of a patient before treatment show enlarged nodal disease (arrows). (B and D) After three cycles of 90Y-daclizumab therapy there was complete resolution of disease in three axillary nodes and almost complete resolution of posterior mediastinal disease.
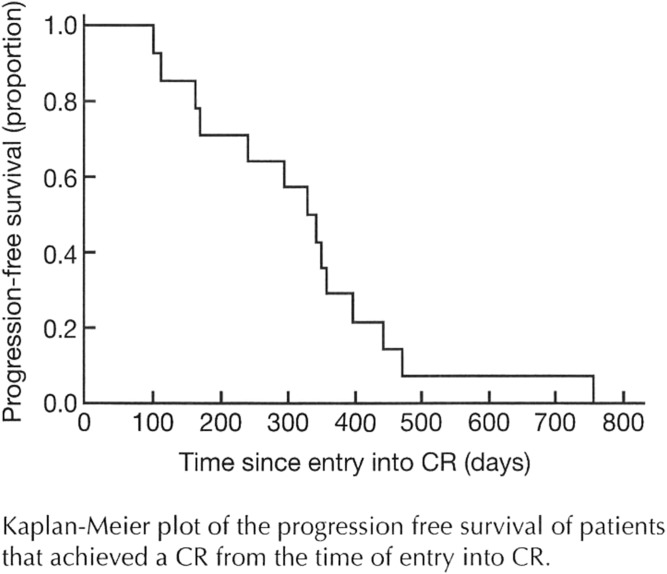
Fig. S1.
Kaplan–Meier plot of the progression-free survival of patients who achieved a CR, shown from the time of entry into CR.
Fig. S2.
Waterfall analysis of best response of 46 refractory or relapsed HL patients treated with 90Y-daclizumab.
Correlative Studies
Impact of Therapy on the Number of Circulating Treg Cells.
The number of circulating Treg cells in the 12 patients of the final group of 16 patients for whom adequate material was available before and after therapy was assessed by the number of CD3+CD25+ (7G7+) FoxP3+ cells before and after 90Y-daclizumab therapy. When assessed by the nonparametric Wilcoxon matched-pair signed-rank test on days 5–7 after administration of 90Y-daclizumab, there was a significant (P = 0.0044) reduction in the number of circulating Treg cells in each of the 12 cases examined, compared with the number pretherapy for the same patient. The reduction in Treg cells probably represents crossfire from radiolabeled antibody circulating in the blood, marrow, or spleen rather than depletion of Treg cells that is specific to antibody binding, because free Treg cells in the circulation would not be effectively exposed to crossfire of the antibody targeting them directly.
Immunohistochemistry to Define Apoptosis by Enumerating Cells Expressing Cleaved Caspase-3.
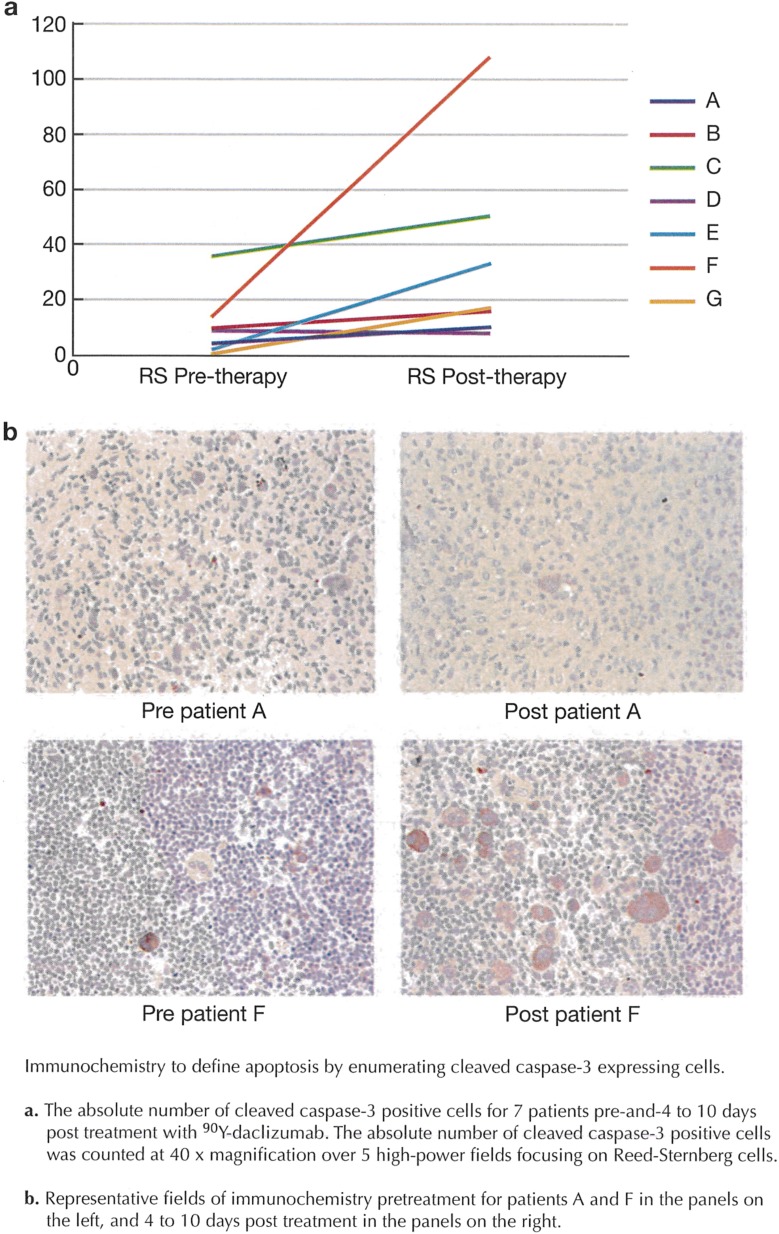
The apoptosis induced by 90Y-daclizumab therapy was defined by enumerating the percentage of cells expressing cleaved caspase-3 before and 4–10 d after treatment. The absolute number of cleaved caspase-3+ cells was counted at 40× magnification over five high-power fields. There was more evidence of apoptosis in the Reed–Sternberg cells in patients biopsied less than 1 wk after treatment than in patients biopsied 7–10 d after treatment (Fig. S3).
Fig. S3.
Immunochemistry to define apoptosis by enumerating cells expressing cleaved caspase-3. (A) The absolute number of cleaved caspase-3+ cells for seven patients before and 4–10 d after treatment with 90Y-daclizumab. The absolute number of cleaved caspase-3+ cells was counted at 40× magnification over five high-power fields focusing on Reed–Sternberg cells. (B) Representative immunochemistry fields for patients A and F before treatment (Left) and 4–10 d after treatment (Right).
Use of Phosphorylated H2AX as a Bioindicator of the Effects of the Exposure of HL Tissue to Radiation.
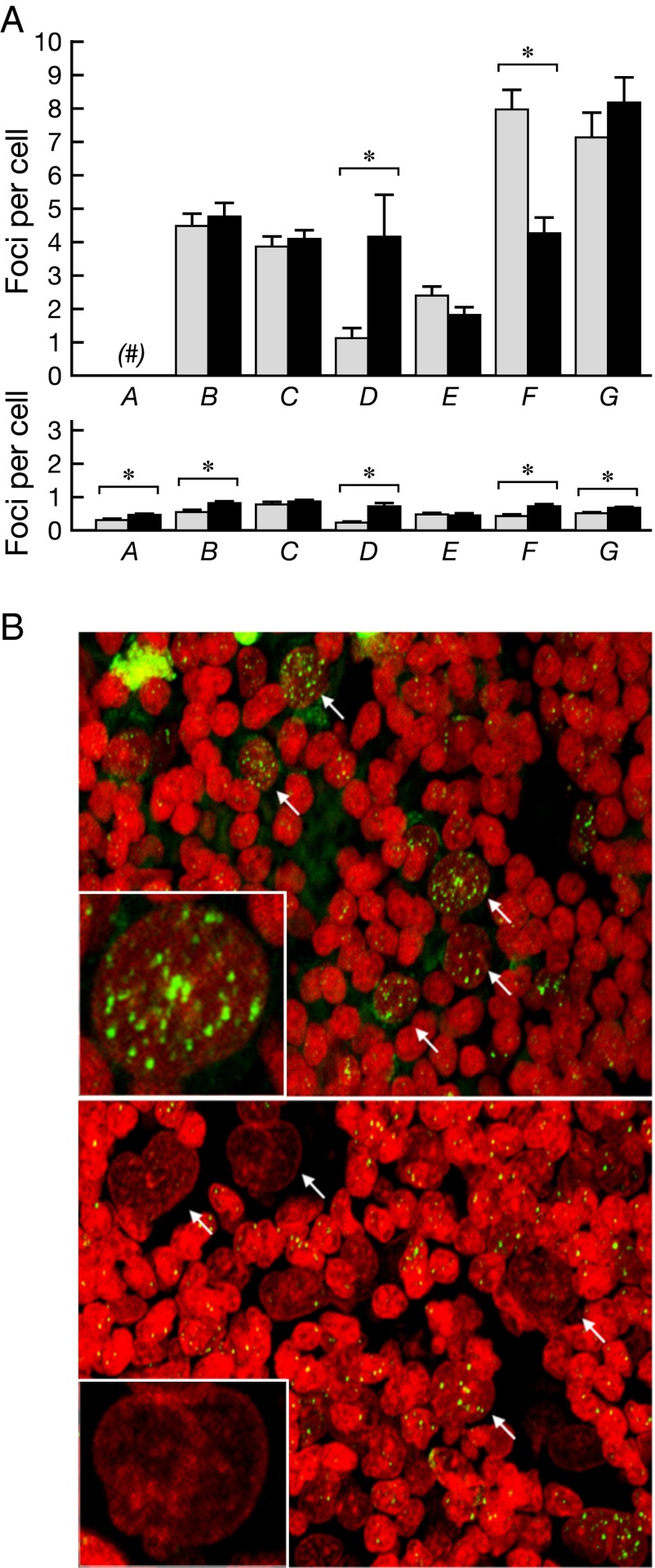
One major outcome from radiation exposure is the formation of DNA double-strand breaks (DSBs). DSBs can be enumerated with a sensitive assay based on the immunohistochemical visualization of phosphorylated H2AX (γ-H2AX) (25–29). γ-H2AX immunostaining was enumerated in both malignant cells and surrounding nonmalignant cells in tumor biopsies before and 4–10 d after 90Y-daclizumab infusions. Very modest increases in γ-H2AX expression were observed in malignant cells, which had 1 to more than 60 foci per cell, compared with the population of surrounding normal cells, which had less than one focus per cell on average (Fig. 4). Increased γ-H2AX incidence after 90Y-daclizumab therapy, as compared with the pretherapy value, was observed in malignant cells in four patients. In two patients, however, the γ-H2AX signal was reduced in malignant cells after 90Y-daclizumab therapy. In contrast to the modest effects on malignant cells, 90Y-induced DNA damage was more evident in nonmalignant cells in the tumor microenvironment, as reflected by the increased expression of γ-H2AX in six of the seven patients examined; in five of these six patients the increase in γ-H2AX expression following 90Y-daclizumab administration reached statistical significance.
Fig. 4.
Analysis of γ-H2AX in lymph node biopsies from patients undergoing 90Y-daclizumab therapy. (A) γ-H2AX incidence in malignant cells (Upper) and surrounding nonmalignant cells (Lower) is shown in six and seven patients, respectively, and is presented as the average number of foci per cell ± SE. Gray bars indicate incidence before therapy; black bars indicate incidence following therapy. Asterisks indicate a statistically significant difference (P < 0.05) in malignant or in surrounding nonmalignant cells before and after treatment. (B) Representative confocal images of biopsies from a patient before (Upper) and after (Lower) 90Y-daclizumab therapy. (Insets) Malignant cells at higher magnification. Note the high variation in the incidence of γ-H2AX foci among malignant cells. Green, γ-H2AX; red, DNA stained with propidium iodide. Arrows point to malignant cells.
Discussion
mAbs are among the most rapidly expanding classes of therapeutics for the treatment of cancer (21). Although not approved in this setting, rituximab (chimeric anti-CD20) is the only commercially available unmodified mAb routinely demonstrating antitumor activity in HL (6, 30). Arming of mAbs by linking them to cellular toxins, drug conjugates such as brentuximab vedotin (11), or radionuclides to target these agents specifically to tumors may provide valuable augmentation of antitumor activity. Indeed the US Food and Drug Administration has approved brentuximab vedotin for the treatment of relapsed HL after failure of autologous stem cell transplantation or after the failure of at least two prior multiagent chemotherapeutic regimens (7). Thirty-two percent of patients with HL had CRs, with a median response duration of 6.7 mo. Nevertheless there is a need for therapeutic agents in patients with refractory or relapsed HL following brentuximab vedotin. One advantage of the use of radiolabeled mAb conjugates for therapy is that, with appropriate choice of radionuclide, radiolabeled mAbs can kill cells at a distance of several cell diameters and thereby may kill antigen-negative tumor cells adjacent to antigen-expressing cells (6, 16, 31–33).
Various mAbs with different antigenic targets have been used to deliver targeted radioimmunotherapy (6, 12–16, 21, 31–33). The current study used daclizumab (humanized anti-Tac, i.e., anti-CD25; Zenapax) armed with the energetic β-particle emitter 90Y. Daclizumab targets the 55-kDa IL-2Rα (CD25) subunit that is constitutively expressed on Treg cells but not on other resting normal cells (18, 34). In contrast, CD25 is overexpressed on certain lymphoid malignancies, on activated T cells involved in autoimmune disorders, and in allograft rejection (16–22, 34). Increased CD25 expression has been demonstrated in anaplastic large-cell lymphoma, adult T-cell leukemia (ATL)/lymphoma, chronic lymphocytic leukemia, cutaneous T-cell lymphoma, hairy cell leukemia, some B-cell non-Hodgkin’s lymphomas, and HL (16–24, 35). Unmodified murine anti-CD25 was evaluated in a trial involving patients with ATL, a malignancy of mature CD4+CD25+ immunosuppressing T lymphocytes (17, 34). Seven of 19 treated patients responded to the antibody (17). In a subsequent study, 9 of 16 evaluable patients with ATL responded to 90Y-labeled murine anti-CD25 (16, 34).
In the present study, 46 patients with relapsed or refractory HL received 10–15 mCi of 90Y-daclizumab every 6–10 wk for up to seven doses. Toxicity in 40 of 46 cases was primarily limited to thrombocytopenia and granulocytopenia. In addition, at a median follow-up of 9 y, 6 of the initial 30 patients who had not had bone-marrow karyotype analyses before 90Y therapy developed MDS, but none of the 16 patients who had pretherapy cytogenetic evaluations developed MDS. The overall incidence of MDS for all 46 patients was 13%. The median time from the initiation of 90Y-daclizumab to the diagnosis of MDS was 37 mo. In one case, retrospective review revealed that the patient had cytogenetic aberrations on chromosomes 5 and 7 following chemotherapy but before systemic radioimmunotherapy with 90Y-daclizumab. Therefore, clinical trial entry criteria were changed to mandate a bone-marrow karyotype analysis before 90Y-daclizumab therapy and the exclusion of patients with aberrations. None of the 16 patients entered with this criterion developed MDS.
Therapy-associated MDS is a serious and well-recognized late complication that typically occurs after extensive exposure to alkylating agents and/or inhibitors of topoisomerase II in the treatment of lymphoid malignancy. The incidence of treatment-related MDS (tMDS) after therapy with the radiolabeled mAbs 90Y-ibritumomab tiuxetan and 131I-tositumomab has been analyzed in two large series (36, 37). Nineteen of 746 patients with non-Hodgkin’s lymphoma treated with ibritumomab tiuxetan developed tMDS or treatment-related acute myeloid leukemia (tAML) 1.9 y (range, 0.4–6.3 y) after radioimmunotherapy, for an incidence of 2.5% (36). Bennett and coworkers (37) reported tMDS/tAML in 35 (3.5%) of 995 patients, 40% of whom had documented preexisting MDS before radioimmunotherapy. No case of tMDS/tAML was reported in any of the 76 patients receiving 131I-tositumomab as initial therapy (38).
In our patients a mean of 9.9% (range, 5.8–21.7%) of the bone-marrow cells expressed CD25 before therapy with 90Y-daclizumab. The red marrow received 172.5 cGy of radiation per 15 mCi dose of 90Y-daclizumab (Table S1). The six patients who developed MDS received a mean of 3.2 doses. As noted above, one of the patients was found to have had aberrations of chromosomes 5 and 7 after chemotherapy but before the initiation of the systemic radioimmunotherapy. The present study was too small to draw meaningful conclusions about the relative roles played by the multiple courses (mean, 6.2) of chemotherapy that patients who developed MDS received before 90Y-daclizumab treatment and the role of the systemic radioimmunotherapy in the pathogenesis of MDS in the patients in this study. Nevertheless this serious complication represents a concern, and on the basis of this observation any subsequent clinical trial should require pretreatment cytogenetic studies of bone-marrow specimens before the initiation of systemic radioimmunotherapy.
In contrast to previous results with immunotoxins and with select alternative antigenic targets identified by radiolabeled antibodies, the response rate to 90Y-daclizumab therapy when used as a single agent was impressive. Among the 46 patients treated with multiple doses of 10–15 mCi 90Y-daclizumab, 14 achieved a complete remission, and 9 manifested a PR. In a separate study of unconjugated antibody using saturating amounts of daclizumab in 22 patients with Hodgkin’s disease, there were no PRs or CRs, and there were only five minor responses that did not meet the PR criteria of ≥50% reduction. Thus, the radionuclide 90Y appears to be critical in the responses observed. One factor that may be involved in the improved response is that, in contrast to most previous systemic radioimmunotherapy trials, we used repeated dosing that permitted an increase in the total dose of radiation delivered to the tumor. This approach can be compared with 90Y-ibritumomab that was capped at a maximum total dose of 32 mCi. However, a comparison of a single large dose with multiple lower doses would be of value. None of the 14 patients who achieved a complete remission did so following a single dose of 90Y-daclizumab; at least two to five administrations were required. A number of additional factors may underlie this greater level of efficacy as compared with immunotherapeutic agents that target other HL antigens. In particular, one limitation in targeting the antigen CD30 is that it is expressed predominantly on HL Reed–Sternberg cells that represent less than 1% of the lymphoid cells in the tumor mass. Thus, relatively few CD30 targets are available to bind the antibody. In contrast, CD25 is expressed by some Reed–Sternberg cells and is overexpressed on the polyclonal rosetting T cells associated with Reed–Sternberg cells (23, 24). This expression greatly increases the number of targets for binding the radiolabeled antibody, thereby increasing the amount of radiation delivered locally to the tumor. Another pivotal advantage is that 90Y has a high β-energy emission maximum, approximately five times greater than that of 131I, and delivers a significantly higher radiation dose to the tumor and within 5–11 mm of the site of decay. Therefore, 90Y-targeted mAbs can kill antigen-negative tumor cells through a crossfire effect from neighboring CD25-expressing T cells that have been targeted by the mAb. This ability to kill non–CD25-expressing malignant cells is pivotally important in the efficacy of 90Y-daclizumab in the majority of patients whose Reed–Sternberg cells did not express CD25 but whose tumor-associated T cells did express this antigen. Another mechanism supported by the γ-H2AX studies that could underlie the increased efficacy observed with the present strategy may be an effect of the β irradiation on normal cells in the tumor microenvironment that nurture the malignant cells in the lymphomatous mass.
Although 14 patients manifested a CR, the lack of cures indicates that monotherapy with this agent is not optimal. In our plans for patients who have not received an autologous bone-marrow transplant, we have initiated a clinical trial that includes the administration of escalating doses of 90Y-daclizumab followed by high-dose chemotherapy using carmustine, etoposide, cytarabine, and melphalan (BEAM) in association with an autologous hematological stem cell transplant using cells harvested from the patient before the administration of 90Y-daclizumab (39). This use of an autologous bone-marrow transplant should permit an escalation of 90Y-daclizumab doses, because the hematological stem cell transplant should ameliorate the otherwise dose-limiting hematological toxicity of the radiolabeled anti-CD25 mAb.
Materials and Methods
This study was approved by the Institutional Review Board of the National Cancer Institute, Protocol 95-C-110. This trial is registered at NCT00001575. All patients gave written informed consent.
Study Population.
Forty-six patients with histologically confirmed HL and expression of CD25 (IL-2Rα) on at least 10% of Reed–Sternberg cells in classical HL or on tumor-infiltrating T cells were entered into the study. (See SI Materials and Methods for further details.)
90Y-111In–Labeled Daclizumab.
Daclizumab (Hoffmann LaRoche, Nutley, NJ) was conjugated with 2-p-isothiocyanatobenzyl-transcyclohexyldiethylenetriamine penta-acetic acid (CHX-A) and was radiolabeled with 90Y for therapy and with 111In for imaging as described previously (16). (See SI Materials and Methods for further details.)
Study Design and Treatment.
This was a single-institution, nonrandomized, open-label phase II trial of up to seven infusions of 90Y-daclizumab in patients with refractory or relapsed HL. Patients without a prior stem cell transplant received 15 mCi 90Y-daclizumab with 5 mg of unlabeled daclizumab as the initial dose; patients with a prior transplant received an initial dose of 10 mCI. (See SI Materials and Methods for further details.)
Use of γ-H2AX as a Bioindicator for the Exposure of HL Tissue to Radiation.
γ-H2AX was used as a bioindicator of the effects on HL tissue of exposure to radiation using procedures described previously (25, 26). (See SI Materials and Methods for further details.)
SI Materials and Methods
Study Population.
Patients who had histologically confirmed classical HL with CD25 (IL-2Rα) expression on at least 10% of the Reed–Sternberg cells or who had either classical or NLP HL with CD25 (IL-2Rα) expression on tumor-infiltrating T cells were eligible. Because of the high incidence of CD25 positivity in T cells rosetting around Reed–Sternberg cells in HL, patients with CD25+ infiltrating T cells were eligible even if the Reed–Sternberg cells were negative.
Patients with stage II–IV HL were eligible if they had relapsed or failed to obtain a complete remission after first-line chemotherapy and had received an autologous bone-marrow transplant or were not eligible for or had refused salvage chemotherapy or an autologous bone-marrow transplantation. Omission of cytotoxic chemotherapy or other systemic therapy of malignancy for 3 wk before entering the trial was required. The patients were required to have an absolute granulocyte count of ≥1,200/μL, a platelet count ≥100,000/μL, and a serum creatinine level ≤2.0 mg/dL.
Patients had to have serum glutamic oxaloacetic transaminase (SGOT) and SGPT levels less than five times the upper limit of normal and bilirubin <3.0 unless the elevation was thought to be caused by HL or Gilbert’s disease. Patients could not have clinical cardiac failure or symptomatic pulmonary dysfunction unless it was caused by the underlying malignancy. Patients had to be at least 18 y old. Women of childbearing potential were tested for pregnancy, and pregnant patients were excluded from the study. Breastfeeding women were not eligible for the study. Patients who were HIV antibody-positive were excluded, as were patients with symptomatic disease that was caused by malignant involvement of the central nervous system or who had an active second primary cancer.
The Protocol followed the principles of the Helsinki Agreement and was approved by the National Cancer Institute internal review board. All patients gave written informed consent.
CD25 Antigen Expression.
Biopsies of patients’ HL lesions were assessed for CD25 expression on Reed–Sternberg cells and on tumor-infiltrating T cells by immunohistochemical staining as described previously (40). Bone marrow also was assessed for CD25 expression. Serum IL-2Rα (CD25) was measured by a specific ELISA (Biolegend, Bethesda, MD) (41).
90Y-Labeled Daclizumab.
Daclizumab (Zenapax) was obtained from Hoffmann-La Roche (Nutley, NJ). Daclizumab is a humanized IgG1 mAb directed against the IL-2–binding site of the human IL-2Rα subunit (CD25, Tac) (18, 34). Daclizumab was conjugated with CHX-A (42–44). Radiolabeling was performed using 90Y for therapy and 111In for imaging as described previously.
One milligram of CHX-A–chelated daclizumab was added to a polypropylene vial that served as a reaction vessel and was allowed to react with 90Y or 111In. Excess diethylene triamine pentaacetic acid (DTPA) (Sigma-Aldrich, St. Louis) then was added to the complex. Unreacted ionic metal isotopes and the daclizumab-bound fraction were separated by size-exclusion HPLC using a TSK-GEL G3000 column. The radioactivity in the final product was more than 98% protein bound as determined by instant TLC using Circa Gel impregnated with glass fiber sheets and by paper chromatography. The radiochemical purity was confirmed by analytical HPLC. The immunoreactivity of the radiolabeled daclizumab was confirmed to be greater than 85% binding to the CD25-expressing Kit-225–Ig leukemic T-cell line. The chelated antibody products passed sterility and pyrogen testing before use.
111In-Labeled Daclizumab Imaging.
Simultaneous with the administration of therapeutic 90Y-daclizumab, 111In-labeled daclizumab was administered to identify biodistribution and tumor targeting. Planar imaging was done 1–168 h post treatment; typically five sets of images were obtained over 7 d. In a majority of 111In administrations, a different chelator, 2-(4-isothiocyanatobenzyl) 6-methyl-diethylenetriamine pentaacetic acid (MX-DTPA), was used instead of CHX-A.
Study Design and Treatment.
This was a single-institution, nonrandomized, open-label phase II trial. Patients without a prior stem-cell transplant received 15 mCi 90Y-daclizumab with 5 mg of unlabeled daclizumab as the initial dose. Patients with a prior transplant received an initial dose of 10 mCi.
The purpose of the administration of 5 mg of unlabeled daclizumab along with 90Y-daclizumab was to reduce the binding of the radiolabeled antibody to circulating soluble IL-2Rα (sIL-2Rα) and thereby increase the proportion of the administered radiolabeled antibody that binds to CD25 expressed on rosetting T cells around Reed–Sternberg cells in the lymphomatous masses. Previously, we analyzed the impact of circulating IL-2Rα (CD25) on the bioactivity of the infused anti-CD25 (anti-Tac) antibody (the murine version of daclizumab) and analyzed the implications for dose selection in antibody immunotherapy (45). The effect of treatment with anti-Tac both as an unmodified antibody and as a carrier of radionuclides was analyzed in more than 100 individuals with CD25-expressing leukemia/lymphoma. Initially we became aware that occasionally surface CD25 antigen was unsaturated on the tumor cells in some patients despite adequate plasma antibody concentrations, and this failure to saturate cellular CD25 receptors correlated with high plasma levels of soluble CD25 (sIL-2Rα, sTac). We subsequently confirmed that sTac (CD25) in vivo could block anti-Tac binding to tumor cells and diminish antibody binding to CD25 (Tac)-expressing cells within tumor masses. Second, the bioactivity of antibody in vivo correlated directly with the amount of antibody infused and inversely with the sTac concentration. Finally, tumor targeting was achieved even in the presence of excess sTac, demonstrating a partition of antibody between soluble and cell-bound antigen. In a study performed to determine the loss in bioactivity from binding by circulating antigen, we demonstrated that, when exceedingly small doses of antibody were infused, each of the binding sites was occupied by the circulating antigen (CD25, sIL-2Rα), and the antibody could not bind to targets expressing cellular CD25. We performed analyses to define the quantity of antibody that had to be delivered to ensure that 90% of the radiolabeled anti-CD25 antibody was bindable after the infusion. These analyses showed that, to achieve 90% bindability of the infused antibody, one must administer 2.5 mg of anti-Tac per 10,000 units/mL of circulating sTac (sIL-2Rα) (one unit equals 3.3 pg). We previously determined that with low levels of serum IL-2Rα (sTac), such as those manifested in the patients with HL (all but two of the patients in this study had serum IL-2Rα levels <10,000 pg/mL) (Table S1), a 5-mg dose of unlabeled daclizumab was sufficient to saturate all sites of circulating IL-2Rα. Finally, when we reviewed the 111In imaging in the previous study, we found that binding of the administered radiolabeled antibody to the tumor cells could be achieved with the low 5-mg dose of unmodified anti-Tac administered in this study.
In summary, on the basis of our previous studies and the principles developed for predicting the binding activity of administered antibody in the presence of soluble antigen, the 5 mg of unmodified daclizumab was deemed optimal to overcome the circulating sTac (CD25, sIL-2Rα) levels without unduly reducing the specific activity of the radiolabeled antibody.
As a safety measure, patients who had undergone a prior stem-cell transplant received an initial dose of 10 mCi 90Y-daclizumab of with 5 mg of unmodified daclizumab. With the initial and last scheduled treatment, patients also received 5 mCi 111In-daclizumab for concurrent imaging of antibody uptake by tumor (46). To enhance the urinary excretion of free 90Y, trisodium calcium diethylenetriaminepentaacetate (Ca-DTPA) (Akorn, Inc., Ann Arbor, MI), 250 mg⋅m−2⋅d−1, was administered i.v. once daily for 3 d beginning immediately after the infusion of 90Y-daclizumab (47). After the first cycle, patients could receive 15 mCi 90Y-daclizumab per cycle every 6–10 wk, to a maximum of seven doses or two doses beyond a complete remission, provided that they did not develop circulating antibodies to daclizumab and achieved a hematological recovery (defined as a platelet count ≥100,000/µL and an absolute neutrophil count ≥1,000/µL) within 10 wk and that the patient’s disease was stable or responding to treatment at the 4-wk posttreatment evaluation point.
The dose of 90Y given as 90Y-daclizumab for the phase II study was determined on the basis of three previous clinical trials (16). We performed a phase I trial of yttrium-labeled anti-Tac (the anti-CD25 mAb, parent antibody of daclizumab) in 16 patients with ATL. On the basis of this phase I/II trial the maximum tolerated dose (MTD) (predominantly in previously untreated patients) was 25 mCi per dose (16). Subsequently, in a phase I/II study of 90Y-labeled humanized anti-Tac mAb (daclizumab) in Tac-expressing ATL, the MTD for the 20 patients in the phase I element was determined to be 25 mCi. Nine responses were observed in these 20 patients. Subsequently a phase I/II study of 90Y-radiolabeled daclizumab was conducted in Tac-expressing malignancies other than ATL. Nine patients were entered into the phase I aspect of this trial, and the MTD was determined to be 15 mCi because prolonged grade III/IV thrombocytopenia was observed at the 25-mCi dose in patients who previously had received multiple courses of chemotherapy and an autologous or allogeneic of bone-marrow stem cell transplant. On the basis of these previous phase I/II trials, we chose the 15-mCi dose for the present study. Patients who had received a previous bone-marrow transplant received 10 mCi as the initial dose. We chose an interval of 6–10 wk between therapy cycles based on our previous observation that maximum hemocytopenia was observed at weeks 5–7. We required that hematological recovery occur within 10 wk because previous studies had shown prolonged thrombocytopenia in patients who did not manifest a hematological recovery of platelet counts within 10 wk (34). We limited the administration to a maximum of seven doses, because in previous studies that permitted nine doses prolonged thrombocytopenia was observed in select patients who received more than seven doses of 90Y-daclizumab (34).
Daclizumab Pharmacokinetics, Dosimetry, and Imaging.
Computer-assisted analysis of images obtained from 111In-daclizumab scintigraphy and serial pharmacokinetic estimates were used for dosimetry. Pre- and posttreatment 111In-daclizumab imaging studies were evaluated for the extent of tumor involvement. Patients receiving 111In-daclizumab underwent serial quantitative planar imaging using a two-headed gamma camera (47). In addition SPECT was performed to assess antibody targeting. Dosimetry estimates were obtained using Medical Internal Radionuclide Dose Committee (MIRDC) schema methods accounting only for the β dose (48), and marrow doses were estimated as previously described by Sgouros (49).
Toxicity and Immunogenicity.
Toxicity was evaluated and scored according to the Cancer Therapy and Evaluation Program (CTEP) Common Toxicity Criteria (CTC) version 3.0. Patients were evaluated for the development of antibodies to daclizumab before the study and 4–6 wk after each treatment using a two-arm capture ELISA (50).
Tumor Response.
Tumor response was defined by the Internal Working Group Criteria (51).
Response was assessed by patient history, physical examination, and CT 4–6 wk after each treatment. In addition, pretreatment and posttreatment 111In-daclizumab imaging studies were evaluated for lymph node visualization.
Correlative Studies.
Immunohistochemistry to define apoptosis by enumerating cells expressing cleaved caspase-3.
For immunohistochemistry on formalin-fixed paraffin-embedded tissue sections, we used cleaved caspase-3 rabbit polyclonal antibody (Cell Signaling, Danvers, MA catalog no. 9661) at a 1:200 dilution. After deparaffinization, the slides were retrieved with citrate buffer (pH 6.0) and incubated for 1 h at room temperature. An automated system (BenchMark XT; Ventana Medical Systems, Oklahoma City, OK) was used for detection.
Use of γ-H2AX as a bioindicator of the effects on HL tissue of exposure to radiation.
Paraffin-embedded skin tissue sections (10 µm) were mounted on superfrosted slides. Sections were dewaxed in xylene, hydrated in a graded alcohol series, and placed in PBS. Slides were kept at 4 °C in 70% ethanol until use. After a 15-min washing in PBS, slides were placed in PBS/0.05% Tween 20 for 10 min and then were treated for 25 min at 95 °C in 0.01 M sodium citrate/0.05% Tween 20 (pH 6). Slides were cooled to room temperature in the citrate solution for 20 min and were placed in PBS/0.05% Tween 20 for 10 min. Staining for γ-H2AX and mounting processes were performed as described previously (25), but all antibody incubations and washing times were increased by 50%. The primary antibody was a mouse monoclonal anti–γ-H2AX antibody (Abcam, Cambridge, MA), and the secondary antibody was a goat anti-mouse Alexa-488–conjugated IgG (Invitrogen). Laser-scanning confocal microscopy was performed with a Nikon PCM 2000 (Nikon Inc.). Eight optical sections (1 µm each) through the thickness of the tissue were imaged and combined in a maximum projection. γ-H2AX foci in nonmalignant cells were counted visually in single optical sections, and combined maximum projection was used to enumerate foci in the bigger malignant cells. The foci were counted visually in 39–300 cancer cells and in more than 1,000 normal cells. All values in this study are expressed as mean ± SE. The significant differences between the groups were analyzed by a Student's t test; a P value of <0.05 was considered significant.
Statistics.
Progression-free survival, overall survival, and response duration were determined from the time of initial treatment. Descriptive statistics were used including the median value for the above parameters. For the development of MDS the crude incidence, the median time from HL diagnosis to MDS diagnosis, the median time from treatment with 90Y to diagnosis of MDS, and the annualized rates of MDS after 90Y treatment were provided.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH, and by the National Institute for Allergy and Infectious Diseases Radiation/Nuclear Countermeasures Program.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 12907.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516107112/-/DCSupplemental.
References
- 1.DeVita VT, Jr, Hubbard SM. Hodgkin’s disease. N Engl J Med. 1993;328(8):560–565. doi: 10.1056/NEJM199302253280808. [DOI] [PubMed] [Google Scholar]
- 2.Canellos GP, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478–1484. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 3.Longo DL, et al. Treatment of advanced-stage Hodgkin’s disease: Alternating noncrossresistant MOPP/CABS is not superior to MOPP. J Clin Oncol. 1991;9(8):1409–1420. doi: 10.1200/JCO.1991.9.8.1409. [DOI] [PubMed] [Google Scholar]
- 4.Diehl V, et al. German Hodgkin’s Lymphoma Study Group Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348(24):2386–2395; dosage error in text corrected in N Engl J Med (2005) 353(7):744. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz N, et al. German Hodgkin’s Lymphoma Study Group; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 6.Schnell R, Borchmann P, Schulz H, Engert A. Current strategies of antibody-based treatment in Hodgkin’s disease. Ann Oncol. 2002;13(Suppl 1):57–66. doi: 10.1093/annonc/13.s1.57. [DOI] [PubMed] [Google Scholar]
- 7.de Claro RA, et al. U.S. Food and Drug Administration approval summary: Brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18(21):5845–5849. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
- 8.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engert A, et al. A phase-I study of an anti-CD25 ricin A-chain immunotoxin (RFT5-SMPT-dgA) in patients with refractory Hodgkin’s lymphoma. Blood. 1997;89(2):403–410. [PubMed] [Google Scholar]
- 10.Kreitman RJ, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18(8):1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 11.Younes A, et al. 2008. Objective responses in a phase I dose-escalation study of SGN-35, a novel antibody-drug conjugate (ADC) targeting CD30, in patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 26(15S): Abstract 8526.
- 12.Vriesendorp HM, et al. Phase I-II studies of yttrium-labeled antiferritin treatment for end-stage Hodgkin’s disease, including Radiation Therapy Oncology Group 87-01. J Clin Oncol. 1991;9(6):918–928. doi: 10.1200/JCO.1991.9.6.918. [DOI] [PubMed] [Google Scholar]
- 13.Vriesendorp HM, et al. Fractionated radiolabeled antiferritin therapy for patients with recurrent Hodgkin’s disease. Clin Cancer Res. 1999;5(10) Suppl:3324s–3329s. [PubMed] [Google Scholar]
- 14.Decaudin D, et al. Radioimmunotherapy (RIT) of refractory or relapsed Hodgkin’s lymphoma (HL) with 90yttrium- labeled antiferritin antibody. Haematologia. 2007;92(Suppl 5):78–79. [Google Scholar]
- 15.Schnell R, et al. Treatment of refractory Hodgkin’s lymphoma patients with an iodine-131-labeled murine anti-CD30 monoclonal antibody. J Clin Oncol. 2005;23(21):4669–4678. doi: 10.1200/JCO.2005.09.098. [DOI] [PubMed] [Google Scholar]
- 16.Waldmann TA, et al. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac. Blood. 1995;86(11):4063–4075. [PubMed] [Google Scholar]
- 17.Waldmann TA, et al. The interleukin-2 receptor: A target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood. 1993;82(6):1701–1712. [PubMed] [Google Scholar]
- 18.Queen C, et al. A humanized antibody that binds to the interleukin 2 receptor. Proc Natl Acad Sci USA. 1989;86(24):10029–10033. doi: 10.1073/pnas.86.24.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldmann TA, et al. Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. J Clin Invest. 1984;73(6):1711–1718. doi: 10.1172/JCI111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchiyama T, Broder S, Waldmann TA. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981;126(4):1393–1397. [PubMed] [Google Scholar]
- 21.Waldmann TA, Morris JC. Development of antibodies and chimeric molecules for cancer immunotherapy. Adv Immunol. 2006;90:83–131. doi: 10.1016/S0065-2776(06)90003-0. [DOI] [PubMed] [Google Scholar]
- 22.Vincenti F, et al. Daclizumab Triple Therapy Study Group Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med. 1998;338(3):161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 23.Agnarsson BA, Kadin ME. The immunophenotype of Reed-Sternberg cells. A study of 50 cases of Hodgkin’s disease using fixed frozen tissues. Cancer. 1989;63(11):2083–2087. doi: 10.1002/1097-0142(19890601)63:11<2083::aid-cncr2820631102>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Hsu SM, Yang K, Jaffe ES. Phenotypic expression of Hodgkin’s and Reed-Sternberg cells in Hodgkin’s disease. Am J Pathol. 1985;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- 25.Redon CE, et al. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS One. 2010;5(11):e15544. doi: 10.1371/journal.pone.0015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonner WM, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8(12):957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lassmann M, et al. In vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2010;51(8):1318–1325. doi: 10.2967/jnumed.109.071357. [DOI] [PubMed] [Google Scholar]
- 28.Sedelnikova OA, Bonner WM. GammaH2AX in cancer cells: A potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5(24):2909–2913. doi: 10.4161/cc.5.24.3569. [DOI] [PubMed] [Google Scholar]
- 29.Löbrich M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci USA. 2005;102(25):8984–8989. doi: 10.1073/pnas.0501895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekstrand BC, et al. Rituximab in lymphocyte-predominant Hodgkin disease: Results of a phase 2 trial. Blood. 2003;101(11):4285–4289. doi: 10.1182/blood-2002-08-2644. [DOI] [PubMed] [Google Scholar]
- 31.Witzig TE, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin’s lymphoma. J Clin Oncol. 2003;21(7):1263–1270. doi: 10.1200/JCO.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 32.Press OW, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329(17):1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 33.Kaminski MS, et al. Radioimmunotherapy of B-cell lymphoma with [131I]anti-B1 (anti-CD20) antibody. N Engl J Med. 1993;329(7):459–465. doi: 10.1056/NEJM199308123290703. [DOI] [PubMed] [Google Scholar]
- 34.Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: A 25-year personal odyssey. J Clin Immunol. 2007;27(1):1–18. doi: 10.1007/s10875-006-9060-0. [DOI] [PubMed] [Google Scholar]
- 35.Strauchen JA, Breakstone BA. IL-2 receptor expression in human lymphoid lesions. Immunohistochemical study of 166 cases. Am J Pathol. 1987;126(3):506–512. [PMC free article] [PubMed] [Google Scholar]
- 36.Czuczman MS, et al. Treatment-related myelodysplastic syndrome and acute myelogenous leukemia in patients treated with ibritumomab tiuxetan radioimmunotherapy. J Clin Oncol. 2007;25(27):4285–4292. doi: 10.1200/JCO.2006.09.2882. [DOI] [PubMed] [Google Scholar]
- 37.Bennett JM, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine I131 tositumomab. Blood. 2005;105(12):4576–4582. doi: 10.1182/blood-2004-12-4690. [DOI] [PubMed] [Google Scholar]
- 38.Kaminski MS, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352(5):441–449. doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 39.Waldmann TA, Sportes C. (Protocol # NCI-12-C-0003) Clinical Trials gov. Identifier NCT01468311. Phase I/II trial of yttrium-90–labeled daclizumab (anti-CD25) radioimmunotherapy with high-dose BEAM chemotherapy and autologous hematopoietic stem cell rescue in recurrent and refractory Hodgkin’s lymphoma.
- 40.Janik JE, et al. Elevated serum-soluble interleukin-2 receptor levels in patients with anaplastic large cell lymphoma. Blood. 2004;104(10):3355–3357. doi: 10.1182/blood-2003-11-3922. [DOI] [PubMed] [Google Scholar]
- 41.Rubin LA, et al. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135(5):3172–3177. [PubMed] [Google Scholar]
- 42.Brechbiel MW, Gansow OA. 1992. Synthesis of C-functionalized derivatives of trans-cyclohexyldiethylenetriaminepenta-acetic acids for labeling of monoclonal antibodies with the bismuth -212 alpha-particle emitter. J Chem Soc, Perkin Trans 1(9):1173–1178.
- 43.Wu C, Gansow OA, Brechbiel MW. Evaluation of methods for large scale preparation of antibody ligand conjugates. Nucl Med Biol. 1999;26(3):339–342. doi: 10.1016/s0969-8051(98)00112-7. [DOI] [PubMed] [Google Scholar]
- 44.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat Rev Drug Discov. 2004;3(6):488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 45.Junghans RP, Carrasquillo JA, Waldmann TA. Impact of antigenemia on the bioactivity of infused anti-Tac antibody: Implications for dose selection in antibody immunotherapies. Proc Natl Acad Sci USA. 1998;95(4):1752–1757. doi: 10.1073/pnas.95.4.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrasquillo JA, et al. Similarities and differences in 111In- and 90Y-labeled 1B4M-DTPA antiTac monoclonal antibody distribution. J Nucl Med. 1999;40(2):268–276. [PubMed] [Google Scholar]
- 47.Kroll H, et al. Excretion of yttrium and lanthanum chelates of cyclohexane 1,2-trans diamine tetraacetic acid and diethylenetriamine pentaacetic acid in man. Nature. 1957;180(4592):919–920. doi: 10.1038/180919b0. [DOI] [PubMed] [Google Scholar]
- 48.Loevinger R, Berman M. 1976. Calculating the absorbed dose from biologically distributed radionuclides (The Society of Nuclear Medicine, New York)
- 49.Sgouros G. Bone marrow dosimetry for radioimmunotherapy: Theoretical considerations. J Nucl Med. 1993;34(4):689–694. [PubMed] [Google Scholar]
- 50.Nussenblatt RB, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: A phase I/II clinical trial. Proc Natl Acad Sci USA. 1999;96(13):7462–7466. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheson BD, et al. NCI Sponsored International Working Group Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]