Abstract
Background:
Tocotrienols have been shown to improve glycemic control and redox balance in an animal study, but their effects on patients with diabetes are unknown. The study aimed to investigate whether tocotrienols improves glycemic control, insulin sensitivity, and oxidative stress in individuals with type 2 diabetes mellitus (T2DM).
Materials and Methods:
This study was a double-blinded, placebo-controlled, randomized trial. A total of 50 patients, aged 35-60 years, with T2DM treated by noninsulin hypoglycemic drugs were randomly assigned to receive either 15 mL/day tocotrienols (200 mg) enriched canola oil (n = 25) or pure canola oil (n = 25) for 8 weeks. Fasting blood sugar (FBS), fasting insulin, total antioxidant capacity (TAC), malondialdehyde (MDA), and homeostatic model assessment for insulin resistance (HOMA-IR) were determined before and after the intervention. The data were compared between and within groups, before and after the intervention.
Results:
Baseline characteristics of participants including age, sex, physical activity, disease duration, and type of drug consumption were not significantly different between the two groups. In tocotrienol enriched canola oil, FBS (mean percent change: –15.4% vs. 3.9%; P = 0.006) and MDA (median percent change: –35.6% vs. 16.3%; P = 0.003) were significantly reduced while TAC was significantly increased (median percent change: 21.4% vs. 2.3%; P = 0.001) compared to pure canola oil. At the end of the study, patients who treated with tocotrienols had lower FBS (P = 0.023) and MDA (P = 0.044) compared to the pure canola oil group. However, tocotrienols had no effect on insulin concentrations and HOMA-IR.
Conclusion:
Tocotrienols can improve FBS concentrations and modifies redox balance in T2DM patients with poor glycemic control and can be considered in combination with hypoglycemic drugs to better control of T2DM.
Keywords: Diabetes mellitus, hyperglycemia, oxidative stress, randomized controlled trial, tocotrienols
INTRODUCTION
Diabetes mellitus (DM), a complex and progressive disease, is associated with higher risk of cardiovascular disease and premature death.[1,2] The antioxidant system is challenged in patients with diabetes by increased oxidative stress due to hyperglycemia, hyperinsulinemia, and insulin resistance.[3] Oxidative stress has been implicated in the pathogenesis and complications of type 2 diabetes.[4,5,6] It has been proposed that antioxidants may improve insulin action and reduce the complications in DM.[7,8,9]
Vitamin E consists of two classes of biologically active substances named tocopherols and tocotrienols, and four vitamers in each class (α, β, γ and δ). Although all tocopherols and tocotrienols have a share structure which possesses antioxidant activity, each member has unique biological actions.[10] Tocotrienols are thought to be more efficient than the α-tocopherol in scavenging free radicals as a result of a better distribution in the fatty layer of cell membrane.[11,12] Some beneficial effects of tocotrienols that are often not exhibited by tocopherols, including hypocholesterolemic,[13] anti-cancer,[14] neuroprotective,[15,16] and anti-inflammatory properties[17] have been reported recently. However, tocotrienols have been less studied than tocopherols, and the most data were derived from in vitro or animal studies.[10] There are very limited data from human intervention studies, so the functional implications of tocotrienols properties are not yet fully determined.
In human studies, tocotrienol-rich fraction (TRF) derived from rice bran oil or palm oil were associated with reduced oxidative stress in healthy participants,[18,19] and improved lipid profile in hypercholesterolemic individuals[13] and patients with diabetes.[20] The synergistic effect of tocotrienols and lovastatin on lipid profile in hypercholesterolemic humans was also demonstrated.[21]
Although their purported properties suggest that tocotrienols could be useful to improve diabetes control and to prevent the development of its chronic complications, the possible benefits of tocotrienols administration as an adjuvant therapy for the treatment of DM, based on a randomized controlled trial, are limited and deserve further investigation.[20,22] We have therefore examined the effects of tocotrienols rich Vitamin E preparation from palm oil on fasting blood sugar (FBS), fasting insulin concentrations, the homeostatic model assessment for insulin resistance (HOMA-IR), total antioxidant capacity (TAC) and malondialdehyde (MDA) in individuals with type 2 DM (T2DM).
MATERIALS AND METHODS
Study design and participants
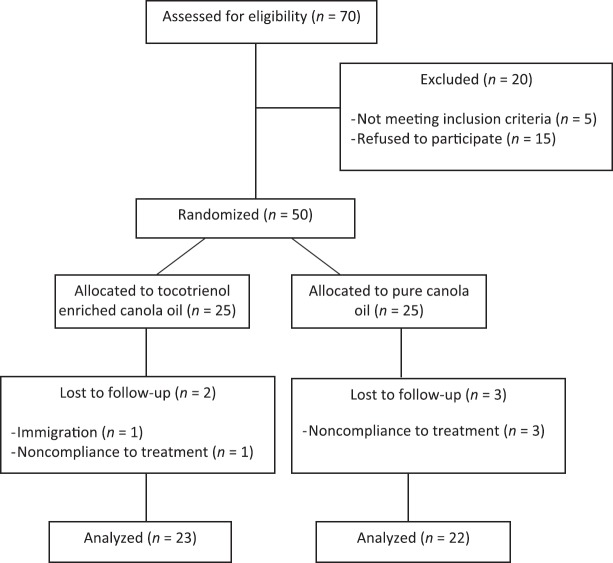
In this randomized, double-blind placebo-controlled trial, 50 patients with type 2 diabetes participated. The participants were recruited from the Endocrinology and Metabolism Center of Iran University of Medical Sciences between November 2011 and April 2012. Applicants could be included in the study if they were aged 35-60 years, had a body mass index (BMI) of <40 kg/m2, and diabetes had been diagnosed at least 1-year before the study based on the American Diabetes Association (FBS ≥126 mg/dL or 2 h-plasma glucose (2h-PG) ≥200 mg/dL or treated with hypoglycemic medications).[4] Pregnant or lactating women, those suffering from renal, liver, thyroid, cancer, inflammatory or infectious disease, treating with insulin, anti-inflammatory or anti-coagulant drugs, smoking, and taking alcohol were excluded from the study. No participants had taken any vitamin or mineral supplements for at least 1-month prior to the study. The objectives and protocol of the study were explained to the participants, who expressed their written informed consent to participate. Five patients, two in tocotrienols enriched canola oil group (one person due to immigration and the other due to changing in treatment protocol) and three in pure canola group (first one due to inability to walk to center, second due to unavailable enough blood serum and the third due to unwilling to end the study), withdrew the study [Figure 1]. No adverse events were reported during the study. To monitor adherence to intervention, a close supervision of all participants was carried out through personal contact weekly. Compliance, assessed by measuring the remaining volume of oil in the returned bottles, was more than 95%.
Figure 1.
Study participants flow chart
The study was approved by the Ethics Committee of the School of Public Health of Tehran University of Medical Sciences and was registered at the Iranian Registry of Clinical Trials (IRCT) as IRCT201008092365N2.
Randomization
Participants were divided into two groups randomly. Randomization was carried out by means of a random number table; for this, an independent coordinator created the randomization list assigning participants to the tocotrienols enriched canola oil or pure canola oil group. Two bottles of oil were provided to each participant for consuming during each 4-week period. Each bottle was returned at the end of 4-week duration. These identical bottles of tocotrienols enriched canola oil and pure canola oil were provided unlabeled and then an independent coordinator labeled these bottles with subject numbers (1-50) using the randomization list. Both the participants and the research team members were blinded to the treatment allocation.
Preparation of tocotrienol enriched canola oil
A palm-based mixture of Vitamin E tocotienol was prepared commercially (Vitrenol, P.T. MUSIM MAS Manufacturer, Indonesia) and it contained approximately 51% tocols (total tocotrienols and tocopherols). The mixture consisted of approximately 38.4% total tocotrienols (including 13.2% α-tocotrienols and 16.6% γ-tocotrienols) and 16% α-tocopherol. In the lab, 29,400 mg Vitrenol added to 810 g canola oil. Thus, a tablespoon of canola oil (15 mL) contained approximately 525 mg Vitrenol, 200 mg total tocotrienols, 69.3 mg α-tocotrienols and 87.15 mg γ-tocotrienols. Adding tocotrienols to canola oil did not change its appearance, taste or smell. Tocotrienols enriched canola oil and pure canola oil was placed into identical bottles.
Interventions
The intervention group received 15 mL/day of tocotrienols enriched canola oil (n = 25), containing 200 mg/day total tocotrienols, and control group received the same amount of pure canola oil for 8 weeks. Participants ingested 15 mL/day of the oil after lunch or dinner, as preferred, by adding a tablespoon of the oil to their cooked foods or salad. Since Vitamin E isomers are sensitive to heat and oxidation, they were requested to avoid using the oil in the cooking process. They were also asked to maintain their usual diet and physical activity throughout the study.
Biochemical evaluations and other measurements
Blood samples were collected before and after the intervention after 10-12 h overnight fast and before taking the hypoglycemic drugs. Blood Serum was obtained by centrifugation at 3000-4000 rpm for 10 min. Serum FBS was measured by the glucose-oxidase method (Pars Azmun kit, Iran) and insulin concentrations were measured by radioimmunoassay (Immunotech kit, France). HOMA-IR was calculated as fasting glucose (mg/dL) × fasting insulin (μIU/mL)/405.
TAC was determined using 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method[23] with minor modification. Bovin serum albumin (BSA), the Trolox equivalent antioxidant activity was used as a standard.[24,25] The assay is based on the generation of a stable blue-green color radical cation (ABTS•+) from a reaction of metmyoglobin and H2O2 producing a radical that interacts with the chromogen ABTS. When the colored ABTS•+ is combined with antioxidants, it is reduced to the colorless form of ABTS. Quenching of absorbance of this species at 734 nm by antioxidants was quantified and then compared to the one from BSA as a standard.[25] Serum MDA concentrations were determined by colorimetric method.[26]
Dietary intakes were monitored by 3-day 24 h food recall, including 2 weeks day and 1 weekend day, at entry and end of the study, and the daily nutrient intakes (energy, carbohydrate, protein, fat, Vitamin C and E, zinc and selenium) were determined using Nutritionist 4 software (N-squared Computing, San Bruno, CA, USA). Physical activity levels were assessed by the International Physical Activity Questionnaire at beginning and end of the study and expressed as low (<600 Met-min/week) and moderate (>600 Met-min/week) physical activity.[27] Body weight and height were measured before and after intervention and BMI was calculated as body weight (kg) divided by height squared (m2).
Statistical analyses
The number of participants estimated for each group was 21 at 80% power and α of 0.05 to detect a difference of 11 mg/dL in FBS concentrations between groups with an standard deviation (SD) of 12.8.[20] To allow for dropouts, it was decided to recruit 25 participants for each group. Analyses were based on the participants who completed the study.
The Kolmogorov-Smirnov test was applied to assess normality of data. Data for continuous variables with normal distribution are presented as mean ± SD while nonnormally distributed data are presented as medians and percentiles 25 and 75 (25th, 75th percentile). Normally distributed data within groups were compared using paired samples t-test and between groups by independent-samples t-test. Comparison of nonnormally distributed data was conducted using Wilcoxon signed ranks and the Mann-Whitney U-test. The percent change for variables was also calculated as (week 8 values − baseline values)/baseline values × 100. Categorical variables are presented as n (%) and compared between two groups by Chi-square test. We used the SPSS software (version 15.0; SPSS Inc., Chicago, IL, USA) for all the statistical analyses. A two-tailed P ≤ 0.05 was considered significant statistically.
RESULTS
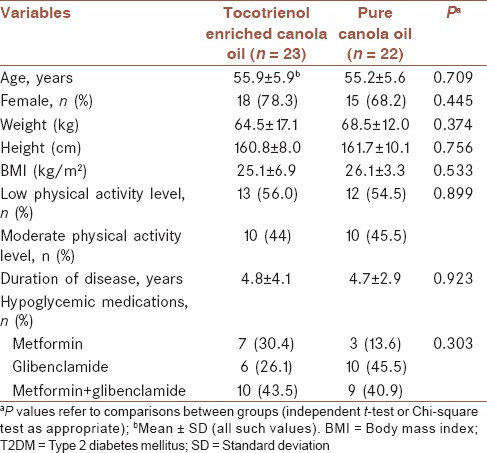
Baseline characteristics of the 45 participants who completed the study are shown in Table 1. There were no significant differences between the groups in regard of age, sex, weight, height, BMI, physical activity, disease duration, and type of drug consumption (P > 0.05). Likewise, no significant changes were observed for weight, BMI, and physical activity levels throughout the study (P > 0.05). The hypoglycemic agents used were metformin (n = 10) and glibenclamide alone (n = 16) or in combination (n = 19) from the beginning of the study, in doses that remained unchanged during the study (P = 0.303).
Table 1.
Baseline characteristics of patients with T2DM treated by tocotrienol enriched canola oil or pure canola oil
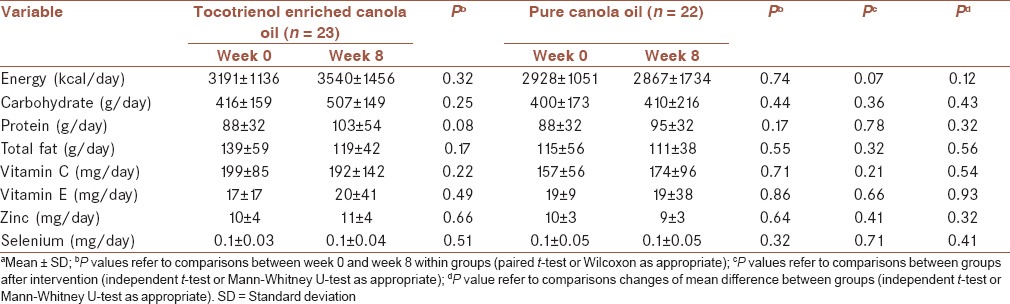
Dietary intake of energy, carbohydrate, protein, fat, Vitamin C and E, zinc and selenium, as determined by the 3-day food recall without considering 15 mL added canola oil, were not significantly different between the two groups before and after the intervention [Table 2].
Table 2.
Changes of dietary intakes of participants before and after the interventiona
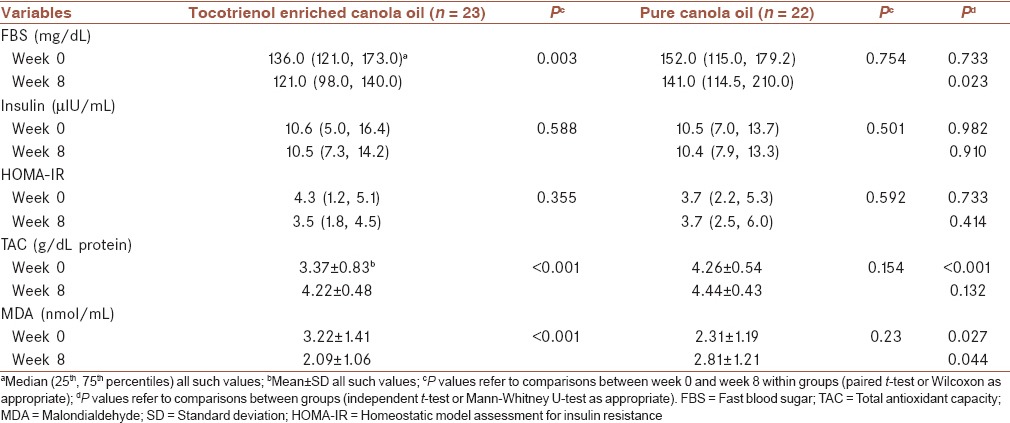
Effects of tocotrienols on glycemic control and oxidative stress are presented in Table 3. FBS, insulin and HOMA-IR were not different between the two groups at the study entry, while TAC was lower)3.37 ± 0.83 vs. 4.26 ± 0.54; P < 0.001) and MDA was higher (3.22 ± 1.41 vs. 2.31 ± 1.19; P < 0.001) in the tocotrienols enriched canola oil group, significantly. Tocotrienols enriched canola oil consumption resulted in significant reductions in FBS (P = 0.003) and MDA (P < 0.001) concentrations compared to baseline values. After 8-week, the FBS and MDA concentrations were significantly lower in tocoterienol enriched canola oil compared with pure canola oil group (P = 0.023 and P = 0.044, respectively). TAC concentration was increased in tocotrienols enriched canola oil compared with baseline values (4.22 ± 0.48 vs. 3.37 ± 0.83; P < 0.001,) but its concentration was not significantly different between two groups at the end of the study (4.22 ± 0.48 vs. 4.44 ± 0.43; P = 0.132). No significant effect was observed regarding insulin concentration and HOMA-IR.
Table 3.
Changes of FBS, insulin, HOMA-IR, TAC, MDA before and after 8 weeks of follow-up between tocotrienol enriched canola oil versus pure canola oil
Percent changes of variables in the two groups are shown in Figure 2. Mean/medians for percent changes during the study were significant in FBS (mean percent change: −15.4% vs. 3.9%; P = 0.006), MDA (median percent change: −35.6% vs. 16.3%; P = 0.003), and TAC (median percent change: 21.4% vs. 2.3%; P = 0.001) when tocotrienols enriched canola oil was compared with pure canola oil.
Figure 2.
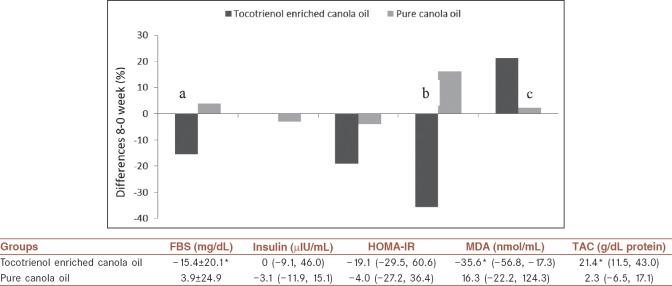
Differences in percent changes from baseline to 8 weeks in tocotrienol enriched canola oil (n = 23) and pure canola oil groups (n = 22). aP = 0.006, bP = 0.003, cP = 0.001 by independent t-test or Mann-Whitney U-test between two groups. Values are median (25th, 75th percentiles) except for FBS which is mean ± standard deviation. *Significantly different between groups (P < 0.05). HOMA-IR = Homeostatic model assessment for insulin resistance; TAC = Total antioxidant capacity; MDA = Malondialdehyde; SD = Standard deviation; FBS = Fast blood sugar
DISCUSSION
Diabetes is associated with substantial premature death from various diseases including cancer, vascular diseases, infectious diseases, and degenerative disease. Continuous associations between fasting glucose concentrations >100 mg/dL and risk of death suggest that hyperglycemia may be directly relevant to premature death in diabetes. These findings also reinforce the need to control and maintain FBS near to normal concentrations in DM.[2]
In our study, 525 mg/day tocotrienols rich Vitamin E, equivalent to 200 mg/day tocotrienols, for 8 weeks reduced FBS by 15.4% in individuals with T2DM; this reduction was also significant compared with the pure canola oil group at end of the study (P = 0.006). TRF supplementation in streptozotocin-induced diabetic (STZ-diabetic) rats caused an improvement in glycemic status by decreasing FBS concentrations and glycated hemoglobin (HbA1C).[28,29] In an earlier intervention study in human, 6 mg/kg TRF treatment, in two divided doses, for 60 days did not affect either fasting plasma glucose or HbA1C in patients with T2DM.[20] Differences in glucose concentrations at baseline in those participants compared with ours may cause the inconsistent findings. The glucose concentrations in those participants were close to normal (mean ± SD: 113.5 ± 11.8 mg/dL) and they were glycemically stable, but our participants had a higher mean glucose concentration of 153.8 ± 55.5 mg/dL. Therefore, it seems that the blood glucose concentration might be a determinant of response to the tocotrienols supplementation and the hypoglycemic effect of tocotrienols are more likely to be observed in individuals with high FBS concentrations or poor glycemic control. The mechanisms of hypoglycemic activity of tocotrienols have not yet fully understood. It has been suggested that tocotrienols (and not tocopherols) can improve insulin sensitivity through activating peroxisome proliferator-activated receptors (PPARs). Binding of tocotrieols to PPAR promotes insulin mediated glucose uptake through increasing the expression of glucose transporter 4.[30] In addition, previous studies indicate improved liver structure and function following Vitamin E[31] or TRF treatment.[32] Therefore, the reduction in FBS observed in our study can also be attributed to improved hepatocellular function and reduced release of glucose from the liver.
Concentrations of antioxidants are lower in diabetes because of excessive production of reactive oxidative specious and increased oxidative stress. A weakened antioxidant defense system, in turn, can intensify oxidative stress.[3] The present study demonstrated that TRF improved oxidative status in diabetes patients; since it significantly increased TAC and decreased lipid peroxidation. Although at baseline the tocotrienols enriched canola oil group appeared to be exposed to higher oxidative stress, as documented by lower TAC and higher MDA, redox status was improved at the end of the study to a larger extent than in the pure canola oil.
In animal studies, tocotrienols administration could increase TAC in hypertensive rats.[33] Furthermore, TRF enhanced the concentrations of Vitamin C and superoxide dismutase activity and prevented an increase in MDA and DNA damage in STZ-induced diabetic rats.[29] In a dose-response study in healthy individuals, the administration of 320 mg TRF was associated with a small significant increase (9.2%) in plasma TAC compared to baseline, in which was not significant compared with untreated group.[34] TRF supplementation in middle-aged and older adults at dose of 160 mg/day for 6 months attenuated serum advanced glycosylation end products and protein carbonyl content, the markers of protein oxidative damage, only in the individuals >50 years. MDA concentrations were also reduced in the >50 years old group after 3 months and remained low thereafter, but the change did not achieve statistical significance.[19] This age-dependent response to TRF supplementation might reflect that TRF supplementation in a population with the well-maintained antioxidant defense could not confer further improvement.
Tocotrienols may reduce the oxidative damage directly by exerting their antioxidant activity and as a consequence of improved counteracting the oxidative damage. In addition, the production of free radicals due to hyperglycemia might also be reduced by tocotrienols administration through a better control of glycemia. Reduced oxidative stress can improve insulin sensitivity, which in turn might decrease plasma triglyceride and free fatty acid through down-regulation of lipolysis. This down-regulation of lipolysis induced by the effect of insulin on adipose tissue might also lead to a decline in lipid peroxidation.[32,35]
Tocotrienols are thought to have greater antioxidant properties than α-tocopherol.[11,12] Indeed, tocotrienols have a unique conformation because of their three double bonds hydrocarbon tail which allows for better distributions in the fatty layers of the cell membrane.[36,37] Only pharmacological doses of α-tocopherol were associated with improving insulin action, inflammatory or oxidative markers.[31,35,38] The tocotrienols rich Vitamin E mixture in the present study contains 38.4% tocotrienols and 12.6% tocopherol. Therefore, the improved redox status in our study does not seem to be attributed to tocopherols. However, tocopherols might affect the responses to tocotrienols; as an example, it has been demonstrated that TRF preparations containing 20% or more α-tocopherol attenuate the hypocholesterolemic effect of γ-tocotrienols.[39]
In this study, we added tocotrienols to canola oil in order to improve its absorption and patient compliance. Because of the low content of tocotrienols in edible natural sources, it does not seem that the dietary sources can provide sufficient amounts of tocotrienols.[40] Thus, promoting intakes of tocotrienols through supplements appears prudent especially in patients with diabetes. Fortification of oil with this vitamin is an appropriate way to increase its intake.
Some limitations of our study should be mentioned. First, we did not measure blood levels of active components of the supplementation to confirm the compliance with treatment. Previous studies indicate that blood concentrations of tocotrienols cannot be detectable in the fasted state, even after supplementation with tocotrienols.[34,41] Since the blood samples were collected after 10-12 h of fasting in the present study, plasma tocotrienols concentrations were not an appropriate marker to confirm treatment compliance in our study. However, plasma tocopherol concentrations could be used instead of tocotrienols. Second, since we did not measured lipid profile in our study, we could not show the possible effect of tocotrienol on lipid metabolism in our study. Finally, not measuring any inflammatory markers and hepatic enzyme concentrations are the other limitations of our study. Therefore, we could not determine the possible mechanism of the effectiveness of tocotrienols in our study.
CONCLUSION
Administration of 525 mg tocotrienols rich Vitamin E or 200 mg total tocotrienols added to canola oil for 8 weeks were well-tolerated and improved blood glucose, antioxidant capacity, and oxidative stress in T2DM patients with poor control of glucose. The optimal doses and duration of TRF administration to show the most favorable effects on T2DM patients remain to be determined. Furthermore, studies with individual tocotrienols are needed since the pure components may lead to different responses as compared to the mixture. Investigations of the effects of tocotrienols on hepatocell function, hepatic enzyme concentrations, and inflammatory markers are also required to elucidate the mechanisms of tocotrienols actions.
Financial support and sponsorship
This study was supported by grants from Vice Chancellor of Research, Iran University of Medical Sciences, M/994 (Tehran, Iran).
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
All authors were involved in the concept and design of the study. MV and NH conducted the study and collected the data, and contributed to manuscript preparation. MV supervised the conduct of the study and data collection, provided advice and edited the manuscript. NM analyzed and interpreted the data, and wrote the manuscript. SEsupervised the conduct of the study. IH assisted with study design and conduct of the study.
Acknowledgments
This study was supported by grants from Vice Chancellor of Research, Iran University of Medical Sciences, M/994 (Tehran, Iran).
REFERENCES
- 1.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–6. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration. Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 4.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease?. The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 6.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Paolisso G, D’Amore A, Giugliano D, Ceriello A, Varricchio M, D’Onofrio F. Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. Am J Clin Nutr. 1993;57:650–6. doi: 10.1093/ajcn/57.5.650. [DOI] [PubMed] [Google Scholar]
- 8.Evans M, Anderson RA, Smith JC, Khan N, Graham JM, Thomas AW, et al. Effects of insulin lispro and chronic vitamin C therapy on postprandial lipaemia, oxidative stress and endothelial function in patients with type 2 diabetes mellitus. Eur J Clin Invest. 2003;33:231–8. doi: 10.1046/j.1365-2362.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- 9.Peerapatdit T, Likidlilid A, Patchanans N, Somkasetrin A. Antioxidant status and lipid peroxidation end products in patients of type 1 diabetes mellitus. J Med Assoc Thai. 2006;89(Suppl 5):S141–6. [PubMed] [Google Scholar]
- 10.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994;234:354–66. doi: 10.1016/0076-6879(94)34105-2. [DOI] [PubMed] [Google Scholar]
- 12.Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161:199–207. doi: 10.1016/s0021-9150(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 14.Miyazawa T, Shibata A, Sookwong P, Kawakami Y, Eitsuka T, Asai A, et al. Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol) J Nutr Biochem. 2009;20:79–86. doi: 10.1016/j.jnutbio.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Sen CK, Rink C, Khanna S. Palm oil-derived natural vitamin E alpha-tocotrienol in brain health and disease. J Am Coll Nutr. 2010;29(3 Suppl):314S–23. doi: 10.1080/07315724.2010.10719846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S, Parinandi NL, Kotha SR, Roy S, Rink C, Bibus D, et al. Nanomolar vitamin E alpha-tocotrienol inhibits glutamate-induced activation of phospholipase A2 and causes neuroprotection. J Neurochem. 2010;112:1249–60. doi: 10.1111/j.1471-4159.2009.06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids. 2009;44:787–97. doi: 10.1007/s11745-009-3326-2. [DOI] [PubMed] [Google Scholar]
- 18.Ladeia AM, Costa-Matos E, Barata-Passos R, Costa Guimarães A. A palm oil-rich diet may reduce serum lipids in healthy young individuals. Nutrition. 2008;24:11–5. doi: 10.1016/j.nut.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Chin SF, Ibahim J, Makpol S, Abdul Hamid NA, Abdul Latiff A, Zakaria Z, et al. Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutr Metab (Lond) 2011;8:42. doi: 10.1186/1743-7075-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baliarsingh S, Beg ZH, Ahmad J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis. 2005;182:367–74. doi: 10.1016/j.atherosclerosis.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AA, Sami SA, Salser WA, Khan FA. Synergistic effect of tocotrienol-rich fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J Nutr Biochem. 2001;12:318–29. doi: 10.1016/s0955-2863(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 22.Wan Nazaimoon WM, Sakinah O, Gapor A, Khalid BA. Effects of palm olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in non-insulin diabetes mellitus patients. Nutr Res. 1996;16:1901–11. [Google Scholar]
- 23.Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 24.Ramanathan B, Jan KY, Chen CH, Hour TC, Yu HJ, Pu YS. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455–60. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 25.Nikseresht S, Etebary S, Karimian M, Nabavizadeh F, Zarrindast MR, Sadeghipour HR. Acute administration of Zn, Mg, and thiamine improves postpartum depression conditions in mice. Arch Iran Med. 2012;15:306–11. [PubMed] [Google Scholar]
- 26.Farvid MS, Jalali M, Siassi F, Hosseini M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care. 2005;28:2458–64. doi: 10.2337/diacare.28.10.2458. [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Wan Nazaimoon WM, Khalid BA. Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays J Pathol. 2002;24:77–82. [PubMed] [Google Scholar]
- 29.Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 2009;64:235–44. doi: 10.1590/S1807-59322009000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang F, Kang Z, Wong C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol Nutr Food Res. 2010;54:345–52. doi: 10.1002/mnfr.200900119. [DOI] [PubMed] [Google Scholar]
- 31.Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR, et al. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004;27:2166–71. doi: 10.2337/diacare.27.9.2166. [DOI] [PubMed] [Google Scholar]
- 32.Wong WY, Poudyal H, Ward LC, Brown L. Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet-fed rats. Nutrients. 2012;4:1527–41. doi: 10.3390/nu4101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newaz MA, Nawal NN. Effect of gamma-tocotrienol on blood pressure, lipid peroxidation and total antioxidant status in spontaneously hypertensive rats (SHR) Clin Exp Hypertens. 1999;21:1297–313. doi: 10.3109/10641969909070850. [DOI] [PubMed] [Google Scholar]
- 34.Rasool AH, Yuen KH, Yusoff K, Wong AR, Rahman AR. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol (Tokyo) 2006;52:473–8. doi: 10.3177/jnsv.52.473. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo MR, Abbatecola AM, Barbieri M, Vietri MT, Cioffi M, Grella R, et al. Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: Impact on insulin action. J Am Coll Nutr. 2008;27:505–11. doi: 10.1080/07315724.2008.10719732. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, et al. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993;32:10692–9. doi: 10.1021/bi00091a020. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida Y, Niki E, Noguchi N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem Phys Lipids. 2003;123:63–75. doi: 10.1016/s0009-3084(02)00164-0. [DOI] [PubMed] [Google Scholar]
- 38.de Oliveira AM, Rondó PH, Luzia LA, D’Abronzo FH, Illison VK. The effects of lipoic acid and α-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Diabetes Res Clin Pract. 2011;92:253–60. doi: 10.1016/j.diabres.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi AA, Pearce BC, Nor RM, Gapor A, Peterson DM, Elson CE. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr. 1996;126:389–94. doi: 10.1093/jn/126.2.389. [DOI] [PubMed] [Google Scholar]
- 40.Sookwong P, Nakagawa K, Yamaguchi Y, Miyazawa T, Kato S, Kimura F, et al. Tocotrienol distribution in foods: Estimation of daily tocotrienol intake of Japanese population. J Agric Food Chem. 2010;58:3350–5. doi: 10.1021/jf903663k. [DOI] [PubMed] [Google Scholar]
- 41.Fairus S, Nor RM, Cheng HM, Sundram K. Postprandial metabolic fate of tocotrienol-rich vitamin E differs significantly from that of alpha-tocopherol. Am J Clin Nutr. 2006;84:835–42. doi: 10.1093/ajcn/84.4.835. [DOI] [PubMed] [Google Scholar]