Abstract
AIM: To investigate the expression of human telomerase reverse transcriptase gene (hTRT) in gastric cancer (GC) and its relevance with cell cycle regulators including P16INK4, cyclin and P53.
METHODS: In situ hybridization (ISH) for hTRT mRNA was performed in 53 cases of gastric cancer and adjacent cancerous tissues. Immunohistochemical staining (S-P method) for hTRT protein, P16INK4, cyclinD1 and P53 was performed in 53 cases of GC and adjacent cancerous tissues.
RESULTS: Of 53 cases of GC, the expression of hTRT mRNA and hTRT protein was significantly higher than the expression of hTRT mRNA and hTRT protein in adjacent canerous tissues (P < 0.01), the positive rates of hTRTmRNA and hTRT protein were 79.2% and 88.6%. There was a stastical difference of the expression of hTRT protein among well differentiated adenocarcinoma, poorly differentiated adenocarcinoma and mucoid carcinoma. And there was a highly significant positive correlation between the expression of hTRT mRNA and hTRT protein (r = 0.625, P < 0.01). However, the expression of hTRT mRNA and its protein in GC were not related with other clinicopathological parameters including gender, age, location and size of neoplasm, invasion depth, lymph node metastasis and clinical stage. There was a significant positive correlation between the expression of hTRT mRNA and cyclinD1 protein (r = 0.350, P < 0.01). There was a significant positive correlation between the expression of cyclinD1 protein and hTRT protein (r = 0.549, P < 0.01), so was between P53 and hTRT protein (r = 0.319, P < 0.05).
CONCLUSION: The expression of hTRT gene is correlated significantly to the specific defects of cell cycle on G1/S check point ; telomerase activity may depend on cell cycle in gastric cancer and it is available to clarify the molecular mechanism of telomerase activity regulation. The expression of hTRT mRNA and hTRT protein in GC is significantly different from the expression of hTRT mRNA and hTRT protein in adjacent cancerous tissue which indicates that these targets are correlated closely to the occurrence of GC and can provide important morphologic index for diagnosis of GC.
INTRODUCTION
Telomerase activity is absent in most normal somatic cells, but has been detected in the tissues of vast majority of human malignant neoplasm by a highly sensitive method of Trap-PCR assay, and the positive of telomerase activity is 85%. Currently, it may be the broadest-spectrum molecular marker of malignant neoplasm[1]. Human telomerase is composed of human telomerase RNA(hTR), human telomerase reverse transcriptase (hTRT/hTERT as well as human telomerase catalytic subunit, hEst2) and human telomerase associated protein (TP) which connects two subunit concerned above[2-5]. High level hTRT expression has been dectected in primary carcinoma, cancer cell lines and tissues which express telomerase activity. There is a consistent correlation between hTRT and telomerase activity. Meanwhile, we can’t detect hEst2/hTRT in cell lines whose telomerase activity is negative and in well differentiated tissues[6-8]. Therefore, Meyseron proposed that the expression of hEst2/hTRT mRNA is a critical step in cell immortalization and tumorigenesis[3].
It has been reported in telomere-telomerase hypothesis that the senescence process of human cell can be divided into two stages, one is the mortality stage 1(M1), another is the mortality stage 2(M2). When telomerase shortens to a critical length 2kb-4kb, the stability of chromosome will be damaged and the cells enter the senescence stage that is M1. At this stage, DNA breaks with activation of P53-dependent or-independent DNA damage pathways, and the DNA damage can also induce products of CDk3 inhibitors such as p21, p27 and lead to the G1 block and eventual death[9]. If certain tumor suppressor genes PRb, P53 or P16 are inactivated and are deprived of normal function, cells will prolong their life span, but usually not to immortalization. Then telomere further decreases to M2 stage. Most cells will die at this stage, but rare cells will survive and become immortalized because of the up-regulation or re-activation of telemerase activity which restores the telomere function and the stability of chromosome[10]. Thus, telomere length and activiation of telomerase closely correlate to life span of cells. Zhu x et al[11] demonstrated that the level of telomerase activity varies with different phases of cell cycle. In this study, we chose hTRT which could represent telomerase activity and markers around the G1/S check point of cell cycle such as P16, cyclinD1, P53 to reveal the interaction between hTRT gene and regulation of cell cycle and to investigate the role of these indexes in the oncogenesis and development of gastric cancer by in situ hybridization method and immunohistochemistry technique.
MATERIALS AND METHODS
Tissue specimens
Tissue spencimens of gastric cancer were obtained from the first affiliated hospital of Anhui Medical University from October 1994 to October 1997. No patient had been treated with anti-neoplasm therapy before surgical removal. 53 patients (42 males, 11 feamles, from 23 to 73 years old, median age 55 years) were as follows: 22 cases of poorly differentiated adenocarcinoma, 26 cases of well differentiated adenocarcinoma (including 18 cases of tubular adenocarcinoma, 8 papillary adenocarcinoma), 5 cases of mucoid carcinoma (including 4 cases of mucinous adeocarcinoma, 1 case of signet-ring cell carcinoma). 53 cases of adjacent cancerous tissues were taken as controls. All specimens were fixed in 10% formalin, embedded in paraffin, cut in serial 4 μm sections and adhered to slides treated by poly-L-Lysine and 0.1% DEPC.
Reagents
hTRT ISH detection kit was purchased from Boster Biological Technology Ltd. The probe labeled by digoxin is made of three sequences of oligocleotide of hTRT: (1) 5’-AGTCAGGCTG GGCCT CAGAG AGCTG AGTAG GAAGG-3’; (2) 5’-GCATG TACGG CTGGA GGTCT GTCAA GGTAG AGACG-3’; (3) 5’-TGCAC ACCGT CTGGA GGCTG TTCAC CTGCA AATCC-3’.
Rabbit polyclonal antibodies against hTRT and P16INK4, monoclonal mouse antibodies against cyclinD1 and P53, and S-P immunohistochemical kit were purchased from Beijing Zhongshan Biological Technology LtD.
In situ hybridization
The specimens were deparaffinized and rehydrated through a graded series of ethanol, and endogenous peroxidase was blocked by using 3% hydrogen peroxide for 10 min. After washed with distilled water treated by 0.1% DEPC three times at 5 min each, the slides were digested with pepsin diluted by 3% citric acid at 37 °C for 15-20 min. 20 μl reagent of pre-hybridization was added to each slide at 37 °C for 2 hours, then 20 μl of probe was hybridized to each slide at 42 °C for 16-20 hours. After hybridization, each slide was washed with 2 × SSC twice at 37 °C for 20 min each, then again with 0.2 × SSC twice at 37 °C for 10 min each. Blocking reagent was dropped to the slides and incubated for 30 min, then the mouse anti-digoxin antibody labeled by biotin was added at 37 °C for 60 min. After washed with 0.5M PBS thrice at 2 min each, the slides were incubated with strept-avidin-biotin complex (SABC) for 20 min at 37 °C, then washed with 0.5M PBS four times at 5 min each. At last, chromogen DAB was added to visualize the reaction products of peroxidase, then the slides were counterstained for nuclei by haematoxylin stain. A negative control was prepared according to the above steps with the probe substituted by 2 × SSC, a positive control showed positive always in repeated experiments. The positive signals of hTRT mRNA expression were stains with brown-yellow color located in cytoplasm and/or nucleus. The percentage of positive cells was determined by 10 areas at high power fields ( × 400) and graded as follows: negative (-); mildly positive (+), the percentage of positive staining was less than 25%, moderately positive (++), the percentage of positive staining was less than 25%-50%; strongly positive (+++), the percentage of positive staining was more than 50%.
Immunohistochemistry
Immunochemical staining was performed by S-P method, anti-hTRT antibody was diluted into 1:100, anti-P16INK4 1:50, anti-cyclinD1 1:50. anti-P53 antibody was the reagent ready to use. A negative control was dyed according to the above method with the primary antibody substituted by animal serum. The positive standard of hTRT protein expression was stains with the brown-yellow color in cell plasma, P53 positive expression was stains in nucleus, the expression of P16 and cyclinD1 was stains in nucleus and(or) cytoplasm. A semi-quantitative evaluation was used to determine positive expression of positive cell by viewing 10 areas at high power field ( × 400)[12]: negative(-), cells were stained less than 10%; mildly positive (+), cells were stained in 11%-25%; moderately positive (++), cells were stained in 26%-50%; strongly positive (+++), cells were stained over 50%. And we regarded the last three grades as positive.
Statistical analyses
The data was copied down by Excel and analyzed by SPSS version 10.0. The χ2 test or Fisher’s exact test (n < 5) was used for statistical analysis, Spearman rank correlation was performed for correlation analysis.
RESULTS
Expression of hTRTmRNA and hTRT protein
The positive signals of hTRTmRNA were brownish-yellow stains located in cytoplasm and/or nucleus (Figure 1, Figure 2). There was barely hTRTmRNA expression in adjacent cancerous tissues, only a few positive cells in dysplasia and intestinal metaplasia of adjacent cancerous tissues. Positive expression of hTRTmRNA was detected in 42 of 53(79.2%) of GC, but only 8 of 53(15.1%) of adjacent cancerous tissues. The expression of hTRTmRNA of GC was significantly higher than those of the adjacent cancerous tissues (P < 0.01) (Table 1).
Figure 1.
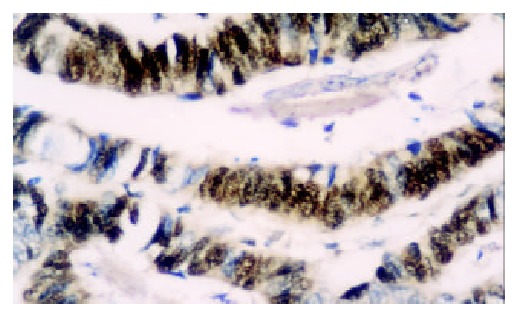
The expression of hTRTmRNA is strongly positive in tubular adenocarcinoma. ISH × 400.
Figure 2.
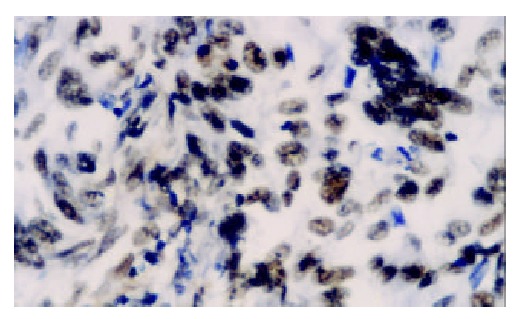
The expression of hTRTmRNA is moderately posi-tive in poorly differentiated adenocarcinoma. ISH × 400.
Table 1.
Expression of hTRTmRNA in histologic pattern of gastric cancer and adjacent cancerous tissue
| Groups | n |
hTRTmRNA |
Positive(%) | P | |||
| (-) | (+) | (++) | (+++) | ||||
| WD | 26 | 5 | 12 | 7 | 2 | 80.8 | 0.067a |
| PD | 22 | 3 | 11 | 8 | 0 | 83.4 | |
| Mucoid GC | 5 | 3 | 1 | 1 | 0 | 40.0 | |
| Adjacent | 53 | 45 | 6 | 2 | 0 | 15.1 | < 0.01b |
: comparison among groups of GC;
: comparison between adjacent cancerous tissue and GC. WD: well differentiated adenocarcinoma; PD: poorly differentiated adenocarcinoma.
The positive signals of hTRT protein were brownish-yellow stains located in cytoplasm and the strength of coloration was directly proportional to positive percentage (Figure 3, Figure 4). There was barely hTRT protein expression in adjacent cancerous tissues, but a few positive cells in dysplasia and intestinal metaplasia of adjacent cancerous tissues. Positive expression of hTRT protein was detected in 47 of 43(88.6%) of GC, but 13 of 53(24.5%) of adjacent cancerous tissues, so there was significant difference between GC and adjacent cancerous tissues (P < 0.01). There was statistical difference of the expression of hTRT protein among groups of different histologic patterns. Further comparison showed no significant difference between well differentiated adenocarcinama and poorly differentiated adenocarcinoma, but there was statistical difference between mucoid carcinoma and well differentiated adenocarcinoma, so was mucoid carcinoma and poorly differentiated adenocarcinoma (Table 2). And there was a highly significant positive correlation between the expression of hTRTmRNA and its protein (r = 0.625, P < 0.01).
Figure 3.
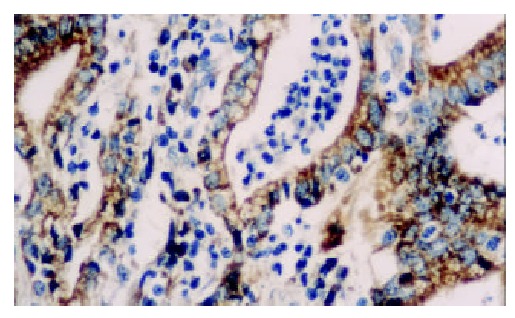
The expression of hTRT protein is strongly positive in papillary adenocarcinoma. S-P × 400.
Figure 4.
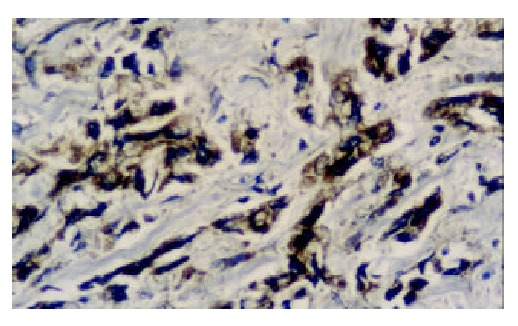
The expression of hTRT protein is moderately posi-tive in poor differentiated adenocarcinoma. S-P × 400.
Table 2.
Expression of hTRT protein in histologic pattern of gastric cancer and adjacent cancerous tissue
| Groups | n |
hTRTmRNA |
Positive(%) | P | |||
| (-) | (+) | (++) | (+++) | ||||
| WD | 26 | 1 | 6 | 15 | 4 | 96.2 | < 0.01a |
| PD | 22 | 2 | 9 | 10 | 1 | 90.9 | |
| Mucoid GC | 5 | 3 | 2 | 0 | 0 | 40.0 | |
| Adjacent | 53 | 40 | 11 | 2 | 0 | 24.5 | < 0.01b |
: comparison among groups of GC;
: comparison between adjacent cancerous tissue and GC.
We grouped the 53 cases of cancer patients who had complete clinical data as gender, age, tumor location and size, lymph node metastasis, invasion depth and clinical staging, and found that the expression of hTRTmRNA and its protein in GC was not related with clinicopathological parameters concerned above (Table 3).
Table 3.
Relationship between the expression of hTRTmRNA, hTRT protein and clinicopathological parameters in gastric cancer
| Parameters | n | hTRTmRNA positive (%) | P | hTRT protein positive(%) | P |
| Gender | |||||
| Male | 42 | 35 (83.3) | 0.309 | 39 (92.9) | 0.180 |
| Female | 11 | 7(63.6) | 8 (72.7) | ||
| Age(years) | |||||
| <55 | 25 | 18 (72.0) | 0.219 | 20 (80.0) | 0.147 |
| ≥ 55 | 28 | 24 (85.7) | 27 (96.4) | ||
| Tumor size(cm) | |||||
| < 5 | 24 | 20 (83.3) | 0.735 | 22 (91.7) | 0.678 |
| ≥ 5 | 29 | 22 (75.9) | 25 (86.2) | ||
| Tumor location | |||||
| Cardia | 26 | 23 (88.5) | 0.128 | 24 (92.3) | 0.529 |
| Body | 16 | 10 (62.5) | 13 (81.3) | ||
| Antrum | 11 | 9 (81.8) | 10 (90.9) | ||
| Lymph node metastasis | |||||
| Absent | 25 | 18 (72.0) | 0.219 | 20 (80.0) | 0.089 |
| Present | 28 | 24 (85.7) | 27 (96.4) | ||
| Invasion depth | |||||
| Not invading serosa | 8 | 7(87.5) | 0.879 | 7 (87.5) | 1.000 |
| Invading serosa | 45 | 35 (77.8) | 40 (88.9) | ||
| Clinical stage | |||||
| I and II | 47 | 37 (78.7) | 1.000 | 41 (87.2) | 0.806 |
| III and IV | 6 | 5 (83.3) | 6 (100.0) |
Correlation between hTRTmRNA, hTRT protein and cell cycle regulators
The positive percentage of P16INK4 expression was 60.3% (32/53) of GC, but 88.6%(47/53) of the adjacent cancerous tissues; the positive percentage of cyclinD1 overexpression was 69.8%(37/53) of GC, but 5.7%(3/53) of adjacent cancerous tissues; the positive expression of P53 was 32 of 53 (60.4%) of GC, but none of adjacent tissues. We analyzed relationship between hTRTmRNA, hTRT protein and cell cycle regulators through Spearman rank correlation. There was a significant positive correlation between the expression of hTRT mRNA and cyclinD1 protein (r = 0.350, P < 0.01), and a significant positive correlation between the expression of cyclinD1 protein and hTRT protein (r = 0.549, P < 0.01), so was P53 and hTRT protein (r = 0.319, P < 0.05). The expression of P16INK4 didn’t correlate with hTRTmRNA and hTRT protein, but the tumor tissues of the group with negative P16INK4 in combination with cyclinD1 overexpression had the strongest positive stains of hTRTmRNA, and the hTRTmRNA overexpression of this group was 79%(10/13). However, the hTRTmRNA overexpression of the group with positive P16INK4 in combination with low-expression of cyclinD1 was 37.5%(3/8), but there was no significant difference between the two groups (P > 0.05).
DISCUSSION
Current studies have proposed that activation of telomerase is a critical step in tumorigenesis of gastric cancer. Jong et al[6] observed telomerase activity in 25 of 27 primary GC and found the up-regulation of hTRTmRNA expression in 26 of 26 GC tissues by RT-PCR analysis. Hiyama et al[13] had detected telomerase activity in 66 samples of primary GC and found that the positive percentage was 85%, but 6% in the adjacent cancerous tissues. In our study, we detected the expression of hTRT gene in 53 GC specimens by ISH and immunohistochemistry, and found that positive percentage of hTRTmRNA and hTRT protein was 79.2% and 88.6% respectively in GC, and was 15.1% and 24.5% in the adjacent cancerous tissues. Meanwhile, the index of hTRT could be located for observation and determined semiquantitatively. So it provided an important morphological marker for detection of GC. It is reported that telomerase activity is associated with histological differentiation and malignant grade of tumor[14,15]. However, telomerase activity doesn’t correlate with age, gender, tumor stage and histological differentiation of gastric cancer[16-19]. In our present study of GC, the expression of hTRTmRNA and hTRT protein was not associated with clinicopathological parameters including gender, age, tumor size, location, lymph node metastasis, invasion depth and clinical staging. There was statistical difference of hTRT protein only between mucoid carcinoma and well differentiated adenocarcinoma, poorly differentiated adenocarcinoma, but our cases of mucoid carcinoma were few. Therefore, we considered that the expression of hTRT gene might be associated with gastric carcinogenesis, but not involved in differentiation and biological property of the tumor. Mild expression of hTRTmRNA and hTRT protein in dysplasia and intestinal metaplasia of adjacent cancerous tissues showed that the expression of hTRT gene might be presented in precancerous lesions or diseases, and the positive cells may be precancerous cells, but it was needed to do further studies to demonstrate the precise significance of this phenomenon.
There are several checkpoints of different function during cell cycle including G1/S checkpoint, S checkpoint and G2/M checkpoint. G1/S checkpoint that is the PRb pathway is especially important to ensure completion of cell cycle events on schedule and to prevent abnormal cells from proliferation to carcinogenesis[20]. At the end of G1 stage, cyclinD1 combined with CDK4/6 is responsible for the phosphorylation of PRB to release an important transcription factor -E2F, which initiates DNA synthesis and drives the cells through G1/S checkpoint. There are two vital inhibitory pathways of G1/S checkpoint, one is that P16INK4 competitively combines with CDK4 against cyclinD1 to suppress CDK4 activity[21,22], the other is that P53 initiates P21 gene to express P21 protein combined with cyclinD1/CDK4 and to inhibit the activity of cyclinD1/CDK4 when DNA is damaged[23-29]. The two pathways both abolish phosphorylation of PRb, and cell cycle arrests at G1 phase. It is reported at the M1/M2 model of telomere-telomerase hypothesis that a cell can’t obtain immortality until it passes through M1/M2stage. When the activity of tumor suppressor proteins such as P53, PRb, P16INK4 are absent, the cells can prolong their life by passing through M1 stage, thus there will provide more chances of activating telomerase for those cells to pass through M2 and obtain immortality[30-33].
There were a few studies about relationship between P16INK4 and telomerase. In the studies of human keratinocytes and mesothelial cells, Dickson et al[34] proposed that the expression of hTRT and loss of P16INK4 were two prerequisites for cell immortalization and carcinogenesis. Landberg et al[35] reported that though down-regulation of P16INK4 was not related to telomerase activity, tumors with low P16INK4 had demonstrated to have high activity of telomerase; down-regulation of P16INK4 alone was insufficient to activation of telomerase, and it was necessary in combination with other cell cycle defects such as over-expression of cyclinD1 or cyclinE. In our study of GC, the expression of P16INK4 was not associated with hTRTmRNA and hTRT protein, but there were two strong positive cases of hTRTmRNA which were absence of P16INK4 expression. So it was coordinate with studies in breast cancer of Landberg. CyclinD1 and P16INK4 locating in up-stream of PRb regulated phosp horylation of PRb and comb ined with CDK competitively, and the function of the two regulators was just contrary. During the progress of carcinogenesis, the abnomal expression of the two was in cooperation. Absence of P16INK4 and overexpression of cyclinD1 always occured at the same time in studies of telomerase. In the investigation of development of UVB-induced tumors in SKH-1 hairless mice, Balasubramanian et al[36] found that telomerase activity increased persistently after exposure to UVB, meanwhile, the expression of cyclinD1 and cyclinE was up-regulated when combined with CDK4, CDK2, and the expression of P16INK4, P21WAF1 and P27KIP1 all changed significantly between tumor and normal epidermis. It was reported that the over expression of cyclinD1 protein was associated with high telomerase level in the studies of breast cancer, and tumors with cyclinD1 or E overexpression in combination with low P16INK4 and normal PRb demonstrated high activity of telomerase[35]. In our study, there was positive correlation between cyclinD1 protein and hTRTmRNA, hTRT protein, the strongest expression of hTRTmRNA existed in the tumor sample which presented the absence of P16INK4 and overexpression of cyclinD1 at the same time. Our data demonstrated that cyclinD1 protein was highly correlated with the expression of hTRT gene. The mechanism may be that the overexpression of cyclinD1 protein accelerates the phosphorylation of PRb and elevates the proliferation rate of abnormal cells, then cell cycle loses regulation. Activated telomerase drives cells to pass through M2 stage to tumorigenesis.If in combination with inactivation of tumor suppressor genes such as the loss of P16INK4, it is especially helpful for activation of telomerase and quickening the pace of carcinogenesis. Therefore, the overexpression of cyclinD1 represents a cell cycle defect correlated with telomerase activity closely. Li et al[37] reported that telomerase activity was inhibited lately in human breast cancer cells after introducing recombinant human P53 to them, and this experiment indicated that activity of telomerase might be regulated by P53 in vivo and the down-regulation of P53 might increase the telomerase activity. In our study, the expression of P53 positively correlated with hTRT protein; the result demonstrated that P53 regulated the telomerase, and it prompted that when the pathway of P53-P21WAF1 was inactive, abnormal cells devoid of braking mechanism and passing through G1/S checkpoint to S stage at which telomerase activity increases giving cells the chance to pass through M2 and achieving immortality that produces specific tumor phenotype.
Our data shows that the expression of hTRT gene is correlated significantly to the specific defects of cell cycle in G1/S checkpoint, which proposes that telomerase activity may depend on cell cycle in gastric cancer and thus helps to clarify the molecular mechanism of telomerase activity regulation.
Footnotes
Surpported by Science and Technology Fund, Governmental Department of Education, Anhui Province, No.99j10091
Edited by Wu XN
References
- 1.Hiyama E, Kodama T, Shinbara K, Iwao T, Itoh M, Hiyama K, Shay JW, Matsuura Y, Yokoyama T. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res. 1997;57:326–331. [PubMed] [Google Scholar]
- 2.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 3.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 4.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 5.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]
- 6.Jong HS, Park YI, Kim S, Sohn JH, Kang SH, Song SH, Bang YJ, Kim NK. Up-regulation of human telomerase catalytic subunit during gastric carcinogenesis. Cancer. 1999;86:559–565. [PubMed] [Google Scholar]
- 7.Meyerson M. Telomerase enzyme activation and human cell immortalization. Toxicol Lett. 1998;102-103:41–45. doi: 10.1016/s0378-4274(98)00278-1. [DOI] [PubMed] [Google Scholar]
- 8.Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558–1561. [PubMed] [Google Scholar]
- 9.Vaziri H, Benchimol S. From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp Gerontol. 1996;31:295–301. doi: 10.1016/0531-5565(95)02025-x. [DOI] [PubMed] [Google Scholar]
- 10.Rogan EM, Bryan TM, Hukku B, Maclean K, Chang AC, Moy EL, Englezou A, Warneford SG, Dalla-Pozza L, Reddel RR. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Kumar R, Mandal M, Sharma N, Sharma HW, Dhingra U, Sokoloski JA, Hsiao R, Narayanan R. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc Natl Acad Sci USA. 1996;93:6091–6095. doi: 10.1073/pnas.93.12.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR. Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis. Hum Pathol. 1993;24:469–476. doi: 10.1016/0046-8177(93)90158-d. [DOI] [PubMed] [Google Scholar]
- 13.Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995;55:3258–3262. [PubMed] [Google Scholar]
- 14.Sharma HW, Sokoloski JA, Perez JR, Maltese JY, Sartorelli AC, Stein CA, Nichols G, Khaled Z, Telang NT, Narayanan R. Differentiation of immortal cells inhibits telomerase activity. Proc Natl Acad Sci USA. 1995;92:12343–12346. doi: 10.1073/pnas.92.26.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan X, Zhang B, Ying J, Jin Y, Hou L. [Expression of telomerase genes in human tumors] Zhonghua Binglixue Zazhi. 2000;29:16–19. [PubMed] [Google Scholar]
- 16.Ahn MJ, Noh YH, Lee YS, Lee JH, Chung TJ, Kim IS, Choi IY, Kim SH, Lee JS, Lee KH. Telomerase activity and its clinicopathological significance in gastric cancer. Eur J Cancer. 1997;33:1309–1313. doi: 10.1016/s0959-8049(97)00113-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang SM, Fang DC, Luo YH, Lu R, Yang JL, Liu WW. The Detec-tion of telomerase activity and subunit in different lesions of gas- tric mucosa. Aizheng. 2001;20:23–27. [Google Scholar]
- 18.Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol. 1999;5:316–319. doi: 10.3748/wjg.v5.i4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586–590. doi: 10.3748/wjg.v8.i4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clurman BE, Roberts JM. Cell cycle and cancer. J Natl Cancer Inst. 1995;87:1499–1501. doi: 10.1093/jnci/87.20.1499. [DOI] [PubMed] [Google Scholar]
- 21.Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, Johnson BE, Skolnick MH. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 22.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 23.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 24.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 25.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 26.Somasundaram K, Zhang H, Zeng YX, Houvras Y, Peng Y, Zhang H, Wu GS, Licht JD, Weber BL, El-Deiry WS. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Martindale JL, Gorospe M, Holbrook NJ. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 1996;56:31–35. [PubMed] [Google Scholar]
- 28.Marchetti A, Doglioni C, Barbareschi M, Buttitta F, Pellegrini S, Bertacca G, Chella A, Merlo G, Angeletti CA, Dalla Palma P, et al. p21 RNA and protein expression in non-small cell lung carcinomas: evidence of p53-independent expression and association with tumoral differentiation. Oncogene. 1996;12:1319–1324. [PubMed] [Google Scholar]
- 29.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 30.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 31.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 32.Velculescu VE, El-Deiry WS. Biological and clinical importance of the p53 tumor suppressor gene. Clin Chem. 1996;42:858–868. [PubMed] [Google Scholar]
- 33.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 34.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landberg G, Nielsen NH, Nilsson P, Emdin SO, Cajander J, Roos G. Telomerase activity is associated with cell cycle deregulation in human breast cancer. Cancer Res. 1997;57:549–554. [PubMed] [Google Scholar]
- 36.Balasubramanian S, Kim KH, Ahmad N, Mukhtar H. Activation of telomerase and its association with G1-phase of the cell cycle during UVB-induced skin tumorigenesis in SKH-1 hairless mouse. Oncogene. 1999;18:1297–1302. doi: 10.1038/sj.onc.1202417. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Cao Y, Berndt MC, Funder JW, Liu JP. Molecular interactions between telomerase and the tumor suppressor protein p53 in vitro. Oncogene. 1999;18:6785–6794. doi: 10.1038/sj.onc.1203061. [DOI] [PubMed] [Google Scholar]


